Summary
Allergic asthma is caused by Th2 cell-type cytokines in response to allergen exposure. Type 2 innate lymphoid cells (ILC2s) are a newly identified subset of immune cells that besides Th2 cells contribute to the pathogenesis of asthma by producing copious amount of IL-5 and IL-13 which cause eosinophilia and airway hyperreactivity (AHR), a cardinal feature of asthma. ILC2s express ICOS, a T cell costimulatory molecule, with currently unknown function. Here we showed that lack of ICOS on murine ILC2s and blocking ICOS:ICOS-Ligand interaction in human ILC2s, reduced AHR and lung inflammation. ILC2s expressed both ICOS and ICOS-Ligand and the ICOS:ICOS-Ligand interaction promoted cytokine production and survival in ILC2s through STAT5 signaling. Thus, ICOS:ICOS-Ligand signaling pathway is critically involved in ILC2 function and homeostasis.
Introduction
Allergic asthma is a chronic inflammatory disease of the airways that is initiated by Th2 cell-type cytokines including IL-5 and IL-13. IL-5 plays an important role in the activation and recruitment of eosinophils to the airways and IL-13 increases goblet cell hyperplasia, mucus production and the sensitivity of airway smooth muscle cells to stimuli and leads to airway hyperreactivity (AHR), a cardinal feature of asthma. Besides Th2 cells, type 2 innate lymphoid cells (ILC2s) can rapidly produce large amounts of Th2 cytokines (Hazenberg and Spits, 2014; Walker et al., 2013) and therefore play an important role in the pathogenesis of asthma.
ILC2s have been discovered as the source of IL-5 and IL-13 production in alymphoid recombination activating gene (RAG) deficient mice, can be activated by IL-25, IL-33, Thymic stromal lymphopoietin (TSLP) and fungal allergens such as Alternaria, contribute to the pathogenesis of asthma and play a role in maintaining airway epithelial integrity through the production of amphiregulin (Barlow et al., 2012; Barlow et al., 2013; Bartemes et al., 2012; Bartemes et al., 2014; Chang et al., 2011; Fallon et al., 2006; Mjosberg et al., 2011; Monticelli et al., 2011; Moro et al., 2010; Neill et al., 2010; Teunissen et al., 2014). ILC2s lack the expression of the markers that are associated with previously identified immune lineages but express CD45, CD90, cytokine receptors IL2Rα, IL-7Rα, IL-33R, c-Kit (mouse) and CRTH2 and CD161 (human) (Barlow et al., 2012; Bartemes et al., 2012; Chang et al., 2011; Fallon et al., 2006; Mjosberg et al., 2011; Monticelli et al., 2011; Moro et al., 2010; Neill et al., 2010). ILC2s do not express RAG and unlike T or B cells act in a non-antigen specific manner, however they express Inducible T-cell COStimulator (ICOS) that is an important costimulatory molecule in T cells (Bartemes et al., 2012; Halim et al., 2014; Hutloff et al., 1999; McAdam et al., 2001).
ICOS is related to the CD28 superfamily and is highly expressed on activated T cells (Hutloff et al., 1999) as well as regulatory T cells and is crucial for the survival and function of T cells, Th2 cell differentiation and for lung inflammatory responses (Coyle et al., 2000; Dong et al., 2001; Gonzalo et al., 2001; McAdam et al., 2000; Nurieva et al., 2003). Binding ICOS to ICOS-Ligand (ICOS-L) activates a cascade of intracellular signaling molecules that prevent apoptosis and lead to the production of cytokines such as IL-4 and IL-13 (Coyle et al., 2000; Dong et al., 2001). To date, ICOS-L has been reported to be expressed by B cells, non-lymphoid and lung epithelial cells but not by T cells or innate lymphoid cells (Qian et al., 2006; Swallow et al., 1999; Watanabe et al., 2008) and is down-regulated upon binding to ICOS as an immunoregulatory mechanism (Watanabe et al., 2008). The functional requirement of ICOS for the function and survival of ILC2 remains to be elucidated.
In this study we evaluated the functional requirement for ICOS and its interaction with ICOS-L in cytokine production and homeostasis of murine and human ILC2s. We found, for the first time, that human and murine ILC2s express both ICOS and ICOS-L. Using gene deficient and humanized mice, we discovered that the ICOS:ICOS-L interaction is crucial for the function and homeostatic survival of ILC2s. We further showed that lack of the ICOS:ICOS-L interaction alters the activation of transcription factor STAT5. Our findings provide new insight into the mechanisms of induction of allergic asthma that could ultimately be used in new therapeutic approaches that target the ICOS:ICOS-L pathway in ILCs.
Results
ICOS deficient mice show reduced IL-33 induced AHR, inflammation and ILC2s
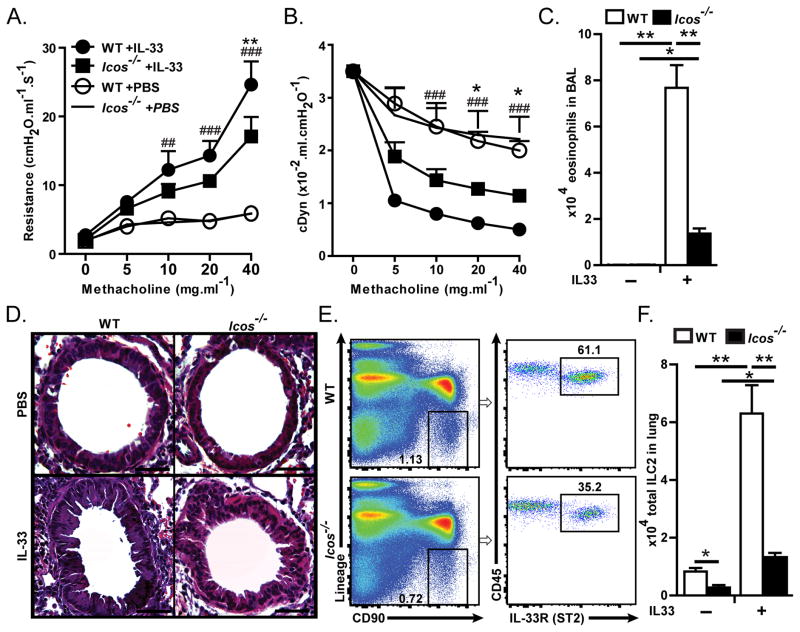
We first addressed whether the expression of ICOS is required for the function of ILC2s by comparing IL-33 induced AHR and airway inflammation in Icos−/− mice with wild type (WT) BALB/c mice. Mice received (intranasal) i.n. of either IL-33 (0.5 μg in 50 μl per mouse) or PBS (50 μl per mouse) on three consecutive days. One day after the last i.n. challenge, lung function was measured by direct measurement of lung resistance and dynamic compliance in anesthetized tracheostomized mice using the FinePointe RC system (Buxco Research Systems), followed by analyzing bronchial alveolar lavage (BAL) and taking lung tissue samples. As expected, i.n. administration of IL-33 significantly increased lung resistance in WT and Icos−/− mice (Figure 1A), however, lung resistance in IL-33-treated Icos−/− mice was significantly lower than IL-33-treated WT mice (Figure 1A), indicating that ICOS is required for IL-33-induced AHR. In agreement with lung resistance, results of dynamic compliance showed improved response in IL-33 treated Icos−/− compared to IL-33 treated WT mice while they showed significantly lower dynamic compliance compared to their PBS-treated counterparts (Figure 1B). IL-33 treatment significantly increased the number of eosinophils in the BAL of WT and Icos−/− mice, although the number of eosinophils in the BAL was reduced in IL-33 treated Icos−/− compared to WT mice, indicating that IL-33 induced inflammation is impaired in the absence of ICOS (Figure 1C–D).
Figure 1. Icos−/− mice exhibit reduced AHR, inflammation and number of ILC2s in response to intranasal IL-33.
BALB/cBYJ or Icos−/− mice were i.n. challenged with recombinant mouse IL-33 or PBS on days 1–3 followed by measurement of lung function and sample withdrawal on day 4. A) Lung resistance and B) dynamic compliance in response to increasing doses of inhaled methacholine. C) Total numbers of eosinophils in BAL, D) Lung histology, E) Flow cytometry analysis of lung ILC2s in PBS treated mice, as defined by lack of expression of lineage markers (CD3e, CD45R, Gr-1, CD11c, CD11b, Ter119, NK1.1, TCR-γδ and FCεRI) and expression of CD90, CD45 and ST2. Dot plots are gated on CD45+ single cells, G) Total number of ILC2s in the lungs of PBS and IL-33 treated WT and Icos−/− mice. Data are representative of 3 independent experiments and bar-graphs shown as mean ± SEM (n=5). (**: P<0.01 Icos−/−+IL33 compared to WT+IL33, *: P<0.05 Icos−/−+IL33 compared to WT+IL33, ###: P<0.005 WT+IL-33 compared to WT+PBS, ##: P<0.01 WT+IL-33 compared to WT+PBS).
IL-33 treatment significantly increased the total number of ILC2s in WT and in Icos−/− mice but the number of ILC2s was lower in Icos−/− mice compared to WT controls in PBS and in IL-33 treated groups (Figure 1E–F). We also quantified the frequency of ILC2s in the bone marrow, small intestine and colon and found that similar to the lungs, the frequency of ILC2s was significantly lower in the aforementioned organs in Icos−/− compared to WT mice (Supplementary figure 1). These results suggest that ICOS is important for the function and homeostasis of ILC2s.
Lack of ICOS increases cell death in ILC2s
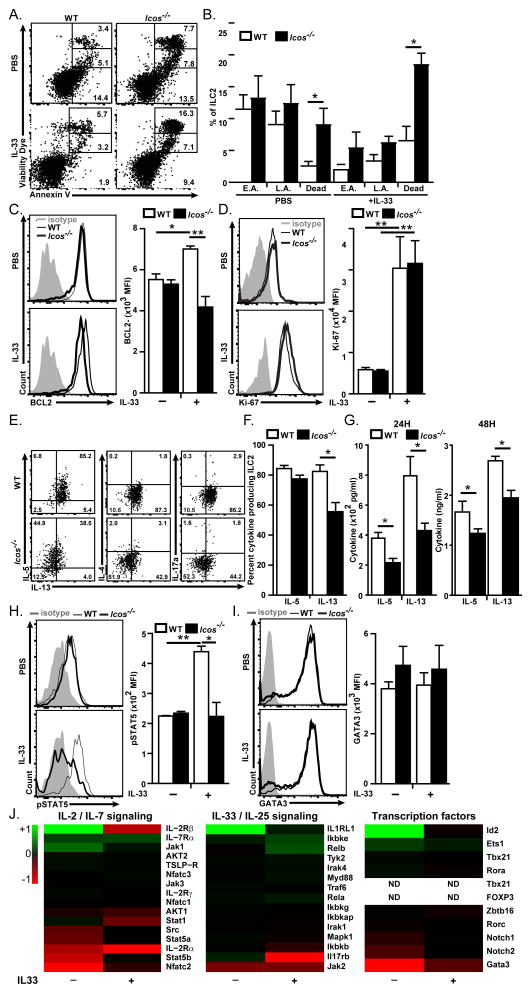
Since we found that the number of ILC2 is lower in Icos−/− mice, we investigated whether lack of ICOS affects the survival, proliferation or migration capacities of ILC2s. Icos−/− and WT mice were i.n. challenged with rm-IL-33 (0.5 μg/mouse) and after 24 hours ILC2s were stained with dead cell discrimination dye, and Annexin V for analyzing cell death and apoptosis. The number of dead cells was significantly increased in PBS-treated Icos−/− mice compare to PBS-treated WT mice (Figure 2A–B). Similarly, the number of dead ILC2s after IL-33-administration was higher in Icos−/− than in WT mice (Figure 2A–B) whereas, the number of early apoptotic and late apoptotic ILC2s was similar in both strains with the same treatments. We further analyzed the expression of anti-apoptotic factor BCL-2 and found that upon stimulation by IL-33, the expression of BCL-2 in WT ILC2s was significantly increased (Figure 2C). Interestingly, the expression of BCL-2 was significantly lower in Icos−/− than in WT ILC2s (Figure 2C). To evaluate whether lack of ICOS affects the proliferation rate of ILC2s we examined the expression of Ki-67 in WT and Icos−/− ILC2s. There was no significant difference between the expression level of Ki-67 in Icos−/− and WT ILC2s in PBS or IL-33 treated mice (Figure 2D). These data suggest that lack of ICOS impairs the survival of ILC2s rather than their proliferation.
Figure 2. Lack of ICOS increases cell death and impairs cytokine production in ILC2s.
BALB/cBYJ and Icos−/− mice received i.n. IL-33 or PBS and after 24 hours lungs were either immediately analyzed for apoptosis, cell death and proliferation or cultured in the presence of IL-33 (20 ng/ml) and Brefeldin A for 6 hours followed by intracellular cytokine analysis. ILC2s were gated based on lineage-, CD45+, CD90.2+, ST2+ and CD25+. A) Dead cell discrimination dye (DCD) and Annexin V staining of ILC2s of WT and Icos−/− mice. EA: early apoptotic (Annexin V+, DCD−), LA: late apoptotic (Annexin V+, DCDint) and dead cells (DCDhi). Numbers show the percentage of the gate, B) The frequency of E.A., L.A. and dead cells within ILC2s, C) Histogram (left panel) and Median fluorescence intensity (right panel) of BCL-2 in ILC2, D) Histogram (left panel) and Median fluorescence intensity (right panel) of Ki-67 in ILC2 as an indication of proliferation, E) Production of IL-5, IL-13, IL-4 and IL-17A in ILC2 of WT and Icos−/− mice. F) Bar graphs presentation of the percent of cytokine producing cells within ILC2s gate, G) ILC2 were purified by FACS and cultured (104 cells/100 μl) in the presence of rm-IL-33 (20 ng/ml), rm-IL-2 (10 ng/ml) and rm-IL-7 (10 ng/ml) for 24 and 48 H. The level of IL-5 and IL-13 produced by purified lung ILC2 as measured by ELISA. Data are representative of at least 3 independent experiments and shown as mean ± SEM (n=5, **: P<0.01, *: P<0.05). H) Histogram (left panel) and median fluorescence intensity (right panel) of phosphorylated STAT5in ILC2s. I) Histogram (left panel) and median fluorescence intensity (right panel) of GATA3 expression in ILC2s. J) Heat plot demonstration of modulation of the depicted genes in FACS purified Icos−/− ILC2s compared to WT ILC2s in steady state and after 3 × i.n. IL-33 administration quantified by Nanostring nCounter technology. Data are ranged −1 to +1 for the max reduced and max increased gene expression respectively.
Since reduced number of ILC2s in the absence of ICOS may be caused by altered migratory behavior of ILC2s we analyzed the expression of chemokine receptors, CCR3, CCR4, CXCR4, CCR6 and CCR7 and adhesion molecules ICAM-I, ICAM-II and Integrin β7 in Icos−/− and WT ILC2s in steady state and after IL-33 stimulation (Supplementary Figure 2A). We found that in steady state Icos−/− ILC2s seem to express higher level of ICAM-II and modestly lower level of CXCR4 than WT ILC2s. After IL-33 stimulation Icos−/− ILC2s show a modest increase in Integrin β7 and in CCR7 expression (Supplementary Figure 2A).
Lack of ICOS impairs cytokine production by ILC2s
We next examined whether ICOS is required for the functional production of cytokines by ILC2s by intracellular measurement of cytokines 24 hours after i.n. rm-IL33 administration and by measurement of cytokines in the supernatant of in vitro cultures of purified ILC2s for 24 and 48 hours. For intracellular staining fixable dead cell discrimination dye was used to assess the cytokine production specifically in live ILC2s. Intracellular cytokine staining data show that production of IL-13 by ILC2s was lower in Icos−/− than WT mice, whereas there was no difference in the production of IL-5 between Icos−/− and WT mice (Figure 2E–F). We did not detect significant production of IL-4 or IL-17a by ILC2s. Interestingly, we observed that the amount of IL-5 and IL-13 in the supernatant of in vitro cultured purified ILC2s was significantly lower in Icos−/− ILC2s than WT ILC2s after 24 and 48 hours of culture (Figure 2G). Taken together, these data suggest that while production of IL-13 is directly affected by lack of ICOS, IL-5 production in Icos−/− is also ultimately lower due to the lower number of viable ILC2s in these mice.
Since we found that survival of ILC2s is reduced in the absence of ICOS we next investigated whether the expression of the receptors that might mediate the survival of ILC2s are altered in Icos−/− mice. We evaluated the expression of CD25, CD127, ST2 and CD117 in ILC2s in Icos−/− and WT mice in the steady state (PBS treated) and after stimulation with IL-33. To confirm the phenotype of Icos−/− mice, the level of ICOS was also evaluated in both strains. Although there was no significant difference between the expression of CD127, ST2 and CD117, the expression of CD25 was increased in Icos−/− mice suggesting an altered sensitivity to IL-2 in the absence of ICOS (Supplementary figure 2B).
Lack of ICOS impairs STAT5 activation
Since we observed that the level of IL-2Rα is increased in ILC2s from Icos−/− mice, we compared the sensitivity of wild type and Icos−/− ILC2s to IL-2 by testing the phosphorylation of STAT5 in response to IL-2 stimulation in ILC2s. Icos−/− and WT mice were challenged i.n. by either rm-IL-33 (0.5 μg/mouse) or PBS. After 24h lung single cells were stimulated with recombinant murine IL2 (100ng/ml) for 30 minutes then ILC2s were analyzed for the expression of phosphoSTAT5. The amount of phosphoSTAT5 in ILC2s from PBS treated WT and Icos−/− mice was similar, IL-33 treated Icos−/− ILC2s show a significantly lower amount of phosphoSTAT5 compared to WT mice (Figure 2H). These results suggest a reduced sensitivity to IL-2 signaling despite higher expression of IL-2Rα in Icos−/− ILC2s.
Next we evaluated the expression of the transcription factor GATA binding protein-3 (GATA-3), which has been associated with development and maintenance of ILC2s (Hoyler et al., 2012; Klein Wolterink et al., 2013). We administered either rm-IL-33 (0.5 μg/mouse) or PBS i.n. to Icos−/− or WT mice and analyzed the expression of GATA-3 in ILC2s after 24 hours. There was no difference in the expression of GATA-3 in ILC2s between Icos−/− and WT in either IL-33 or PBS treatments (Figure 2I). These results suggest that the GATA-3 pathway in ILC2s is not affected by the lack of ICOS.
To further investigate the mechanism of reduced cell survival and impaired cytokine production in Icos−/− ILC2s we analyzed the gene expression profile of naïve and IL-33 stimulated WT and Icos−/− ILC2s using Nanostring® technology as described in experimental procedure. Nanostring nCounter allows direct measurement of the abundance of transcripts (Schmitt et al., 2014). The evaluated genes are categorized and shown in 3 panels. I) genes that are mainly involved in IL-2/IL-7 signaling pathway, II) genes that are involved in IL-33/IL-25 signaling pathway and III) transcription factors (Figure 2J). As demonstrated in IL-2/IL-7 signaling pathway panel, the expression of STAT5b, STAT5a, NFATc2 was reduced in naïve Icos−/− ILC2s while this reduction is less pronounced after IL-33 stimulation. Given the implication of STAT5 and NFATc2 for cell survival and that we found phosphorylation of STAT5 is reduced in the absence of ICOS this finding may explain the reduced number and higher rate of death in Icos−/− ILC2s in steady state. The expression of IL-2Rα gene seems to be reduced in steady state and after IL-33 stimulation in Icos−/− ILC2s while we found the increased expression by flow cytometry. This seemingly conflicting findings may suggest a difference in translation rate of IL-2Rα between Icos−/− and WT ILC2s since protein amount is measured by flow cytometry whereas Nanostring analyzes gene expression. The expression of IL-2Rβ seems to be increased in steady state in Icos−/− ILC2. The most notable differences in IL-33/IL-25 signaling were increased IL-33R in steady state and decreased IL-17RB receptor gene expression and a modest decrease in JAK2 signaling in naïve and IL-33 stimulated states (Figure 2J, middle panel). In the transcription factor panel, gene expression of GATA-3 seemed to be increased in Icos−/− ILC2 while Id2 expression was decreased in steady state. However, we did not find a significant difference in GATA-3 protein level by flow cytometry. Thus, lack of ICOS affects mainly IL-2 signaling pathway.
Adoptively transferred Icos−/− ILC2s fail to induce AHR in alymphatic hosts
To confirm our findings and to eliminate the effect of bystander cells including T cells subsets which can express IL-33 receptor (Kim et al., 2012; Lohning et al., 1998), we first transferred purified ILC2s (1.5 × 104/mouse) from Icos−/− or WT mice into Rag2−/−Il2rg−/− mice followed by i.n. administration of either IL-33 (0.5 μg/mouse) or PBS on 3 consecutive days and evaluation of lung function and inflammation a day after the last i.n. treatment (Supplementary figure 3A). I.n. IL-33 administration leads to significantly increased lung resistance and inflammation and decreased lung compliance in the recipients of WT ILC2s but not in the recipients of Icos−/− ILC2s (Supplementary Figure 3B–D). Lung resistance and dynamic compliance are significantly lower in IL-33 treated recipients of Icos−/− ILC2s compare to recipients of WT-ILC2s (Supplementary Figure 3B–D). The number of ILC2s in the lungs of recipients of Icos−/− ILC2s was significantly lower than the recipients of WT ILC2s (Supplementary Figure 3E).
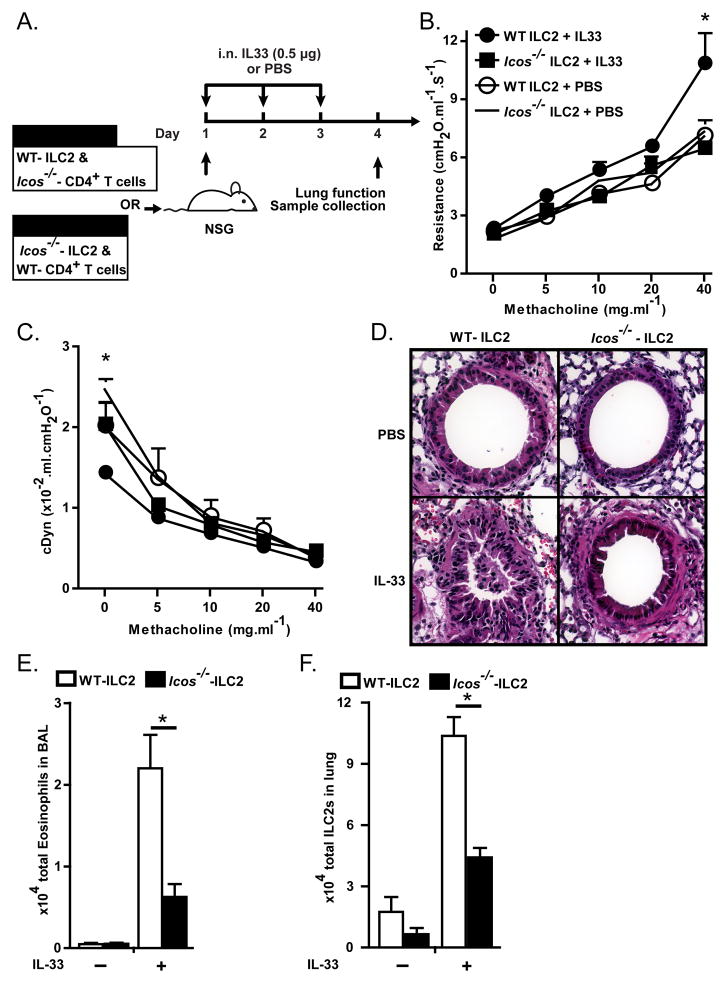
To exclude the effects of host and further address the role of bystander T cells in the observed phenotype of Icos−/− ILC2s, we transferred either purified WT ILC2s (WT ILC2 group, 1.5 × 104/mouse) combined with Icos−/− CD4+ T cells (3 × 106 cells/mouse) or Icos−/− ILC2s (Icos−/− group, 1.5 × 104/mouse) combined with WT CD4+ T cells (3 × 106 cells/mouse) into alymphoid mice. Similar to the aforementioned experiment upon adoptive cell transfer, mice received 3 i.n. administration of IL-33 on consecutive days followed by dissection one day after the last i.n. treatment (Figure 3A). As expected IL-33 treatment lead to a significant increase in lung resistance, inflammation and eosinophil count in BAL of WT ILC2 recipient group but not in Icos−/− ILC2 recipient group (Figure 3B, D–E). Lung dynamic compliance shows a significant decline in basal level after IL-33 administration with no further decrease in WT ILC2 recipient group (Figure 3C). The number of ILC2s in the lungs and all other measured parameters was significantly lower in Icos−/− ILC2s recipients compared to WT ILC2 recipient group after i.n. IL-33 administration (Figure 3B–F). Taken together, these data indicate that expression of ICOS by ILC2s is required for the function and survival of ILC2s in vivo.
Figure 3. Icos−/− ILC2s fail to induce AHR and lung inflammation.
A) ILC2s and splenic CD4+ T cells were purified from BALB/cBYJ and Icos−/− mice using FACS. WT ILC2s (1.5 × 104 cells/mouse) combined with splenic Icos−/− CD4+ T cells (3 × 106 cells/mouse) or Icos−/− ILC2s combined WT CD4+ T cells were injected into NSG mice intravenously followed by 3 intranasal challenges with rm-IL-33 or PBS on 3 consecutive days. One day after the last challenge lung function was measured and samples were collected. B) Lung resistance and C) dynamic compliance. D) Lung histology after PBS or IL-33 treatment. E) Total eosinophils in bronchoalveolar lavage in PBS or IL-33 treated mice, F) Total ILC2s in lungs after PBS or IL-33 treatment. Data are representative of 2 independent experiments and shown as mean ± SEM (n=3–5, *: P<0.05).
Blocking ICOS in RAG2−/− mice hinders IL-33-induced AHR and lung inflammation
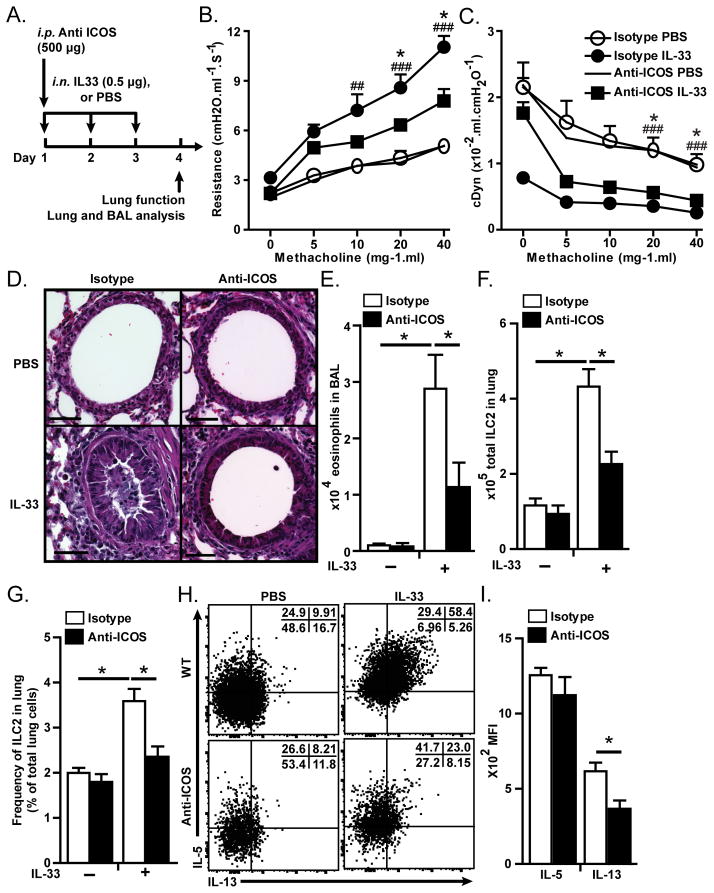
Next we examined whether blocking ICOS-ICOS-L binding leads to the same results as we observed in Icos−/− mice. We examined the effects of anti-ICOS blocking antibody on IL-33 induced AHR and lung inflammation in Rag2−/− mice that lack mature B and T cells. Rag2−/− mice received either anti-ICOS (500 μg/mouse, clone: 7E.17G9) or Rat IgG2b (isotype matched control) intraperitoneally on day 1 and each group also received i.n. either IL-33 (0.5 μg/mouse) or PBS on day 1–3 followed by measurement of lung function and sample acquisition on day 4 (Figure 4A). Lung function data shows that IL-33 i.n. increases lung resistance (Figure 4B) and decreases dynamic compliance (Figure 4C) in WT mice. In contrast, lung resistance in IL-33 treated mice that received anti-ICOS was significantly lower and dynamic compliance higher than in IL-33 treated isotype control receiving mice (Figure 4B–C). Lung histology showed that IL-33 treatment led to thickening of epithelium and increased inflammatory cells in isotype control but not anti-ICOS treated mice (Figure 4D). Similarly, the number of eosinophils in BAL was significantly higher in IL-33 treated than in PBS treated mice, but significantly lower in anti-ICOS treated than isotype treated mice (Figure 4E). Anti-ICOS antibody significantly reduced the number and frequency of ILCs compared to isotype control (Figure 4F–G). We also analyzed the cytokine production in ILC2s by intracellular staining and found that anti-ICOS administration significantly reduced the production of IL-13 but not IL-5 than isotype control (Figure 4H–I). These results indicate that binding of ICOS to ICOS-L is required for efficient function in ILC2s.
Figure 4. Blocking ICOS inhibits AHR and lung inflammation in RAG2 deficient mice.
A) Rag2−/− mice received 500 μg/mouse anti-mouse ICOS blocking antibody or rat IgG2b (isotype control) on day 1 and received rm-IL-33 or PBS intranasally on day 1 to 3 followed by measurement of lung function, and analyses of bronchoalveolar lavage and lung histology on day 4. B) Lung resistance and C) dynamic compliance. D) Lung histology. E) Total numbers of eosinophils in bronchoalveolar lavage. F) Total number and G) frequency of ILC2s. H) Dot plot of intracellular cytokine production by lung ILC2s after 4 hour of culture in the presence of Brefeldin A. I) Median fluorescence intensity of intracellular IL-5 and IL-13 in lung ILC2s. Data are representative of at least three independent experiments and are presented as mean ± SEM (n=5)(*: P<0.05 anti-ICOS+IL33 compared to isotype+IL33, ###: P<0.005isotype+IL-33 compared to isotype+PBS, ##: P<0.01 isotype+IL-33 compared to isotype+PBS).
ICOS is required for the induction of allergen-induced AHR and lung inflammation
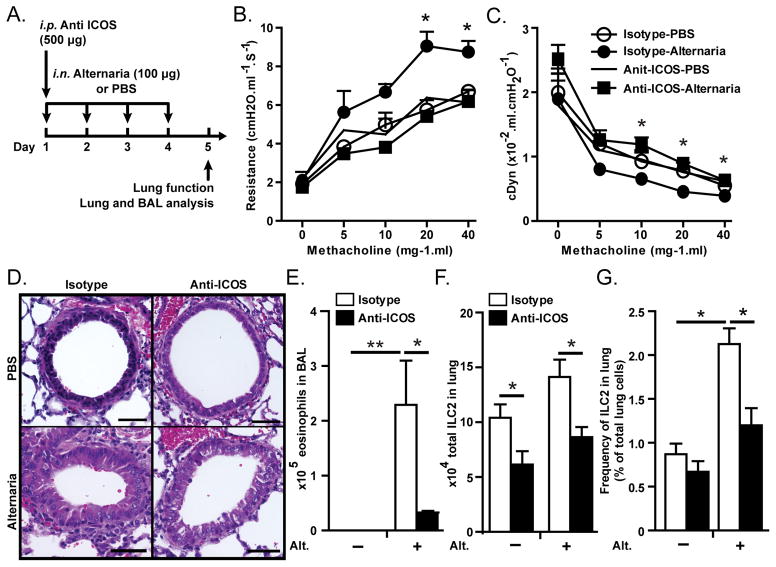
We next investigated whether ICOS on ILC2s is required for the induction of AHR and lung inflammation induced by a clinically relevant allergen. Rag2−/− mice received i.p. either anti-ICOS or Rat IgG2b on day 1 and i.n. extract of Alternaria alternata (100 μg/mouse) on day 1–4 followed by measurement of lung function and sample withdrawal on day 5 (Figure 5A). As shown in figure 5B–C administration of Alternaria induced AHR, as evident by increased lung resistance and decreased dynamic compliance, only in isotype treated but not anti-ICOS treated mice (P<0.05 at dose 40). Lung histology showed an increased in thickening of the epithelium and increased number of inflammatory cells in Alternaria-treated isotype receiving but not anti-ICOS receiving mice (Figure 5D). The number of eosinophils was increase in the BAL of Alternaria treated WT mice but it was lower in anti-ICOS treated than in isotype treated mice (Figure 5E). In addition, the total number of ILC2s was significantly lower in anti-ICOS receiving than in isotype receiving mice (Figure 5F). These results suggest that ICOS plays an important role in the function of ILC2 in response to clinically relevant allergens.
Figure 5. Blocking ICOS inhibits Alternaria-induced AHR and lung inflammation.
A) Rag2−/− mice received intraperitoneal injection of anti-mouse ICOS blocking antibody or rat IgG2b on day 1 and received extract of Alternaria alternata or PBS intranasally on day 1 to 4 followed by measurement of lung function, and BAL and lung histology on day 5. B) Lung resistance and C) dynamic compliance in response to increasing doses of methacholine. D) Lung histology, E) Total numbers of eosinophils in bronchoalveolar lavage. F) Total number of lung ILC2s. Data are representative of at least three independent experiments and are presented as mean ± SEM (n=4, **: P<0.01, *: P<0.05).
ILC2s express functional ICOS-L
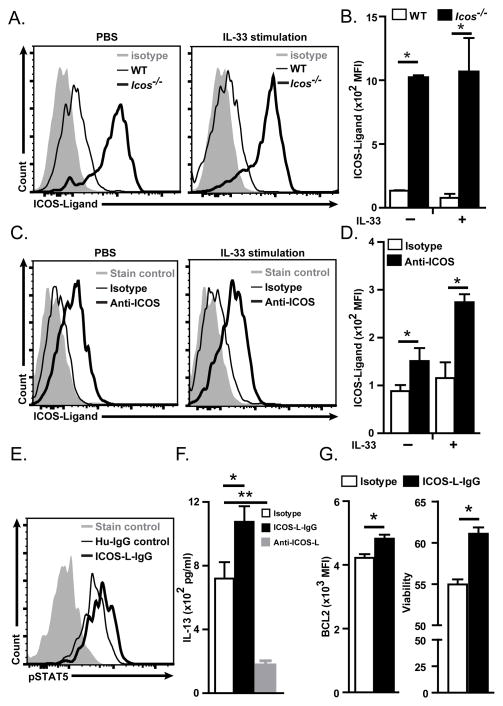
Next, we examined whether ILC2s express ICOS-L. We administered rm-IL33 (0.5 μg/mouse) or PBS i.n. to WT and Icos−/− mice and analyzed ILC2s for the expression of ICOS-L by flow cytometry after 24 hours. Whereas WT ILC2s expressed low amounts of ICOS-L, Icos−/− ILC2s expressed high amounts of ICOS-L in both PBS and IL-33 treated mice (Figure 6A–B). Since it has been shown that ICOS-L is down-regulated in APCs upon binding to ICOS (Watanabe et al., 2008), we hypothesized that ICOS-L is down-regulated in WT ILC2s upon binding to ICOS. To test our hypothesis we cultured ILC2s from PBS and IL-33 treated mice in the presence of anti-ICOS (10 μg/ml,) or Rat IgG2b (10 μg/ml) for 24 hours. We found that while a low level of ICOS-L expression could be detected in cultured ILC2s in the presence of isotype control, the expression of ICOS-L was significantly increased in cultured ILC2s in the presence of anti-ICOS antibody (Figure 6C–D).
Figure 6. Murine ILC2s express ICOS-L.
A) Histogram of expression of ICOS-L by ILC2s in BALB/cBYJ (thin line) and in Icos−/− (thick line) mice 24 hours after i.n. IL-33 or PBS administration. The level of isotype-matched stain control is shown as gray filled histogram. B) Median fluorescence intensity of ICOS-L in wild type and Icos−/− mice in i.n. IL-33 or PBS treated mice. C) Histogram of expression of ICOS-L by lung ILC2s in BALB/cBYJ mice 24 hours after administration of blocking anti-ICOS (thick line) or rat IgG2a (thin line) in PBS or IL-33 treated mice. D) Median fluorescence intensity of ICOS-L in lung ILC2s of BALB/cBYJ mice treated with blocking anti-ICOS (black bars) or rat IgG2a isotype control (white bars) antibody after PBS or IL-33 administration. E) Histogram of the level of phosphorylated STAT5 24 hours after in vitro culture of purified ILC2s in the present of plate-bound ICOS-L IgG or human-IgG as isotype control. F) Production of IL-13 by purified ILC2s (104/100 μl) after 24 hours culture in the present of plate-bound ICOS-L IgG or human-IgG and rm-IL-2 (20 ng/ml), rm-IL-7 (20 ng/ml) and rm-IL-33 (20 ng/ml) as measured by ELISA. G). Purified WT ILC2s were cultured in the absence of stimuli for 24 hours then cultured in the present of plate-bound ICOS-L IgG or human-IgG and rm-IL-2 (20 ng/ml), rm-IL-7 (20 ng/ml) and rm-IL-33 (20 ng/ml). Expression of BCL-2 (left panel) and viability (right panel) as measured by flow cytometry. Data are representative of at least three independent experiments and are presented as mean ± SEM (n=3–4, **: P<0.01, *: P<0.05).
To confirm the functionality of ICOS-L in ILC2s we evaluated phosphoSTAT5 and production of IL-13 by purified cultured ILC2s in the presence of plate bound mouse ICOS-L-IgG (5μg/ml) fusion protein or plate bound human-IgG (isotype control). The culture media was supplemented with rm-IL2 (100 ng/ml), rm-IL-33 (100 ng/ml) and IL-7 (20 ng/ml). PhosphoSTAT5 was increased in the presence of ICOS-L-IgG as compared to isotype control (Figure 6E). Moreover, purified ILC2s produce a significantly higher amount of IL-13 in the presence of ICOS-L-IgG than in the presence of isotype control while IL-13 production is dramatically reduced in the presence of anti-ICOS-L mAb (Figure 6F).
To address whether ICOS: ICOS-L interaction is cis or trans we co-cultured Icos−/− ILC2s with WT ILC2s in the presence of anti-ICOS blocking or isotype-matched antibodies. Since ICOS-L is down-regulated upon interaction with ICOS we used ICOS-L expression as an indication of ICOS:ICOS-L binding. We found that ICOS-L expression in the presence of anti-ICOS blocking antibody is lower than that of isotype-matched control suggesting that trans interaction occurs. Moreover, the readily lower level of ICOS-L expression in WT ILC2s than in Icos−/− ILC2s may suggest cis transaction as well (Supplementary figure 4).
We next addressed whether ICOS:ICOS-L binding leads to increased BCL2 expression and ILC2s survival by testing the stimulatory effects of exogenous plate bound ICOS-L-IgG on ILC2s in vitro. As shown in figure 6A, ICOS-L-IgG stimulation leads to increased expression of BCL2 and enhanced survival of WT ILC2s. These data show that ILC2s express ICOS-L in addition ICOS and that expression of ICOS-L plays a functional role in ILC2s cytokine production and survival.
Human peripheral ILC2s express functional ICOS and ICOS-L
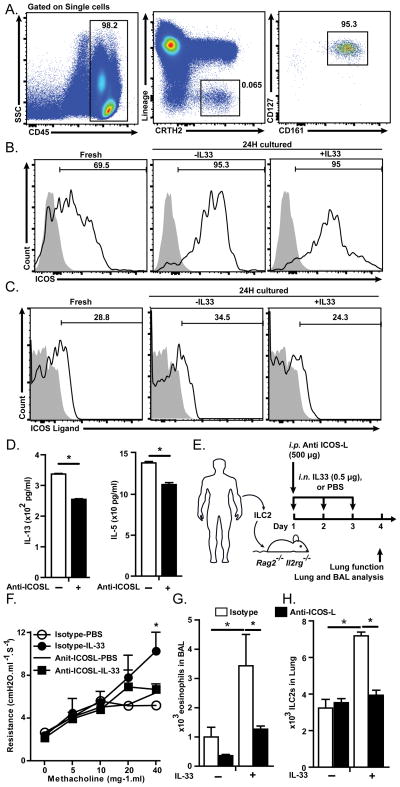
We next investigated whether human ILC2s express ICOS and ICOS-L and whether ICOS and ICOS-L play a crucial role in the function of human ILC2s. Peripheral blood from healthy donors was collected and gated for ILC2s based on the lack of expression of human lineage markers (CD3, CD14, CD16, CD19, CD20, CD56, CD235a, CD1a, CD123) and expression of CD45, CRTH2, CD127 and CD161 (Figure 7A), and then analyzed for the expression of ICOS and ICOS-L (Figure 7B–C, left panels). Alternatively, human peripheral blood mononuclear cells (PBMCs) were cultured in the presence of recombinant human (rh)-IL-2 (20 ng/ml), rh-IL-7 (20 ng/ml) in the presence or absence of rh-IL-33 (20 ng/ml) for 24 hours and expression of ICOS and ICOS-L was evaluated by flow cytometry (Figure 7B–C, right panels). We found that ICOS is expressed by human peripheral ILC2s at steady state and its expression is increased upon in vitro culture with IL-2 and IL-7, while IL-33 stimulation does not affect the expression of ICOS by human ILC2s (Figure 7B). Similar to mouse ILC2s, we found that human peripheral ILC2s express ICOS-L at a low basal level that is modestly increased after in vitro stimulation by IL-2 and IL-7, while it is modestly decreased by IL-33 (Figure 7C).
Figure 7. Human peripheral ILC2s express ICOS and ICOS-L and blocking their interaction reduces cytokine production by ILC2s.
Human peripheral blood mononuclear cells were isolated using Ficoll gradient isolation and cultured in the presence or absence of recombinant human IL-33 (20 ng/ml), IL-2 (10 ng/ml) and IL-7 (20 ng/ml) for 24 hours. A) Human peripheral ILC2s were gated on single cells, CD45+ CRTH2+ Lineage−, CD127+ and CD161+ cells, B) Expression of ICOS and C) ICOS-L by human peripheral ILC2 in freshly isolated (left panels), or cultured in the absence or presence of rh-IL-33 (20 ng/ml, middle and right panels) for 24 hours. Stain isotype control is shown in gray filled histogram. D) Human ILC2s were purified from PBMCs using FACS and cultured (104/ml) in the presence of rhuman-IL-33 (20 ng/ml), IL-2 (10 ng/ml) and IL-7 (20 ng/ml) for 24 hours. Data are representative of 4 individual donors. E) Human peripheral ILC2s were purified using FACS, cultured with rh-IL2 (20 ng/ml) and rh-IL-7 (20 ng/ml) for 48 hours then adoptively transferred into 2 group of Rag2−/−Il2rg−/− mice receiving either anti-human and anti-mouse ICOS-L (500 μg/mouse) or isotype control (500 μg/mouse) on day 1. Both groups received either rh-IL-33 (0.5 μg/mouse) or PBS i.n. on day 1–3 followed by dissection on day 4. F) Lung resistance. G) Total numbers of eosinophils in BAL. H) Total numbers of human ILC2s in the lungs of humanized mice (n=3–4).
To evaluate the functional requirement of ICOS-ICOS-L binding for cytokine production by human ILC2s, we purified ILC2s from PBMCs and cultured in the presence of (rh)-IL-2 (20 ng/ml), rh-IL-7 (20 ng/ml) and rh-IL-33 (20 ng/ml) in the presence of blocking anti-human-ICOS-L antibody (10 μg/ml, clone: 9F.8A4) or isotype control for 72 hours followed by measurement of IL-13 and IL-5 in the supernatant. The results showed that blocking ICOS-ICOS-L interaction significantly reduces production of IL-13 and IL-5 by human cultured ILC2s (Figure 7D).
To further confirm the functional requirement of ICOS-ICOS-L interaction for human ILC2s, we purified ILC2s form human PBMCs and after 3 days culture in vitro in the presence of (rh)-IL-2 (20 ng/ml) and rh-IL-7 (20 ng/ml) adoptively transferred ILC2s through the tail vain to Rag2−/− Il2rg−/− mice, that lack T,B and NK cells and ILCs. Then mice received either anti-human-ICOS-L plus anti-mouse-ICOS-L (clone: 9F.8A4 and 16F.7E5, 500 μg/mouse each) or isotype control on day 1 and either i.n. rh-IL-33 (1 μg/mouse) or PBS on days 1–3 (Figure 7E). On day 4, lung function was measured as described above and assessment of BAL was performed. IL-33 treatment induces AHR in mice that received isotype control while IL-33 failed to induce AHR in mice that received anti-ICOS-L antibodies (Figure 7F). IL-33 treatment significantly increases the number and frequency of eosinophils in BAL only in isotype control but not anti-ICOS-L treated mice (Figure 7G). The number and frequency of eosinophils are significantly lower in mice that received anti-ICOS-L than in recipients of isotype control (Figure 7G). Taken together, these results indicate that human peripheral ILC2s express both ICOS and ICOS-L and that ICOS:ICOS-L interaction plays a crucial role for the function of human ILC2s.
Discussion
In this study we demonstrate that ICOS is required for ILC2-mediated induction of airway hyperreactivity and inflammation in murine models and human ILC2-reconstituted mice. We showed that mouse and human ILC2s expressed both ICOS and ICOS-L and that ICOS: ICOS-L interaction was required for efficient ILC2 function and provides a survival signal for ILC2. We further demonstrated that blocking ICOS:ICOS-L interaction impairs STAT5 signaling and IL-13 production in ILC2s.
In the absence of ICOS, IL-33 or Alternaria induce lower AHR and lung inflammation. We used the IL-33 based model to test the functional requirement for ICOS in cytokine production and survival of ILC2s since IL-33 and IL-25 have been previously shown to induce ILC2-mediated AHR and lung inflammation in RAG-deficient mice with IL-33 being more potent that IL-25 (Barlow et al., 2012; Barlow et al., 2013; Chang et al., 2011; Fort et al., 2001). We used Alternaria since it is a common fungus in the environment and an allergen in humans and has been shown to cause allergic inflammation in mice independent of adaptive immunity (Doherty et al., 2013; Kim et al., 2014). Our results suggest that besides AHR, ILC2s-mediated induction of lung inflammation and eosinophilia depend on ICOS. In line with our findings, it has been shown that ICOS is required for T cells mediated lung inflammatory responses (Gonzalo et al., 2001).
We observed a reduced numbers of ILC2s in Icos−/− compare to WT mice which suggests that ICOS plays an important role in survival and/or proliferation of ILC2s. By analyzing ILC2s apoptosis and death, we found that the frequency of dead cells was increased in the absence of ICOS while the frequency of early and late apoptotic ILC2s was comparable. Similarly we found that the expression of anti-apoptotic factor, BCL-2, was significantly lower in Icos−/− than in WT ILC2s. Evaluation of ILC2s proliferation by measuring the expression of Ki-67, a protein associated with cell proliferation, revealed that the rate of proliferation of ILC2s is similar in Icos−/− and WT mice. These findings suggest that while ICOS provides a survival signal for ILC2s, it is not essential for proliferation of ILC2s. In agreement with our findings in ILC2s, it has been shown that ICOS substantially contributes to survival of effector T cells upon antigen exposure, however, Burmeister, et al, have also observed a role of ICOS in T cell expansion (Burmeister et al., 2008).
ICOS substantially contributes to cytokine production by ILC2s. Using intracellular staining and gating on the live cells, we found that upon stimulation with IL-33, production of IL-13 is significantly reduced in the absence of ICOS, while the production of IL-13 and IL-5 in the supernatant is reduced upon in vitro stimulation in the absence of ICOS. Similarly, it has been shown that ICOS plays an important role of the production of IL-13 and IL-4 in T cells (Dong et al., 2001). These data indicate that IL-13 production is directly affected by lack of ICOS however, due to the higher rate of ILC2 death the sum production of IL-5 is also reduced in the absence of ICOS. This is in agreement with the reduced AHR and BAL eosinophilia in Icos−/− mice which reflect the in vivo amounts of IL-13 and IL-5 respectively.
We show that mouse and human ILC2s express functional ICOS-L and that ICOS:ICOS-L interaction is required for survival and cytokine production of ILC2s. Expression of both ICOS and ICOS-L by ILC2s explains the reduced cytokine production after blocking ICOS:ICOS-L interaction in purified in vitro cultures of ILC2s. We found that blocking ICOS:ICOS-L interaction increases the expression of ICOS-L in WT mice, while Icos−/− ILC2s readily express high levels of ICOS-L. These findings suggest that ICOS-L is down-regulated upon binding to ICOS. In agreement with our observations, it has previously been shown that ICOS:ICOS-L interaction leads to down-regulation of ICOS-L in B cells (Watanabe et al., 2008). To our knowledge this is the first report of expression of ICOS and ICOS-L by the same type of cells. Since it has been reported that ILC2s (previously reported as nuocytes) may express MHC-II (Neill et al., 2010) and we observed that they express ICOS-L, further investigation is needed to determine whether these cells can engage in antigen presentation activities.
Several reports have shown that GATA-3 is expressed by ILC2s and plays a crucial role in development, maintenance and function of ILC2s (Hoyler et al., 2013; Hoyler et al., 2012; Klein Wolterink et al., 2013; Mjosberg et al., 2012). and IL-13 production by ILC2s has been associated with high level of expression of GATA-3 (Liang et al., 2012). However, when we compared the expression of GATA-3 in WT with Icos−/− ILC2s at steady state and after IL-33 stimulation, we found comparable levels of GATA-3 suggesting that impairment of IL-13 production by Icos−/− ILC2s is caused by a mechanism other than the reduction of GATA-3 expression.
We found that STAT-5 signaling was impaired in Icos−/− ILC2s. The expression of phospho-STAT5 in Icos−/− ILC2s was substantially lower than in WT ILC2s. This finding suggests that lack of ICOS leads to alteration in IL-2 and IL-7 signaling. Interestingly, STAT5 is not only required for IL-2 mediated cell survival and Th2 differentiation but it is also required for efficient production of IL-13 in T cells and mast cells (Antov et al., 2003; Junttila et al., 2013; Moriggl et al., 1999; Zhu et al., 2003). We further investigated ICOS signaling using plate bound ICOS-L-Fc, that consists of mouse ICOS-L fused with Fc part of human IgG, and found that ICOS-L-Fc increases the amount of phospho-STAT5 and leads to higher production of IL-13. Taken together, our data suggest that ICOS plays an important role in the ILC2 cell survival and cytokine production through the IL-2 and STAT-5 signaling pathway which may be unique for ILC2s.
In addition, we introduce a humanized mouse model for the first time, in which human peripheral ILC2s are adoptively transferred to Rag2−/−Il2rg−/− mice and i.n. administration of hu-IL-33 causes AHR and inflammation. Human IL-5 has been shown to activate murine eosinophils emphasizing the feasibility of using humanized mice in eosinophilic inflammatory studies (Ingley et al., 1991; Tavernier et al., 1995). This mouse model provides a unique platform for studying the contribution of ILC2s to human asthma and evaluating the efficacy of potential therapeutic targets in preclinical studies. Using this model we show that ICOS:ICOS-L interaction is required for efficient function of human ILC2s in vivo. These findings underscore the importance of the ICOS signaling pathway for efficient function of human ILC2s and demonstrate that our findings in mouse ILC2s are of high importance for and translatable to clinical studies.
In conclusion, our study demonstrates that human and mouse ILC2s express ICOS and ICOS-L and that ICOS:ICOS-L interaction provides a survival signal for ILC2s and is required for efficient cytokine production by ILC2s. We show that the STAT5 signaling pathway is impaired in Icos−/− ILC2s while it is enhanced through ICOS signaling leading to higher cytokine production. This may provide an explanation for the lower rate of survival and cytokine production in the absence of ICOS or blockade of ICOS:ICOS-L signaling. We introduce a humanized mouse model where human ILC2s drive AHR in mice and using this system we show that ICOS:ICOS-L interaction is required for optimal function of human ILC2s in vivo. Since ILC2s are the only cells that both express ICOS and ICOS-L, our findings set the stage for designing new therapeutic approaches for asthma where ILC2s can be targeted, for instance by dual specific antibodies that recognize ICOS and ICOS-L.
Experimental procedures
Mice and in vivo experiments
ICOS deficient mice (C.129S4-Icostm1Shr/J) were obtained from Arlene Sharpe (Harvard Medical School, Boston, Massachusetts). RAG2 deficient (C.B6(Cg)-Rag2tm1.1Cgn/J), RAG2 IL-2Rg deficient (C;129S4-Rag2tm1.1Flv Il2rgtm1.1Flv/J) breeder pairs and BALB/cBYJ experimental mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). NSG experimental mice were purchased from the Jackson Laboratory. RAG2 deficient, RAG2 GC deficient and ICOS deficient mice were bred in our animal facility at USC. 5–8 week old age-matched female mice were used in the studies. In vivo and in vitro ILC2s stimulation are described in details in supplementary experimental procedures.
Analysis of intracellular factors by flow cytometry
Cytokines production was assessed using BD Cytofix/Cytoperm containing BD Golgi-Plug (BD biosciences) according to manufacturer’s instructions and staining with anti-IL-5, anti-IL-13, anti-IL4 and anti-IL-17a antibodies as mentioned above.
GATA-3 and BCL-2 expression was assessed by fixing and permeabilizing cells using Fixation/Permeabilization buffer kit (eBioscience) according to manufacturer’s instructions followed by anti-GATA-3 and anti-BCL-2 antibodies as mentioned above.
The expression of phosphorylated STAT5 was measure after stimulation of cells with rm-IL-2 (100 ng/ml) in the presence or absence of rm-IL-33 (100 ng/ml) for 30 minutes followed by surface staining and fixation and permeabilization using BD Cytofix™ Fixation Buffer and BD Phosflow™ Perm Buffer III according to manufacturer’s instructions and staining with anti-mouse pSTAT5 as mentioned above.
Humanized mice and purification of humanILC2
For human peripheral ILC2, peripheral blood mononuclear cells (PBMCs) were first isolated from human fresh blood as described in details in supplementary experimental procedure. Purified human ILC2s were cultured with rh-IL2 (20 ng/ml) and rh-IL-7 (20 ng/ml) for 3 days then adoptively transferred to Rag2−/− Il2rg−/− mice (2 x104 cells/mouse) followed by i.n. administration of recombinant human IL-33 (0.5 μg/mouse) or PBS i.n. on day 1–3. On day 1 mice received either anti-human (clone: 9F.8A4, 500 μg/mouse) + anti-mouse ICOS-L (clone: 16F.7E5, 500 μg/mouse) or isotype-matched control (500 μg/mouse). On day 4 lung function was measured and BAL was performed and analyzed. Anti-ICOS-L antibodies were generated by Dr. Gordon Freeman as described elsewhere (Akbari et al., 2002).
Cytokine measurement in the supernatant
Human IL-5 ELISA MAX™ Deluxe was purchased from Biolegend, Ready-SET-Go!® ELISA for human IL-13, mouse IL-5 and IL-13 were purchased from eBioscience and the level of cytokines were measured according to the manufacturer’s instructions.
Measurement of Lung Function
Lung function was evaluated by direct measurement of lung resistance and dynamic compliance in restrained tracheostomized mechanically ventilated mice using the FinePointe RC system (Buxco Research Systems, Wilmington, NC) under general anesthesia as described before (Kerzerho et al., 2013).
Collection of bronchoalveolar lavage (BAL) fluid and lung histology
Mice were euthanized after evaluating lung function, the trachea was intubated and lungs were washed three times with 1 ml of PBS then the cells were harvest as previously described (Kerzerho et al., 2013). Relative and absolute cell numbers in the BAL were calculated using flow cytometry as described in supplementary experimental procedure. After taking BAL lung histology slides were made as described previously (Kerzerho et al., 2013). Histology pictures were acquired using a KeyenceBZ-9000 microscope (Keyence, Itasca, IL) and analyzed using BZ-II Image Analysis Application (Keyence, Itasca, IL).
Gene expression analysis using Nanostring® nCounter technology
The difference in the abundance of scripts between the purified Icos−/− and WT ILC2s were analyzed using Nonostring nCounter technology as described in supplementary experimental procedure. Heat plots were generated using R statistical software.
Statistical analysis
Experiments were repeated at least three times (N=4–6 each) and data are shown as the representative of 3 independent experiments. AHR data were analyzed by repeated measurements of general linear model. Al other data were analyzed using JMP statistical software (Cary, NC) by Student’s t-Tests and confirmed by Mann–Whitney U. For multi-group comparison we used one way ANOVA with Bonferroni post-hoc test.
Supplementary Material
Acknowledgments
Studies described in this article were financially supported by National Institutes of Health Public Health Service Grant R01 AI 066020, R01 ES 021801, R21 ES 024707 (O. A.) and P01 AI 056299 (G. J. F. and A. H. S.). Hadi Maazi is supported by American Association of Immunology (AAI) Careers in Immunology Fellowship.
Footnotes
Author contributions
H. M. performed experiments, analyzed the data and wrote the manuscript. N. P., I. S. and D. R. helped in performing experiments. Y. S. contributed to revising the manuscript. P. S. contributed to data interpretation. G. J. F. and A. H. S. contributed to improving manuscript revision, study guidance and provided experimental tools. O.A. supervised the studies, contribute to data interpretation, and improvement of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- Antov A, Yang L, Vig M, Baltimore D, Van Parijs L. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. Journal of immunology (Baltimore, Md : 1950) 2003;171:3435–3441. doi: 10.4049/jimmunol.171.7.3435. [DOI] [PubMed] [Google Scholar]
- Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–198. e191–194. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. Journal of immunology (Baltimore, Md : 1950) 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671–678. e674. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, Kroczek RA, Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. Journal of immunology (Baltimore, Md: 1950) 2008;180:774–782. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- Gonzalo JA, Tian J, Delaney T, Corcoran J, Rottman JB, Lora J, Al-garawi A, Kroczek R, Gutierrez-Ramos JC, Coyle AJ. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat Immunol. 2001;2:597–604. doi: 10.1038/89739. [DOI] [PubMed] [Google Scholar]
- Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenberg MD, Spits H. Human innate lymphoid cells. Blood. 2014 doi: 10.1182/blood-2013-11-427781. [DOI] [PubMed] [Google Scholar]
- Hoyler T, Connor CA, Kiss EA, Diefenbach A. T-bet and Gata3 in controlling type 1 and type 2 immunity mediated by innate lymphoid cells. Current opinion in immunology. 2013;25:139–147. doi: 10.1016/j.coi.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Ingley E, Cutler RL, Fung MC, Sanderson CJ, Young IG. Production and purification of recombinant human interleukin-5 from yeast and baculovirus expression systems. European journal of biochemistry/FEBS. 1991;196:623–629. doi: 10.1111/j.1432-1033.1991.tb15858.x. [DOI] [PubMed] [Google Scholar]
- Junttila IS, Watson C, Kummola L, Chen X, Hu-Li J, Guo L, Yagi R, Paul WE. Efficient cytokine-induced IL-13 production by mast cells requires both IL-33 and IL-3. J Allergy Clin Immunol. 2013;132:704–712. e710. doi: 10.1016/j.jaci.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerzerho J, Maazi H, Speak AO, Szely N, Lombardi V, Khoo B, Geryak S, Lam J, Soroosh P, Van Snick J, Akbari O. Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J Allergy Clin Immunol. 2013;131:1048–1057. 1057.e1041–1042. doi: 10.1016/j.jaci.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Lund S, Baum R, Rosenthal P, Khorram N, Doherty TA. Innate type 2 response to Alternaria extract enhances ryegrass-induced lung inflammation. International archives of allergy and immunology. 2014;163:92–105. doi: 10.1159/000356341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, Savage PB, McKenzie AN, Smith DE, Rottman JB, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216–227. e211–216. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein Wolterink RG, Serafini N, van Nimwegen M, Vosshenrich CA, de Bruijn MJ, Fonseca Pereira D, Veiga Fernandes H, Hendriks RW, Di Santo JP. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10240–10245. doi: 10.1073/pnas.1217158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. Journal of immunology (Baltimore, Md : 1950) 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, te Velde AA, Fokkens WJ, van Drunen CM, Spits H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriggl R, Topham DJ, Teglund S, Sexl V, McKay C, Wang D, Hoffmeyer A, van Deursen J, Sangster MY, Bunting KD, et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10:249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Duong J, Kishikawa H, Dianzani U, Rojo JM, Ho I, Flavell RA, Dong C. Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity. 2003;18:801–811. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- Qian X, Agematsu K, Freeman GJ, Tagawa Y, Sugane K, Hayashi T. The ICOS-ligand B7-H2, expressed on human type II alveolar epithelial cells, plays a role in the pulmonary host defense system. Eur J Immunol. 2006;36:906–918. doi: 10.1002/eji.200535253. [DOI] [PubMed] [Google Scholar]
- Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, Banchereau J, Ueno H. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- Tavernier J, Tuypens T, Verhee A, Plaetinck G, Devos R, Van der Heyden J, Guisez Y, Oefner C. Identification of receptor-binding domains on human interleukin 5 and design of an interleukin 5-derived receptor antagonist. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5194–5198. doi: 10.1073/pnas.92.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen MB, Munneke JM, Bernink JH, Spuls PI, Res PC, Te Velde A, Cheuk S, Brouwer MW, Menting SP, Eidsmo L, et al. Composition of Innate Lymphoid Cell Subsets in the Human Skin: Enrichment of NCR ILC3 in Lesional Skin and Blood of Psoriasis Patients. The Journal of investigative dermatology. 2014 doi: 10.1038/jid.2014.146. [DOI] [PubMed] [Google Scholar]
- Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nature reviews. Immunology. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Takagi Y, Kotani M, Hara Y, Inamine A, Hayashi K, Ogawa S, Takeda K, Tanabe K, Abe R. Down-regulation of ICOS ligand by interaction with ICOS functions as a regulatory mechanism for immune responses. Journal of immunology (Baltimore, Md : 1950) 2008;180:5222–5234. doi: 10.4049/jimmunol.180.8.5222. [DOI] [PubMed] [Google Scholar]
- Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.