Abstract
Background
Anesthetic cardioprotection reduces myocardial infarct size following ischemia-reperfusion injury. Currently, the role of microRNA in this process remains unknown. MicroRNAs are short, non-coding nucleotide sequences that negatively regulate gene expression through degradation or suppression of messenger RNA. In this study, we uncovered the functional role of microRNA-21 (miR-21) upregulation after anesthetic exposure.
Methods
MicroRNA and messenger RNA expression changes were analyzed by quantitative real-time polymerase chain reaction in cardiomyocytes after exposure to isoflurane. Lactate dehydrogenase release assay and propidium iodide staining were conducted after inhibition of miR-21. miR-21 target expression was analyzed by Western Blot. The functional role of miR-21 was confirmed in vivo in both wild type and miR-21 knockout mice.
Results
Isoflurane induces an acute upregulation of miR-21 in both in vivo and in vitro rat models (n = 6, 247.8 ± 27.5% and 258.5 ± 9.0%), which mediates protection to cardiomyocytes through downregulation of programmed cell death protein 4 (PDCD4) messenger RNA (n = 3, 82.0 ± 4.9% of control group). This protective effect was confirmed through knockdown of miR-21 and PDCD4 in vitro. Additionally, the protective effect of isoflurane was abolished in miR-21 knockout mice in vivo, with no significant decrease in infarct size compared to non-exposed controls (n = 8, 62.3 ± 4.6% and 56.2 ± 3.2%).
Conclusions
We demonstrate for the first time that isoflurane mediates protection of cardiomyocytes against oxidative stress via a miR-21/PDCD4 pathway. These results reveal a novel mechanism by which the damage done by ischemia-reperfusion injury may be decreased.
Introduction
Cardiovascular disease is one of the leading causes of morbidity and mortality worldwide. Myocardial ischemia-reperfusion (I/R) injury alone accounts for approximately 450,000 deaths per year in the United States.1 Therapies to reduce infarct size are predicted to increase patient survival rates, as final infarct size after an I/R event has been directly correlated to patient prognosis for future cardiac events.2
One way to reduce the size of infarct prior to an I/R injury is through the use of preconditioning. Exposure to volatile anesthetics, such as isoflurane, has been shown to decrease myocardial infarct size in vivo and increase cell viability after oxidative stress in vitro.3–7 Previously, we have shown that anesthetic preconditioning with isoflurane affected expression levels of nitric oxide synthase and heat shock proteins, as well as decreased levels of reactive oxygen species, resulting in increased cell viability.3,8 However, knowledge of the underlying mechanisms remains incomplete. In this study, we investigate whether microRNAs are playing a role in isoflurane-mediated protection of cardiomyocytes.
MicroRNAs are 18–22 nucleotide sequences that suppress protein expression through degradation or suppression of messenger RNA (mRNA). MicroRNAs have previously been linked to the cardioprotective effects of ischemic, hypoxic, and heat shock preconditioning.9–11 In 2009, the Zhang lab showed that overexpression of miR-21, similar to the upregulation observed during ischemic preconditioning, significantly decreased infarct size after acute myocardial infarction.9 The Abdellatif lab has shown that miR-199a is a key regulator of hypoxia-triggered pathways, through actions on Hif-1α and Sirt1.10 MicroRNAs -378 and -711 are suppressed during both ischemic and heat shock preconditioning, resulting in increased expression of heat shock protein 70 and increased cell viability.12 So far, no studies have been done on microRNAs regarding anesthetic protection of cardiomyocytes.
Recent studies have also shown that isoflurane, sevoflurane, and other anesthetics can influence microRNA expression profiles in the liver and in neurons,13–15 suggesting that isoflurane exposure may influence microRNA in cardiomyocytes as well. Thus, in this study, we used in vivo and in vitro rat models to investigate, for the first time, the role of miR-21 in isoflurane-mediated preconditioning. We hypothesized that isoflurane protects cardiomyocytes against oxidative stress through upregulation of miR-21.
Materials and Methods
Housing and Care of Rats and Mice
All experimental procedures and protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin (Milwaukee, Wisconsin; approval number 2602; 12-19-2011), and the investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (National Institutes of Health Publication No. 85-23, revised 1996). All animals were housed with free access to food and water. Male Wistar rats (Charles River Laboratories International, Inc., Wilmington, MA) weighing between 300–400 g were used for in vivo microRNA screening. For in vitro microRNA screening, time pregnant Sprague Dawley female rats (Jackson Laboratory, Bar Harbor, ME) were housed in separate cages until gestation was complete. Male B6129SF1/J wild-type (WT) mice (Jackson Laboratory) and miR-21 knockout mice (Jackson Laboratory) weighing between 25–29 g were used for in vivo functional studies.
Neonatal Rat Cardiomyocyte Cell Culture
Neonatal cardiomyocytes were isolated as described our in previous studies.8,16 Left ventricles were harvested from litters of 1–2 day old Sprague Dawley rat pups. Two litters were pooled per experiment to decrease variation within species, as well as to ensure that enough tissue was isolated for Western Blots and time course studies. Cells were isolated using serial digestion with collagenase and pancreatin (Sigma-Aldrich, St. Louis, MO) and plated on gelatin-coated dishes at 1.0 × 106 cells per 60 mm dish or 2.7 × 105 cells per well in a 12-well plate.* Cells were cultured under these conditions for one week prior to isoflurane exposure.
Exposure of neonatal rat cardiomyocyte culture to isoflurane
Each dish of neonatal rat cardiomyocytes was randomly assigned to the isoflurane -exposed or control group. Isoflurane (Baxter Healthcare Corporation, Deerfield, IL) exposure was performed in a sealed chamber placed in a 37°C incubator for thirty minutes at a concentration of 0.5 mM. The same protocol has been established in our previous studies and mirrors a clinically relevant dosage.17,18 Control cells remained in the same incubator throughout the experiment. Isoflurane-exposed cells were removed from the sealed chamber and placed with control cells for 15 minutes to allow for washout of the volatile anesthetic before RNA isolation.
Exposure of Wistar rats to isoflurane
Each rat was randomly assigned to the isoflurane-exposed or control group. Rats were exposed to thirty minutes of 0.5 mM of isoflurane in a sealed plexiglass unit. Isoflurane was delivered using a VetEquip funnel-fill vaporizer. A 32% O2 environment was monitored using the Criticare Systems Poet IQ anesthesia gas monitor. Control animals were exposed to 32% O2 for thirty minutes. Left ventricles were harvested after a fifteen minute washout period in the original housing units. Left ventricles were pooled in groups of three for each experiment to decrease variation between groups of animals on the twelve arrays used for microRNA screening.
Total RNA isolation and complementary DNA (cDNA) synthesis
Total RNA was isolated using the miRNeasy Mini Kit (Qiagen Inc., Valencia, CA). Cells were homogenized in QIAzol lysis reagent. Afterwards, chloroform was added and samples were centrifuged in order to separate aqueous and organic phases. The aqueous phase was extracted and combined with ethanol in order to facilitate RNA binding to miRNeasy Mini spin columns. Samples were washed twice in buffer and total RNA was eluted using RNase-free water. cDNA was synthesized by adding RNA to a mixture of miScript Reverse Transcriptase Mix, 10× miScript Nucleics Mix, and 5× miScript HiFlex Buffer (Qiagen). The mixture was incubated at 37°C for 60 minutes, then at 95° C for 5 minutes in order to convert RNA to cDNA.
MicroRNA analysis by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
MicroRNA profiling was conducted using the miFinder miScript microRNA Polymerase Chain Reaction (PCR) Array (Qiagen) according to the manufacturer’s instructions. These arrays contain 84 lyophilized microRNA primers distributed in single wells along with four housekeeping genes (RNAs Rnu-6, U87, 4.5 S-V1, and Y1). Samples without reverse transcriptase were run to confirm that no genomic DNA was present in the sample. The microRNAs present in this assay represent 84 of the most abundantly expressed microRNAs across all mammalian tissues. A total of twelve microRNA arrays were run for in vivo rat hearts, and twelve for in vitro cardiomyocytes: six control plates, and six exposed to isoflurane, as designed in previous microRNA screening studies.19 Differentially expressed microRNAs were validated in technical triplicate using miScript Primer Assays for miR-21 (Qiagen, Lot Nos. 117241738 and 117259379). qRT-PCR was conducted using the BioRad iCycler Real-Time PCR Detection System. cDNA was diluted and combined with QuantiTect SYBR Green PCR Master Mix and miScript Universal Primer using low-adhesion pipette tips (VWR, Radnor, PA). Data collected from these experiments defined a threshold cycle number (Ct) of detection for the microRNAs present in each sample. MicroRNAs were excluded from the study if Ct values were greater than or equal to 35. Primer specificity was tested by running qRT-PCR analysis with miScript Primer Assays specific for miR-21 in a solution containing only synthetic miR-21 mimic (Qiagen), as well as by size fractionation of stem-loop RT-PCR products on 2% agarose gel. Melt curve denaturation levels also occurred in the predicted 70° – 80° C range (Supplemental Digital Content 1, Figure).
Knockdown of miR-21 expression
The neonatal rat cardiomyocytes were transfected with doses ranging from 1 nM to 25 nM of locked nucleic acid (LNA)-modified anti-miR-21 (Exiqon, Skelstedet, Denmark) using the Lipofectamine 2000 transfection system (Life Technologies, Carlsbad, CA). The LNAs are comprised of a single ribose ring connected by a methylene bridge at the 2’-O and 4’-C atoms, allowing for the nucleotides to bind in an optimal conformation. In order to transfect the cardiomyocytes, LNAs were incubated briefly with the Lipofectamine reagent to allow for anti-miR incorporation into lipid bubbles. The mixture was then exposed to cells for 20 hours. Scrambled LNA anti-miRs (Exiqon) were used as a control. After an exposure to anti-miR-21, cells were lysed and tested for miR-21 expression using qRT-PCR analysis as described under “MicroRNA analysis by quantitative reverse transcriptase PCR” in this methods section.
Lactate dehydrogenase (LDH) release assay and Propidium iodide (PI) Staining
Cardiomyocytes were exposed to 0.5 mM of isoflurane in an anesthetic chamber, as described under “Exposure of neonatal rat cardiomyocyte culture to isoflurane” in this methods section, while the control plates were kept in a 37° C incubator. After a 15 minute washout period, 50 µM of hydrogen peroxide was used to cause oxidative stress to the cardiomyocytes. After six hours of exposure to hydrogen peroxide, LDH release was measured by absorbance of indicator dyes as calculated by the Epoch spectrophotometer (Biotek, Winooski, VT). After media samples were taken for LDH analysis, cells were stained with propidium iodide (Life Technologies). Nuclei were stained with Hoechst 33342 (Life Technologies) at a concentration of 1:800 diluted in cell culture media. 1.0 mg/ml of propidium iodide solution was diluted in media at a concentration of 1:500. Hoechst-positive nuclei were counted as a control using fluorescence microscopy and PI positive cells were taken as a percent of the control.
Short interfering RNA (siRNA) knockdown of PDCD4
Cardiomyocytes were transfected with 10 nM of PDCD4 Stealth RNAi™ siRNA Select RNAi (Life Technologies, Oligo ID: RSS329752) using the Lipofectamine 2000 (Life Technologies) transfection system for 24 hours. PDCD4 knockdown of mRNA expression was confirmed by qRT-PCR analysis, as described under “MicroRNA analysis by quantitative reverse transcriptase PCR” in this methods section. Knockdown of PDCD4 protein expression was confirmed by Western Blot. Negative control cells were transfected with Stealth RNAi™ siRNA Negative Control Med GC (Life Technologies).
mRNA analysis of miR-21 targets by qRT-PCR
miR-21 mRNA targets PDCD4 and phosphatase and tensin homologue (PTEN) were investigated using rat RT2 PCR Assays (SABiosciences, Valencia, CA) according to the manufacturer’s instructions. Total RNA was extracted using the Qiagen RNeasy kit as described under “Total RNA isolation and cDNA synthesis” in this methods section and cDNA was prepared using the RT2 First Strand Kit (Qiagen). RNA was combined with genomic DNA Elimination Buffer and incubated at 42°C for 5 minutes to ensure cDNA purity. Samples were chilled on ice for 1 minute, then mixed with 5× RT Buffer 3, Primer and External Control Mix, RT Enzyme Mix 3, and water. cDNA was diluted and combined with RT2 SYBR Green/Fluorescein Master Mix and distributed evenly across the wells of a 96-well plate in technical triplicates. qRT-PCR was conducted using the BioRad iCycler Real-Time PCR Detection System. Samples were exposed to an initial 95°C hot-start activation step, followed by 40 cycles of 94°C denaturation and 60°C annealing/extension phases. Data collected from these experiments defined Ct values of the mRNAs present in each sample.
Expression levels of the housekeeping genes beta-actin and beta-2 microglobin were used as controls to normalize samples. Samples without reverse transcriptase were run to confirm that no genomic DNA was present in the sample. mRNAs with Ct values greater than or equal to 35 were excluded from the study.
Western Blot Analysis
Cells were lysed and sonicated in RIPA lysis buffer (Cell Signaling, Danvers, MA) containing phosphatase inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Lysates were centrifuged at 10,000×g for 10 minutes at 4°C. Pellets were discarded and the total protein concentration of the supernatants was determined by DC Protein Assay Reagents Package kit (Bio-Rad, Hercules, CA). The samples were boiled for 5 minutes at 97°C. 25 µg of protein was loaded per lane for SDS-PAGE gel separation and then transferred to nitrocellulose membrane. Membranes were blocked with Blocking Buffer (Thermo Scientific, Waltham, MA) and then incubated overnight at 4°C with primary antibodies against PTEN, actin (Cell Signaling), or PDCD4 (Rockland Immunochemicals, Limerick, PA). Following the incubation of membranes with secondary antibodies conjugated to horseradish peroxidase (Cell Signaling) for 1 hour at room temperature. Labeled proteins were detected with chemiluminescence detection reagent (Cell Signaling). The membrane was transferred to x-ray film and exposed. The intensity of the protein bands in films was quantified using ImageJ software and the data was reported as % of control.
Myocardial Ischemia/Reperfusion Injury in vivo
Myocardial I/R injury in vivo was produced by occluding the left coronary artery, as previously described.20,21 WT and miR-21 knockout mice were randomly divided into isoflurane exposed or control groups. After instrumentation was completed, all mice were stabilized for 30 minutes and subjected to 30 minutes of coronary artery occlusion followed by 2 hours of reperfusion. Isoflurane was administered at 1.0 minimum alveolar concentration for 30 minutes via an isoflurane-specific vaporizer (Ohio Medical Instruments, Cinncinati, OH) followed by a 15 minute washout period prior to coronary artery occlusion. Control mice were not exposed to isoflurane. The infarct area was delineated by perfusing the coronary arteries with 2,3,5-triphenyltetrazolium chloride via the aortic root, and the area at risk was delineated by perfusing phthalo blue dye (Heucotech Ltd., Fairless Hills, PA) into the aortic root after tying the coronary artery at the site of the previous occlusion.
Statistical Analysis
All rats, mice, and neonatal rat cardiomyocyte cell culture dishes were randomly assigned to isoflurane or control groups, as well as scramble or transfected groups. Western blot analysis was conducted blindly, with samples divided into randomly numbered groups. Values are expressed as means ± Standard Error of the Mean. T-test confirmed statistically significant differences between microRNAs in single groups. We used an Analysis of Variance test followed by a Tukey test to compare microRNAs in multiple groups. P values less than 0.05 (two-tailed) were considered to be significant. MicroRNA Arrays were analyzed using the miScript microRNA PCR Array Data Analysis Web Portal (SABiosciences) and the Benjamini and Hochberg procedure was applied to control the False Discovery Rate in our samples.22 Three microRNAs (miRs-137, -142, and -196b) had a single data point greater than two standard deviations from the mean and were considered to be outliers. After these data points were discarded, the isoflurane exposed groups were no longer significantly different from the control. All other statistical analysis was performed using Sigma Plot 12.0 (Systat Software, Inc., San Jose, CA).
Results
Isoflurane exposure causes changes in expression levels of microRNAs
Array-based analyses investigated the effect of isoflurane on expression levels of 84 microRNAs in thirty-six in vivo rat hearts pooled into groups of three and in vitro cultured neonatal rat cardiomyocytes from twenty-four litters of Sprague-Dawley pups pooled into two litters per group (for a total of n = 6 per group). Eleven microRNAs were found to be differentially expressed between isoflurane and control groups. All eleven microRNAs were upregulated with a Benjamini-Hochberg adjusted p value to control the False Discovery Rate at 0.05 level (Fig. 1A).22 Bioinformatics analysis (Ingenuity Pathway Analysis Software, Redwood City, CA) was used to nominate a candidate for further functional studies. MicroRNAs were investigated that directly targeted proteins in cell signaling pathways previously reported to mediate anesthetic preconditioning. These microRNAs were compared to the in vivo and in vitro array data, as well as against known literature.
Fig. 1.
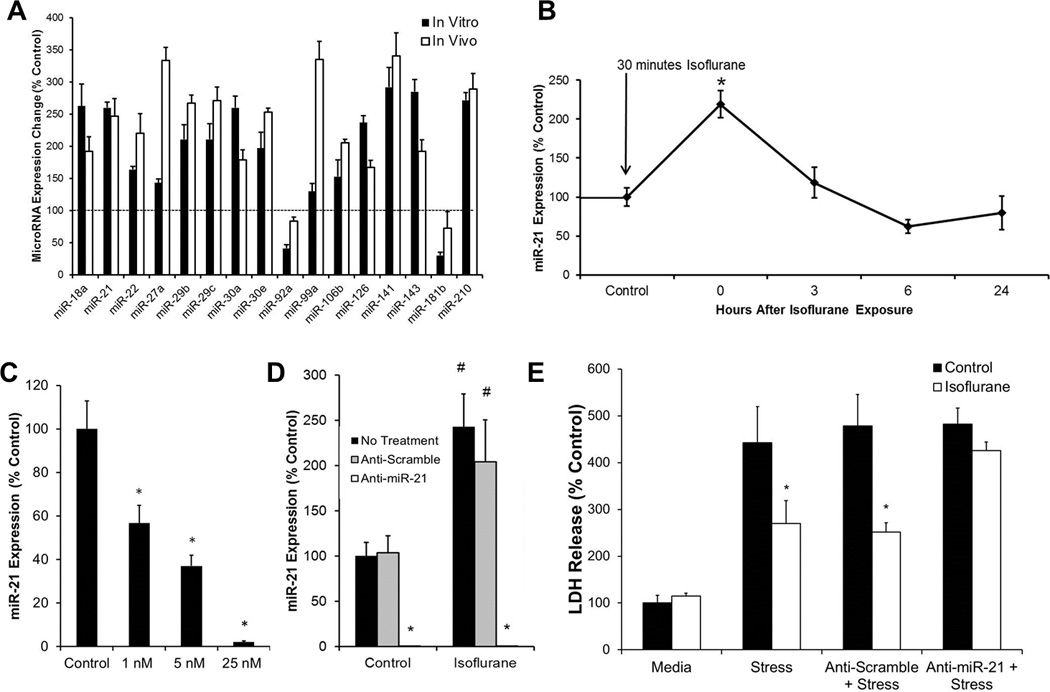
MicroRNAs play an important role in isoflurane-mediated protection of cardiomyocytes. (A) Isoflurane exposure causes changes in expression levels of microRNAs. MicroRNA expression changes were measured by quantitative real-time polymerase chain reaction in thein vitro neonatal rat cardiomyocyte model (black bars) and in the in vivo rat model (white bars) after exposure to thirty minutes of isoflurane followed by a fifteen minute washout period. n = 6; All data = P < 0.05 compared with control without isoflurane exposure.
(B) miR-21 is transiently upregulated by isoflurane in cardiomyocytes. miR-21 increased significantly after a 30 minute exposure to isoflurane, followed by a 15 minute washout period. miR-21 expression was measured 3, 6, and 24 hours after exposure. Expression decreased over time and returned to baseline after 3 hours. n = 6, *P < 0.05 compared to non-isoflurane exposed control. (C) Anti-miR-21 transfection dose-dependently knocks down expression of miR-21. Neonatal rat cardiomyocytes were treated with 1 nM, 5 nM, and 25 nM of transfection reagent for twenty hours. miR-21 expression was decreased in a dose-dependent manner and significantly knocked down in the 25 nM transfection group. n = 6, *P < 0.05 compared with control. (D) Anti-miR-21 transfection knocks down expression of miR-21 after isoflurane exposure. Cultured neonatal rat cardiomyocytes were treated with media, 25 nM of microRNA scramble inhibitor, or 25 nM of anti-miR-21 for twenty hours. miR-21 expression was increased in non-treated and scramble-transfected groups following isoflurane exposure. miR-21 expression was significantly knocked down in both control and isoflurane-exposed groups. n = 6, *P < 0.05 compared with non-treated and scramble transfected controls. #P < 0.05 compared with non-isoflurane exposed controls. (E) Anti-miR-21 abolishes the protective preconditioning effect of isoflurane. miR-21 transfected cells showed no significant difference in lactate dehydrogenase (LDH) release between isoflurane and control groups. Cells were exposed to 50 µM of hydrogen peroxide for six hours. Non-treated and scramble-transfected controls showed significantly decreased lactate dehydrogenase (LDH) release when exposed to 30 minutes of isoflurane prior to hydrogen peroxide exposure. n = 6, *P < 0.05 compared to controls.
MicroRNA-21 emerged as the strongest candidate for further functional studies, as it was linked to multiple cardioprotective cell signaling pathways, exerting effects on PDCD4, Akt, and PTEN.9,23–25 Additionally, miR-21 amplified at the lowest Ct values of all candidate microRNAs identified, indicating a higher relative abundance, and potentially, a more robust functional effect.
miR-21 is transiently upregulated in cardiomyocytes
Samples were taken following thirty minutes of isoflurane exposure, as well as after 3, 6, and 24 hour time points to determine miR-21 expression changes over time. At thirty minutes following isoflurane exposure, miR-21 was significantly upregulated (219.2% ± 17.1%). Three hours after the initial upregulation, miR-21 expression returned to baseline values. (Fig. 1B). qRT-PCR sample purity was confirmed by melt curve analysis and the presence of a single PCR product after size fractionation on a 4% agarose gel. (Supplemental Digital Content 1, Figure)
Dose-dependent knockdown of miR-21 expression with Lipofectamine
Neonatal rat cardiomyocytes were exposed to 20 hours of multiple doses of LNA-modified anti-miR-21 or scramble antagomirs. qRT-PCR analysis revealed a dose-dependent response of miR-21 knockdown at 1 nM (56.7% ± 8.1%), 5 nM (37.0% ± 4.9%), and 25 nM (2.1% ± 0.6%) of transfection reagent (Fig. 1C).
Anti-miR-21 transfection with Lipofectamine knocks down expression of miR-21 after isoflurane exposure
Neonatal rat cardiomyocytes were exposed to 20 hours of 25 nM anti-miR-21 or scramble antagomirs. qRT-PCR analysis revealed similar baseline levels of miR-21 between non-treated and scramble-transfected groups. Non-treated and scramble transfected groups also exhibited upregulation of miR-21 following isoflurane exposure similar to previous experiments. In anti-miR-21 transfected cells, both the isoflurane exposed (0.01 ± 0.002) and control cells (0.10% ± 0.06%) exhibited significantly decreased expression of miR-21 compared to non-transfected controls (Fig. 1D), confirming a complete knockdown.
Knockdown of miR-21 abolishes the preconditioning effect of isoflurane
Measurements of cell viability following six hours of oxidative stress with 50 µM hydrogen peroxide revealed an increased LDH release in non-transfected (442.3% ± 77.0%), scramble transfected (478% ± 66.9%), and miR-21 transfected (481.9% ± 34.9%) cells compared to non-stressed control cells. Exposure to isoflurane decreased LDH release in non-transfected (269.8% ± 49.2%) and scramble-transfected (251% ± 20.5%) cells, but not anti-miR-21 transfected (425% ± 18.1%) cells (Fig. 1E).
Knockdown of miR-21 abolishes the isoflurane-induced downregulation of PDCD4, but not PTEN
mRNA expression of miR-21 targets PDCD4 and PTEN were evaluated via qRT-PCR. PDCD4 mRNA expression was significantly decreased after isoflurane exposure. Western blot analysis also revealed that cells exposed to isoflurane exhibited significantly decreased expression of PDCD4 protein when compared to controls. Transfection with anti-miR-21 increased the endogenous levels of PDCD4 and abolished the isoflurane-induced downregulation of PDCD4 (Fig. 2A).
Fig. 2.
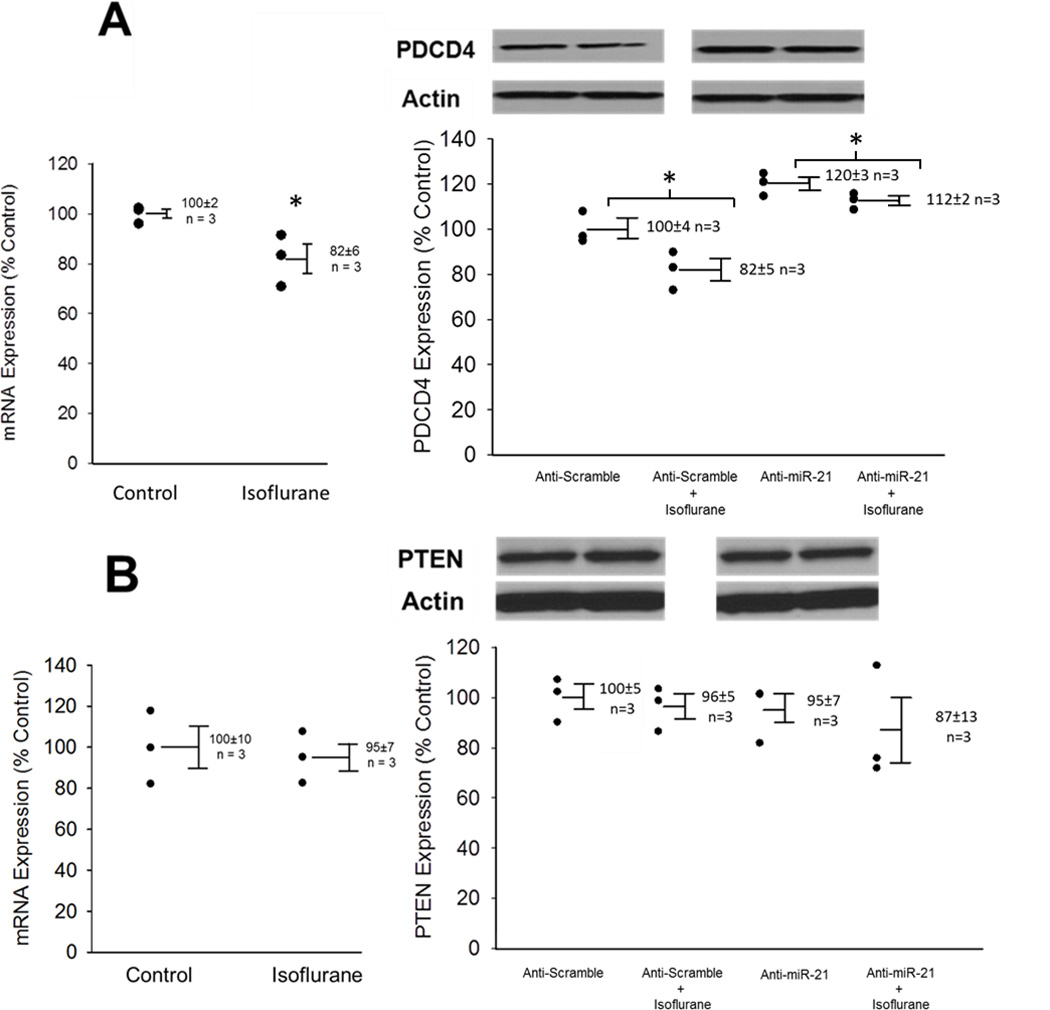
Anti-miR-21 abolishes the isoflurane-induced downregulation of programmed cell death protein 4 (PDCD4), but not phosphatase and tensin homolog (PTEN). (A) PDCD 4 expression. Cells were exposed to 30 minutes of isoflurane followed by a 15 minute washout period. Messenger RNA (mRNA) expression changes as measured by quantitative real-time polymerase chain reaction in cardiomyocytes and protein expression levels as determined by Western blot. In anti-scramble transfected cells, miR-21 expression was significantly decreased. In anti-miR-21 transfected cells, endogenous levels of PDCD4 were increased, and the isoflurane-induced downregulation of PDCD4 was abolished. n = 3, *p<0.05 compared to non-isoflurane controls. (B) PTEN expression. In anti-scramble and anti-miR-21 transfected cells, isoflurane did not influence PTEN expression at either mRNA or protein levels. In addition, miR-21 knocking down did not change endogenous PTEN expression. n = 3.
Both mRNA and Western blot analysis revealed no change in PTEN expression after isoflurane exposure. Anti-miR-21 transfection also had no effect on endogenous PTEN expression (Fig. 2B).
PDCD4 siRNA transfection with Lipofectamine knocks down expression of PDCD4
Neonatal rat cardiomyocytes were exposed to 10 nM of PDCD4 siRNA or medium guanine/cytosine content negative control siRNA. qRT-PCR analysis revealed that PDCD4 mRNA expression after siRNA knockdown was reduced to 25.7% ± 3.0% of control values (Fig. 3A). Western blot analysis showed that PDCD4 protein expression was reduced to 8.8% ± 1.3% of control values (Fig. 3B).
Fig. 3.
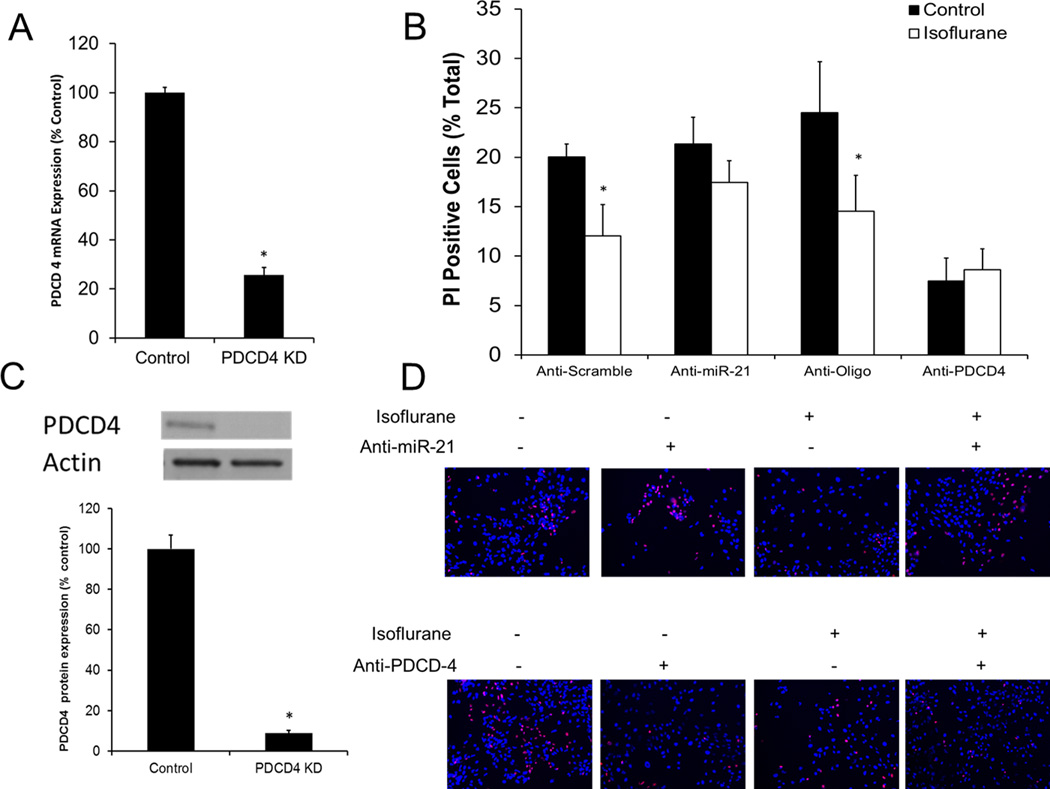
Programmed cell death protein 4 (PDCD4) small interfering RNA (siRNA) transfection knocks down expression of PDCD4. Neonatal rat cardiomyocytes were transfected with 10 nM of PDCD4 siRNA or the negative control. Twenty-four hours of transfection significantly decreased (A) siRNA knockdown of PDCD4 messenger RNA (mRNA) expression. n = 3, *p<0.05 compared to negative control transfected cells.(B) Anti-miR-21 knockdown and PDCD4 knockdown impair isoflurane preconditioning. All cells were exposed to six hours of 50 µM hydrogen peroxide. Anti-miR-21 abolished the cardioprotective effect of isoflurane, while PDCD4 knockdown mimicked the effect of isoflurane in non-exposed cells. n = 6, *p<0.05.(C) siRNA knockdown of PDCD4 protein expression. n = 3, *p<0.05 compared to negative control transfected cells. (D) Representative images from propidium iodide (PI) staining.
PDCD4 inhibition mimics the preconditioning effect of isoflurane in non-treated cells
Measurements of cell viability following six hours of oxidative stress with 50 µM hydrogen peroxide revealed that anti-PDCD4 transfection caused a protective effect in cardiomyocytes that mimicked the protective effect of isoflurane. Oligo-transfected cardiomyocytes were preconditioned with isoflurane (11.8% ± 1.9% PI-positive cells compared to 21.3% ± 3.6% of cells not exposed to isoflurane), while there was no significant difference in the protective effect of isoflurane in PDCD4 transfected cells (7.5% ± 2.3% control cells compared to 8.6% ± 2.1% isoflurane-exposed). As a comparison, anti-miR-21 transfected cells were also stained with propidium iodide. Scramble transfected cells were successfully preconditioned with isoflurane (10.6% ± 2.2% compared to 20.0% ± 1.3% non-isoflurane exposed cells). Transfection with 25 nM of anti-miR-21 abolished the protective effect of isoflurane (21% ± 2.7% compared to 17.5% ± 2.2%) (Fig. 3C). Representative PI staining images show decreased cell viability after miR-21 knockdown, and increased cell viability after PDCD4 knockdown (Fig. 3D).
miR-21 knockout abolishes isoflurane-induced decreases in infarct size
There were no significant differences in area at risk between WT and miR-21 knockout mice subjected to I/R injury with or without isoflurane exposure (Fig. 4A). Coronary occlusion followed by reperfusion resulted in an infarct size of 59.1 ± 4.2% and 62.3 ± 4.6% in WT and miR-21 knockout mice, respectively. Pretreatment of WT mice with isoflurane significantly decreased infarct size (44.1 ± 2.9%). However, disruption of miR-21 abolished isoflurane-induced decreases in infarct size (56.2 ± 3.2%) (Fig. 4B).
Fig. 4.
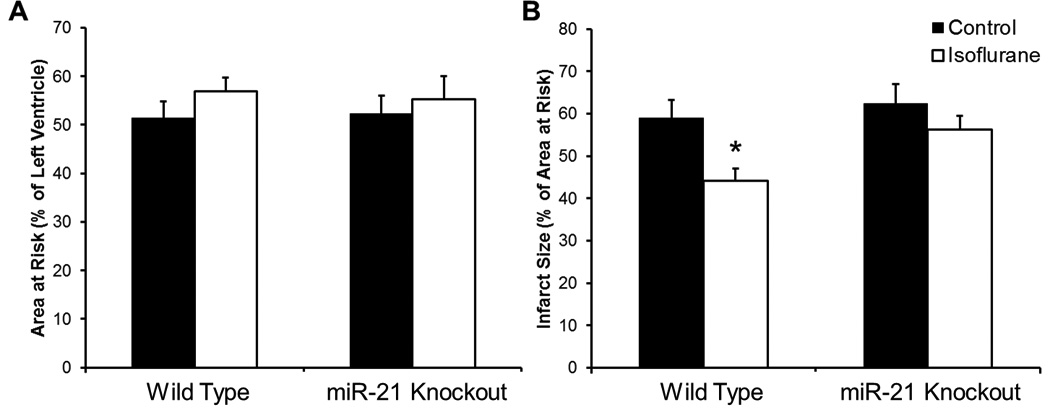
Isoflurane preconditioning is abolished in miR-21 knockout mice. Wild type (WT) and miR-21 knockout mice were exposed to 30 minutes of coronary artery occlusion followed by 2 hours of reperfusion with and without isoflurane exposure. (A) Area at risk measurements. There were no significant differences between area at risk in control or isoflurane exposed WT and miR-21 knockout mice. n = 8/group. (B) Isoflurane preconditioning is abolished in miR-21 knockout mice. WT mice were successfully preconditioned with isoflurane, while miR-21 knockout mice showed no significant difference between control and isoflurane exposed groups. n = 8/group, *p<0.05.
Discussion
In this study, we showed for the first time that isoflurane induces upregulation of miR-21, and that this upregulation is linked to protection of cardiomyocytes. Lipofectamine-mediated knockdown of miR-21 completely abolished the protective effect of isoflurane and resulted in increased expression of PDCD4, a well-established target of miR-21.23 Inhibition of PDCD4 resulted in a protective effect on control cells that mimicked the protective effect on isoflurane-treated cardiomyocytes. However, not all miR-21 targets that we investigated were suppressed. PTEN, a protein suppressed by miR-21 during ischemic postconditioning,24 showed no difference in expression levels after isoflurane exposure or miR-21 knockdown during our study. From this, we conclude that anesthetic preconditioning acts through a miR-21/PDCD4-mediated pathway. This protective effect was correlated in vivo, with miR-21 knockout mice failing to show significant decreases in infarct size after exposure to isoflurane when compared to control mice.
Our array results showed that preconditioning with isoflurane for 30 minutes altered the profiles of microRNAs in cardiomyocytes (Fig. 1). We used a Benjamini-Hochberg adjusted p value for false discovery rate, and found that 16 microRNAs were differentially expressed. When a more conservative Bonferroni correction is applied, one third of the microRNAs remain significant. In particular, isoflurane upregulated members of the miR-30 family, as well as miR-210 and miR-21. These microRNAs have been previously linked to cardioprotective pathways.26–28 The miR-30 family (miR-30a, b, c, d, and e) have recently been shown to be downregulated in response to hydrogen peroxide-induced stress in both neonatal rat ventricular cells and rat aorta vascular smooth muscle cells.28 miR-210 has been shown to be upregulated in cardiomyocytes under hypoxic conditions, and confers cardioprotection.27,28 Specially, acute upregulation of miR-21 was involved in ischemic and hypoxic protective signaling mechanisms in multiple cell types including cardiomyocytes.15,24,29 In the heart, acute upregulation of miR-21 has been shown to decrease myocardial infarct size, increase cell viability, and indirectly upregulate expression of numerous cardioprotective proteins.25,29 Upregulation of miR-21 has been linked to the protective effects of ischemic preconditioning in both an in vivo rat model and cultured cardiomyocytes.9,23 In other organs, miR-21 has been linked to protection as well. In 2013, Jia et al. showed that upregulation of miR-21 contributed to the protective effect of xenon preconditioning before ischemia-reperfusion injury in the mouse kidney model.15 These studies, as well as the link to multiple cell signaling pathways involved in anesthetic preconditioning, led us to focus our study on the dissection of the important role of miR-21 in isoflurane-mediated cardiomyocyte protection.
Following isoflurane exposure, miR-21 initially increased and then decreased back to baseline levels over a twenty-four hour period (Fig. 1B). Knocking down miR-21 attenuated the isoflurane-induced decrease of LDH in cardiomyocytes exposed to hydrogen peroxide (Fig. 1E), suggesting that acute upregulation of miR-21 after isoflurane exposure is sufficient to contribute to protection of cardiomyocytes. Several studies conflict on the long term effects of miR-21 upregulation, but an acute upregulation is well established as protective.30,31
Our results revealed that exposure to isoflurane decreased expression of both PDCD4 mRNA and protein levels compared to non-exposed controls. This decrease was abolished when miR-21 was knocked down. Endogenous levels of PDCD4 protein were raised, and the decrease following isoflurane exposure was not significant compared to non-exposed controls (Fig. 2A). Additionally, inhibition of PDCD4 expression led to protection in non-isoflurane exposed cardiomyocytes that was similar to the preconditioning effect of isoflurane (Fig. 3). It should be noted that the magnitude of PDCD4 suppression was greater than the downregulation seen after isoflurane exposure, so it is not surprising that the amount of protection conferred is greater. This suggests that PDCD4 is the downstream target mediated by miR-21 upregulation. PDCD4 is widely studied as a tumor suppressor,32 however, it has recently emerged as a target of miR-21 with powerful downstream effects. In renal I/R injury, miR-21 knockdown increased renal I/R injury accompanied by upregulation of PDCD4.33 In a cardioprotective response to chronic oxidative stress, neonatal cardiomyocytes were shown to upregulate expression of miR-21. Inhibition of miR-21 was linked to an increase in expression of PDCD4, and decreased cell viability.23
Interestingly, not all reported miR-21 targets previously implicated in preconditioning pathways were influenced by the presence of miR-21 or isoflurane. PTEN has been widely shown to be a direct target of miR-21 that is involved in cardioprotection, and was initially hypothesized to be a main contributor to isoflurane-induced cardioprotection. Ischemic postconditioning showed a significant upregulation miR-21 in mouse hearts which correlated with a significant upregulation in the PTEN/Akt signaling pathway.24 Extensive investigation of PTEN was conducted in our model, at multiple time points up to six hours after exposure to isoflurane. However, all time points failed to show a significant change in PTEN expression after isoflurane exposure or miR-21 knockdown. Specifically, thirty minutes of isoflurane exposure failed to decrease PTEN mRNA or protein levels compared to control values. miR-21 knockdown also failed to influence both endogenous and isoflurane-exposed levels of PTEN compared to control values (Fig. 2B).
To ensure that the results seen in vitro correlated to in vivo conditions, we tested whether the miR-21 knockout mouse could be successfully preconditioned with isoflurane. miR-21 knockout mice are created using a targeting vector which replaces the 93 bp precursor to miR-21 with a pGK-gb2 loxP/FRT-flanked neomycin resistance cassette.34 These mice are healthy and viable, and are capable of cardiac remodeling in response to stress.35 However, when exposed to isoflurane, these mice displayed no decrease in infarct size after I/R injury when compared to non-isoflurane exposed controls. Both the in vivo and in vitro data provide direct evidence that miR-21 is playing a critical role in the cardioprotection against I/R injury mediated by isoflurane.
One limitation of this study is the use of neonatal rat cardiomyocytes and hydrogen-peroxide-induced stress to dissect the important roles of miR-21 in isoflurane-induced protection of cardiomyocytes. This in vitro model may not be able to completely recapitulate the in vivo environments. However, the results found in neonatal rat cardiomyocytes parallel our work in the miR-21 knockout mouse, as well as previous work in our lab, studying anesthetic preconditioning in whole animal models, isolated heart preparations, and in human induced pluripotent stem cell-derived cardiomyocytes.36–38 Additionally, neonatal rat cardiomyocytes allow for a broad range of studies and an in-depth view of microRNA signaling pathways in a cardiomyocyte-specific model. When compared to isolated adult rat cardiomyocytes, neonatal myocytes have been shown to maintain a phenotype and contractile profile comparable with in situ hearts during I/R injury.39
The use of hydrogen peroxide to mediate reactive oxygen species (ROS) induced injury has also been used in previous studies to investigate the role of microRNAs in ischemic preconditioning, and in anesthetic preconditioning studies done previously in our lab.16,23,40 ROS-induced stress plays an important role not only in I/R injury, but also myocardial infarction, heart failure, and hypertrophy.41–44 Understanding the changes in expression levels of genes after ROS-induced injury can provide important insight into the pathogenesis of these diseases.
In conclusion, the results obtained in this study reveal for the first time that isoflurane directly affects microRNA expression profiles in cardiomyocytes, and that miR-21 is acting to protect cardiomyocytes after isoflurane exposure, most likely through its actions on PDCD4. This shows that while isoflurane-induced cardioprotection shares many pathways with ischemic, hypoxic, and heat shock preconditioning, the mechanisms are not identical. Investigating unique, isoflurane-mediated cardioprotective pathways may lead to mechanisms by which infarct size after ischemia reperfusion injury may be decreased, leading to improved patient outcomes following cardiac events.
Supplementary Material
Supplemental Digital Content 1, Figure. miScript primer assays selectively target expression of miR-21. (A) miR-21 quantitative real-time polymerase chain reaction (qRT-PCR) run only with synthetic miR-21 mimic. miR-21 mimics amplify when run with miScript primer assays specific for miR-21. Melt curve occurs in the 70° – 80° C range, as shown by (B) Melt curve (C) Melt Peak and (D) Melt Temperature Analysis. n = 6. (E) miR-21 RT-PCR products generated by stem-loop RT-PCR size fractionated on 2% agarose gel. Samples include M: 100 bp marker, and two samples of Rnu-6 (housekeeping gene, 107 bp) and miR-21 (122 bp).
Acknowledgements
The authors gratefully acknowledge the statistical services of Qun Xiang, M.S. and Aniko Szabo, Ph.D. of the Biostatistics Consulting Service at the Medical College of Wisconsin, supported in part, by the National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, Maryland, USA through Grant Number 8UL1TR000055.
Supported by grants P01GM066730 and R01HL034708 from the National Institutes of Health, Bethesda, Maryland, USA and by FP00003109 from Advancing Healthier Wisconsin Research and Education Initiative Fund, Milwaukee, Wisconsin, USA (to Dr. Zeljko J. Bosnjak), and by grant R01GM112696 from the National Institutes of Health, Bethesda, Maryland, USA (to Dr. Xiaowen Bai).
Footnotes
Part of this work has been presented at the American Society of Anesthesiologists meeting, San Francisco, California, USA, October 15, 2013.
The authors declare no competing interests.
Abcam neonatal rat cardiomyocyte harvest protocol. Accessed January 3, 2014. Available at: http://www.abcam.com/ps/pdf/protocols/neonatal_rat_cardiomyocyte_harvest.pdf
References
- 1.Najjar SS, Rao SV, Melloni C, Raman SV, Povsic TJ, Melton L, Barsness GW, Prather K, Heitner JF, Kilaru R, Gruberg L, Hasselblad V, Greenbaum AB, Patel M, Kim RJ, Talan M, Ferrucci L, Longo DL, Lakatta EG, Harrington RA. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: A randomized controlled trial. JAMA. 2011;305:1863–1872. doi: 10.1001/jama.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lonborg J, Vejlstrup N, Kelbaek H, Holmvang L, Jorgensen E, Helqvist S, Saunamaki K, Ahtarovski KA, Botker HE, Kim WY, Clemmensen P, Engstrom T. Final infarct size measured by cardiovascular magnetic resonance in patients with ST elevation myocardial infarction predicts long-term clinical outcome: An observational study. Eur Heart J Cardiovasc Imaging. 2013;14:387–395. doi: 10.1093/ehjci/jes271. [DOI] [PubMed] [Google Scholar]
- 3.Amour J, Brzezinska AK, Weihrauch D, Billstrom AR, Zielonka J, Krolikowski JG, Bienengraeber MW, Warltier DC, Pratt PF, Jr, Kersten JR. Role of heat shock protein 90 and endothelial nitric oxide synthase during early anesthetic and ischemic preconditioning. Anesthesiology. 2009;110:317–325. doi: 10.1097/ALN.0b013e3181942cb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedlic F, Sepac A, Pravdic D, Camara AK, Bienengraeber M, Brzezinska AK, Wakatsuki T, Bosnjak ZJ. Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane: Roles of ROS and Ca2+ Am J Physiol Cell Physiol. 2010;299:C506–C515. doi: 10.1152/ajpcell.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stadnicka A, Marinovic J, Bienengraeber M, Bosnjak ZJ. Impact of in vivo preconditioning by isoflurane on adenosine triphosphate-sensitive potassium channels in the rat heart: Lasting modulation of nucleotide sensitivity during early memory period. Anesthesiology. 2006;104:503–510. doi: 10.1097/00000542-200603000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol. 2007;292:C1583–C1590. doi: 10.1152/ajpcell.00221.2006. [DOI] [PubMed] [Google Scholar]
- 7.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: Reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–370. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Leucker TM, Bienengraeber M, Muravyeva M, Baotic I, Weihrauch D, Brzezinska AK, Warltier DC, Kersten JR, Pratt PF., Jr Endothelial-cardiomyocyte crosstalk enhances pharmacological cardioprotection. J Mol Cell Cardiol. 2011;51:803–811. doi: 10.1016/j.yjmcc.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lishmanov Iu B, Maslov LN, Khaliulin IG, Zhang Y, Pei J. [Role of heat shock proteins, aldose reductase, Bcl-2 protein and microRNA in the mechanism of delayed preconditioning of heart] Ross Fiziol Zh Im I M Sechenova. 2010;96:472–488. [PubMed] [Google Scholar]
- 12.Yin C, Salloum FN, Kukreja RC. A novel role of microRNA in late preconditioning: upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circ Res. 2009;104:572–575. doi: 10.1161/CIRCRESAHA.108.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa M, Tanaka S, Arai M, Genda Y, Sakamoto A. Differences in microRNA changes of healthy rat liver between sevoflurane and propofol anesthesia. Anesthesiology. 2012;117:1245–1252. doi: 10.1097/ALN.0b013e3182746676. [DOI] [PubMed] [Google Scholar]
- 14.Cao L, Feng C, Li L, Zuo Z. Contribution of microRNA-203 to the isoflurane preconditioning-induced neuroprotection. Brain Res Bull. 2012;88:525–528. doi: 10.1016/j.brainresbull.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia P, Teng J, Zou J, Fang Y, Zhang X, Bosnjak ZJ, Liang M, Ding X. miR-21 contributes to xenon-conferred amelioration of renal ischemia-reperfusion injury in mice. Anesthesiology. 2013;119:621–630. doi: 10.1097/ALN.0b013e318298e5f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamnicki-Abegg M, Weihrauch D, Pagel PS, Kersten JR, Bosnjak ZJ, Warltier DC, Bienengraeber MW. Isoflurane inhibits cardiac myocyte apoptosis during oxidative and inflammatory stress by activating Akt and enhancing Bcl-2 expression. Anesthesiology. 2005;103:1006–1014. doi: 10.1097/00000542-200511000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Hirata N, Shim YH, Pravdic D, Lohr NL, Pratt PF, Jr, Weihrauch D, Kersten JR, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane differentially modulates mitochondrial reactive oxygen species production via forward versus reverse electron transport flow: Implications for preconditioning. Anesthesiology. 2011;115:531–540. doi: 10.1097/ALN.0b013e31822a2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bienengraeber M, Pellitteri-Hahn M, Hirata N, Baye TM, Bosnjak ZJ, Olivier M. Quantitative characterization of changes in the cardiac mitochondrial proteome during anesthetic preconditioning and ischemia. Physiol Genomics. 2013;45:163–170. doi: 10.1152/physiolgenomics.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funari VA, Winkler M, Brown J, Dimitrijevich SD, Ljubimov AV, Saghizadeh M. Differentially expressed wound healing-related microRNAs in the human diabetic cornea. PLoS One. 2013;8:e84425. doi: 10.1371/journal.pone.0084425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge ZD, Ionova IA, Vladic N, Pravdic D, Hirata N, Vasquez-Vivar J, Pratt PF, Jr, Warltier DC, Pieper GM, Kersten JR. Cardiac-specific overexpression of GTP cyclohydrolase 1 restores ischaemic preconditioning during hyperglycaemia. Cardiovasc Res. 2011;91:340–349. doi: 10.1093/cvr/cvr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge ZD, van der Hoeven D, Maas JE, Wan TC, Auchampach JA. A(3) adenosine receptor activation during reperfusion reduces infarct size through actions on bone marrow-derived cells. J Mol Cell Cardiol. 2010;49:280–286. doi: 10.1016/j.yjmcc.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green GH, Diggle PJ. On the operational characteristics of the Benjamini and Hochberg False Discovery Rate procedure. Stat Appl Genet Mol Biol. 2007;6 doi: 10.2202/1544-6115.1302. Article 27. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol. 2009;47:5–14. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu Y, Wan L, Fan Y, Wang K, Bu L, Huang T, Cheng Z, Shen B. Ischemic postconditioning-mediated miRNA-21 protects against cardiac ischemia/reperfusion injury via PTEN/Akt pathway. PLoS One. 2013;8:e75872. doi: 10.1371/journal.pone.0075872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinkel R, Penzkofer D, Zuhlke S, Fischer A, Husada W, Xu QF, Baloch E, van Rooij E, Zeiher AM, Kupatt C, Dimmeler S. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation. 2013;128:1066–1075. doi: 10.1161/CIRCULATIONAHA.113.001904. [DOI] [PubMed] [Google Scholar]
- 27.Mutharasan RK, Nagpal V, Ichikawa Y, Ardehali H. microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects. Am J Physiol Heart Circ Physiol. 2011;301:H1519– H1530. doi: 10.1152/ajpheart.01080.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen M, Ma G, Yue Y, Wei Y, Li Q, Tong Z, Zhang L, Miao G, Zhang J. Downregulation of the miR-30 family microRNAs contributes to endoplasmic reticulum stress in cardiac muscle and vascular smooth muscle cells. Int J Cardiol. 2014;173:65–73. doi: 10.1016/j.ijcard.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Haider KH, Idris NM, Kim HW, Ahmed RP, Shujia J, Ashraf M. MicroRNA-21 is a key determinant in IL-11/Stat3 anti-apoptotic signalling pathway in preconditioning of skeletal myoblasts. Cardiovasc Res. 2010;88:168–178. doi: 10.1093/cvr/cvq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac injury and protection. Mol Pharmacol. 2011;80:558–564. doi: 10.1124/mol.111.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 32.Vikhreva PN, Shepelev MV, Korobko EV, Korobko IV. Pdcd4 tumor suppressor: Properties, functions, and possible applications in oncology. Molecular Genetics, Microbiology and Virology. 2010;25:47–55. [Google Scholar]
- 33.Xu X, Kriegel AJ, Liu Y, Usa K, Mladinov D, Liu H, Fang Y, Ding X, Liang M. Delayed ischemic preconditioning contributes to renal protection by upregulation of miR-21. Kidney Int. 2012;82:1167–1175. doi: 10.1038/ki.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma X, Kumar M, Choudhury SN, Becker Buscaglia LE, Barker JR, Kanakamedala K, Liu MF, Li Y. Loss of the miR-21 allele elevates the expression of its target genes and reduces tumorigenesis. Proc Natl Acad Sci U S A. 2011;108:10144–10149. doi: 10.1073/pnas.1103735108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patrick DM, Montgomery RL, Qi X, Obad S, Kauppinen S, Hill JA, van Rooij E, Olson EN. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120:3912–3916. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tampo A, Hogan CS, Sedlic F, Bosnjak ZJ, Kwok WM. Accelerated inactivation of cardiac L-type calcium channels triggered by anaesthetic-induced preconditioning. Br J Pharmacol. 2009;156:432–443. doi: 10.1111/j.1476-5381.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.An J, Rhodes SS, Jiang MT, Bosnjak ZJ, Tian M, Stowe DF. Anesthetic preconditioning enhances Ca2+ handling and mechanical and metabolic function elicited by Na+-Ca2+ exchange inhibition in isolated hearts. Anesthesiology. 2006;105:541–549. doi: 10.1097/00000542-200609000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Canfield SG, Sepac A, Sedlic F, Muravyeva MY, Bai X, Bosnjak ZJ. Marked hyperglycemia attenuates anesthetic preconditioning in human-induced pluripotent stem cell-derived cardiomyocytes. Anesthesiology. 2012;117:735–744. doi: 10.1097/ALN.0b013e3182655e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamashita N, Nishida M, Hoshida S, Kuzuya T, Hori M, Taniguchi N, Kamada T, Tada M. Induction of manganese superoxide dismutase in rat cardiac myocytes increases tolerance to hypoxia 24 hours after preconditioning. J Clin Invest. 1994;94:2193–2199. doi: 10.1172/JCI117580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogbi M, Johnson JA. Protein kinase Cepsilon interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning. Biochem J. 2006;393:191–199. doi: 10.1042/BJ20050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lefer DJ, Granger DN. Oxidative stress and cardiac disease. Am J Med. 2000;109:315–323. doi: 10.1016/s0002-9343(00)00467-8. [DOI] [PubMed] [Google Scholar]
- 42.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takano H, Zou Y, Hasegawa H, Akazawa H, Nagai T, Komuro I. Oxidative stress-induced signal transduction pathways in cardiac myocytes: Involvement of ROS in heart diseases. Antioxid Redox Signal. 2003;5:789–794. doi: 10.1089/152308603770380098. [DOI] [PubMed] [Google Scholar]
- 44.Berg K, Jynge P, Bjerve K, Skarra S, Basu S, Wiseth R. Oxidative stress and inflammatory response duringand following coronary interventions for acute myocardial infarction. Free Radic Res. 2005;39:629–636. doi: 10.1080/10715760400028027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1, Figure. miScript primer assays selectively target expression of miR-21. (A) miR-21 quantitative real-time polymerase chain reaction (qRT-PCR) run only with synthetic miR-21 mimic. miR-21 mimics amplify when run with miScript primer assays specific for miR-21. Melt curve occurs in the 70° – 80° C range, as shown by (B) Melt curve (C) Melt Peak and (D) Melt Temperature Analysis. n = 6. (E) miR-21 RT-PCR products generated by stem-loop RT-PCR size fractionated on 2% agarose gel. Samples include M: 100 bp marker, and two samples of Rnu-6 (housekeeping gene, 107 bp) and miR-21 (122 bp).


