Abstract
Background
We have shown previously that inhaled anesthetics disrupt the interaction between the second postsynaptic density protein-95, Drosophila disc large tumor suppressor, and zonula occludens-1 (PDZ) domain of postsynaptic density protein-95 (PSD-95) and the C-terminus of N-methyl-D-aspartate receptor subunits NR2A and NR2B. Our data indicate that PDZ domains may serve as a molecular target for inhaled anesthetics. However, the underlying molecular mechanisms remain to be illustrated.
Methods
Glutathione S-transferase pull-down assay, co-immunoprecipitation and yeast two-hybrid analysis were used to assess PDZ domain-mediated protein-protein interactions in different conditions. Nuclear magnetic resonance spectroscopy was used to investigate isoflurane-induced chemical shift changes in the PDZ1–3 domains of PSD-95. A surface plasmon resonance-based BIAcore assay was used to examine the ability of isoflurane to inhibit the PDZ domain-mediated protein-protein interactions in real time.
Results
Halothane and isoflurane dose dependently inhibited PDZ domain-mediated interactions between PSD-95 and Shaker-type potassium channel Kv1.4 and between α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunit GluA2 and its interacting proteins— glutamate receptor interacting protein or protein interacting with c kinase 1. However, halothane and isoflurane had no effect on PDZ domain-mediated interactions between γ-aminobutyric acid, type B receptor and its interacting proteins. The inhaled anesthetic isoflurane mostly affected the residues close to or in the peptide binding groove of PSD-95 PDZ1 and PDZ2 (especially PDZ2), while barely affecting the peptide binding groove of PSD-95 PDZ3.
Conclusion
These results suggest that inhaled anesthetics interfere with PDZ domain-mediated protein-protein interactions at several receptors important to neuronal excitation, anesthesia and pain processing.
Introduction
Anesthetics have been used in surgical procedures for well over a hundred years, but the molecular mechanism that underlies inhalational anesthesia is still poorly understood. Ion channels and receptors at synapses of the central nervous system (CNS) have been highlighted as potential targets for inhaled anesthetics1–5. In the CNS, these ion channels and receptors are linked to their downstream signaling pathways through PDZ domain-mediated protein-protein interactions. The PDZ domain can recognize and bind to specific amino acid sequences, including those at the C-termini of receptors and ion channels6–8. Our previous studies have shown that knockdown of PDZ domain-containing scaffolding protein postsynaptic density protein-95 (PSD-95) facilitates isoflurane anesthesia9 and inhibits chronic pain behaviors10;11. PSD-95, a member of the membrane-associated guanylate kinase family of proteins that assemble protein complexes at synapses, is composed of three tandem PDZ domains (PDZ1–3) at the N-terminus, and guanylate kinase and Src homology 3 domains12–14. The first two PDZ domains of PSD-95 can bind the NR2A and NR2B subunits of N-methyl-D-aspartate (NMDA) receptors, neuronal nitric oxide synthase (nNOS), Shaker-type potassium channel Kv1.4, and Src family tyrosine kinase7;15–17. Furthermore, we have demonstrated that PDZ domain-mediated interactions between PSD-95 and NMDA receptors or nNOS are disrupted by clinically relevant concentrations of inhaled anesthetic halothane18. By injecting cell-permeable peptide Tat-PSD-95 PDZ2 (comprising the second PDZ domain of PSD-95) intraperitoneally into mice, we also showed that disrupting PSD-95 PDZ domain-mediated protein-protein interactions reduces the threshold for halothane anesthesia19 and diminishes chronic inflammatory pain20. Taken together, these results suggest that the PDZ domain at synapses of the CNS may be an important molecular target for inhaled anesthetics and that alteration of PDZ domain-mediated protein-protein interactions may contribute to the molecular mechanism of inhaled anesthesia. However, it is unclear whether and how inhaled anesthetics interact with different PDZ domains in the CNS.
In the present study, we utilized nuclear magnetic resonance (NMR) and other state-of-the-art techniques to examine in greater detail the nature of the anesthetic interaction with the PDZ protein binding domains.
Materials and Methods
Glutathione S-transferase (GST) Pull-down Assay
GST and GST fusion proteins (GST-PSD-95 PDZ2 and GST-Kv1.4 C-terminus 100) were prepared in BL21 (Novagen, Darmstadt, Germany) with glutathione sepharose 4B (Amersham Pharmacia Biotech Inc., Piscataway, NJ) as an affinity resin for purification. All animal experiments were conducted with the approval of the Animal Care and Use Committee at Johns Hopkins University, Baltimore, Maryland, USA and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Membrane-bound proteins were extracted from mouse cerebral cortex and solubilized. Mouse cerebral cortex was lysed in homogenizing buffer [10 mM Tris-HCl (pH 8.0), 5 mM MgCl2, 250 mM sucrose, 2 mM EGTA, 1 mM sodium orthovanadate, 1 mM DTT, 1 mM phenylmethanesulfonylfluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 20 μg/ml pepstatin A] with a rotor-stator Polytron homogenizer (Brinkmann Instruments, Westbury, NY). The homogenized solution was centrifuged at 900 × g for 15 min, and the supernatant was removed and centrifuged again at 37,000 × g for 40 min. The precipitate was solubilized with resuspension buffer [500 mM Tris-HCl, 1% (vol/vol) TritonX-100, and 1% (wt/vol) sodium deoxycholate] and centrifuged at 37,000 × g for 20 min. The supernatant contained the membrane-bound proteins. For binding experiments, glutathione sepharose 4B (40 μl) was pre-incubated with 1 nmol of GST or GST fusion protein for 30 min, and then the membrane-bound proteins (400 μg) were mixed with the GST fusion protein at room temperature for 1 h in the presence of different concentrations of halothane delivered in an incubation chamber (described under “Yeast Two-hybrid Analysis”). After the resin was washed five times with washing buffer (500 mM NaCl, 0.1% Triton X-100, 1 mM phenylmethanesulfonylfluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 20 μg/ml pepstatin A in phosphate-buffered saline), the bound proteins were eluted in SDS-PAGE sample buffer, separated by electrophoresis, and detected by immunoblotting with anti-PSD-95 (Chemicon, Temecula, CA), anti-Kv1.4 (Chemicon), and anti-GST (Santa Cruz Biotechnology Inc., Santa Cruz, CA) antibodies.
Co-immunoprecipitation Assay
The membrane-bound proteins were prepared as described above. The affinity-purified rabbit anti-Kv1.4 antibody (4 μg) was incubated with 100 μl of protein A-sepharose slurry for 1 h. The solubilized membrane proteins (400 μg) were then added to the sepharose beads, and the mixture was incubated with different concentrations of isoflurane for 2 h at room temperature. The mixture was washed once with 1% Triton X-100 in immunoprecipitation buffer [containing (in mM): 137 NaCl, 2.7 KCl, 4.3 Na2HPO4, 1.4 KH2PO4, 5 EGTA, 1 sodium vanadate, 10 sodium pyrophosphate, 50 NaF, and 0.1 phenylmethylsulfonyl fluoride plus 20 U/ml Trasylol], twice with 1% Triton X-100 in immunoprecipitation buffer plus 300 mM NaCl, and three times with immunoprecipitation buffer. The bound proteins were separated by SDS-PAGE and detected by immunoblotting with anti-PSD-95 and anti-Kv1.4 antibodies. As a positive control (input), 40 μg of the solubilized membrane proteins were loaded onto the gel. The specificity of the anti-Kv1.4 antibody was verified by preincubation with Kv1.4 fusion peptide.
Yeast two-hybrid analysis
The two partners in each interaction were subcloned into EcoRI and BamHI sites of the yeast vectors pGADT7 and pGBKT7 (Clontech Laboratories Inc., Mountain View, CA), respectively. The yeast reporter strain AH109 (Clontech Laboratories Inc.) was transformed with the pGBT9 and pGAD424 plasmids. Protein-protein interactions were evaluated as described in our previous study18 by growth of yeast on selective agar plates sealed in incubator chambers containing different concentrations of isoflurane, which was introduced into the chambers by its conventional calibrated anesthesia vaporizer (OHIO Medical Co., Gurnee, IL) for 30 min of equilibrium with air at 3 l/min. Anesthetic concentration within the chamber was determined with a Capnomac Ultima analyzer (Datex-Engstrom, Helsinki, Finland), and the yeast medium/gas partition coefficient for isoflurane at 30°C was determined by gas chromatography (Hewlett Packard 5890 series II plus, Agilent Technologies, Inc., Santa Clara, CA) according to the method described previously18;21. Concentration of halothane or isoflurane in the medium was calculated in millimolar units. The amount of yeast growth was quantified by measuring the average intensity of the yeast on the selective agar plates with Image-J software (National Institute of Mental Health, Bethesda, MD).
NMR investigation
The PDZ123 domain of Rat source PSD95 protein [residues 61–39322, PDZ1 61-151, PDZ2 155-247, PDZ3 302-393] was incorporated into the pET28 vector and expressed within E. Coli. The N-terminus of the protein was extended with His Tag for facilitating the Ni affinity column purification. The 15N labeled sample was at concentration of 5–6.8 mg/ml or 125–170 μM in elution buffer (50mM NaH2PO4/Na2HPO4 pH8.0, 300 mM NaCl, and 250 mM imidazole). The samples were aliquoted about 1 ml and saved at −80°C. For NMR experiments, the aliquoted sample was exchanged to NMR buffer (50 mM NaH2PO4/Na2HPO4 pH6.7, 50 mM NaCl) through diafiltration; the protein concentration was about 100 μM. The peptides composed of the last 20 amino acids from the C-terminal of NR2A (LNSCSNRRVYKKMPSIESDV) and NR2B (FNGSSNGHVYEKLSSIESDV) were synthesized and purified by United Peptide Corporation as lyophilized powder. The peptides were dissolved with NMR buffer to 10 mM (~ 2.5% w/v) in clean glass vial and visually checked for solubility. The reported binding affinity for PDZ-domain C-terminal peptide normally ranges from 10−8 M to 10−6 M23. A peptide containing the C-terminal nine amino acid residues of NR2B binds PDZ1, PDZ2, and PDZ3 of SAP102 (a PSD-95 family protein) with EC50 values of 30 nM, 6 nM, and 1 μM, respectively24. In our experiments, the peptides were titrated up to 310 μM. The isoflurane concentration was calibrated by 19F NMR with an external reference of trifluoroacetic acid. 20 μl D2O and 2 μl 100 mM DSS were added to each sample (~ 500 μl) for NMR signal locking and reference, respectively. NMR spectra were recorded at 298K on a Bruker-BioSpin Avance 600 spectrometer using a cryoprobe. The chemical-shift perturbation was quantified as the combined 1H and 15N chemical-shifts change with the following equation
Surface plasmon resonance analysis
The synthetic peptide corresponding to the C-terminal tail of NR2B was synthesized (Invitrogen, Carlsbad, CA) with an additional cysteine residue for thiol coupling to a biosensor CM5 chip (BIAcore, Uppsala, Sweden)18;25. The peptide was immobilized to the biosensor CM5 chip surface by using the standard maleimide coupling with m-maleimidobutyryloxy-sulfosuccinimide ester (sulfo-GMBS). First, the matrix surface was activated by injecting a 1:1 mixture of 0.1 M N-hydroxysuccinimide and 0.4 M 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide at a flow rate of 10 μl/min at 25°C for 10 min. Second, amine groups were introduced with 10 M ethylenediamine solubilized in 0.1 M sodium borate (pH 8.5) for 10 min. Third, maleimide groups were introduced with 50 mM sulfo-GMBS solubilized in 0.1 M sodium borate (pH 8.5) for 20 min and then 100 μl of NR2B peptide (100 μM) was injected and immobilized onto the matrix. The remaining active sites of the matrix were blocked with 50 mM cysteine. PSD-95 PDZ2 was subcloned into pET28a (Stratagene, La Jolla, CA) and expressed in BL21. Expressed proteins were purified with nickel NTA agarose (Qiagen, Valencia, CA). The interaction between PSD-95 PDZ2 and NR2B C-terminal peptide was analyzed on a BIAcore 2000 system (BIAcore). PSD-95 PDZ2 (20 μM) was injected with different concentrations of isoflurane over the NR2B peptide-coupled surface at a flow rate of 5 μl/min. Anesthetic concentrations were determined by head-space sampling from an aliquot of the anesthetic-containing solution. Sensorgrams were recorded and normalized by subtracting the baseline resonance unit value. The baseline sensorgram was obtained by injecting PSD-95 PDZ2 over a non-protein, blocked surface. Between successive measurements, the surface was regenerated with 50 mM phosphoric acid (3 min contact time). The analysis of kinetic parameters was performed with BIAevaluation version 3.0 software according to the manufacturer’s instructions.
Statistical analysis
Data from yeast two-hybrid experiments were expressed as means ± standard deviation and statistically analyzed with SigmaStat version 3.1 software (Systat Software Inc., Chicago, IL) by using one-way analysis of variance followed by Student-Newman-Keul’s method. The nature of hypothesis testing is two-tailed. The sample sizes were selected based on our previous study18. Statistical significance was set at P < 0.05.
Results
Effects of Halothane and Isoflurane on PDZ Domain-mediated Interactions between NMDA Receptor-interacting Protein PSD-95 and Kv1.4
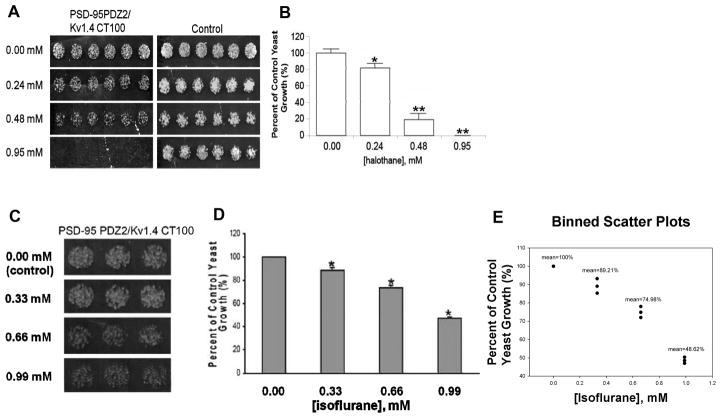
Our previous studies showed that PDZ domain-mediated protein-protein interactions between PSD-95 and NMDA receptors or nNOS are disrupted by clinically relevant concentrations of inhaled anesthetics 18. Here, we used a yeast two-hybrid approach to investigate whether halothane and isoflurane also disrupt PDZ domain-mediated interactions between PSD-95 and potassium channel Kv1.4. In the absence of anesthetic, the second PDZ domain of PSD-95 (PSD-95 PDZ2) interacted with the C-terminal tail of Kv1.4 (Kv1.4 CT100), as evidenced by the growth of the yeast cells harboring both pGADT7-PSD-95 PDZ2 and pGBKT7-Kv1.4 CT100 fusion protein plasmids in synthetic dropout agar plates lacking adenine, leucine, tryptophan, and histidine (Fig. 1). However, the yeast cell growth was slowed by halothane (Fig. 1, A and B) and isoflurane (Fig. 1, C–E) in a dose-dependent manner, indicating that these anesthetics dose-dependently inhibit PDZ domain-mediated protein-protein interactions between PSD-95 and Kv1.4. Compared to isoflurane, halothane disrupted the interactions with a stronger potency. A high but still clinically relevant concentration of halothane (0.95 mM) completely blocked the yeast cell growth (Fig. 1, A and B). We verified that halothane itself was not cytotoxic to yeast cells by growing the same strain on low-stringency media (synthetic dropout agar plates that lack leucine and tryptophan and are selective for the yeast cells co-transformed with pGADT7 and pGBKT7) in the presence of the same concentrations of halothane (Fig. 1A); halothane did not inhibit the growth of the control yeast cells.
Fig. 1. Halothane and isoflurane dose-dependently disrupt PDZ domain-mediated interactions between PSD-95 and potassium channel Kv1.4 in a yeast two-hybrid system.
(A and B) The effect of halothane on the growth of yeast cells harboring pGADT7-PSD-95 PDZ2 and pGBKT7-Kv1.4 CT100 (n = 6). (A) Halothane dose-dependently inhibited the yeast growth. (B) Yeast growth is shown as a percent of control, relative to halothane concentration. *p < 0.05 and **p < 0.01 vs. 0.00 mM halothane. (C–E) The effect of isoflurane on the growth of yeast cells harboring pGADT7-PSD-95 PDZ2 and pGBKT7-Kv1.4 CT100 (n = 3). (C) Isoflurane dose-dependently inhibited the yeast growth. (D) Yeast growth is shown as a percent of control, relative to isoflurane concentration. *p < 0.05 vs. 0.00 mM isoflurane. (E) Binned scatter plots of (C). CT100, C-terminal 100 amino acids; PDZ, postsynaptic density protein-95, Drosophila disc large tumor suppressor, and zonula occludens-1; PSD-95, postsynaptic density protein-95.
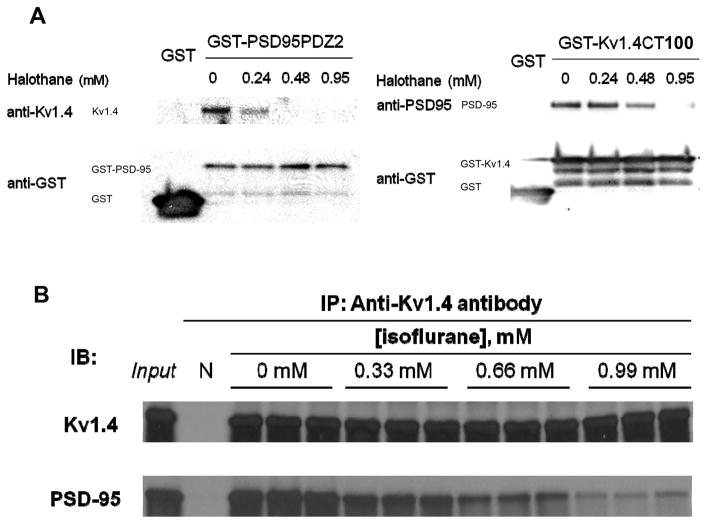
Next, we used GST pull-down and co-immunoprecipitation assays to further confirm the yeast two-hybrid results. GST-PSD-95 PDZ2 and GST-Kv1.4 CT100, but not GST alone, precipitated Kv1.4 and PSD-95 (Fig. 2A), in the absence of halothane. However, halothane dose-dependently inhibited the binding of Kv1.4 to GST-PSD-95 PDZ2 and the binding of PSD-95 to GST-Kv1.4 CT100 (Fig. 2A). To determine whether inhaled anesthetics disrupt PDZ domain-mediated protein-protein interactions in a physiological setting, we used a co-immunoprecipitation assay to detect in vivo binding of PSD-95 to Kv1.4. We found that immunoprecipitation of Kv1.4 by its specific antibody resulted in co-precipitation of PSD-95 under normal conditions but that isoflurane dose-dependently inhibited the co-precipitation of PSD-95 (Fig. 2B). These results suggest that inhaled anesthetics may disrupt the physiological complex of PSD-95 and Kv1.4 in the CNS.
Fig. 2. Halothane and isoflurane dose-dependently disrupt the association between PSD-95 and potassium channel Kv1.4 in the forebrain.
(A) The GST pull-down assay showed that halothane dose-dependently inhibits the interaction between PSD-95 PDZ2 and Kv1.4 C-terminus. The bottom panels indicate equal amounts of loading of GST-fusion proteins. (B) The co-immunoprecipitation assay showed that isoflurane dose-dependently disrupts the association between PSD-95/SAP90 and Kv1.4 in vivo. The specificity of the anti-Kv1.4 antibody was verified by preincubation with Kv1.4 fusion peptide (N). As a positive control (input), 40 μg of the solubilized membrane proteins were loaded onto the gel. IP, immunoprecipitation; IB, immunoblotting; GST, glutathione S-transferase; PSD-95, postsynaptic density protein-95.
Effects of Halothane and Isoflurane on PDZ Domain-mediated Interactions Between α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) Receptor and AMPA Receptor-interacting proteins protein interacting with c kinase 1 (PICK1) or glutamate receptor interacting protein (GRIP)
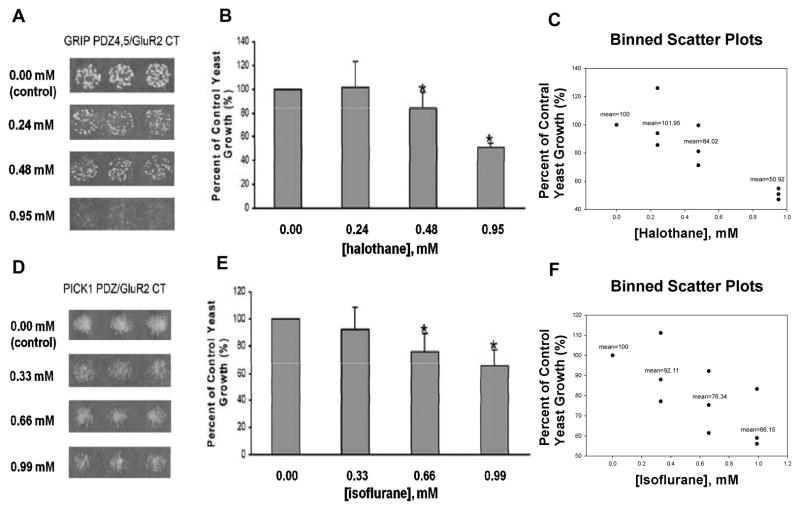
To test whether inhaled anesthetics also disrupt AMPA receptor-involved PDZ domain-mediated protein-protein interactions, we investigated the effects of halothane and isoflurane on interactions between the AMPA receptor GluA2 subunit and GRIP or PICK1 in a yeast two-hybrid system. Similar to our previous studies 18 and the results described above for PSD-95, halothane and isoflurane dose-dependently inhibited the growth of yeast cells harboring both pGADT7-GluR2 C-terminus and pGBKT7-GRIP PDZ4,5 or pGBKT7-PICK1 PDZ fusion protein plasmids (Fig. 3). As in our other studies, halothane was more potent that isoflurane at disrupting these PDZ domain-mediated protein-protein interactions (Fig. 3).
Fig. 3. Halothane and isoflurane dose-dependently disrupt PDZ domain-mediated interactions between AMPA receptor subunit GluR2 and GRIP or PICK1 in a yeast two-hybrid system.
(A–C) The effect of halothane on the growth of yeast cells harboring pGADT7-GluR2 CT and pGBKT7-GRIP PDZ4,5 (n = 3). (A) Halothane dose-dependently inhibited the yeast growth. (B) Yeast growth is shown as a percent of control, relative to halothane concentration. *p < 0.05 vs. 0.00 mM halothane. (C) Binned scatter plots of (A). (D–F) The effect of isoflurane on the growth of yeast cells harboring pGADT7-GluR2 CT and pGBKT7-PICK1 PDZ (n = 3). (D) Isoflurane dose-dependently inhibited the yeast growth. (E) Yeast growth is shown as a percent of control, relative to isoflurane concentration. *p < 0.05 vs. 0.00 mM isoflurane. (F) Binned scatter plots of (D). CT, C-terminus; GRIP, glutamate receptor interacting protein; PICK1, protein interacting with c kinase 1.
Isoflurane dose-dependently disrupts PDZ domain-mediated interactions between PSD-95 and NR2B in a yeast two-hybrid system
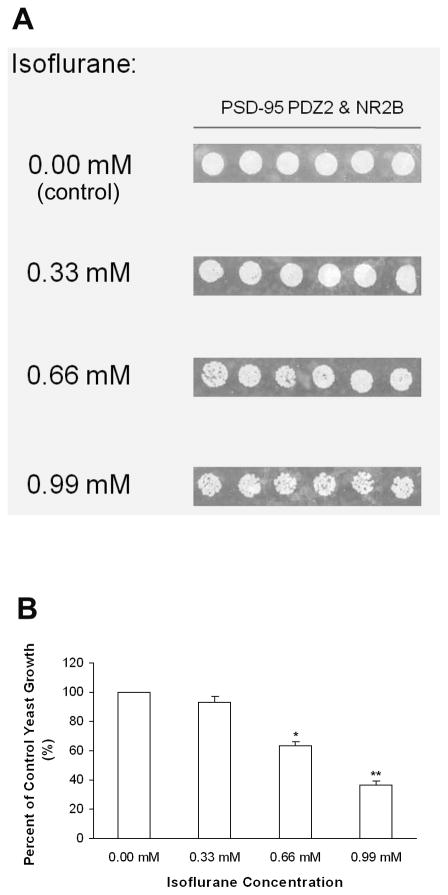
We also used a yeast two-hybrid approach to determine the effect of isoflurane on the interaction of PSD-95 PDZ2 with NR2B C-terminus (C-terminal 20 amino acids). In the absence of isoflurane, PSD-95 PDZ2 interacted with the C-terminal tail of NMDA receptors NR2B subunit, as evidenced by the growth of the yeast cells harboring both pGADT7-PSD-95 PDZ2 and pGBKT7-NR2B C-terminus fusion protein plasmids in synthetic dropout agar plates lacking adenine, leucine, tryptophan, and histidine (Fig. 4A). However, the yeast cell growth was slowed by isoflurane (0.33 mM, 0.66 mM, and 0.99 mM) in a dose-dependent manner (Fig. 4, A and B), indicating that isoflurane dose-dependently inhibits PDZ domain-mediated protein-protein interactions between PSD-95 and NR2B. To verify that isoflurane itself is not cytotoxic to yeast cells, we grew the same strain on low-stringency media (synthetic dropout agar plates that lack leucine and tryptophan and are selective for the yeast cells co-transformed with pGADT7 and pGBKT7) in the presence of the same concentrations of isoflurane. It was shown that isoflurane did not inhibit the growth of the control yeast cells.
Fig. 4. Isoflurane dose-dependently disrupts PDZ domain-mediated interactions between PSD-95 and NR2B in a yeast two-hybrid system.
(A) The effect of isoflurane on the growth of yeast cells harboring pGADT7-PSD-95 PDZ2 and pGBKT7-NR2B C-terminus. Note that isoflurane dose-dependently inhibited the yeast growth. (B) Yeast growth is shown as a percent of control, relative to isoflurane concentration (n = 6). *P < 0.05 and **P < 0.01 vs. 0.00 mM isoflurane. PSD-95, postsynaptic density protein-95.
Isoflurane dose-dependently disrupts the association between PSD-95 and NR2B in real time
We further used a surface plasmon resonance-based BIAcore assay to examine the ability of isoflurane to inhibit PSD-95 PDZ domain-mediated protein-protein interactions in real time. Binding of PSD-95 PDZ2 to NR2B C-terminus was measured in real time as an increase in resonance units. In the absence of isoflurane, 10 μM PSD-95 PDZ2 formed a complex with immobilized NR2B C-terminal peptide as indicated by a large increase in resonance unit level (Fig. 5). Interestingly, the inclusion of isoflurane in the mobile phase inhibited the equilibrium binding in a dose-dependent manner (Fig. 5). These results suggest that isoflurane reduces the number of sites available for the binding between PSD-95-PDZ2 and NR2B C-terminus, thus preventing the formation of the PDZ domain-mediated protein-protein complex.
Fig. 5. Isoflurane dose-dependently disrupts the association between PSD-95 and NR2B in real time.
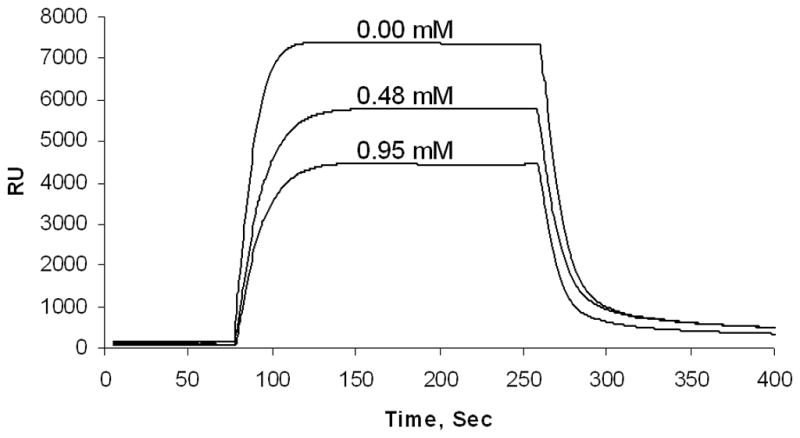
Surface Plasmon resonance analysis revealed that isoflurane dose-dependently reduces the real-time binding of PSD-95 PDZ2 to NR2B C-terminal peptide, as illustrated by superimposed sensorgrams. RU, resonance units.
Effects of Halothane and Isoflurane on PDZ Domain-mediated Interactions Between the GABAB Receptor and GABAB Receptor-interacting Proteins PAPIN and Mupp1
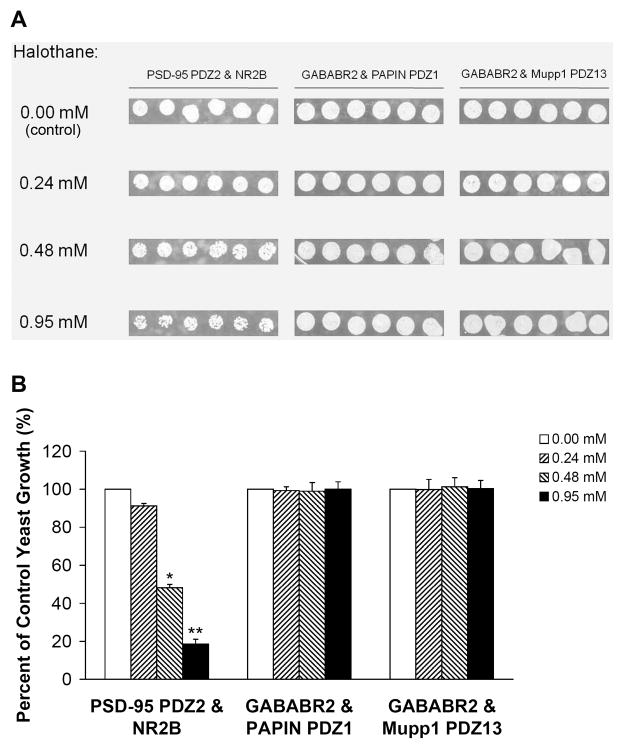
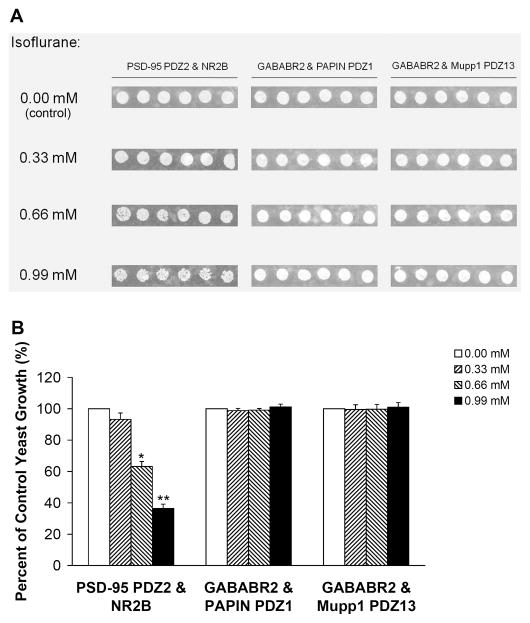
Different PDZ domain-mediated protein-protein interactions exist in the CNS. To define whether inhaled anesthetics disrupt PDZ domain-mediated interactions between the GABAB Receptor subunit 2 (GABABR2) and GABAB receptor-interacting proteins PAPIN and Mupp1, we investigated the effects of halothane and isoflurane on these interactions in a yeast two-hybrid system. We used the PDZ interaction between PSD-95 PDZ2 and NR2B as a positive control. Although halothane and isoflurane dose-dependently inhibited the growth of yeast cells harboring both pGADT7-PSD-95 PDZ2 and pGBKT7-NR2B C-terminus fusion protein plasmids (Figs. 6 and 7), treatment with the same concentrations of the two inhaled anesthetics had no effect on the growth of yeast cells harboring pGADT7-GABABR2 and pGBKT7-PAPIN PDZ1 or pGBKT7-Mupp1 PDZ13 fusion protein plasmids (Figs. 6 and 7). Likewise, the yeast cells grew normally in the absence of the inhaled anesthetics (Figs. 6 and 7).
Fig. 6. Halothane has no effect on PDZ domain-mediated protein-protein interactions between GABAB receptor subunit 2 and PAPIN or Mupp1 in a yeast two-hybrid system.
(A) No concentration of halothane tested inhibited the growth of yeast cells harboring pGADT7-GABABR2 and pGBKT7-PAPIN PDZ1 or pGBKT7-Mupp1 PDZ13. Consistent with our previous study15, halothane dose-dependently inhibited the growth of yeast cells harboring pGADT7-PSD-95 PDZ2 and pGBKT7-NR2B C-terminus, which were used as a positive control. (B) Yeast growth is shown as a percent of control, relative to halothane concentration (n = 6). *p < 0.05 and **p < 0.01 vs. 0.00 mM halothane. PSD-95, postsynaptic density protein-95; GABABR2, GABAB receptor subunit 2.
Fig. 7. Isoflurane has no effect on PDZ domain-mediated interactions between GABAB receptor subunit 2 and PAPIN or Mupp1 in a yeast two-hybrid system.
(A) No concentration of isoflurane tested inhibited the growth of yeast cells harboring pGADT7-GABABR2 and pGBKT7-PAPIN PDZ1 or pGBKT7-Mupp1 PDZ13. Consistent with our previous study15, isoflurane dose-dependently inhibited the growth of yeast cells harboring pGADT7-PSD-95 PDZ2 and pGBKT7-NR2B C-terminus, which were used as a positive control. (B) Yeast growth is shown as a percent of control, relative to isoflurane concentration (n = 6). *p < 0.05 and **p < 0.01 vs. 0.00 mM isoflurane. PSD-95, postsynaptic density protein-95; GABABR2, GABAB receptor subunit 2.
Isoflurane dose-dependently induces chemical shift changes in the three PDZ domains of PSD-95
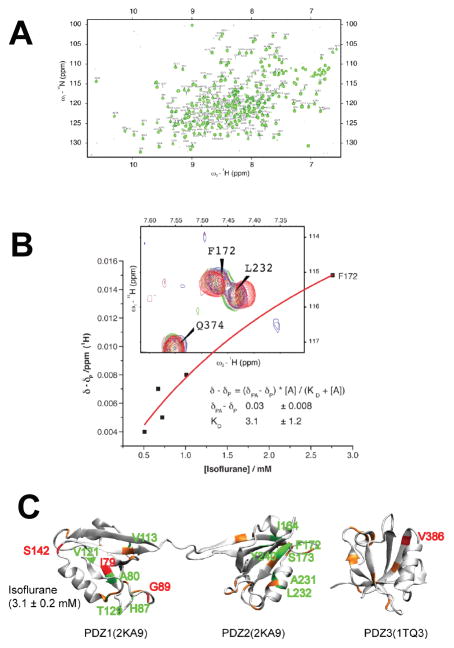
Fig. 8A shows a representative 15N-1H Heteronuclear Single Quantum Coherence (HSQC) NMR spectrum of PDZ1-3, in which 230 peaks are assigned to individual residues based on previous publications26;27. Those residues without reliable assignments or with weak intensity are labeled in lower case. To reveal an underlying cause of isoflurane perturbation on PSD-95 PDZ domain-mediated protein-protein interactions, we studied the interaction of isoflurane to the three PDZ domains of PSD-95 (PDZ1, PDZ2, and PDZ3) using NMR chemical shift as a probe. For each residue, its 15N and 1H chemical shifts can be affected by changes in the local chemical environment, such as ligand binding or the binding induced alteration in protein conformations. Upon titrating different concentrations of isoflurane into a PDZ1–3 sample, a number of residues showed chemical shift changes in an isoflurane-concentration dependent manner in the 15N-1H HSQC spectra (Fig. 8B). The fitting of chemical shift changes as a function of isoflurane concentrations provided a disassociation constant (Kd) of ~3 mM, suggesting a low affinity binding of isoflurane to PDZ1–3. The combined chemical shift changes of 15N and 1H quantified the anesthetic perturbation. We found that isoflurane affected all three PDZ domains, but PDZ2 had the most affected residues, while PDZ3 had the least (Fig. 8C). The affected residues in PDZ1 and PDZ2 were mostly in, or close to, their peptide binding groove, whereas very few residues close to the binding groove in PDZ3 were affected (Fig. 8C). These findings are valuable for understanding how anesthetics perturb PDZ domain-mediated protein-protein interactions.
Fig. 8. Isoflurane concentration-dependent changes in chemical shift of the PDZ domains of PSD-95.
(A) A representative 15N-1H HSQC spectrum of the 15N-labeled PDZ1–3 of rat source PSD-95 (residues 61–393) showing 230 assigned residues. (B) Representative 1H chemical shift changes of residues in the PDZ1–3 as a function of isoflurane concentrations. (C) Structures of PDZ1–3 mapped with the combined chemical shifts ( ) induced by isoflurane. Residues experienced chemical shift changes are highlighted in red (Δδ≧ 0.03 ppm), green (Δδ ~ 0.02–0.03ppm), and orange (Δδ ~ 0.01–0.02 ppm). Isoflurane concentration was calibrated with the external reference of trifluoroacetic acid. PDZ, postsynaptic density protein-95, Drosophila disc large tumor suppressor, and zonula occludens-1.
NMR spectral changes in the PDZ1–3 domains of PSD-95 produced by binding peptides NR2A/2B and/or isoflurane
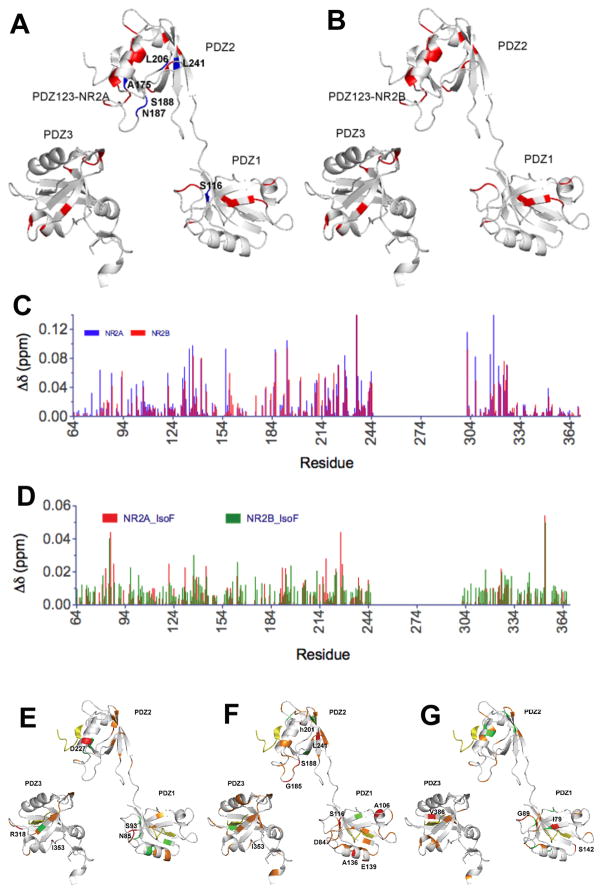
To further determine whether and how isoflurane perturbs peptide binding to PDZ1–3, we characterized NMR spectral changes of the PDZ1–3 domains of PSD-95 produced by binding of peptides NR2A-c20 or NR2B-c20 in the absence and presence of isoflurane. We found that NR2A-c20 and NR2B-c20 disturbed the motion of PSD-95 PDZ1–3. Upon peptide binding, dozens of NMR peaks in the 1H-15N HSQC NMR spectra of PDZ1–3 exhibit considerable intensity changes and some of them even disappeared from the spectra (Supplemental Digital Content 1, Figures 1 and 2; Fig. 9, A and B). The NMR peak intensity changes are often associated with changes in protein motions. The reduced intensity or disappearance of peaks signified that the peptide binding probably drove the residues into intermediate time-scale motions28–32. Significant chemical shift changes along with peptide binding were also observed in the 1H-15N HSQC NMR spectra of PDZ1–3 (Supplemental Digital Content 1, Figures 1 and 2; Fig. 9, C and D). The combined 1H and 15N chemical-shift changes were mapped onto the structures of PDZ1–3 (Fig. 9, E–G).
Fig. 9. Changes in PDZ1–3 of PSD-95 produced by binding of peptides NR2A-c20 or NR2B-c20 in the absence or presence of isoflurane.
(A and B) The structure of PDZ1–3 of PSD-95 showing residues with diminished (left) and enhanced (right) NMR peak intensities after binding to NR2A-c20 (A) or NR2B-c20 (B). Color codes: blue – changed only by NR2A-c20 binding; red- changed only by NR2B-c20 binding; green-changed in both cases of binding. The same color codes are applied to all figures here. (C) The combined 1H and 15N chemical shift changes in PDZ1–3 of PSD-95 upon NR2A-c20 (blue) or NR2B-c20 (red) binding. (D) The combined 1H and 15N chemical shift changes induced by isoflurane in PDZ1–3 of PSD-95 bound with NR2A-c20 (red) or NR2B-c20 (green). (E–G) The structure of PDZ1–3 of PSD-95 showing isoflurane-disturbed residues (Δδ≧ 0.04 ppm) upon binding of NR2A-c20 (E), NR2B-c20 (F) or no peptide present (G). Concentrations used are 100 μM for PDZ1–3 of PSD-95, 310 μM for peptides, and 3 mM for isoflurane. PDZ, postsynaptic density protein-95, Drosophila disc large tumor suppressor, and zonula occludens-1.
In the presence of peptides NR2A-c20 or NR2B-c20, isoflurane disturbed more extended regions of PSD-95 PDZ1–3 than in the absence of the peptides as evidenced by chemical shift changes in PDZ-peptide complexes upon adding isoflurane of ~3 mM (Supplemental Digital Content 1, Figures 3 – 6). Furthermore, the combined chemical shift showed that isoflurane introduced larger disturbance on the NR2A-c20 complex, but more extended disturbance on the NR2B-c20 complex (Fig. 9, E–G). Several residues, including A106 and S116 of PDZ1 and S188, A201, and L241 of PDZ2, even disappeared in the spectrum of the NR2A-c20 complex. The disturbance of isoflurane on the PDZ-peptide complex could be beyond the binding groove (βB and αB). For the NR2A-c20 complex, the residues most perturbed by isoflurane were not within the binding groove, as was the case for the NR2B-c20 complex. Collectively, isoflurane interacts with residues outside of the peptide-binding region once either one of the peptides bound to PDZ1–3.
Discussion
Cumulative evidence suggests that inhaled anesthetics act on multiple targets in the CNS33–35. It has been hypothesized that to achieve their anesthetic effect, they primarily modulate membrane-associated proteins that are involved in synaptic transmission3. Within synapses, ion channels regulate ionic flow across the cellular membrane, thereby influencing the presynaptic release of neurotransmitters and altering postsynaptic excitability. Several ion channels have been reported to contribute to the physiological actions of anesthetics 3. Some, including nicotinic acetylcholine, serotonin type 3, GABAA, glycine, NMDA, and AMPA receptors, are sensitive to inhaled anesthetics at clinically effective concentrations 33;36–38. The alteration of potassium channel function has also been reported to be involved in the central effects of inhaled anesthetics 39;40. In the CNS, PDZ domain-mediated protein-protein interactions have been identified in different signaling complexes at synapses and are critical to synaptic organization and for the activity of several excitatory receptors16;41–45. Our previous studies have shown that clinically relevant concentrations of inhaled anesthetics dose-dependently inhibit PDZ domain-mediated interactions between PSD-95 and NMDA receptors or nNOS, but have no effect on the non-PDZ domain-mediated interaction between the guanylate kinase domain of PSD-95 and Src homology 3 domain of SAP10218. We have also shown that disrupting PDZ domain-mediated protein-protein interactions by systemic injection of the cell-permeable peptide Tat-PSD-95 PDZ2 reduces the threshold for halothane anesthesia19. In the present study, we show that halothane and isoflurane inhibit PDZ domain-mediated interactions between PSD-95 and Shaker-type potassium channel Kv1.4 and between AMPA receptor subunit GluR2 and GRIP or PICK1 and that isoflurane inhibits PDZ domain-mediated interactions between PSD-95 and NMDA receptors. A previous study46 has shown that mutations of the homologous shaker channel in flies causes anesthetic resistance. The discrepancy may be due to the difference of properties of potassium channels between Drosophila and mammalian species. For instance, it has been reported that the potassium channel in Drosophila differs kinetically from mammalian species by exhibiting a faster inactivation time course47. We further demonstrate that isoflurane mostly affects the residues close to or in the peptide binding groove of PDZ1 and PDZ2 of PSD-95, while barely affecting the peptide binding groove of PDZ3. Among three PDZ domains of PSD-95, PDZ2 domain has the most residues affected by isoflurane. Given that our present studies are investigating PDZ domain-mediated protein-protein interactions in in vitro preparations, our experiments are only suggestive of conditions in synapses, and actions in neuronal milieu could be different. Yet our combined studies of plasmon resonance, yeast two-hybrid, GST pulldown and co-immunoprecipitation as well as our previous in vivo minimum alveolar anesthetic concentration studies are all consistent with a functional effect of anesthetic disruption of PDZ domain-mediated protein-protein interactions being important to the anesthetic state.
As binding partners of PDZ domains, NR2B-c20 and NR2A-c20 interact with the PDZ domains of PSD-95. Due to the long length of both peptides, their interactions with the PDZ domains extend beyond the peptide-binding groove, which is formed by αB, βB, and the carboxylate binding loop7 of the PDZ domain. The extended region includes βA (E65, K162), βB-βC link (T83, I88, G89, G177, G179), βC (I100, S339), αA (K202, G345, L349), βD (G209, V215, V362), and βE (L367, r368). The “disappeared” and “shifted” residues partially overlapped with the residues forming the three peptide binding groves of PSD-95 PDZ1–3, which include R70, G74, L75, G76, F77, S78, I79, G81, T97, H130, L137, and G141 in PDZ1; K165, G169, L170, G171, F172, S173, I174, G176, T192, H225, L232, and Y236 in PDZ2; R318, G322, L323, G324, F325, N326, I327, G329, S339, H372, L379, and G383 in PDZ3. This divergence from the peptide-binding groove could come from at least three different sources. First, the real structure could be different from the model used here, the simple combination of PDZ1, PDZ2, and PDZ3; Second, the peptides used in the model are shorter than what we used in our experiments, which should cause extended disturbance on the PSD-95 PDZ1–3; Third, some “disappeared” and “shifted” residues, which were not assigned by the current 1H-15N HSQC NMR spectra, could be other disturbed residues in the groove. About half of the residues in the groove of PDZ1 were affected by the peptides binding, while three quarters of the residues of PDZ2 were affected. These results suggest that the binding of NR2A-c20 and NR2B-c20 to the three PDZ domains of PSD-95 is not equivalent and PDZ2 may be the primary target of the peptides.
Our yeast two-hybrid analysis and surface plasmon resonance assay further demonstrates that the inhaled anesthetic isoflurane disrupts the PDZ domain-mediated interaction between PSD-95 and NMDA receptors. PDZ domain-mediated protein-protein interactions provide a framework for the assembly of multiprotein signaling complexes at synapses. These PDZ proteins coordinate and guide the flow of regulatory information, and regulate receptor and ion channel activities. One of the best-understood PDZ-domain proteins at synapses is PSD-95, a modular protein highly enriched in the postsynaptic density. In our yeast two-hybrid experiments, the growth of the yeast cells harboring both pGADT7-PSD-95 PDZ2 and pGBKT7-NR2B C-terminus was slowed by isoflurane (0.33 mM, 0.66 mM, and 0.99 mM) in a dose-dependent manner. Our surface plasmon resonance assay showed that the inclusion of isoflurane in the mobile phase inhibited the equilibrium binding of PSD-95 PDZ2 to NR2B C-terminus in real time. These results suggest that the inhaled anesthetic isoflurane can inhibit the binding of PSD-95 with NMDA receptors and thereby interrupt relevant downstream signaling. Taken together, our data indicate that the ability of anesthetics to disrupt neuronal signaling pathways through inhibition of PDZ domain interactions plays a significant role in anesthesia itself. Different experimental conditions certainly affect quantitative data for peptide or isoflurane binding. For example, a protein-crowding effect may exist in cells, but not in NMR sample tubes; the isolated PDZ2 is not comparable to the intact PDZ1–3; plasmon resonance experiments need to have the peptide immobilized. All of these may render variances in quantification of binding affinities.
We also investigated the effects of halothane and isoflurane on PDZ domain-mediated protein-protein interactions in GABA receptor signaling. We found that inhaled anesthetics had no effect on PDZ domain-mediated interactions between the GABAB receptor and its interacting proteins PAPIN and Mupp1, suggesting that inhaled anesthetics have differential effects on different PDZ domain-mediated protein-protein interactions in the CNS.
In conclusion, PDZ domain-mediated protein-protein interactions in NMDA/AMPA receptor signaling and Kv1.4 channels, but not GABA receptor signaling, can be inhibited by inhaled anesthetics. Our data also indicate that PSD-95 PDZ1–3 domains can interact with isoflurane, of which PDZ2 is the most affected domain. Thus, inhaled anesthetics may affect synaptic transmission by binding to PDZ domains, which can be considered as a new molecular target for inhaled anesthetics. The common inhibition seen on pull-down, yeast two hybrid, and NMR (all controlled experiments) as well as a functional change in minimum alveolar anesthetic concentration, provide support for our hypothesis and for a functional role for the anesthetic-PDZ interaction in anesthesia.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the National Institutes of Health (Bethesda, Maryland) grants of R01 GM049111 (R.A.J.) and R01 GM056257 (P.T.).
The authors thank Dr. Randy A. Hall, Ph.D., Professor, in the Department of Pharmacology at Emory University School of Medicine (Atlanta, GA) for generously providing GABABR2, PAPIN PDZ1, and Mupp1 PDZ13 cDNA constructs.
Footnotes
The authors declare no competing interests.
Reference List
- 1.Franks NP. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 2.Solt K, Forman SA. Correlating the clinical actions and molecular mechanisms of general anesthetics. Curr Opin Anaesthesiol. 2007;20:300–6. doi: 10.1097/ACO.0b013e32816678a5. [DOI] [PubMed] [Google Scholar]
- 3.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–24. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- 4.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 5.Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Garner CC, Nash J, Huganir RL. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 2000;10:274–80. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- 7.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–40. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 8.Nourry C, Grant SG, Borg JP. PDZ domain proteins: Plug and play! Sci STKE. 2003:RE7. doi: 10.1126/stke.2003.179.re7. [DOI] [PubMed] [Google Scholar]
- 9.Tao YX, Johns RA. Effect of the deficiency of spinal PSD-95/SAP90 on the minimum alveolar anesthetic concentration of isoflurane in rats. Anesthesiology. 2001;94:1010–5. doi: 10.1097/00000542-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Tao F, Tao YX, Gonzalez JA, Fang M, Mao P, Johns RA. Knockdown of PSD-95/SAP90 delays the development of neuropathic pain in rats. Neuroreport. 2001;12:3251–5. doi: 10.1097/00001756-200110290-00022. [DOI] [PubMed] [Google Scholar]
- 11.Tao F, Tao YX, Mao P, Johns RA. Role of postsynaptic density protein-95 in the maintenance of peripheral nerve injury-induced neuropathic pain in rats. Neuroscience. 2003;117:731–9. doi: 10.1016/s0306-4522(02)00801-1. [DOI] [PubMed] [Google Scholar]
- 12.Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–42. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 13.Kornau HC, Seeburg PH, Kennedy MB. Interaction of ion channels and receptors with PDZ domain proteins. Curr Opin Neurobiol. 1997;7:368–73. doi: 10.1016/s0959-4388(97)80064-5. [DOI] [PubMed] [Google Scholar]
- 14.Kistner U, Wenzel BM, Veh RW, Cases-Langhoff C, Garner AM, Appeltauer U, Voss B, Gundelfinger ED, Garner CC. SAP90, a rat presynaptic protein related to the product of the Drosophila tumor suppressor gene dlg-A. J Biol Chem. 1993;268:4580–3. [PubMed] [Google Scholar]
- 15.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–8. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 16.Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–8. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 17.Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc Natl Acad Sci U SA. 1999;96:435–40. doi: 10.1073/pnas.96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang M, Tao YX, He F, Zhang M, Levine CF, Mao P, Tao F, Chou CL, Sadegh-Nasseri S, Johns RA. Synaptic PDZ domain-mediated protein interactions are disrupted by inhalational anesthetics. J Biol Chem. 2003;278:36669–75. doi: 10.1074/jbc.M303520200. [DOI] [PubMed] [Google Scholar]
- 19.Tao F, Johns RA. Effect of disrupting N-methyl-d-aspartate receptor-postsynaptic density protein-95 interactions on the threshold for halothane anesthesia in mice. Anesthesiology. 2008;108:882–7. doi: 10.1097/ALN.0b013e31816c8a8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao F, Su Q, Johns RA. Cell-permeable peptide Tat-PSD-95 PDZ2 inhibits chronic inflammatory pain behaviors in mice. Mol Ther. 2008;16:1776–82. doi: 10.1038/mt.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allott PR, Steward A, Mapleson WW. Determination of halothane in gas, blood, and tissues by chemical extraction and gas chromatography. Br J Anaesth. 1971;43:913–8. doi: 10.1093/bja/43.10.913. [DOI] [PubMed] [Google Scholar]
- 22.Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: Molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–76. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- 23.Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Muller BM, Kistner U, Kindler S, Chung WJ, Kuhlendahl S, Fenster SD, Lau LF, Veh RW, Huganir RL, Gundelfinger ED, Garner CC. SAP102, a novel postsynaptic protein that interacts with NMDA receptor complexes in vivo. Neuron. 1996;17:255–65. doi: 10.1016/s0896-6273(00)80157-9. [DOI] [PubMed] [Google Scholar]
- 25.Chou CL, Sadegh-Nasseri S. HLA-DM recognizes the flexible conformation of major histocompatibility complex class II. J Exp Med. 2000;192:1697–706. doi: 10.1084/jem.192.12.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piserchio A, Pellegrini M, Mehta S, Blackman SM, Garcia EP, Marshall J, Mierke DF. The PDZ1 domain of SAP90. Characterization of structure and binding. J Biol Chem. 2002;277:6967–73. doi: 10.1074/jbc.M109453200. [DOI] [PubMed] [Google Scholar]
- 27.Ukpabi N, Sharma S, Spaller MR, Tsang P. (1)H, (15)N and (13)C backbone and side chain assignments of PSD-95 PDZ3 protein. J Biomol NMR. 2004;30:111–2. doi: 10.1023/B:JNMR.0000042953.83397.8e. [DOI] [PubMed] [Google Scholar]
- 28.Bondarenko V, Mowrey D, Liu LT, Xu Y, Tang P. NMR resolved multiple anesthetic binding sites in the TM domains of the alpha4beta2 nAChR. Biochim Biophys Acta. 2013;1828:398–404. doi: 10.1016/j.bbamem.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canlas CG, Cui T, Li L, Xu Y, Tang P. Anesthetic modulation of protein dynamics: Insight from an NMR study. J Phys Chem B. 2008;112:14312–8. doi: 10.1021/jp805952w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frueh DP, Arthanari H, Koglin A, Vosburg DA, Bennett AE, Walsh CT, Wagner G. Dynamic thiolation-thioesterase structure of a non-ribosomal peptide synthetase. Nature. 2008;454:903–6. doi: 10.1038/nature07162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mowrey DD, Liu Q, Bondarenko V, Chen Q, Seyoum E, Xu Y, Wu J, Tang P. Insights into distinct modulation of alpha7 and alpha7beta2 nicotinic acetylcholine receptors by the volatile anesthetic isoflurane. J Biol Chem. 2013;288:35793–800. doi: 10.1074/jbc.M113.508333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao BD. Nuclear magnetic resonance line-shape analysis and determination of exchange rates. Methods Enzymol. 1989;176:279–311. doi: 10.1016/0076-6879(89)76016-x. [DOI] [PubMed] [Google Scholar]
- 33.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–14. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 34.el-Maghrabi EA, Eckenhoff RG, Shuman H. Saturable binding of halothane to rat brain synaptosomes. Proc Natl Acad Sci U SA. 1992;89:4329–32. doi: 10.1073/pnas.89.10.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckenhoff RG, Johansson JS. Molecular interactions between inhaled anesthetics and proteins. Pharmacol Rev. 1997;49:343–67. [PubMed] [Google Scholar]
- 36.Franks NP, Lieb WR. Which molecular targets are most relevant to general anaesthesia? Toxicol Lett. 1998;100–101:1–8. doi: 10.1016/s0378-4274(98)00158-1. [DOI] [PubMed] [Google Scholar]
- 37.Narahashi T, Aistrup GL, Lindstrom JM, Marszalec W, Nagata K, Wang F, Yeh JZ. Ion channel modulation as the basis for general anesthesia. Toxicol Lett. 1998;100–101:185–91. doi: 10.1016/s0378-4274(98)00184-2. [DOI] [PubMed] [Google Scholar]
- 38.Mennerick S, Jevtovic-Todorovic V, Todorovic SM, Shen W, Olney JW, Zorumski CF. Effect of nitrous oxide on excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 1998;18:9716–26. doi: 10.1523/JNEUROSCI.18-23-09716.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berg-Johnsen J, Langmoen IA. Isoflurane hyperpolarizes neurones in rat and human cerebral cortex. Acta Physiol Scand. 1987;130:679–85. doi: 10.1111/j.1748-1716.1987.tb08192.x. [DOI] [PubMed] [Google Scholar]
- 40.Nicoll RA, Madison DV. General anesthetics hyperpolarize neurons in the vertebrate central nervous system. Science. 1982;217:1055–7. doi: 10.1126/science.7112112. [DOI] [PubMed] [Google Scholar]
- 41.Wakamori M, Ikemoto Y, Akaike N. Effects of two volatile anesthetics and a volatile convulsant on the excitatory and inhibitory amino acid responses in dissociated CNS neurons of the rat. J Neurophysiol. 1991;66:2014–21. doi: 10.1152/jn.1991.66.6.2014. [DOI] [PubMed] [Google Scholar]
- 42.Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–24. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- 43.Roche KW, Ly CD, Petralia RS, Wang YX, McGee AW, Bredt DS, Wenthold RJ. Postsynaptic density-93 interacts with the delta2 glutamate receptor subunit at parallel fiber synapses. J Neurosci. 1999;19:3926–34. doi: 10.1523/JNEUROSCI.19-10-03926.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen NA, Brenman JE, Snyder SH, Bredt DS. Binding of the inward rectifier K+ channel Kir 2. 3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996;17:759–67. doi: 10.1016/s0896-6273(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 45.Becamel C, Figge A, Poliak S, Dumuis A, Peles E, Bockaert J, Lubbert H, Ullmer C. Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J Biol Chem. 2001;276:12974–82. doi: 10.1074/jbc.M008089200. [DOI] [PubMed] [Google Scholar]
- 46.Tinklenberg JA, Segal IS, Guo TZ, Maze M. Analysis of anesthetic action on the potassium channels of the Shaker mutant of Drosophila. Ann NY Acad Sci. 1991;625:532–9. doi: 10.1111/j.1749-6632.1991.tb33884.x. [DOI] [PubMed] [Google Scholar]
- 47.Christie MJ, Adelman JP, Douglass J, North RA. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989;244:221–4. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.