Abstract
Background
Racial disparities in care and outcomes contribute to mortality and morbidity in children however the role in pediatric Crohn’s disease (CD) is unclear. In this study, we compared cohorts of Black and White children with CD to determine the extent race is associated with differences in readmissions, complications, and procedures among hospitalizations in the United States.
Methods
Data were extracted from the Pediatric Health Information System (January 1, 2004–June 30, 2012) for patients ≤21 years of age hospitalized with a diagnosis of CD. White and Black cohorts were randomly selected in a 2:1 ratio by hospital. The primary outcome was time from index hospital discharge to readmission. The most frequent complications and procedures were evaluated by race.
Results
There were 4377 patients. Black children had a shorter time to first readmission and higher probability of readmission (p=0.009), and a 16% increase in risk of readmission compared to White children (p=0.01). Black children had longer length of stay and higher frequency of overall and late (30 days–12 months post discharge) readmissions (p<0.001). During index hospitalization, more Black children had perianal disease and anemia (p<0.001). During any hospitalization, Black children had higher incidence of perianal disease, anemia, and vitamin D deficiency, and greater number of perianal procedures, endoscopies, and blood product transfusion (p<0.001).
Conclusions
There are differences in hospital readmissions, complications, and procedures among hospitalized children related to race. It is unclear whether these differences are due to genetic differences, worse intrinsic disease, adherence, access to treatment, or treatment disparities.
Keywords: inflammatory bowel disease, disparities, race, pediatrics
INTRODUCTION
Inflammatory bowel disease (IBD), which includes Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic, relapsing, inflammatory disorder that primarily affects the digestive tract of approximately 1.4 million people in North America,1, 2 with 25% being diagnosed in childhood. IBD carries a significant burden of disease given its rising incidence,3–5 the significant associated morbidity, psychosocial effects6–8 and the healthcare related costs9–11.
Disparities in care and outcomes contribute to mortality and morbidity in various conditions in children including asthma, congenital heart disease, diabetes, and ADHD.12–19 The Institute of Medicine defines a disparity as “racial or ethnic difference in the quality of healthcare that is not due to access-related factors or clinical needs, preference and appropriateness of intervention”.20
Health disparity research in adult IBD and other disease processes suggest that minority status, female gender and low socioeconomic status are related to poorer outcomes.21, 22 There are numerous differences between Black and White IBD patients in regards to health care utilization practices and disease impact upon daily activities,23 as well as evidence of racial differences regarding characteristics of IBD, including more severe disease among minority patients.24, 25 Disproportionately high rates of IBD-related hospitalizations and mortality among non-Hispanic Blacks as compared to non-Hispanic Whites occur, even after taking into account differences in IBD prevalence across racial groups.26 Bowel resection among hospitalized adult CD patients varies by race, health insurance status and gender.27 A systematic review of 40 studies reported evidence for race and SES-based disparities in IBD care, especially with regard to surgical care and in-hospital mortality risk.28
We suspect healthcare disparities also exist in pediatric IBD, which may contribute to variation in care, increased costs and poorer outcomes, however data in children is limited. The effects of race on hospital admissions in children with CD are unknown. Our study begins to address this gap by evaluating racial differences between Black children and White children with CD who are hospitalized. The main objectives of our study were to determine 1.) if Black children hospitalized for CD are more likely to be readmitted and have longer length of stay (LOS) compared to White children 2.) if corticosteroids, biologic agents and total parenteral nutrition (TPN) usage differs between races and 3.) if there are differences in CD related complications and procedures between races.
MATERIALS AND METHODS
Data Source
The Pediatric Health Information System (PHIS) database is a large, administrative database established in 2002 (with archived data since 1992) by Children’s Hospital Association (formerly known as Child Health Corporation of America). PHIS contains comprehensive inpatient data from 44 not-for-profit children’s hospitals in the United States, which represents about 25% of pediatric centers in the U.S. and the majority of the tertiary care centers.
PHIS provides direct access to the data and viewers can select which hospitals and networks to compare. Encrypted medical record numbers (MRN) allow longitudinal tracking of patients over time within an institution. Standardized data is collected on demographics, diagnoses, procedures, interventions, outcomes and resource utilization for all pediatric patients admitted to participating hospitals. Data elements are abstracted and coded using PHIS data quality guidelines (de-identified at submission and reliability/validity checks are performed before database inclusion).
Study Cohort
The study population included pediatric patients (age ≤21years) who were admitted to a PHIS affiliated hospital between January 1, 2004 and June 30, 2012. Patients entered the study cohort on the day of admission of their index hospitalization (first hospitalization in the study period) and were followed through subsequent admissions during the entire study period. Patients were included in the study population if they had a primary or secondary diagnosis of CD (555.×) and if both race and gender were reported. A hospitalization was excluded if a patient was admitted and discharged on the same day, or if a patient was admitted for an ostomy take down (CPT 44625, ICD 9 procedure code 46.5×) as this procedure was considered an elective, planned admission. Other exclusion criteria included: missing race, multiple races, Hispanic ethnicity, missing gender, or gender mismatch among two or more encounters. A subset of eligible patients was randomly selected at the hospital level to achieve a 2:1 ratio of Whites to Blacks which increased the number of patients in the study (relative to parity). Whereas some hospital sites had more Whites than Blacks, a few sites had a disproportionately higher number of Blacks due to their local population demographics. In order to maintain this ratio at each hospital site, the number of Blacks was reduced using random selection at a given site where the number of Whites was less than twice the number of Blacks. No matching based on potentially confounding variables was performed; rather, the influence of potential confounds was controlled for statistically.
Variables
Basic demographics included: gender, age, race, ethnicity, payor, median neighborhood income, and hospital location. We included only patients who reported a single race for analysis. Payor status was assigned to 1 of 3 groups: commercial insurance, Medicaid, or other (APPENDIX A). Additional variables collected included admission and discharge dates, discharge ID, MRN (encrypted), LOS, and number of readmissions.
Secondary predictors included payor status, and median neighborhood income. Regional divisions (Northeast, South, Midwest, and West) were derived from the state in which the hospital resided and then assigned to a region based on the 2010 Census Regions and Divisions of the United States (www.census.gov). The median neighborhood incomes were based upon patients’ actual zip code at initial hospitalization.
Treatment variables included corticosteroids, biologic agents and TPN use. We collected the number of IBD-related complications and abdominal surgical procedures during the index hospitalization by generating a list of the top 200 procedural codes and complications in the study cohort and grouped them along clinical grounds. (APPENDIX B)
Outcomes
The primary outcome was the time from index hospital discharge to readmission as it was more indicative of poor post-hospitalization care and/or poor self-management. We also felt an admitted population would increase the likelihood of similar disease severity between groups at study entry (i.e. both cohorts demonstrated significant enough disease to warrant hospitalization) and assure that the patient had established care within a pediatric medical center, although admission criteria vary and several factors could potentially affect the decision to admit a patient, (e.g., access to care, reliability of follow-up, etc.). Readmissions were divided into 2 categories: early (< 30 days from initial discharge) and late (30 days to 1 year after initial discharge). Secondary outcomes included median LOS and the number of readmissions. Secondary predictors included payor status and the median neighborhood income associated with zip code.
Study Design
This is a retrospective review of patients who were hospitalized with a diagnosis of CD. To assess the validity of the data extracted from PHIS, a subset of the main cohort was created, consisting of 113 CD patients (37 Black and 76 White) from Nationwide Children's Hospital (NCH). Chart reviews were conducted for this group, and the results were compared to the data extracted from PHIS. Variables examined included: race, CD diagnosis, payor status, and corticosteroids, biologic agents (infliximab, adalimumab, and certolizumab), and TPN.
Statistical Analyses
To examine the validity of data extracted from PHIS, percent agreement was calculated between PHI data and the corresponding data obtained via medical record review at NCH. Baseline and demographic characteristics were summarized using standard descriptive statistics. Effect sizes were calculated using Cohen’s d to describe the magnitude of the difference between races. Categorical data were compared between races using either the Chi-Square test of independence (for 2×2 comparisons) or the exact binomial test (for comparing White to Black frequencies to the expected 2:1 distribution). Continuous data (e.g., LOS) were compared using nonparametric Wilcoxon two-sample tests. A Cox proportional hazards model was constructed to assess the risk of readmission over time between Black and White children. The proportionality assumption of the Cox model was examined and validated with a Kaplan-Meier survival curve. The Kaplan-Meier survival analysis with a log-rank test was then used to compare the probability of readmission in the first year after index hospitalization by race. Multivariate logistic regression models were constructed to assess the influence of the following predictors on readmission status (yes versus no): age at first admission, gender, geographical region (West, Midwest, South, and Northeast), corticosteroid use, biologic agent use, TPN use, insurance payor (Medicaid, commercial, and other), and income. This was calculated for the total number of readmissions, readmission within 1 year, readmission after 1 year, and LOS. Similar multivariate logistic regression models were constructed to assess the IBD-related complications and procedures which occurred during the index hospitalization, and for any hospitalization.
For each set of analyses, we assumed a critical alpha of 0.05 and made Bonferroni corrections for multiple testing where appropriate. We expected that small effects would be magnified by our large study population and considered an effect size of >0.15 to be clinically meaningful. All statistical analyses were conducted using SAS 9.3 (by SAS Institute Inc., Cary, NC, USA). This study was approved by the NCH institutional review board.
RESULTS
PHIS validation/NCH cohort
Overall, agreement between data extracted from PHIS and data from NCH documentation was high. For race, corticosteroid usage, and TPN usage, the PHIS and NCH records agreed for over 99% and payor status percent agreement was about 94%.
Descriptive Statistics
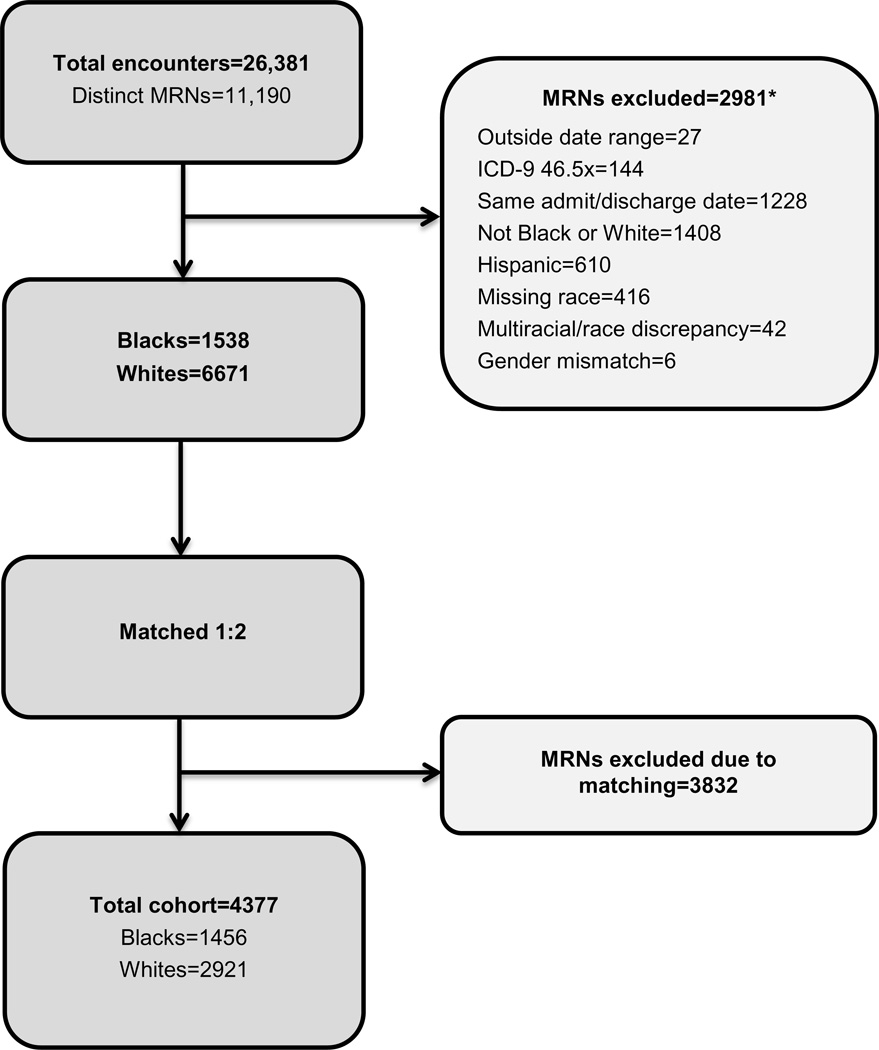
The initial query included 26,381 total encounters and 11,190 distinct patients, with a final cohort of 1456 Black children and 2921 White children (Figure 1). We found no significant differences in either gender or geographical region between the Black and White cohorts. Compared with White children, Black children were slightly older at the first admission, were more likely to have Medicaid as their primary payor, and had a lower median neighborhood income. (Table 1)
FIGURE 1.
Cohort Section Procedures and Exclusion Criteria (*not mutually exclusive categories).
TABLE 1.
Demographic Characteristics by Race
| Variable | Black n (%) |
White n (%) |
p value1 |
|---|---|---|---|
| Total | 1456 (33) | 2921 (67) | |
| Gender | |||
| Male | 781 (54) | 1545 (53) | 1 |
| Female | 673 (46) | 1373 (47) | |
| Age at 1st admission (years) | 14.6±3.4 (median: 15.1) | 14.1±3.7 (median: 14.7) | <0.001 |
| Region | |||
| Midwest | 381 (26) | 765 (26) | NA |
| Northeast | 267 (18) | 536 (18) | |
| South | 731 (50) | 1465 (50) | |
| West | 77 (5) | 155 (5) | |
| Payor2 | |||
| Commercial | 378 (26) | 1305 (45) | <0.001 |
| Medicaid | 626 (43) | 462 (16) | |
| Other | 406 (28) | 1056 (36) | |
| Missing | 46 (3) | 98 (3) | |
| Median neighborhood income (at 1st admission)3. | $39,855±$15,764 (median: $36,423) | $53,599±$21,155 (median: $49,763) | <0.001 |
Bonferroni corrected p value;
See Appendix A;
Based on 2010 United States Census Data compared to reported zip code
LOS
Black children had slightly longer LOS (6.8±7.1 days, median=5 days) than White children (6.3±8.9 days, median=4 days) (p<0.001, d=0.07) although the effect size was small.
Percent of Early and Late Readmissions
Using the exactly binomial test, there was no significant difference in the percentage of Black vs. White children who had an early readmission (154 (11%) vs. 327 (11%), ns), however a larger percentage of Black children had a late readmission (410 (28%) vs. 677 (23%), p=0.002). During the overall study period, Black children had a higher frequency of readmissions (1.4±2.6, median=0) than White children (0.9±1.9, median=0) (p<0.001, d=0.19). Black children also had a higher number of readmissions after 1 year compared to White children (3.1±3.2 vs. 2.4±2.4, <0.001, d=0.22).
Time to readmission
A Kaplan-Meier survival analysis with a log-rank test shows that the probability of being readmitted was essentially identical for Blacks and Whites during the first 100 days, and after 100 days White patients had a statistically lower probability of readmission (p=0.009). A Cox proportional hazards model shows that in the first year after index admission, Black children had a 16% increase in risk of readmission compared to White children (p=0.01). (Figure 2)
FIGURE 2.
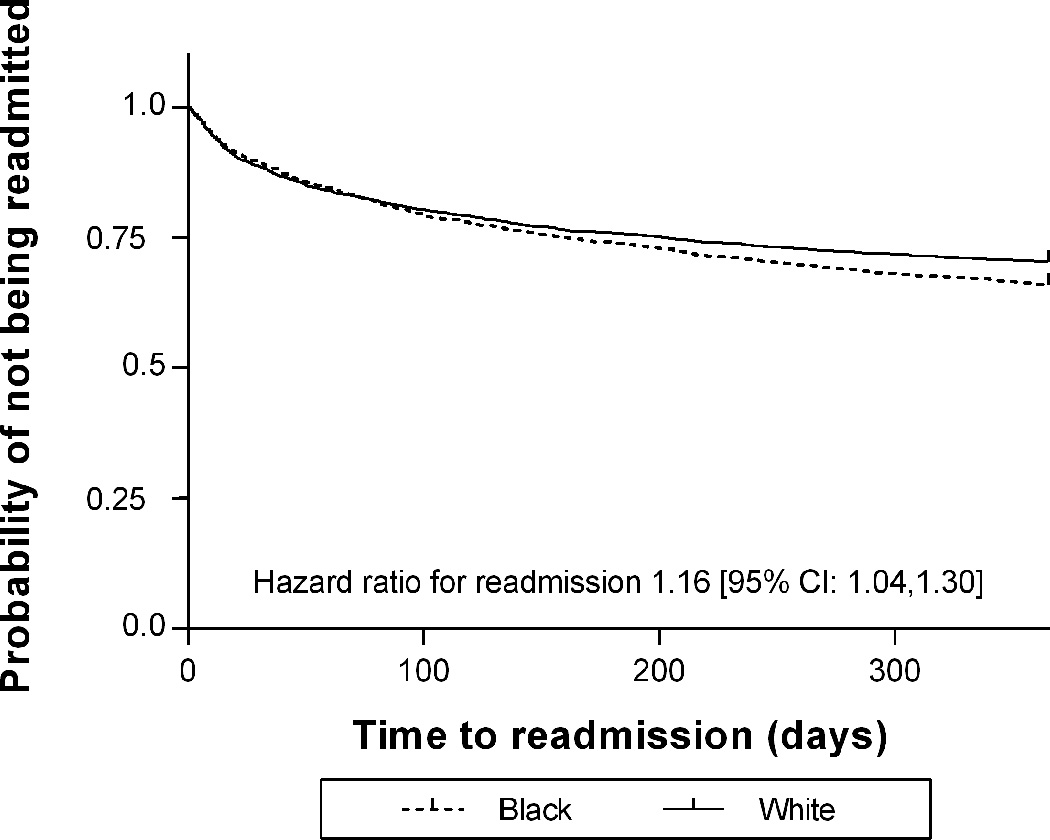
Time to first readmission, according to race, in children hospitalized with Crohn’s disease. The graph depicts the curves are very close together in time period [0, 100], after 100 days, it appears that White patients had slightly less probability of readmission (Kaplan-Meier analysis with Log-Rank test, p=0.009; Cox proportional hazards p=0.01).
Medication Use
Regarding the inpatient medical treatment, we found that more Blacks received biologic agents during any hospitalization, during the index hospitalization and also during late readmissions. More Blacks received corticosteroids during late readmissions. (Table 2)
TABLE 2.
Medications Received by Race
| Variable | Black n (%) |
White n (%) |
p value1 |
|---|---|---|---|
| Any | |||
| Corticosteroid | 1025 (70) | 1977 (68) | 0.81 |
| Biologic agent | 473 (33) | 642 (22) | 0.001 |
| TPN | 399 (27) | 719 (25) | 0.56 |
| Index | |||
| Corticosteroid | 943 (65) | 1786 (61) | 0.23 |
| Biologic agent | 278 (19) | 405 (14) | 0.001 |
| TPN | 262 (18) | 508 (17) | 1 |
| Early (<30 days) | |||
| Corticosteroid | 103 (7) | 234 (8) | 1 |
| Biologic agent | 34 (2) | 74 (3) | 1 |
| TPN | 36 (3) | 95 (3) | 1 |
| Late (30 days-1yr) | |||
| Corticosteroid | 287 (20) | 416 (14) | 0.001 |
| Biologic agent | 130 (9) | 169 (6) | 0.001 |
| TPN | 89 (6) | 149 (5) | 1 |
Bonferroni corrected p value; TPN=total parenteral nutrition
TPN=total parenteral nutrition
Predictors of Readmission
In a multivariate model, race was a significant predictor of readmission (OR=1.28 95% CI [1.09, 1.50], p=0.009). Predictive factors of overall readmission included corticosteroid usage (OR=1.53, 95% CI [1.32, 1.78], p<0.001) and TPN usage (OR=1.32, 95% CI [1.10, 1.59], p=0.009) during the index hospitalization, and patients with “Other” insurance status (OR=1.62, 95% CI [1.38, 1.91], p<0.001). Predictive factors of early readmission included older age (OR=1.05 per year older, 95% CI [1.02–1.09], p=0.01) and TPN usage (OR=1.54, 95% CI [1.18, 2.00], p=0.004) during index hospitalization. The predictive factors of late readmission are corticosteroids given during the index hospitalization (OR=1.38, 95% CI [1.16, 1.64], p=0.0009).
IBD-related Complications and Procedures
During the index hospitalization, there was no significant difference between Black and White children for procedures, however more Black patients had perianal disease (8.6% vs. 5.8%, p=0.011) and anemia (34.2% vs. 25.4%, p<0.002). Among the most prevalent diagnosis and procedure groups during any hospitalization, there was a higher percentage of Black patients with perianal disease (14.6 vs. 8.3%, p<0.002), anemia (42.6% vs. 31.1%, p<0.002), vitamin D deficiency (2.3% vs. 0.9%, p=0.002), and who underwent perianal/perineal procedures (8.5% vs. 5.0%, p<0.001), endoscopies (52.1% vs. 46.4%, p=0.004)), and received blood product transfusions (14.2% vs. 10.9%, p=0.023) than Whites. (Tables 3 and 4). Even after controlling for gender, income, and type of insurance in multivariate regression models, race remained as a significant predictor of the above complications and procedures.
TABLE 3.
Diagnoses at Index Hospitalization and Any Hospitalization
| Index Hospitalization | Any Hospitalization | |||||
|---|---|---|---|---|---|---|
| Black n (%) |
White n (%) |
p value1 | Black n (%) |
White n (%) |
p value | |
| Anemia | 498 (34.2) | 742 (25.4) | <0.002 | 620 (42.6) | 908 (31.1) | <0.002 |
| Nutrition complications2 | 252 (17.3) | 474 (16.2) | 1 | 344 (23.6) | 596 (20.4) | 0.24 |
| Dehydration/hypovolemia | 139 (9.5) | 295 (10.1) | 1 | 243 (16.7) | 458 (15.7) | 1 |
| Infection (others) | 140 (9.6) | 263 (9.0) | 1 | 278 (19.1) | 481 (16.5) | 0.51 |
| Obstruction | 107 (7.3) | 253 (8.7) | 1 | 223 (15.3) | 406 (13.9) | 1 |
| Perianal disease | 125 (8.6) | 170 (5.8) | 0.011 | 213 (14.6) | 243 (8.3) | <0.002 |
| Other | 68 (4.7) | 185 (6.3) | 0.38 | 130 (8.9) | 291 (10) | 1 |
| Electrolyte anomalies | 66 (4.5) | 138 (4.7) | 1 | 118 (8.1) | 223 (7.6) | 1 |
| Intra-abdominal abscess | 37 (2.5) | 110 (3.8) | 0.48 | 86 (5.9) | 187 (6.4) | 1 |
| C. difficile infection | 33 (2.3) | 103 (3.5) | 0.32 | 70 (4.8) | 179 (6.1) | 1 |
| Fistula | 50 (3.4) | 77 (2.6) | 1 | 105 (7.2) | 156 (5.3) | 0.24 |
| Perforation/peritonitis | 21 (1.4) | 57 (2.0) | 1 | 36 (2.5) | 91 (3.1) | 1 |
| Bacteremia/sepsis | 21 (1.4) | 44 (1.5) | 1 | 50 (3.4) | 88 (3.0) | 1 |
| Short stature | 7 (0.5) | 31 (1.1) | 0.64 | 14 (1.0) | 54 (1.8) | 0.32 |
| Vitamin D deficiency | 17 (1.2) | 12 (0.4) | 0.08 | 34 (2.3) | 25 (0.9) | 0.002 |
| Venous Thromboembolism | 7 (0.5) | 8 (0.3) | 1 | 17 (1.2) | 20 (0.7) | 1 |
n=4377 (Black n=1456; White n=2921);
Bonferroni corrected p values were adjusted within outcome group (index and any hospitalization);
Including malnutrition, failure to thrive, weight loss
TABLE 4.
Procedures at Index Hospitalization and Any Hospitalization
| Index Hospitalization | Any Hospitalization | |||||
|---|---|---|---|---|---|---|
| Black n (%) |
White n (%) |
p value1 | Black n (%) |
White n (%) |
p value | |
| Endoscopies | 626 (43.0) | 1135 (38.9) | 0.10 | 759 (52.1) | 1355 (46.4) | 0.004 |
| Blood product transfusion | 134 (9.2) | 200 (6.8) | 0.08 | 207 (14.2) | 319 (10.9) | 0.023 |
| Colectomy2 | 84 (5.8) | 195 (6.7) | 1 | 244 (16.8) | 447 (15.3) | 1 |
| Perianal/perineal procedures | 59 (4.1) | 92 (3.1) | 1 | 124 (8.5) | 146 (5.0) | <0.001 |
| Resection/anastomosis | 37 (2.5) | 109 (3.7) | 0.41 | 113 (7.8) | 234 (8.0) | 1 |
| Abdominal surgery3 | 40 (2.7) | 96 (3.3) | 1 | 116 (8.0) | 230 (7.9) | 1 |
| Appendectomy | 28 (1.9) | 70 (2.4) | 1 | 51 (3.5) | 114 (3.9) | 1 |
| Wound/lesion related | 28 (1.9) | 59 (2.0) | 1 | 78 (5.4) | 136 (4.7) | 1 |
| Ostomy4 | 18 (1.2) | 61 (2.1) | 0.47 | 66 (4.5) | 136 (4.7) | 1 |
| Central access | 22 (1.5) | 45 (1.5) | 1 | 42 (2.9) | 86 (2.9) | 1 |
| Intra-abdominal fistula repair | 4 (0.3) | 19 (0.7) | 1 | 24 (1.6) | 51 (1.7) | 1 |
| Cholecystectomy | 2 (0.1) | 7 (0.2) | 1 | 6 (0.4) | 15 (0.5) | 1 |
n=4377 (Black n=1456; White n=2921);
Bonferroni correctedp values were adjusted within outcome group (index and any hospitalization);
Partial, subtotal, total;
General or lysis of adhesions;
Small and/or large intestine
DISCUSSION
To date, few studies have investigated healthcare disparities in CD, and most existing studies have focused on adult populations.27, 29–31 Of the few studies investigating racial disparities in pediatric CD, most have either utilized smaller sample sizes,32 discussed disparities in IBD without distinction between CD and UC,33 focused only on UC,34 or examined sociodemographic factors other than race that might be associated with disparities.35, 36
Using a large and diverse cohort extracted from the PHIS database, our study investigated evidence of racial disparities in the treatment and outcomes of hospitalized White and Black pediatric patients with CD. Our findings support that racial disparities exist among children and adolescents with CD, but to what degree this represents a healthcare disparity or a disparity due to genetic and/or environmentally determined differences in disease is still unknown. We found several statistically significant differences between races, including important outcomes such as hospital readmissions; however the effect sizes were small. This suggests that although one may not readily identify these racial differences at a practice level due to the small effect size, they may be important at the population level. The etiology of these racial differences must be understood in order to design practice level interventions that can reduce population level disparities. In our study, Black children had a 1.5 times higher frequency of readmissions compared to White children.
Our large, regionally diverse cohort also allowed for the detection of other less common events and adds valuable information to the sparse literature on pediatric IBD race disparities. Our study supports the findings noted by Schaefer et al, that race did not influence the risk of bowel surgery.37 However, we found that Black children were more likely to have anemia, vitamin D deficiency, endoscopic procedures, blood product transfusions and treatment with corticosteroids and biologic agents compared to White children.
Some of the observed differences in our study are likely attributable to intrinsic differences in disease between Blacks and Whites. For example, the increase in perianal procedures is likely secondary to the increased rate of perianal disease in Blacks.24, 38, 39 However, other differences may reflect disparities in care, although biologic differences can’t be excluded. For instance, while the increased usage of corticosteroids and biologic agents among Black children could suggest a more severe disease course that may be attributable to worse intrinsic disease in Black children, it is equally possible that Black patients were more ill at the time of hospital admissions secondary to delays in seeking care or impaired access to care, thus requiring more intensive therapy. This point may also be strengthened by the fact that Black children were slightly older at the first admission than White children, which could represent a subtle marker of diminished access to medical care or a delay in disease recognition from a community health level. Unfortunately, there are no validated measures in PHIS to address disease severity as a potential explanatory factor.
We found that Black children had a lower median income and were more likely to have Medicaid. Financial barriers to outpatient care and self-management, such as transportation, medication and nutrition needs, may have influenced the disparities seen in our study. In support of this hypothesis, a recently published study found that Black children with asthma were twice as likely to be readmitted as White children and this difference was partially explained by financial and social hardship.40
It has been noted in other chronic diseases that the role of biology in health disparities is often modest and there are many other factors which contribute to disparities in care.14–16, 40–42 Some of these factors include SES status, access to care, payor status, health care system inefficiencies, health literacy, adherence, and provider biases. We suspect that this is also true in pediatric IBD.
The limitations of our study include that the results may not be generalizable to all pediatric centers, the PHIS database is not weighted for extrapolation to national estimates and misclassification errors can also occur since the data were obtained from an administrative database. The strengths of this study include that it is a large, regionally diverse population and we minimized confounding by severity by focusing on a hospitalized cohort which restricted the study population to patients with moderate/severe disease.
There are significant differences in hospital readmissions, medication usage and both medical and surgical complications of children with CD which are related to race. It is unclear whether this is due to disparities in care, or phenotypic variation in disease between racial groups, although both likely play a role. We propose that non-biologic factors are likely contributors to these differences and may include factors such as differences in adherence, access or treatment, and may be influenced by financial considerations. Thus, future investigations should examine frequency of follow up care, adherence, access to care, disease severity and distribution, medication treatment differences and provider bias. Further primary research endeavors and standardization of care are needed to ultimately improve healthcare delivery and minimize healthcare disparities among minorities.42, 43 Minimizing complications and cost of care would have a profound effect on quality of life and healthcare expenditures.
Supplementary Material
ACKNOWLEDGEMENT
Dr. Dotson and this project were supported by the NASPGHAN Foundation/Crohn’s and Colitis Foundation of America Young Investigator Development Award. Dr. Kappelman was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (K08 DK088957). The study sponsors had no role in the study design or the collection, analysis, and interpretation of data. The project described was supported by Award Number Grant UL1TR001070 from the National Center For Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Advancing Translational Sciences or the National Institutes of Health. The authors would also like to thank Dr. Rebecca R. Andridge, PhD for her assistance with reviewing this paper and providing statistical expertise.
Dr. Crandall is a consultant and speaker for Abbott and receives research support from AbbVie. Dr. Kappelman is a consultant for AbbVie and receives research support from Janssen and AbbVie.
Footnotes
Disclosures: The remaining authors have no financial relationships relevant to this article to disclose. The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Loftus EV, Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1–20. doi: 10.1016/s0889-8553(01)00002-4. [DOI] [PubMed] [Google Scholar]
- 2.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 3.Benchimol EI, Fortinsky KJ, Gozdyra P, et al. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423–439. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 4.Malaty HM, Fan X, Opekun AR, et al. Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatr Gastroenterol Nutr. 2010;50:27–31. doi: 10.1097/MPG.0b013e3181b99baa. [DOI] [PubMed] [Google Scholar]
- 5.Benchimol EI, Guttmann A, Griffiths AM, et al. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58:1490–1497. doi: 10.1136/gut.2009.188383. [DOI] [PubMed] [Google Scholar]
- 6.Mackner LM, Greenley RN, Szigethy E, et al. Psychosocial issues in pediatric inflammatory bowel disease: report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2013;56:449–458. doi: 10.1097/MPG.0b013e3182841263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackner LM, Bickmeier RM, Crandall WV. Academic achievement, attendance, and school-related quality of life in pediatric inflammatory bowel disease. J Dev Behav Pediatr. 2012;33:106–111. doi: 10.1097/DBP.0b013e318240cf68. [DOI] [PubMed] [Google Scholar]
- 8.Greenley RN, Hommel KA, Nebel J, et al. A meta-analytic review of the psychosocial adjustment of youth with inflammatory bowel disease. J Pediatr Psychol. 2010;35:857–869. doi: 10.1093/jpepsy/jsp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bewtra M, Su C, Lewis JD. Trends in hospitalization rates for inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol. 2007;5:597–601. doi: 10.1016/j.cgh.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen GC, Tuskey A, Dassopoulos T, et al. Rising hospitalization rates for inflammatory bowel disease in the United States between 1998 and 2004. Inflamm Bowel Dis. 2007;13:1529–1535. doi: 10.1002/ibd.20250. [DOI] [PubMed] [Google Scholar]
- 11.Heaton PC, Tundia NL, Schmidt N, et al. The National Burden of Pediatric Hospitalizations for Inflammatory Bowel Disease: Results from the 2006 Kids' Inpatient Database. J Pediatr Gastroenterol Nutr. 2011 doi: 10.1097/MPG.0b013e318239bc79. [DOI] [PubMed] [Google Scholar]
- 12.Turner D, Simpson P, Li SH, et al. Racial disparities in pediatric intensive care unit admissions. South Med J. 2011;104:640–646. doi: 10.1097/SMJ.0b013e3182296e52. [DOI] [PubMed] [Google Scholar]
- 13.Nembhard WN, Salemi JL, Ethen MK, et al. Racial/Ethnic disparities in risk of early childhood mortality among children with congenital heart defects. Pediatrics. 2011;127:e1128–e1138. doi: 10.1542/peds.2010-2702. [DOI] [PubMed] [Google Scholar]
- 14.Berry JG, Bloom S, Foley S, et al. Health inequity in children and youth with chronic health conditions. Pediatrics. 2010;126(Suppl 3):S111–S119. doi: 10.1542/peds.2010-1466D. [DOI] [PubMed] [Google Scholar]
- 15.Howell E, Decker S, Hogan S, et al. Declining child mortality and continuing racial disparities in the era of the Medicaid and SCHIP insurance coverage expansions. Am J Public Health. 2010;100:2500–2506. doi: 10.2105/AJPH.2009.184622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokoska ER, Bird TM, Robbins JM, et al. Racial disparities in the management of pediatric appenciditis. J Surg Res. 2007;137:83–88. doi: 10.1016/j.jss.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Freedman J, Guller U, Benjamin DK, et al. National trends in health care utilization and racial and socioeconomic disparities in pediatric pyogenic arthritis. J Pediatr Orthop. 2006;26:709–715. doi: 10.1097/01.bpo.0000229973.78565.02. [DOI] [PubMed] [Google Scholar]
- 18.Nwomeh BC, Chisolm DJ, Caniano DA, et al. Racial and socioeconomic disparity in perforated appendicitis among children: where is the problem? Pediatrics. 2006;117:870–875. doi: 10.1542/peds.2005-1123. [DOI] [PubMed] [Google Scholar]
- 19.Braveman P, Barclay C. Health disparities beginning in childhood: a life-course perspective. Pediatrics. 2009;124(Suppl 3):S163–S175. doi: 10.1542/peds.2009-1100D. [DOI] [PubMed] [Google Scholar]
- 20.Institute of Medicine. Committee on Understanding and Eliminating Racial and Ethnic Disparities in Heatlh Care. In: Smedley BDSA, Nelson AR, editors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, D.C.: The National Academies Press; 2003. p. 764. [PubMed] [Google Scholar]
- 21.Flasar MH, Johnson T, Roghmann MC, et al. Disparities in the use of immunomodulators and biologics for the treatment of inflammatory bowel disease: a retrospective cohort study. Inflamm Bowel Dis. 2008;14:13–19. doi: 10.1002/ibd.20298. [DOI] [PubMed] [Google Scholar]
- 22.Mangat BK, Evaschen C, Lee T, et al. Ethnic variation in the annual rates of adult inflammatory bowel disease in hospitalized patients in Vancouver, British Columbia. Can J Gastroenterol. 2011;25:73–77. doi: 10.1155/2011/640920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straus WL, Eisen GM, Sandler RS, et al. Crohn's disease: does race matter? The Mid-Atlantic Crohn's Disease Study Group. Am J Gastroenterol. 2000;95:479–483. doi: 10.1111/j.1572-0241.2000.t01-1-01531.x. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen GC, Torres EA, Regueiro M, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: characterization of a large North American cohort. Am J Gastroenterol. 2006;101:1012–1023. doi: 10.1111/j.1572-0241.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 25.Basu D, Lopez I, Kulkarni A, et al. Impact of race and ethnicity on inflammatory bowel disease. Am J Gastroenterol. 2005;100:2254–2261. doi: 10.1111/j.1572-0241.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen GC, Chong CA, Chong RY. National estimates of the burden of inflammatory bowel disease among racial and ethnic groups in the United States. J Crohns Colitis. 2013 doi: 10.1016/j.crohns.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen GC, Bayless TM, Powe NR, et al. Race and health insurance are predictors of hospitalized Crohn's disease patients undergoing bowel resection. Inflamm Bowel Dis. 2007;13:1408–1416. doi: 10.1002/ibd.20200. [DOI] [PubMed] [Google Scholar]
- 28.Sewell JL, Velayos FS. Systematic review: The role of race and socioeconomic factors on IBD healthcare delivery and effectiveness. Inflamm Bowel Dis. 2013;19:627–643. doi: 10.1002/ibd.22986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen G, LaVeist T, Harris M, et al. Racial disparities in utilization of specialist care and medications in inflammatory bowel disease: P-0064. Inflamm Bowel Dis. 2009;15:S25. doi: 10.1038/ajg.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson JF, 3rd, Dhere T, Repaka A, et al. Crohn's disease in an African-American population. Am J Med Sci. 2008;336:389–392. doi: 10.1097/MAJ.0b013e31816a5c06. [DOI] [PubMed] [Google Scholar]
- 31.Sewell JL, Inadomi JM, Yee HF., Jr Race and inflammatory bowel disease in an urban healthcare system. Dig Dis Sci. 2010;55:3479–3487. doi: 10.1007/s10620-010-1442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eidelwein AP, Thompson R, Fiorino K, et al. Disease presentation and clinical course in black and white children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:555–560. doi: 10.1097/MPG.0b013e3180335bb3. [DOI] [PubMed] [Google Scholar]
- 33.Huang JS, Tobin A, Tompane T. Clinicians poorly assess health literacy-related readiness for transition to adult care in adolescents with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012;10:626–632. doi: 10.1016/j.cgh.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Kelley-Quon LI, Tseng CH, Jen HC, et al. Postoperative complications and health care use in children undergoing surgery for ulcerative colitis. J Pediatr Surg. 2012;47:2063–2070. doi: 10.1016/j.jpedsurg.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Kappelman MD, Porter CQ, Galanko JA, et al. Utilization of healthcare resources by U.S. children and adults with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:62–68. doi: 10.1002/ibd.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benchimol EI, To T, Griffiths AM, et al. Outcomes of pediatric inflammatory bowel disease: socioeconomic status disparity in a universal-access healthcare system. J Pediatr. 2011;158:960–967. e1–e4. doi: 10.1016/j.jpeds.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer ME, Machan JT, Kawatu D, et al. Factors that determine risk for surgery in pediatric patients with Crohn's disease. Clin Gastroenterol Hepatol. 2010;8:789–794. doi: 10.1016/j.cgh.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 38.Deveaux PG, Kimberling J, Galandiuk S. Crohn's disease: presentation and severity compared between black patients and white patients. Dis Colon Rectum. 2005;48:1404–1409. doi: 10.1007/s10350-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 39.Markowitz J, Grancher K, Rosa J, et al. Highly destructive perianal disease in children with Crohn's disease. J Pediatr Gastroenterol Nutr. 1995;21:149–153. doi: 10.1097/00005176-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Beck AF, Huang B, Simmons JM, et al. Role of Financial and Social Hardships in Asthma Racial Disparities. Pediatrics. 2014 doi: 10.1542/peds.2013-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan PL, Staff J, Hillemeier MM, et al. Racial and ethnic disparities in ADHD diagnosis from kindergarten to eighth grade. Pediatrics. 2013;132:85–93. doi: 10.1542/peds.2012-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schechter MS, Margolis P. Improving subspecialty healthcare: lessons from cystic fibrosis. J Pediatr. 2005;147:295–301. doi: 10.1016/j.jpeds.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 43.Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998;280:1000–1005. doi: 10.1001/jama.280.11.1000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.