Abstract
We present here that Heat shock protein 90 (Hsp90) inhibitor 17-(allylamino)-17-demethoxygeldanamycin (17AAG), when topically applied to mouse skin, inhibits ultraviolet radiation (UVR)-induced development of cutaneous squamous cell carcinoma (SCC). In these experiments, DMSO:acetone (1:40 v/v) solution of 17AAG (500nmol) was applied topically to mouse skin in conjunction with each UVR exposure (1.8 kJ/m2). The UVR source was Kodacel-filtered FS-40 sun lamps (approximately 60% UVB and 40% UVA). In independent experiments with three separate mouse lines (SKH-1 hairless mice, wild-type FVB, and PKCε overexpressing transgenic FVB mice), 17AAG treatment increased the latency and decreased both the incidence and multiplicity of UVR-induced SCC. Topical 17AAG alone or in conjunction with UVR treatments elicited neither skin nor systemic toxicity. 17AAG-caused inhibition of SCC induction was accompanied by decrease in UVR-induced: 1) hyperplasia, 2) Hsp90β-PKCε interaction, 3) expression levels of Hsp90β, Stat3, pStat3Ser727, pStat3Tyr705, pAktSer473 and matrix metalloproteinase (MMPs). The results presented here indicate that topical Hsp90 inhibitor 17AAG is effective in prevention of UVR-induced epidermal hyperplasia and SCC. One may conclude from the preclinical data presented here that topical 17AAG may be useful for prevention of UVR-induced inflammation and cutaneous SCC either developed in UVR exposed or organ transplant population.
Keywords: PKCε, SCC, UVR, Hsp90, 17AAG
Introduction
Skin cancer is the second most commonly occurring cancer in the US, and about 1.3 million new cases of non-melanoma skin cancer are diagnosed annually (Anonymous Skin Cancer; American Cancer Society, 2014). The most common non-melanoma forms of human skin cancer include cutaneous squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) (Goldman, 1998; Anonymous, 2014). BCC is a localized tumor and can be successfully treated surgically, while SCC can metastasize to lymph nodes and lungs (Anonymous, 2014). Chronic UVR exposure is the most common etiologic factor linked to the development of SCC. It is noteworthy that organ transplant patients have a high incidence of SCC (Lindelof et al., 2000; Ramsey et al., 2007; Bath-Hextall et al., 2007; Bangash and Colegio, 2012;). However, the molecular mechanisms linked to SCC formation are not well defined. We found that PKCε activation plays an important role in UVR-induced signal transduction pathways to the development of SCC (Wheeler et al., 2004; Wheeler et al., 2005; Verma et al., 2006; Sand et al., 2010;). The chaperone Hsp90 mediates the maturation and stabilization of PKCε (Gould et al., 2009).
Hsp90 is a highly conserved protein and comprises about 2% of the total number of expressed cell proteins (Whitesell et al., 2012; Den and Lu, 2012; Scaltriti et al., 2012). Molecular chaperone Hsp90 is required for the stability and maturation of client proteins, essential for cell transformation, proliferation, and survival (Bracher and Hartl, 2006; Taipale et al., 2012; Miyata et al., 2013). Mammalian cells contain three types of Hsp90s: cytosolic Hsp90, mitochondrial Trap-1, and glucose-regulated protein 94 (Grp94) of the endoplasmic reticulum. Each of the Hsp90s including the bacterial homolog HtpG hydrolyzes ATP and undergoes similar conformational changes. Unlike the other forms of Hsp90, cytosolic Hsp90 function is dependent on a battery of co-chaperone proteins that regulate the ATPase activity of Hsp90 or direct Hsp90 to interact with specific client proteins (Zuehlke and Johnson, 2010). Two cytosolic Hsp90 isoforms (Hsp90α and Hsp90β) have been reported in humans and mice. These are encoded by two separate genes, (gene Hsp90aa1) and (gene Hsp90ab1). Cytosolic Hsp90 isoforms are highly homologous and exhibit 85.8% sequence identity and 93.4% similarity. Hsp90β is constitutively expressed while the expression of Hsp90α is tissue-specific (Sreedar et al., 2004). Tumor cell oncogenic proteins require Hsp90 for their activity. Thus, Hsp90 appears to be a potential molecular target for cancer prevention and treatment. (Stebbins et al., 1997; Neckers, 2002; Soti et al., 2005; Neckers, 2006; Biamonte et al., 2010). Consequently, several Hsp90 inhibitors are being evaluated for treatment of various human cancers (Stebbins et al., 1997; Lindelof et al., 2000; Neckers, 2002; Soti et al., 2005; Neckers, 2006; Solit et al., 2008; Heath et al., 2008; Usmani et al., 2009; Biamonte et al., 2010). However, Hsp90 inhibitors have never been investigated for prevention and treatment of cutaneous SCC. The first Hsp90 inhibitor used clinically was geldanamycin, which failed in clinical trials due to liver toxicity. Second generation derivatives such as 17-allylamino-demethoxygeldanamycin (17-AAG) do not cause liver toxicity and are currently being evaluated in phase II clinical trials (Heath et al., 2008; Biamonte et al., 2010; Hubbard et al., 2011; Gartner et al., 2012; Pacey et al., 2012).
In this communication, we evaluated the effects of topically applied 17-AAG on UVR-induced development of cutaneous SCC in mice. Topical applications of 17AAG in conjunction with UVR exposures increased the latency and decreased both the incidence and multiplicity of cutaneous SCC. 17AAG-caused suppression of UVR-induced SCC preceded inhibition of Hsp90β-PKCε interaction, expression levels of Hsp90β, Stat3, pStat3Ser727, pStat3Tyr705, pAktSer473, MMPs, and epidermal hyperplasia.
Results
1. PKCε is a client protein of Hsp90β
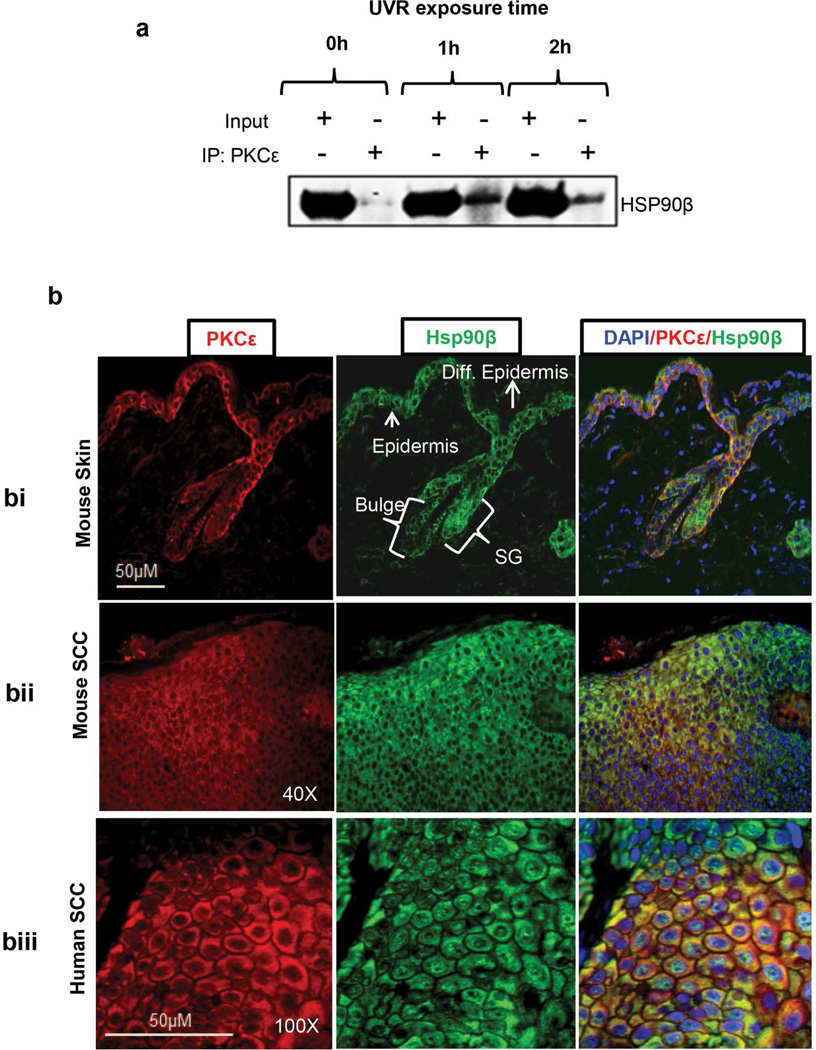
In this experiment (Figure 1), PKCε transgenic mice were exposed once to UVR (4kJ/m2). PKCε-Hsp90β interaction was analyzed at 1 and 2 h post UVR treatment by co-immunoprecipitation and Western blotting. As shown in Figure 1a, PKCε co-immunoprecipitates with Hsp90β. PKCε-Hsp90β interaction was enhanced as early as 1 h post UVR treatment. To confirm the association of PKCε with Hsp90β, we determined the co-localization of PKCε and Hsp90β by double immunofluorescence staining. In this experiment (Figure 1b), 5-µm thick skin sections from paraffin-fixed samples from the indicated specimens were used. Localization of PKCε and Hsp90β is indicated by the presence of red and green fluorescence, respectively. The yellow fluorescence indicates co-localization and association of PKCε with Hsp90β. The co-localization of PKCε with Hsp90 was observed in mouse epidermis (Figure 1bi), UVR-induced SCC in SKH-1 hairless mice (Figure 1bii) and human SCC (Figure 1biii). The expression of PKCε and Hsp90β was seen in epidermis, sebaceous gland, and bulge region of hair follicle. However, the expression of PKCε and Hsp90β was lost in differentiated epidermis keratinocytes.
Figure 1. PKCε is a client protein of Hsp90β.
(a) UVR treatment increases the interaction of PKCε with Hsp90β. PKCε-overexpressing transgenic mice (line 224) (n=3) were exposed to UVR (4 kJ/m2) once. Mice were sacrificed 1 h or 2 h post UVR exposure. Epidermal protein extracts were prepared and pooled as described before (Aziz et al., 2007). 100 microgram of epidermal cell lysate protein was immunoprecipitated (IP) using PKCε antibody and immunoblotted with the Hsp90β antibody. (b) Co-localization of PKCε with Hsp90β. Co-localization of PKCε with Hsp90β in the indicated paraffin-fixed specimen was determined by double immunofluorescence as described (Aziz et al., 2007). The fluorescent pictures were captured through TRITC channel (red color), FITC channel (green color), and DAPI (Blue color) in a Nuance Fluorescent microscope. DAPI/PKCε/Hsp90β is the merge images of DAPI, FITC and TRITC channel. PKCε-overexpressing transgenic mouse skin harvested 24 h post UVR-exposure (1.8 kJ/m2) (bi), UVR induced SCC in SKH-1 hairless mice (bii) and Human SCC (biii). Shown is the representative picture selected from five different specimens.
2. Topical 17AAG inhibited UVR-induced SCC, which was accompanied by decrease in: 1) hyperplasia, and 2) Hsp90β-PKCε interaction and 3) Hsp90β, PKCε, Stat3, pStat3Ser727, pStat3Tyr705, Akt, pAktSer473 and MMPs expression levels (Figure 3)
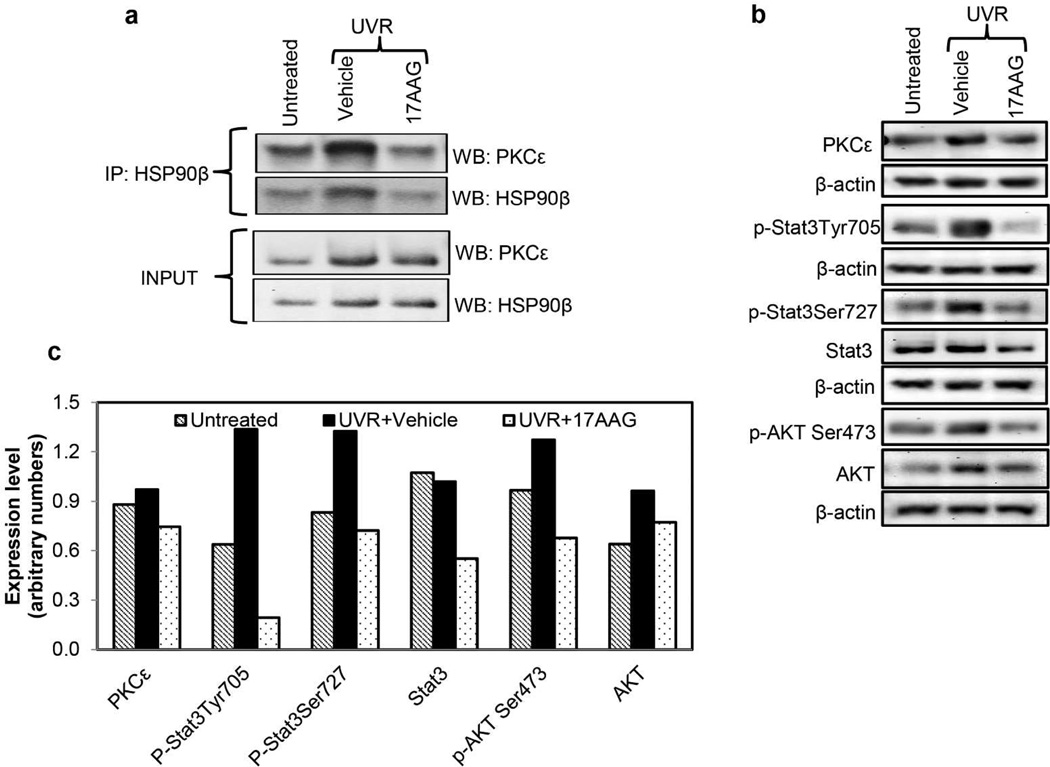
Figure 3. 17AAG inhibits UVR-induced Hsp90β-PKCε interaction and pStat3Tyr705, pStat3Ser727, Stat3 and pAktSer473 expression levels.
(a) 17AAG inhibits Hsp90β-PKCε interaction. PKCε-overexpressing transgenic mice (line 224) were exposed once to UVR (4 kJ/m2). 17AAG (500 nmol) or the vehicle was applied topically both before and after UVR exposure. Mice were sacrificed 24 h post-UVR exposure. There were two mice per treatment point. Dorsal skin was excised and epidermal cell lysates were prepared as described before (Aziz et al., 2007). Pooled epidermal cell lysate was analyzed for PKCε-Hsp90β interaction by immunoprecipitation/Western blot analyses. (b,c) 17AAG inhibits protein levels of Stat3, pStat3Tyr705, pStat3Ser727, and pAktSer473. PKCε-overexpressing transgenic mice (line 224) were exposed once to UVR (2kJ/m2). 17AAG or the vehicle was applied topically both before and after UVR exposure. Mice were sacrificed at 24h post-UVR exposure. There were three mice per treatment point. Dorsal skin was excised and pooled. Epidermal cell lysates were prepared as described (Aziz et al., 2007). Pooled epidermal cell lysate were analyzed for the expression level o f the indicated proteins by the Western blot analysis. (c) Shown is the quantitation of the western blot shown in figure 3b. Experiments were repeated three times with similar results.
17AAG is an ATP antagonist and inhibits Hsp90 ATPase activity (Stebbins et al., 1997). Inhibition of Hsp90 ATPase activity by 17AAG results in affecting the maturity and stability of its client proteins including PKCε (Gould et al., 2009). This led us to hypothesize that disruption of UVR-induced interaction of Hsp90β oncogenic client proteins by Hsp90 inhibitor 17AAG will result in the prevention of UVR-induced SCC. To test this hypothesis, 17AAG was applied topically to skin in conjunction with each UVR exposure. Noteworthy observations are as follows:
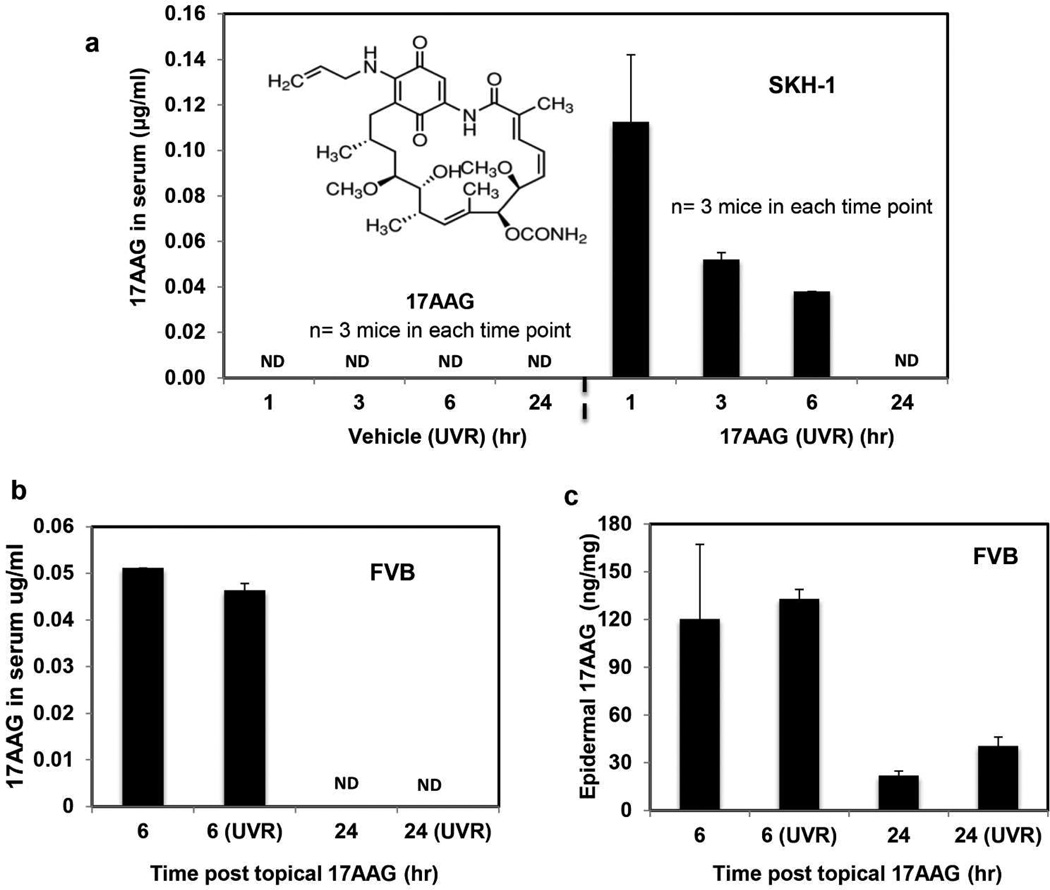
Topically applied 17AAG is distributed both in epidermis and serum. In all the foregoing experiments, stock solution of 17AAG (100mM) was prepared in DMSO and then diluted with acetone (DMSO:acetone: 1:40 v/v) to obtain desired dose for topical application to skin. As a prelude to evaluating the biochemical and biological effects of topical 17AAG on UVR exposed skin, we determined its distribution in skin and serum. In these experiments (Figure 2), 17AAG solution (DMSO:acetone: 1:40 v/v) was applied alone or in conjunction with either acute or chronic UVR exposures. A time course of topically applied 17AAG on epidermal and serum levels is shown in Figure 2a. In this experiment (Figure 2), SKH-1 hairless mice received topical 17AAG treatment in conjunction with each three weekly UVR exposures for 25 weeks. Mice were sacrificed for serum 17AAG analysis at 1, 3, 6 and 24 h after last UVR exposure. Maximum serum 17AAG level was detected as early as 1 h post topical 17AAG application (Figure 2a). In a separate acute UVR exposure experiment with FVB mice, the level of 17AAG was analyzed in both serum and epidermis at 6 and 24 h post 17AAG application (Figure 2b–c). Both serum (Figure 2b) and epidermal 17AAG (Figure 2c) levels were detectable at 6 h after 17AAG treatment. In both experiment with SKH-1 and FVB mice, serum (Figure 2a and b) and epidermal 17AAG (Figure 2c) levels reached to minimum value at 24 h post 17AAG treatment. UVR treatment does not appear to affect either the serum or the epidermal 17AAG level (Figure 2a–c).
Topical 17AAG treatment suppressed Hsp90β-PKCε interaction and expression levels of Hsp90β, Stat3, pStat3Ser727, pStat3Tyr705, Akt, pAktSer473. In this experiment (Figure 3), PKCε-overexpressing FVB transgenic mice (line 224) were exposed once to UVR. 17AAG (500 nmol) or the vehicle was applied topically both before and after UVR exposure. Mice were sacrificed 24 h post UVR exposure. UVR exposure enhanced Hsp90β-PKCε interaction, and 17AAG treatment in conjunction with UVR exposure suppressed PKCε-Hsp90β interaction (Figure 3a). Also, 17AAG treatment in conjunction with UVR inhibited Stat3, pStat3Ser727, pStat3Tyr705 and pAktSer473 expression levels, but the PKCε and Akt expression levels were minimally suppressed (Figure 3b and c). Similar results were observed in several repeated experiments.
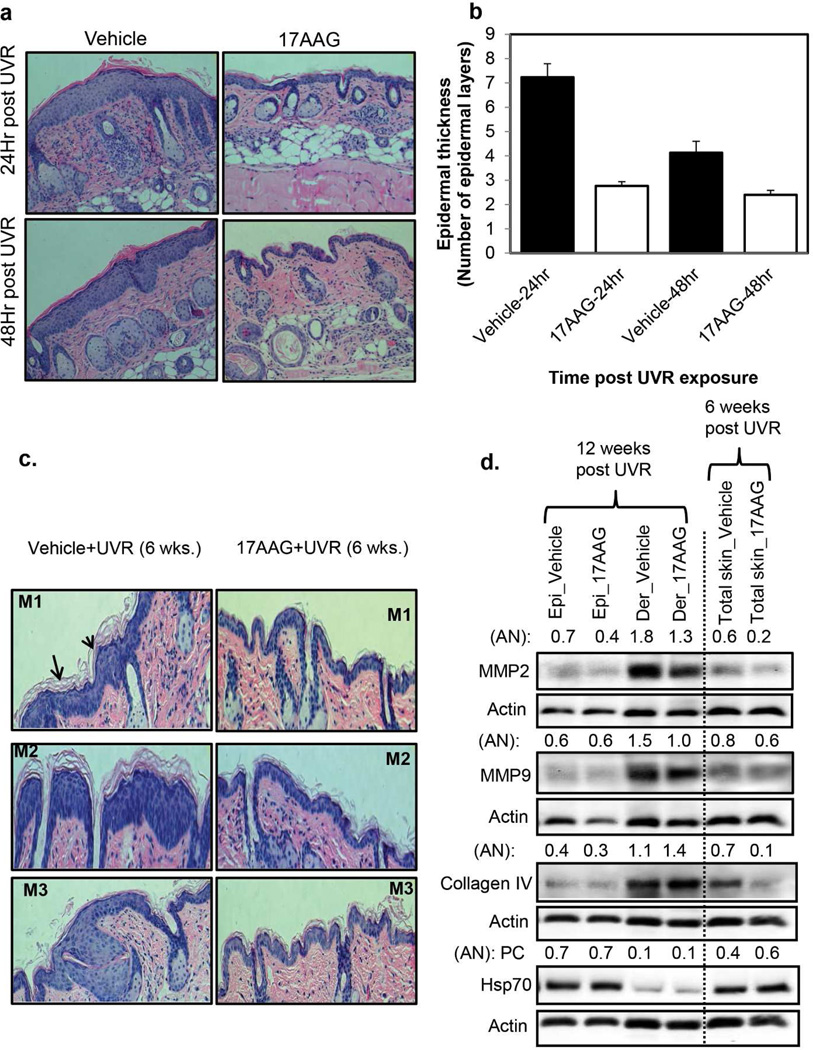
Topical 17AAG inhibited UVR-induced hyperplasia in SKH-1 mice. In this experiment, groups of SKH-1 hairless mice were exposed four times to UVR (4 kJ/m2, Monday, Wednesday, Friday, and Monday). 17AAG (500 nmol) or the vehicle was applied topically after each UVR exposure. The mice were sacrificed at 24 h and 48 h after the fourth UVR exposure. The dorsal skin was removed and fixed in 10% formalin for the analysis of epidermal hyperplasia. 17AAG treatment inhibited UVR-induced hyperplasia as indicated by significant (p<0.01) inhibition of epidermal thickness (Figure 4a, and b).
17AAG-caused inhibition of UVR-induced hyperplasia in SKH-1 mice is accompanied by a decrease in Collagen IV and matrix metalloproteinase (MMPs), the markers of skin wrinkle formation. UVR-induced increased expression of MMPs and subsequent degradation of collagen are linked to the process of skin wrinkle formation (Inomata et al., 2003). Also, Hsp70 has been shown to play a role in UVR-induced wrinkle formation (Shin et al., 2012, Matsuda et al., 2013). To obtain clues about the anti-wrinkling property of 17AAG, we determined the effects of topical 17AAG on UVR-induced expression levels of MMPs, Collagen IV, and Hsp70 in SKH-1 mice. In this experiment (Figure 4c, d), SKH-1 hairless mice were exposed to UVR (2 kJ/m2) thrice weekly (Monday, Wednesday, and Friday) for 6 or 12 weeks. 17AAG (500 nmol) or the vehicle was applied topically after each UVR exposure. The mice were sacrificed at 24 h post 6 or 12 weeks UVR exposures. 17AAG-caused inhibition of UVR-induced hyperplasia (Figure 4c) accompanied by decrease in the protein levels of MMP2, MMP9, and Collagen IV and slight increase in the protein level of Hsp70 in whole skin at 6 weeks post UVR (Figure 4d). MMP2, MMP9, and Collagen IV are predominantly expressed in dermal part of the skin. To evaluate specific change in the dermal and epidermal portions of the skin, we separately analyzed the effect of 17AAG on the expression levels of MMP2, MMP9, and Collagen IV in epidermal and dermal protein lysates at 24 h post 12 weeks UVR exposures (Figure 4d). 17AAG treatment resulted in decrease in the protein levels of MMP2, MMP9 in dermis. However, 17AAG treatment slightly increased the level of Collagen IV in the dermis at 12 weeks post UVR. Hsp70 expression level was affected neither in epidermis nor dermis at 12 weeks post UVR (Figure 4d). 17AAG treatment also inhibited UVR-induced proliferative marker PCNA (data not shown). Similar results (Figure 4) were obtained in three separate repeat independent experiments.
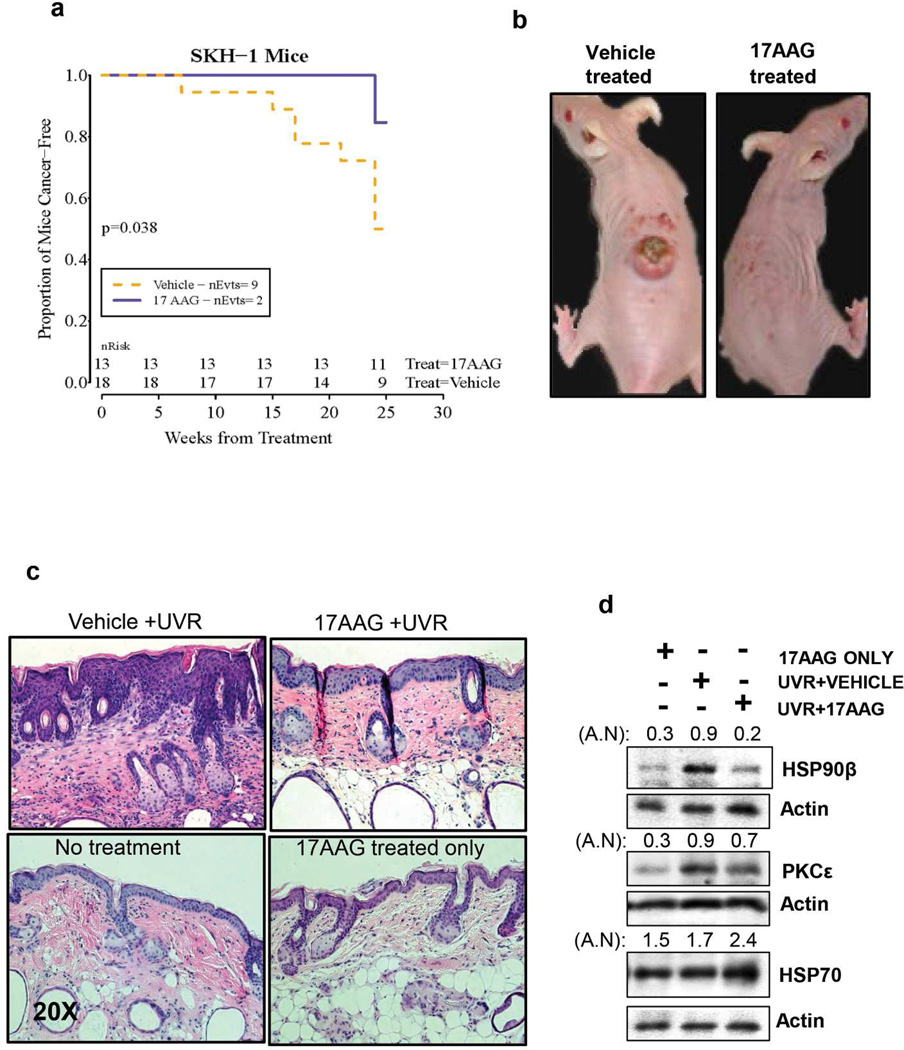
Topical 17AAG treatment inhibited UVR-induced development of SCC. An initial tumor induction experiment was performed with SKH-1 hairless mice. The Kaplan-Meier (product-limit) “survival” curve across time is displayed (Figure 5a). Topical 17AAG treatment increased SCC latency by 17 weeks and significantly (p=0.038) inhibited SCC incidence in SKH-1 hairless mice (Figure 5a). 17AAG-caused inhibition of UVR-induced SCC (Figure 5a and b) was accompanied by inhibition of UVR-induced hyperplasia (Figure 5c), Hsp90β and PKCε expression levels and an increase in Hsp70 level (Figure 5d). Also, topical applications of 17AAG alone did not cause gross morphological changes in mice skin (Figure 5c).
Figure 2. Topically applied 17AAG to skin is distributed both in epidermis and serum.
(a) The SKH-1 hairless mice (6–7 weeks old) were exposed to UVR (1.8 kJ/m2) three times weekly (Monday, Wednesday and Friday). The mice in the vehicle group (n=3) received topical treatment of 200 µl vehicle (DMSO:acetone: 1:40 v/v) before and after UVR exposures. The mice in 17AAG group (n=3) received freshly prepared 500 nmol of 17AAG (DMSO:acetone: 1:40 v/v) before and after each UVR exposure. All mice were treated for 25 weeks and sacrificed at 1, 3, 6 and 24 h post last UVR exposure. Blood samples were collected to analyze serum 17AAG by HPLC. (b, c) In a separate experiment, wild type FVB mice (6–7 weeks old) were treated once topically with either 17AAG alone (n=4) or in conjunction with a single UVR (1.8 kJ/m2) exposure (n=4). Mice of both the groups were sacrificed at 6 h (n=2) and 24 h (n=2) post 17AAG treatment. Blood samples were collected and epidermal protein lysates were prepared for 17AAG analysis. To prepare epidermal lysate, epidermis was scraped and homogenized in the lysis buffer. For 17AAG analysis, 50µl of epidermal lysate was used. Bar graph illustrates the serum (a,b) and the epidermal (c) 17AAG levels. The epidermal 17AAG values were normalized with total protein concentration.. Values shown in all bar graphs are mean±SE. ND: Not detectable. Inset: Chemical structure of 17AAG.
Figure 4. 17AAG-caused inhibition of UVR-induced hyperplasia in SKH1 mice accompanied by decreased expression of matrix metalloproteinase (MMPs).
Groups of SKH-1 mice (n=3) were exposed either four times to UVR (4kJ/m2) or 6 and 12 weeks (2kJ/m2) thrice weekly (Monday, Wednesday, and Friday). Vehicle or 17AAG (500 nmol) was applied post each UVR exposure. (a,b) The mice were sacrificed at 24 h and 48 h after the fourth UVR exposure. For histochemistry, skin specimen were fixed in 10% neutral buffered formalin for 24 h and embedded in paraffin for sectioning. The figure 4a and b illustrate epidermal hyperplasia and epidermal thickness respectively in vehicle and 17AAG treated SKH-1 mice. Each value in Figure b is the mean±SE of 10 measurements per section of two samples from each mouse. (c) In another experiment, mice were sacrificed at 24 h post 6 and 12 weeks UVR exposures. The figure 4c illustrates epidermal hyperplasia in vehicle and 17AAG treated SKH-1 mice. (d) Expression levels of MMP-2, MMP-9, Collagen IV, and Hsp70 in epidermis, dermis and total skin at 6 and 12 weeks post UVR exposures (pooled samples, n=3). AN: arbitrary number of the quantitation of the Western blots. Epi: epidermis. Der: dermis, M1, M2 and M3 are sections from different mice. Experiments were repeated three times with similar results.
Figure 5. 17AAG inhibits UVR-induced hyperplasia and development of SCC in SKH-1 hairless mice.
The SKH-1 hairless mice (6–7 week old) were exposed to UVR. Mice were exposed to UVR (1.8 kJ/m2) three times weekly (Monday, Wednesday and Friday). The mice in the vehicle group (n=18) received topical treatment of 200 µl vehicle (DMSO:acetone: 1:40 v/v) before and after each UVR exposure. The mice in the treatment group (n=13) received freshly prepared 500 nmol of 17AAG (DMSO:acetone: 1:40 v/v) before and after each UVR exposure. Carcinomas were recorded grossly as downward-invading lesions, which were confirmed histologically. (a) nEvts is the number of mice at risk at the time points listed. The log-rank statistic is computed from the expected number of deaths and actual number in each group. The Kaplan-Meier (product-limit) “survival” curve across time is displayed. (b) Representative photographs of the mice at the end of the experiment. (c) The mice were sacrificed and part of the uninvolved dorsal skin was fixed in 10% neutral-buffered formalin for histological examination. H&E stained uninvolved dorsal skin sections from mice receiving indicated treatments. (d) A part of the uninvolved skin from two mice was also used to prepare total epidermal lysate. Protein level of PKCε, Hsp90β and Hsp70 was determined by Western blot analysis. AN denotes arbitrary number of each Western blot.
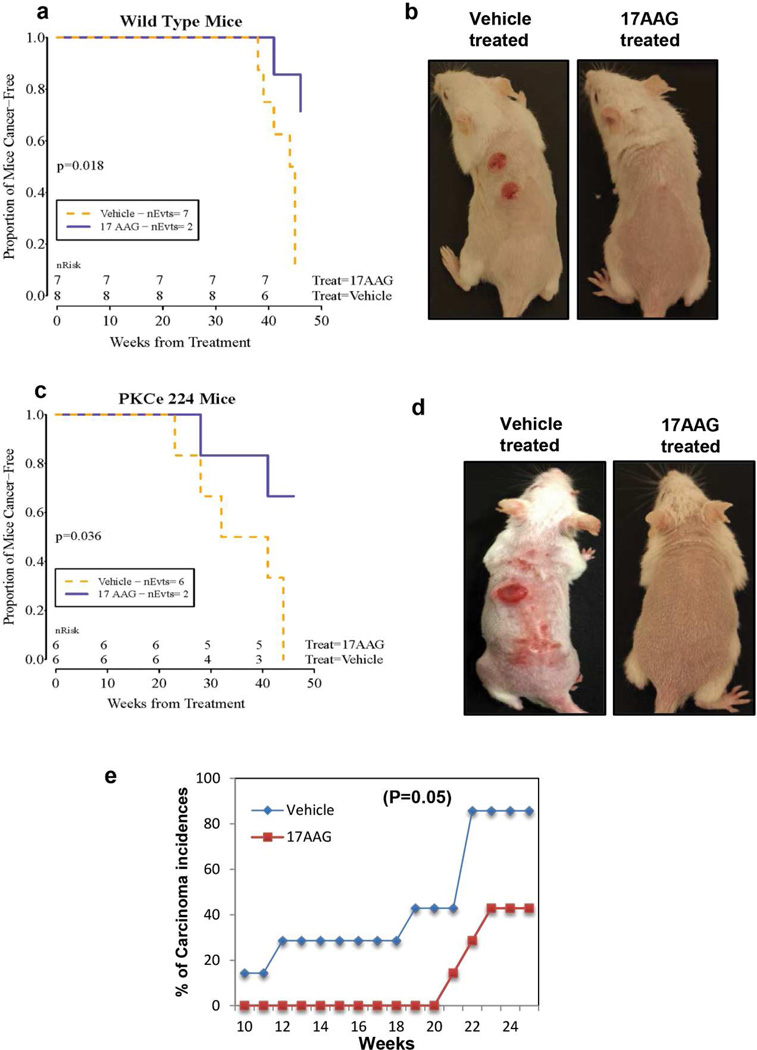
The effects of topical 17AAG treatments on UVR-induced development of SCC were further investigated in PKCε overexpressing transgenic FVB/N mice. We have previously reported that epidermal PKCε level dictates the susceptibility of transgenic mice to the development of SCC elicited either by repeated exposures to UVR or by the DMBA-TPA tumor promotion protocol (Reddig et al., 2000; Jansen et al., 2001a; Jansen et al., 2001b; Wheeler et al., 2003; Wheeler et al., 2004; Verma et al., 2006; Aziz et al., 2007; Sand et al., 2010). As compared to wild type littermates, PKCε over-expressing transgenic mice exhibited decrease in tumor latency and increased in SCC multiplicity (Wheeler et al., 2004; Sand et al., 2010). Again, topical applications of Hsp90 inhibitor 17AAG in conjunction with UVR exposures significantly (p = 0.036) inhibited the development of SCC in both PKCε overexpressing transgenic FVB/N mice and their wild-type littermates (p =0.018) (Figure 6).
Figure 6. 17AAG inhibits UVR-induced development of SCC in FVB/N PKCε overexpressing transgenic mice, FVB/N wildtype and SKH-1 hairless mice.
(a–d) The wildtype FVB/N (n=15) and PKCε-overexpressing transgenic mice (line 224) (TG) (n=12) (6–7 week old) were exposed to UVR (1.8 kJ/m2) three times weekly (Monday, Wednesday and Friday). The mice in the vehicle groups (WT, n=8 and TG, n=6) received topical treatment of 200µl vehicle (DMSO:acetone: 1:40 v/v) before and after UVR each exposure. The mice in treatment groups (WT, n=7 and TG, n=6) received freshly prepared 500 nmol of 17AAG (DMSO:acetone: 1:40 v/v) before and after each UVR exposure. Carcinomas were recorded grossly as downward-invading lesions, which were confirmed histologically. (a,c) nEvts is the number of mice at risk at the time points listed. The log-rank statistic is computed from the expected number of deaths and actual number in each group. (b,d) Representative photographs of indicated mice at the end of the experiment. (e) 17AAG inhibits UVR-induced SCC in SKH-1 mice even when applied post each UVR exposure. A total of 22 SKH-1 hairless mice (6–7 week old) were divided into two groups and treated as described under Figure.5a except 17AAG was applied post each UVR exposure. Line graph is showing % of carcinoma incidence in vehicle and 17AAG treated mice.
In all of the above described tumor induction experiments (Figures 5 and 6), 17AAG was applied before and after each UVR exposure. We further explored the possibility that the inhibitory effects of 17AAG on UVR-induced development of SCC are not attributable to a sunscreen property. In this experiment, 17AAG was applied post each UVR exposure (Figure 6e). Topical 17AAG treatments post UVR exposures increased SCC latency by 10 weeks and inhibited more than 50% SCC incidence (Figure 6e). However, the difference in the SCC incidence in the vehicle and 17AAG treated mice was marginally significant (p =0.05).
Discussion
Cutaneous SCC is the second most common cancer in the United States, with an excess of 200,000 new cases each year (Anonymous, 2014). SCC is mainly caused by chronic UVR exposures. UVR is a complete carcinogen, which both initiates and promotes carcinogenesis. UVR initiates photocarcinogenesis by directly damaging DNA (Berton et al., 1997; de Gruiji et al., 2001). The tumor promotion component of UVR carcinogenesis, which involves clonal expansion of the initiated cells, is probably mediated by aberrant expression of genes altered during tumor initiation. Specific examples are the observations that UVR treatment results in AP-1 activation (Huang et al., 1996), up-regulation of the expression levels of p53 (Ziegler et al., 1994), ornithine decarboxylase (ODC) (Rosen et al., 1990), COX2 (Isoherranem et al., 1999), TNFα, and a wide variety of cytokines and growth factors (Enk et al., 1995), MMPs, Stat3, and PKCε (Aziz et al., 2007). Many of these molecular regulators of UVR carcinogenesis require Hsp90 for their maturity, stability and activity. These several lines of independent evidence prompted us to test the hypothesis that treatment of Hsp90 inhibitor in conjunction with UVR exposures will prevent development of SCC. We now present that Hsp90 inhibitor 17AAG, when applied topically to skin in conjunction with each UVR exposure, increased the latency and decreased both the incidence and multiplicity of UVR-induced cutaneous SCC.
Topically applied 17AAG (500 nmol in acetone/DMSO vehicle (DMSO:acetone: 1:40 v/v) to skin is rapidly distributed both in epidermis and serum (Figure 2). Since both epidermis and serum level of 17AAG declined at 24 h post treatment, topically applied 17AAG appears to has a short half-life (Figure 2). The pharmacokinetic of 17AAG in patients and mice has been reported (Egorin et al., 2001; Goetz et al., 2005; Weigel et al., 2007; Bagatell et al., 2007; Saif et al., 2013). In these reports 17AAG was administered intravenously or orally. 17AAG is more bioavailable when given intraperitoneally than when given orally. The half-life of 17AAG in these reports varied from 3–4 hours (Egorin et al., 2001; Goetz et al., 2005; Weigel et al., 2007; Bagatell et al., 2007; Saif et al., 2013).
About 200 oncogenic proteins have been identified as clients of Hsp90 (Bracher et al., 2006; Taipale et al., 2012; Miyata et al., 2013). However, UVR-induced mouse epidermal protein clients of Hsp90β remain to be identified. Results from reciprocal co-immunoprecipitation experiments (Figures 1, 3) indicate interaction of PKCε with Hsp90β. UVR treatment increases the interaction of PKCε with Hsp90β (Figure 1). UVR exposure of mouse skin resulted in increased expression of PKCε, possibly due to its increased synthesis (data not shown). Newly synthesized PKC undergoes well-ordered sequential phosphorylation for activation (Kazanietz et al., 2000) and Hsp90 binds newly synthesized PKCε, a required step in its maturation and enzyme stability (Gould et al., 2009). UVR treatment-caused increased interaction of PKCε with Hsp90β appears to be the result of increased expression of newly synthesized PKCε. Topical application of 17AAG to skin inhibited UVR-induced Hsp90β-PKCε interaction (Figure 2). However, 17AAG did not appreciably affect PKCε protein level.
UVR exposures-caused skin inflammation, epidermal hyperplasia and alteration in extra-cellular matrix (ECM) (e.g; damage to collagen and elastic fibers, low collagen expression, and disruption of epidermal membrane) are associated with skin wrinkle formation (Talwar et al, 1995; Imokawa, 2009). UVR exposure has been shown to increase the expression level of matrix metalloproteinases (MMP1, 2, 3, and 9) both in mouse and human skin (Inomata et al., 2003; Rabe et al., 2006). Inhibition of MMP-2 and MMP-9 has been shown to protect from UVB-induced wrinkle formation and photoaging (Matsuda et al., 2013, Fischer et al., 1996, Jung et al., 2010). In accord these reports, we found that UVR exposure induced an increased expression of MMP2 and MMP-9 in the skin of SKH-1 mice. Topical 17AAG treatment in conjunction with UVR exposure suppressed UVR-induced increased MMP2 and MMP9 expression level (Figure 4d). These results indicate that 17AAG treatment may protect the skin from UVR-caused wrinkle formation and photoaging via inhibition of MMP2 and MMP9. Topical 17AAG treatment with each UVR exposure effectively inhibited SCC development. Since 17AAG has similar inhibitory activity when was applied post-UVR treatment, inhibitory effects of 17AAG cannot be attributable only to sunscreen property. The mechanism by which topical 17AAG inhibits UVR-induced SCC is unclear. UVR-induced development of SCC accompanies activation of several oncogenic signal transduction pathways (Hubbard et al., 2011). A few examples will be cited. It has been shown that activation of MAP kinases (ERKs, p38), transcription factors (c-fos, cjun, AP1, NF-κB, Stat3, and NFAT) and constitutive expression of pStat3Ser727, pStat3Tyr705 (Aziz et al., 2007) are linked with UVR-induced skin carcinogenesis (Huang et al., 1996; Hubbard et al., 2011; Zhang et al., 2001; Sand et al., 2012). UVR-activated oncogenic signal transduction pathway (Hubbard et al., 2011) proteins are clients of Hsp90. Further experimentation is needed to define the relative contribution of these proteins client of Hsp90 in 17AAG-caused inhibition of UVR-induced development of SCC.
In summary, cutaneous SCC either developed in UVR exposed or organ transplant population is a significant health problem (Lindelof et al., 2000; Ramsay et al., 2007; Bath-Hextall, et al., 2007). Our results indicate that Hsp90 may be a potential molecular target for the prevention of UVR-induced development of cutaneous SCC. 17AAG can be formulated in cream for human use for the prevention of SCC either developed in UVR exposed or organ transplant population.
Materials and Methods
Chemicals, Antibodies, and Assay Kits
Antibodies to PKCε, Hsp90β, Hsp70, MMP2, MMP9, Stat3 and β-actin were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). Antibodies to pStat3Ser727 and pStat3Tyr705 were from BD Biosciences (San Jose, CA). Antibodies to Akt, and pAktSer473 were purchased from Cell Signaling (Danver, MA) and to collagen IV from abcam (Cambridge, MA). Anti-mouse, anti-goat, and anti-rabbit secondary antibodies were purchased from Thermo Scientific (Rockford, IL). 17-AAG was purchased from LC Laboratories (Woburn, MA). 17AAG has >99% purity determined by HPLC analysis. Kodacel filters were purchased from Unique Photo Inc. (Fairfield, NJ), UV lamps were purchased from National Biologicals Corporation, (Beachwood, OH), and UVX-radiometer from UVP, (Upland, CA).
Mice and UVR Treatment
SKH-1 hairless mice were purchased from Charles River Laboratories (Wilmington, MA). PKCε transgenic mice were generated as described previously (Reddig et al., 2000). The UVR source was Kodacel-filtered FS-40 sunlamps (approximately 60% UV-B and 40% UV-A). Mice were exposed to UVR from a bank of six Kodacel-filtered sunlamps. UVR dose was routinely measured using UVX-radiometer. All of the animal protocols were approved by the University’s Research Animal Resources Committee in accordance with the NIH Guideline for the Care and Use of Laboratory Animals.
Western Blot Analysis
Mouse skin was excised and scraped to remove subcutaneous fat. The epidermis was scraped off on an ice-cold glass plate and homogenized in lysis buffer [50 mmol/L HEPES, 150 mmol/L NaCl, 10% glycerol, 1% Triton X-100, 1.5 mmol/L MgCl2, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 200 µmol/L Na3VO4, 200 µmol/L NaF, and 1 mmol/L EGTA (final pH 7.5)]. The homogenate was centrifuged at 14,000g for 30 min at 4°C. Epidermal cell lysate proteins were fractionated on 10% criterion precast SDS–polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA). The protein was transferred to 0.45 µm Hybond-P polyvinylidene difluoride (PVDF) transfer membrane (Amersham Life Sciences, Piscataway, NJ). The membrane was then incubated with the indicated antibody followed by a horseradish peroxidase secondary antibody (Thermo Scientific), and the detection signal was developed with Amersham's enhanced chemiluminescence reagent and using FOTO/Analyst Luminary Work Station (Fotodyne Inc.). The Western blots were quantitated by densitometric analysis using TotalLab Nonlinear Dynamic Image analysis software (Nonlinear USA, Inc., Durham, NC).
Immunoprecipitation Protocol
Epidermal lysates were prepared as for Western blot analysis. 100 µg of epidermal lysate was incubated with 10 µg of the indicated antibody. The total volume of the lysate/antibody mixture was adjusted to 1,000 µL with lysis buffer to allow for appropriate mixing and rotated at 4°C overnight. Lysate/antibody mixture was then mixed with 50 µL of protein agarose A/G (sc-2003 Santa Cruz Biotechnology, Santa Cruz, CA) for 6 h. Lysate/antibody/protein A/G agarose mixture was then centrifuged at 8,000g for 10 min to sediment the protein A/G agarose. The pellet was washed with 0.1% tween in PBS and then sedimented at 8,000g for 10 min three times to wash any non-specific binding from the pellet. After three washes the immunoprecipitate was boiled for 5 min in 20 µL Protein Loading Buffer Blue (Cat # EC-886, National Diagnostics, Atlanta, GA). Immunoprecipitates were then treated as described above under Western Blot analysis method.
HPLC Analysis of 17-AAG in serum and mice epidermis
The dorsal areas of the indicated mice (6–7 week old) were shaved and depilated one day before the treatment. 17AAG stock (100 mM) was prepared in DMSO and freshly reconstituted in acetone to a desired concentration at the time of treatment. 17AAG or vehicle (200µl) was applied topically to skin either alone or in conjunction with UVR exposures. Blood samples were collected to detect 17AAG in serum. To prepare epidermal lysate, epidermis was removed and homogenized with the lysis buffer. 17-AAG levels in the serum and mouse epidermis were analyzed by HPLC (Shin, et al., 2012).
Acknowledgements
We are thankful to Thomas Havighurst for his help in the statistical analysis of the tumor induction data. This study was supported by NIH CA35368 grant.
Abbreviations
- PKCε
Protein Kinase C epsilon
- SCC
squamous cell carcinoma
- UVR
ultraviolet radiation
Footnotes
Conflict of interest: None
References
- Anonymous Skin Cancer; American Cancer Society. Cancer facts and figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- Aziz MH, Manoharan HT, Verma AK. Protein kinase C epsilon, which sensitizes skin to sun's UV radiation-induced cutaneous damage and development of squamous cell carcinomas, associates with Stat3. Cancer Res. 2007;67:1385–1394. doi: 10.1158/0008-5472.CAN-06-3350. [DOI] [PubMed] [Google Scholar]
- Bagatell R, Gore L, Egorin MJ, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-N-allylamino-17-demethoxygeldanamycin in pediatric patients with recurrent or refractory solid tumors: a pediatric oncology experimental therapeutics investigators consortium study. Clin Cancer Res. 2007;13:1783–1788. doi: 10.1158/1078-0432.CCR-06-1892. [DOI] [PubMed] [Google Scholar]
- Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354–376. doi: 10.1007/s11864-012-0195-3. [DOI] [PubMed] [Google Scholar]
- Bath-Hextall F, Leonardi-Bee J, Somchand N, et al. Interventions for preventing non-melanoma skin cancers in high-risk groups. Cochrane Database Syst Rev. 2007;17:CD005414. doi: 10.1002/14651858.CD005414.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton TR, Mitchell DL, Fischer SM, Locniskar MF. Epidermal proliferation but not quantity of DNA photodamage is correlated with UV-induced mouse skin carcinogenesis. J Invest Dermatol. 1997;109(3):340–347. doi: 10.1111/1523-1747.ep12335984. [DOI] [PubMed] [Google Scholar]
- Biamonte MA, Van de Water R, Arndt JW, et al. Heat shock protein 90: inhibitors in clinical trials. J Med Chem. 2010;53:3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- Bracher A, Hartl FU. Hsp90 structure: when two ends meet. Nat Struct Mol Biol. 2006;13:478–480. doi: 10.1038/nsmb0606-478. [DOI] [PubMed] [Google Scholar]
- de Gruijl FR, van Kranen HJ, Mullenders LH. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J Photochem Photobiol B. 2001;63(1–3):19–27. doi: 10.1016/s1011-1344(01)00199-3. [DOI] [PubMed] [Google Scholar]
- Den RB, Lu B. Heat shock protein 90 inhibition: rationale and clinical potential. Ther Adv Med Oncol. 2012;4:211–218. doi: 10.1177/1758834012445574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorin MJ, Zuhowski EG, Rosen DM, et al. Plasma pharmacokinetics and tissue distribution of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) in CD2F1 mice1. Cancer Chemother Pharmacol. 2001;47:291–302. doi: 10.1007/s002800000242. [DOI] [PubMed] [Google Scholar]
- Enk CD, Sredni D, Blauvelt A, et al. Induction of IL-10 gene expression in human keratinocytes by UVB exposure in vivo and in vitro. J Immunol. 1995;1(9):4851–4856. 154. [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Gartner EM, Silverman P, Simon M, et al. A phase II study of 17-allylamino-17-demethoxygeldanamycin in metastatic or locally advanced, unresectable breast cancer. Breast Cancer Res Treat. 2012;131:933–937. doi: 10.1007/s10549-011-1866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz MP, Toft D, Reid J, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol. 2005;23:1078–1087. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- Goldman GD. Squamous cell cancer: a practical approach. Semin Cutan Med Surg. 1998;17:80–95. doi: 10.1016/s1085-5629(98)80002-3. [DOI] [PubMed] [Google Scholar]
- Gould CM, Kannan N, Taylor SS, et al. The chaperones Hsp90 and Cdc37 mediate the maturation and stabilization of protein kinase C through a conserved PXXP motif in the C-terminal tail. J Biol Chem. 2009;284:4921–4935. doi: 10.1074/jbc.M808436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath EI, Hillman DW, Vaishampayan U, et al. A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2008;14:7940–7946. doi: 10.1158/1078-0432.CCR-08-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ma Wy, Bowden GT, et al. Ultraviolet B-induced activated protein-1 activation does not require epidermal growth factor receptor but is blocked by a dominant negative PKClambda/iota. J Biol Chem. 1996;271:31262–31268. doi: 10.1074/jbc.271.49.31262. [DOI] [PubMed] [Google Scholar]
- Hubbard J, Erlichman C, Toft DO, et al. Phase I study of 17-allylamino-17 demethoxygeldanamycin, gemcitabine and/or cisplatin in patients with refractory solid tumors. Invest New Drugs. 2011;29:473–480. doi: 10.1007/s10637-009-9381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G. Mechanism of UVB-induced wrinkling of the skin: paracrine cytokine linkage between keratinocytes and fibroblasts leading to the stimulation of elastase. J Investig Dermatol Symp Proc. 2009;14:36–43. doi: 10.1038/jidsymp.2009.11. [DOI] [PubMed] [Google Scholar]
- Inomata S, Matsunaga Y, Amano S. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J Invest Dermatol. 2003;120:128–134. doi: 10.1046/j.1523-1747.2003.12021.x. [DOI] [PubMed] [Google Scholar]
- Isoherranen K, Punnonen K, Jansen C, et al. Ultraviolet irradiation induces cyclooxygenase-2 expression in keratinocytes. Br J Dermatol. 1999;140(6):1017–1022. doi: 10.1046/j.1365-2133.1999.02897.x. 1999. [DOI] [PubMed] [Google Scholar]
- Jansen AP, Dreckschmidt NE, Verwiebe EG, et al. Relation of the induction of epidermal ornithine decarboxylase and hyperplasia to the different skin tumor-promotion susceptibilities of protein kinase C alpha, -delta and -epsilon transgenic mice. Int J Cancer. 2001a;93:635–643. doi: 10.1002/ijc.1395. [DOI] [PubMed] [Google Scholar]
- Jansen AP, Verwiebe EG, Dreckschmidt NE, et al. Protein kinase C-epsilon transgenic mice: a unique model for metastatic squamous cell carcinoma. Cancer Res. 2001b;61:808–812. [PubMed] [Google Scholar]
- Jung SK, Lee KW, Kim HY, et al. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem Pharmacol. 2010;79:1455–1461. doi: 10.1016/j.bcp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanietz MG. Eyes wide shut: protein kinase C isozymes are not the only receptors for the phorbol eopster tumor promoters. Molecular Carcinogenesis. 2000;28:5–11. doi: 10.1002/(sici)1098-2744(200005)28:1<5::aid-mc2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513–519. [PubMed] [Google Scholar]
- Matsuda M, Hoshino T, Yamakawa N, et al. Suppression of UV-Induced Wrinkle Formation by Induction of HSP70 Expression in Mice. Journal of Investigative Dermatology. 2013;133:919–928. doi: 10.1038/jid.2012.383. [DOI] [PubMed] [Google Scholar]
- Miyata Y, Nakamoto H, Neckers L, et al. The Therapeutic Target Hsp90 and Cancer Hallmarks. Curr Pharm Des. 2013;19:347–365. doi: 10.2174/138161213804143725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8:S55–S61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- Neckers L. Chaperoning oncogenes: Hsp90 as a target of geldanamycin. Handb Exp Pharmacol. 2006;172:259–277. doi: 10.1007/3-540-29717-0_11. [DOI] [PubMed] [Google Scholar]
- Pacey S, Gore M, Chao D, et al. A Phase II trial of 17-allylamino, 17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Invest New Drugs. 2012;30:341–349. doi: 10.1007/s10637-010-9493-4. [DOI] [PubMed] [Google Scholar]
- Rabe JH, Mamelak AJ, McElgunn PJ, et al. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2006;55:1–19. doi: 10.1016/j.jaad.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Ramsay HM, Reece SM, Fryer AA, et al. Seven-year prospective study of nonmelanoma skin cancer incidence in U.K. renal transplant recipients. Transplantation. 2007;84:437–439. doi: 10.1097/01.tp.0000269707.06060.dc. [DOI] [PubMed] [Google Scholar]
- Reddig PJ, Dreckschmidt NE, Zou J, et al. Transgenic mice overexpressing protein kinase C epsilon in their epidermis exhibit reduced papilloma burden but enhanced carcinoma formation after tumor promotion. Cancer Res. 2000;60:595–602. [PubMed] [Google Scholar]
- Rosen CF, Gajic D, Drucker, et al. Ultraviolet radiation induction of ornithine decarboxylase in rat keratinocytes. Cancer Res. 1990;1(9):2631–2635. 50. [PubMed] [Google Scholar]
- Saif MW, Erlichman C, Dragovich T, et al. Open-label, dose-escalation, safety, pharmacokinetic, and pharmacodynamic study of intravenously administered CNF1010 (17-(allylamino)-17-demethoxygeldanamycin [17-AAG]) in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71:1345–1355. doi: 10.1007/s00280-013-2134-9. [DOI] [PubMed] [Google Scholar]
- Sand JM, Aziz MH, Dreckschmidt NE, et al. PKCepsilon overexpression, irrespective of genetic background, sensitizes skin to UVR-induced development of squamous-cell carcinomas. J Invest Dermatol. 2010;130:270–277. doi: 10.1038/jid.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand JM, Hafeez BB, Aziz MH, et al. Ultraviolet radiation and 12-O-tetradecanoylphorbol-13-acetate-induced interaction of mouse epidermal protein kinase C ε with Stat3 involve integration with Erk1/2. Mol Carcinog. 2012;51:291–302. doi: 10.1002/mc.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaltriti M, Dawood S, Cortes J. Molecular pathways: targeting hsp90--who benefits and who does not. Clin Cancer Res. 2012;18:4508–4513. doi: 10.1158/1078-0432.CCR-11-2138. [DOI] [PubMed] [Google Scholar]
- Shin HC, Cho H, Lai TC. Pharmacokinetic Study of 3-in-1 Poly(ethylene glycol)-block-Poly(D,L-Lactic Acid) Micelles Carrying Paclitaxel, 17-allylamino-17-demethoxygeldanamycin and rapamycin. Journal of Controlled Release. 2012;163:93–99. doi: 10.1016/j.jconrel.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MH, Seo JE, Kim YK, et al. Chronic heat treatment causes skin wrinkle formation and oxidative damage in hairless mice. Mech Ageing Dev. 2012;133:92–98. doi: 10.1016/j.mad.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Solit DB, Osman I, Polsky D, et al. Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin Cancer Res. 2008;14:8302–8307. doi: 10.1158/1078-0432.CCR-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sõti C, Nagy E, Giricz Z, et al. Heat shock proteins as emerging therapeutic targets. Br J Pharmacol. 2005;146:769–780. doi: 10.1038/sj.bjp.0706396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedhar AS, Kalmar E, Csermely P, et al. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett. 2004;562:11–15. doi: 10.1016/s0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- Stebbins CE, Russo AA, Schneider C, et al. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- Taipale M, Krykbaeva I, Koeva M, et al. Quantitative analysis of hsp90-client interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar HS, Griffiths CE, Fisher GJ, et al. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105:285–290. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- Usmani SZ, Bona R, Li Z. 17AAG for HSP90 inhibition in cancer--from bench to bedside. Curr Mol Med. 2009;9:654–664. doi: 10.2174/156652409788488757. [DOI] [PubMed] [Google Scholar]
- Verma AK, Wheeler DL, Aziz MH, et al. Protein kinase Cepsilon and development of squamous cell carcinoma, the nonmelanoma human skin cancer. Mol Carcinog. 2006;45:381–388. doi: 10.1002/mc.20230. [DOI] [PubMed] [Google Scholar]
- Weigel BJ, Blaney SM, Reid JM, et al. A phase I study of 17-allylaminogeldanamycin in relapsed/refractory pediatric patients with solid tumors: a Children's Oncology Group study. Clin Cancer Res. 2007;13:1789–1793. doi: 10.1158/1078-0432.CCR-06-2270. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Li Y, Verma AK. Protein kinase C epsilon signals ultraviolet light-induced cutaneous damage and development of squamous cell carcinoma possibly through Induction of specific cytokines in a paracrine mechanism. Photochem Photobiol. 2005;81:9–18. doi: 10.1562/2004-08-12-RA-271. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Martin KE, Ness KJ, et al. Protein kinase C epsilon is an endogenous photosensitizer that enhances ultraviolet radiation-induced cutaneous damage and development of squamous cell carcinomas. Cancer Res. 2004;64:7756–7765. doi: 10.1158/0008-5472.CAN-04-1881. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Ness KJ, Oberley TD, et al. Protein kinase Cepsilon is linked to 12-O-tetradecanoylphorbol-13-acetate-induced tumor necrosis factor-alpha ectodomain shedding and the development of metastatic squamous cell carcinoma in protein kinase Cepsilon transgenic mice. Cancer Res. 2003;63:6547–6555. [PubMed] [Google Scholar]
- Whitesell L, Santagata S, Lin NU. Inhibiting HSP90 to treat cancer: a strategy in evolution. Curr Mol Med. 2012;12:1108–1124. doi: 10.2174/156652412803306657. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Jonason AS, Leffell DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;22–29(6508):773–776. doi: 10.1038/372773a0. 372. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu G, Dong Z. MSK1 and JNKs mediate phosphorylation of STAT3 in UVA-irradiated mouse epidermal JB6 cells. J Biol Chem. 2001;276:42534–42542. doi: 10.1074/jbc.M106044200. [DOI] [PubMed] [Google Scholar]
- Zuehlke A, Johnson JL. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers. 2010;93:211–217. doi: 10.1002/bip.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]