Abstract
Background
Chronic post-surgical pain (CPSP), a significant public health problem, occurs in 10-50% of patients undergoing major surgery. Acute pain induces endogenous analgesia termed conditioned pain modulation (CPM), and the strength of CPM preoperatively predicts the likelihood of CPSP. The relationship between CPM and recovery from surgery has not been examined in preclinical models.
Methods
CPM was assessed in individual rats and correlated with each animal’s time course of recovery of hypersensitivity following partial spinal nerve ligation (pSNL). The role of descending noradrenergic pathways in the spinal cord to mechanisms of CPM and recovery was tested using idazoxan to block noradrenergic receptors or antidopamine β hydroxylase conjugated saporin (DβH-saporin) to ablate these pathways. Behavioral hypersensitivity, static weight bearing and spinal glial activation were measured after pSNL.
Results
The strength of CPM varied over two-fold between individuals and was directly correlated with the slope of recovery from hypersensitivity after surgery (P < 0.0001, r = 0.660). CPM induced release of norepinephrine in the spinal cord and was partially blocked by intrathecal idazoxan or DβH-saporin. DβH-saporin also slowed recovery and enhanced spinal glial activation following pSNL surgery. Ongoing activation of these pathways was critical to sustained recovery, since intrathecal DβH-saporin given 7 weeks after recovery reinstituted hypersensitivity, while having no effect in animals without previous surgery.
Conclusions
Collectively, these studies provide a clear back-translation from clinical observations of CPM and CPSP and suggest that the ability to engage ongoing descending endogenous noradrenergic signaling may be critical in determining time course of recovery from hypersensitivity after surgery.
INTRODUCTION
Chronic postsurgical pain (CPSP) occurs in 10-50% of patients undergoing major surgery and results in pain sufficient to negatively impact quality of life.1 Psychosocial and genetic factors have been shown to contribute to the risk of CPSP,2-4 as do procedures with a high risk of nerve injury such as limb amputation, thoracotomy and breast surgery.1 Individual differences in pain processing impart risk as well, especially the strength of conditioned pain modulation (CPM) in which analgesia is induced diffusely over the body from an intense, focal pain stimulus. Low preoperative CPM correlates with a greater incidence of CPSP following thoracotomy5 and abdominal surgery.6 Whether the association between preoperative CPM and risk of CPSP reflects causality and the mechanisms by which this might occur are unknown. In the current study, we use a rodent postoperative pain model with a nerve injury component to examine for the first time the relationship between preoperative CPM and time course of recovery from surgery and examine one potential mechanism involved in this relationship.
Central nervous system responses to nerve injury which facilitate pain, including spinal neural and glial activation and descending facilitation, have been the focus of neuroscience research for the past 15 yr, yet their relevance to the clinical condition is unknown. Descending inhibition, in contrast, has been less studied, yet the relationship between CPM and CPSP observed clinically suggests a clear relevance. Acute pain activates pontospinal noradrenergic pathways7 resulting in antinociception in acute, incisional, and inflammatory pain models,8 although the role of this pathway in CPM has been less examined. Disruption of noradrenergic neurotransmission may increase9,10 or decrease11 hypersensitivity from nerve injury depending on which projections are ablated. We hypothesized that CPM depends at least in part on descending noradrenergic inhibition and that recovery from the acute and sustained pain after surgery might mechanistically depend on this pathway.
Finally, recovery of normal behavior after several types of stress reflects a new homeostasis from a balance of counteracting mechanisms.12,13 Several studies suggest that the transition from acute to chronic pain after injury involves a disruption in the balance of homeostatic mechanisms with a decrease in inhibitory and an increase in facilitatory (excitatory) influences at multiple levels of the neuroaxis. Alternatively, resolution of pain from tissue injury might involve a more complex upregulation of both inhibitory and facilitatory mechanisms. For example, inflammation and injury often induce pain facilitation which is latent in the absence of a subsequent injury, as exemplified by inflammation induced hyperalgesic priming in primary sensory afferents14-16 or latent sensitization of spinal cord circuits following inflammation and injury induced spinal release of endogenous opioids.17 Inhibitory systems which counterbalance these excitatory responses following injury have received less attention18. We hypothesize that recovery from hypersensitivity after peripheral nerve injury might involve a new homeostasis requiring ongoing activation of descending noradrenergic tone.
MATERIALS AND METHODS
Animal procedures
A total of 113 male Sprague–Dawley rats (Harlan Industries, Indianapolis, IN), weighing 250–300 g, were used for experiments. All studies conformed to the Wake Forest University Guidelines on the ethical use of animals, and studies were performed under Animal Care and Use Committee (Winston-Salem, North Carolina) approval. Animals were housed under a 12-h light–dark cycle, with food and water ad libitum.
Drugs
For studies involving CPM, capsaicin (8-methyl-n-vanillyl-6-nonenamide, Sigma Chemical Co, St. Louis, MO) was prepared fresh in a vehicle solution of 10% ethanol, 10% Tween-80, and 80% sterile saline to a concentration of 3 mg/ml. Idazoxan (Tocris Bioscience, Bristol, United Kingdom) was prepared in sterile saline and passed through a 0.2 μm filter. All intrathecal drug injections were made via percutaneous lumbar puncture. Successful puncture of the dura was confirmed by the presence of a tail flick. Anti-dopamine β hydroxylase conjugated to saporin (DβH-saporin) and control immunoglobulin G (IgG) -saporin were obtained from Advanced Targeting Systems, San Diego, CA and injected in a dose of 5 μg, intrathecally.
Assessment of conditioned pain modulation
CPM was assessed using a previously described procedure developed in rats.19,20 CPM was elicited by subdermal injection of capsaicin (150 μg in a volume of 50 μl) administered on a fore paw during brief isoflurane (3.5%) general anesthesia. This procedure produces a transient, intense stimulation of transient receptor potential vanilloid-1 expressing sensory afferents resulting in local irritation and inflammation of the injected paw as well as a more prolonged widespread analgesia of regions of the body not impacted by the injection. The local, algesic effect of capsaicin lasts several minutes while the analgesia lasts approximately 1 h. The degree of analgesia in the hindpaw was assessed using the Randall Selitto paw pressure test. At each time point paw pressure thresholds were assessed twice on the plantar aspect of both hindpaws and the results were averaged. For pharmacological studies, spinal drugs were administered 5 min prior to injection of capsaicin into the forepaw (n = 6 rats per group). For ablative studies, rats were randomly assigned to treatment groups and DβH-saporin or IgG-saporin were delivered 14 days prior to CPM testing (n = 8 rats per group). A total of 20 rats were assessed for CPM prior to peripheral nerve injury for individual trajectory of recovery studies, however one rat died following spinal nerve ligation and was excluded from correlative analysis.
Partial L5 spinal nerve ligation (pSNL)
We chose partial pSNL to study recovery because this surgical injury has previously been shown to produce a transient mechanical hypersensitivity that resolves by 5-10 weeks postsurgery.21 It also has a nerve injury component which may better reflect the incidental nerve injury that accompanies some types of surgical procedures in humans. A 3 cm incision was made along the right dorsal surface near the spine using aseptic conditions, penetrating underlying muscles. The 6th lumbar transverse process was removed and the caudal 1/3 of the L5 spinal nerve was ligated using 8-0 nylon sutures (Ethicon catalog # BV130-4, Somerville, NJ).
Assessment of mechanical sensitivity
Paw withdrawal thresholds to mechanical stimuli were determined using von Frey filament application. Briefly, rats were placed in individual clear acrylic chambers with a plastic mesh floor and allowed to acclimate to the test environment at least 30 min prior to testing. Filaments were applied to the bending point for 6 s, and a brisk paw withdrawal was considered a positive response. Withdrawal threshold was determined using an up–down statistical method.22
Incapacitance testing
Static weight bearing was measured using an incapacitance apparatus (Linton Instrumentation, Norfolk, United Kingdom). A clear acrylic box with an inclined plane was placed over the two footpads allowing independent measurement of the weight that the rat applied on each hindlimb. Rats were acclimated to the apparatus a minimum of three times prior to surgery. Static weight bearing was assessed prior to pSNL and for several days after surgery (postsurgical days 1, 2, 4, 6, 8,10,12,14,16, 18, 20). A minimum of three readings were obtained for each paw and the values averaged for each paw. In the absence of hindlimb injury, rats typically apply an equal weight on both hindlimbs.
Impact of spinal depletion of noradrenergic fibers on CPM and recovery from pSNL
We used anti-DβH-saporin to ablate spinal noradrenergic fibers. Anti-DβH-saporin binds to the membrane-bound form of DβH when it is transiently exposed to the extracellular space during norepinephrine release.23,24 Anti-DβH-saporin containing vesicles are internalized and retrogradely transported to the cell body where the ribosome inactivating protein, saporin, disrupts protein synthesis leading to cell death.25 Previous studies have shown that complete ablation of spinally projecting locus coeruleus (LC) neurons occurs 7-14 days after intrathecal injection of anti-DβH-saporin.10,26 The dose of anti-DβH-saporin used in the current study resulted in > 95% depletion of spinal noradrenergic fibers.10,26,27
For CPM studies, rats were randomized to receive spinal injection of anti-DβH-saporin (5 μg, intrathecally, n = 8) or control IgG-saporin (5 μg, intrathecally, n = 8), 14 days prior to testing. Following behavioral analysis, spinal cord tissue was collected and processed for immunohistochemistry to verify depletion of noradrenergic fibers. For postoperative recovery studies, rats were randomized to receive spinal injection of anti-DβH-saporin (5 μg, intrathecally, n = 7/8, one rat died due to postsurgical complications) or control IgG-saporin (5 μg, intrathecally, n = 8), 14 days prior to pSNL. Mechanical withdrawal thresholds were obtained on rats prior to pSNL and for several days following surgery (postsurgical days 1, 2, 4, 6, 8,10,12,14,16, 18, 20, 24, 28, 35, 42, 49, 56, 63, and 70) in the ipsilateral and contralateral paws. Rats were examined for shifts in weight bearing on the same days prior to assessing mechanical withdrawal thresholds. The person conducting behavior was blinded to treatment group. Following behavioral analysis, spinal cord tissue was collected and processed for immunohistochemical analysis to examine glial activation and verify depletion of noradrenergic fibers (see below under Immunohistochemistry). In a third experiment to determine if disruption of spinal noradrenergic fibers reinstates mechanical hypersensitivity in recovered rats, mechanical withdrawal thresholds were assessed prior to pSNL and for several days following surgery. Beginning 91 days after pSNL, when mechanical hypersensitivity had resolved, rats were randomized to receive injections of DβH-saporin (5 μg, intrathecally, n = 6) or control IgG-saporin (5 μg, intrathecally, n = 6) and were assessed behaviorally 7 and 14 days after injection. Following final behavioral analysis (106 days after pSNL), spinal cord tissue was collected and analyzed for norepinephrine content to verify depletion of noradrenergic fibers. Values were also compared to norepinephrine content from spinal cord of normal rats (n = 3) and pSNL rats 21 days after surgery (n = 6).
Relationship between CPM and postoperative recovery of mechanical hypersensitivity from pSNL
In separate experiments, we used 19/20 rats (one rat died due to postsurgical complications) to examine the relationship between preoperative CPM and postoperative recovery from hypersensitivity. Mechanical withdrawal thresholds were determined 1, 2, 4, 6, 8, 10,12,14,16, 18, 20, 24, 28, 35, 42, 49, 56, 63, and 70 days following pSNL surgery in the ipsilateral and contralateral paws. Behavioral assessment of preoperative CPM and postoperative trajectory of recovery from mechanical hypersensitivity were conducted by independent investigators so that the assessment of postoperative recovery could be completed in a blinded manner. Growth curve modeling was applied to individual rat’s mechanical withdrawal thresholds over time to obtain a trajectory of recovery for comparison to preoperative CPM responses (see below under Statistical Analysis).
Immunohistochemistry
Spinal cords were collected 70 days following pSNL in rats previously administered DβH-saporin (5 μg, intrathecally, n = 7) or control IgG-saporin (5 μg, intrathecally, n = 8) from the behavioral studies mentioned above. Briefly, rats were anesthetized with sodium pentobarbital (intraperitoneal injection; 100 mg/kg), the thorax was opened, and 0.1 M phosphate buffered saline (PBS, pH 7.4) followed by fixative (4% paraformaldehyde in 0.1 M PBS, pH 7.4) was perfused through the left ventricle with a peristaltic pump (20 ml/min). The spinal cord was removed and immersed in fixative for 12 h at 4° C followed by immersion in 30% sucrose at 4°C for cryoprotection until ready to be sectioned. Spinal cord cross sections (40 μm) were cut on a cryostat. For labeling noradrenergic fibers an antibody against DβH (mouse anti-rat DβH, Millipore, Billerica, MA) was used. For assessing spinal glial activation, an antibody against glial fibrillary acidic protein (GFAP; mouse anti-rat GFAP, Sigma-Aldrich) was used to label astrocytes and an antibody against ionized calcium-binding adapter molecule 1 (IBA1; rabbit anti-rat IBA1, Wako, Richmond, VA) to label microglia. Spinal cord sections were processed free floating and incubated over night at 4°C with primary antibody. Primary antibodies were diluted in a solution consisting of PBS containing 1% normal donkey serum and 0.1% Triton X-100. Sections were washed in PBS and incubated in appropriate secondary antiserum including CY3-conjugated donkey anti-rabbit IgG (1:600, Jackson Immunoresearch, West Grove, PA) and CY2-conjugated donkey anti-mouse IgG (1:200, Jackson Immunoresearch) for 2 h at room temperature. Finally, the sections were washed thoroughly in PBS, mounted on plus-slides, air-dried, dehydrated in ethanol, cleared in xylene, and cover slipped with DPX mounting media (Sigma-Aldrich) at room temperature.
Spinal microdialysis and norepinephrine content
On the day of experiment, anesthesia was induced with 2% isoflurane and then maintained with 1.25–1.5% isoflurane with spontaneous ventilation during the study. The L3–L6 level of spinal cord was exposed by the T13-L1 laminectomy. A microdialysis probe (outer diameter = 0.22 mm, inner diameter = 0.20 mm, length = 1 mm, CX-I-8-01, EICOM Co, San Diego, CA) was inserted from just lateral to the right dorsal root 1 h prior to the study and perfused with Ringer’s solution (1.0 μL/min). Fractions were collected every 30 min for 2.5 h starting 1 h prior to capsaicin injection and samples were kept at −80 °C until assayed for norepinephrine using high performance liquid chromatography with electrochemical detection as previously described.28
For spinal norepinephrine content, rats were killed by deep isoflurane anesthesia followed by decapitation. The spinal cord was quickly removed and the lumbar enlargement (L4-6) was rapidly hemisected into left and right sides on ice, then frozen in dry ice cooled 2-methylbutane. After treatment with 0.1 M perchloric acid, the spinal tissues were homogenized on ice and centrifuged. Supernatants were collected and norepinephrine content was measured by high-pressure liquid chromatography with electrochemical detection as previously described.28
Image analysis and quantification
Tissue sections were examined with fluorescent microscopy and images of ipsilateral and contralateral L4-5 dorsal spinal cord were captured with a charged coupled device digital camera attached to the microscope using a 10X objective at a resolution of 1,600 × 1,200 pixels. For semi-quantitative analysis of immunofluorescence levels, a square with a fixed area (250 × 250 μm2) covering the region of laminae I-II was positioned in the lateral, central, and medial aspects of the spinal cord dorsal horn. The number of pixels occupied by immunoreactive cells within a defined threshold was measured using image analysis software (Image J; NIH Image, National Institutes of Health, Bethesda, MD). Results from these three areas of the spinal cord were summed for each spinal cord section analyzed. Immunofluorescent measurements of IBA1-immunoreactivity (IR) and GFAP-IR were obtained from a minimum of 5 L4/5 spinal cord sections per rat and averaged. Prior to imaging and quantification of spinal cord tissue, sections corresponding to individual animals were coded so that the individual performing quantification was blinded to group.
Statistical analysis
Spinal immunohistochemistry and norepinephrine content data were not normally distributed and are presented as median [25th, 75th percentile]. Statistical analysis of these data was performed using Mann-Whitney rank sum test or Kruskal Wallis ANOVA on ranks with comparisons between groups conducted using Student –Newman-Keuls method, as appropriate. All other data were normally distributed and are presented as mean ± SD. Weight bearing data are expressed as percentage of weight on the paw ipsilateral to surgery. Behavioral time course data for (CPM over minutes, weight bearing after pSNL over days, and withdrawal threshold after pSNL over weeks) were analyzed using a two-way repeated measures (RM) ANOVA, assessing main effects of treatment and time followed by Bonferroni contrasts using SigmaPlot (Version 11.0; Systat Inc., San Jose, CA). Where appropriate, corrected p values were indicated for multiple comparisons.
In addition to traditional 2-way repeated measures ANOVA analysis, we also applied a recently described and more powerful growth curve modeling approach to define the trajectory of recovery from hypersensitivity after pSNL 29. Briefly, using the PROC MIXED procedure in SAS (Version 9.2, Cary, NC), withdrawal threshold over time data were fit to a linear or a quadratic model, the choice being made using Bayesian Information Criteria. In the current group populations, the data were best fit to a linear model over time, described by an intercept (modeled withdrawal threshold immediately following pSNL surgery) and slope (linear rate of change in withdrawal threshold after pSNL surgery). The intercept and slope estimates were allowed to vary across individual rats (random effects) and as a function of treatment group or condition (fixed effects). The primary inferences of the study involve examining if experimental condition impacts some aspect of the change process. To estimate these effects, group and intervention (anti-DβH-saporin) were entered as level-2 predictors of intercept and slope parameters (i.e., as group*parameter interaction) as previously described29.
In addition to providing a secondary analysis to compare anti-DβH-saporin to IgG-saporin groups, growth curve analysis was also used to examine the relationship between CPM and time course of recovery. For the latter, we applied Pearson Product Moment correlation to the strength of preoperative CPM in individual animals to that animal’s modeled slope of recovery. All the hypothesis testing is two-tailed with p < 0.05. considered significant.
RESULTS
CPM in rodents is partially mediated by descending spinal noradrenergic pathways
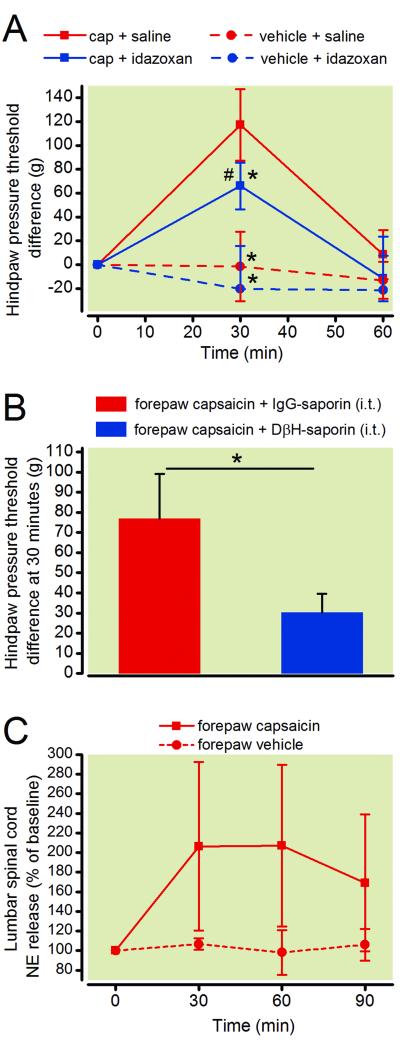
Intradermal injection of capsaicin into the forepaw resulted in a large increase in withdrawal threshold to mechanical pressure in the hindpaw in vehicle treated animals, with a peak effect 30 min after capsaicin injection (fig. 1A). Two way repeated measures ANOVA showed a significant group × time interaction (F6, 40 = 18.76; p < 0.001). Spinal blockade of α2 adrenergic receptors with idazoxan reduced CPM magnitude compared to saline control, but did not eliminate it (fig. 1A, p = 0.002). Withdrawal threshold did not change over time in animals receiving forepaw vehicle injection instead of capsaicin (fig. 1A). In a separate group of rats, spinal ablation of noradrenergic neurons with DβH-saporin (5 μg) delivered fourteen days prior to testing also partially reduced CPM magnitude compared to IgG-saporin treated controls (fig. 1B, p < 0.05). Baseline hindpaw pressure thresholds prior to forepaw capsaicin injection were not significantly different between DβH-saporin and IgG saporin treated rats (130.3 ± 4 g vs. 133.1 ± 3 g, p = 0.54, Student’s t-test) indicating that ablation of noradrenergic pathways does not induce a hypernociceptive state.
Figure 1.
Endogenous analgesia was measured in rats by assessing conditioned pain modulation (CPM). A, Hindpaw withdrawal paw pressure thresholds (test stimuli) were assessed in rats after injection of capsaicin (150 μg/ 50 μl) or vehicle solution into the forepaw (conditioning stimuli). Rats received spinal injections of the α2 adrenergic receptor antagonist idazoxan (30μg, i.t.) or saline solution via lumbar puncture 10 minutes prior to forepaw capsaicin to examine the contribution of noradrenergic mechanisms in this model of CPM. Two-way ANOVA with Bonferroni multiple comparisons. Values represent mean ± SD with n=6 per group. # p < 0.025 vs. pre-capsaicin baseline. * p < 0.0125 vs. capsaicin + saline group. B, Spinal ablation of noradrenergic fibers with DβH-saporin (5 μg, i.t.) 14 days prior to testing also partially reduced CPM. Student’s t-test, * p < 0.05. Values represent mean ± SD with n=8 for the IgG-saporin group and n=8 for the DβH-saporin treated group. C, Time course of spinal norepinephrine release in the lumbar region of the spinal cord following injection of capsaicin into the forepaw. Two-way ANOVA with Bonferroni multiple comparisons. Values represent mean ± SD with n=6 for the capsaicin group and n=4 for the vehicle treated group. cap = capsaicin; DβH = dopamine β hydroxylase; IgG = immunoglobulin G; i.t. = intrathecal; NE = norepinephrine
We also examined the release of norepinephrine in the lumbar spinal cord in response to forepaw capsaicin injection using spinal microdialysis. Norepinephrine levels were significantly increased in the lumbar spinal cord for 60 min following injection of capsaicin but not vehicle solution (fig. 1C, p < 0.05). Two way RM ANOVA did not show significant group × time interaction (F3, 27 = 2.943; p = 0.051), however we did observe a main effect of group (F1, 9 = 7.109; p < 0.05) and time (F3, 9 = 3.202; p < 0.05).
Disrupting spinal noradrenergic fibers prior to surgery prolongs mechanical hypersensitivity but not shifts in weight bearing
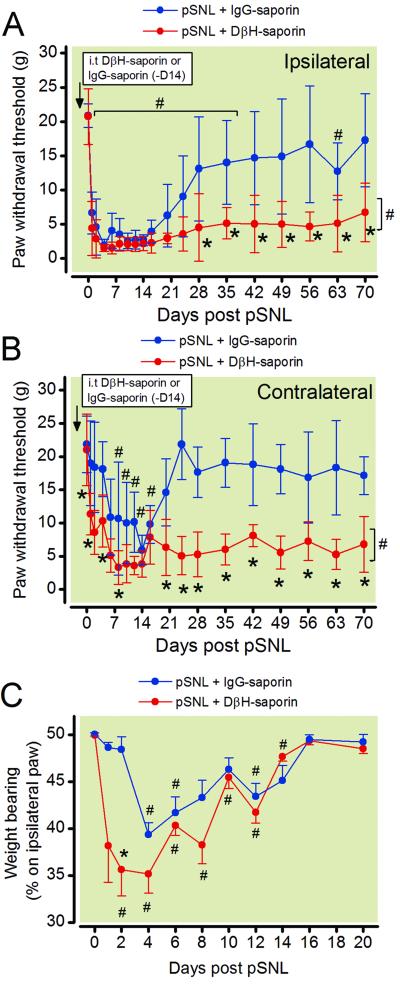
In order to determine the contribution of descending spinal noradrenergic systems to resolution of postoperative hypersensitivity we administered DβH-saporin (5 μg) 14 days prior to surgery. We conducted partial L5 spinal nerve ligation and measured paw withdrawal thresholds in the ipsilateral and contralateral paw longitudinally until 70 days postsurgery. Two-way RM ANOVA showed a significant group × time interaction for the ipsilateral paw withdrawal thresholds (F18, 233 = 5.266; p < 0.001) and contralateral paw withdrawal thresholds (F18, 233 = 3.377; p < 0.001). Mean withdrawal thresholds did not differ between groups prior to pSNL surgery, 14 days after administration of DβH-saporin or IgG-saporin (fig. 2A, p = 0.948) indicating that depletion of spinal noradrenergic fibers did not alter baseline mechanical withdrawal thresholds similar to previous reports.10,27 Rats that received control IgG-saporin prior to surgery developed mechanical hypersensitivity in the ipsilateral paw until 35 days postsurgery (fig. 2A, p<0.05). Contralateral paw withdrawal thresholds were transiently reduced in IgG –saporin treated rats until day 16 postsurgery (fig. 2B, p < 0.05). In contrast, DβH-saporin treated rats had reduced withdrawal thresholds until at least 70 days postsurgery in the ipsilateral paw as well as an earlier onset and longer duration of contralateral hypersensitivity (fig. 2B).
Figure 2.
Time course of mechanical hypersensitivity and static weight bearing following partial L5 spinal nerve ligation (pSNL). Rats received intrathecal treatment with DβH-saporin or control IgG saporin 14 days prior to surgery and were assessed for mechanical hypersensitivity with von Frey filaments and assayed for shifts in weight bearing on the ipsilateral hindpaw. Mechanical withdrawal thresholds were reduced in the ipsilateral (A) and contralateral (B) paw of DβH-saporin treated rats. Two-way RM ANOVA with Bonferroni multiple comparisons. * p < 0.003 for within time point comparison to pSNL + IgG saporin values or # p < 0.003 for within treatment group comparisons to Pre-surgery baseline value. Brackets indicate the data points which withdrawal thresholds were significantly different from Pre-surgery values. The percentage body weight shifted to the ipsilateral paw was reduced in pSNL rats from both groups for approximately 2 weeks (C). A significantly greater shift in weight bearing was present in DβH-saporin compared to IgG saporin treated rats particularly at early time points. Two-way RM ANOVA with Bonferroni multiple comparisons. * p < 0.003 for within time point comparison to pSNL + IgG saporin values or # p < 0.003.Values represent mean± SEM with n=7 for DβH-saporin and n=8 for IgG saporin group. DβH = dopamine β hydroxylase; IgG = immunoglobulin G; i.t. = intrathecal; RM = repeated measures
In the same set of rats, we also assessed static weight bearing on the hindpaws as a non-evoked measure of postoperative pain. Two-way RM ANOVA showed a significant group × time interaction for the percentage of weight bearing on the ipsilateral paw (fig. 2C, F10, 130 = 6.5; p < 0.001). Both groups of pSNL rats previously treated with DβH-saporin or IgG saporin demonstrated a significant reduction in weight bearing on the ipsilateral paw until 12 days after surgery. pSNL DβH-saporin treated rats had significantly greater shifts in weight bearing during earlier time points (Day 1, 2, and 8) postoperatively compared to pSNL IgG-saporin treated rats (p < 0.001).
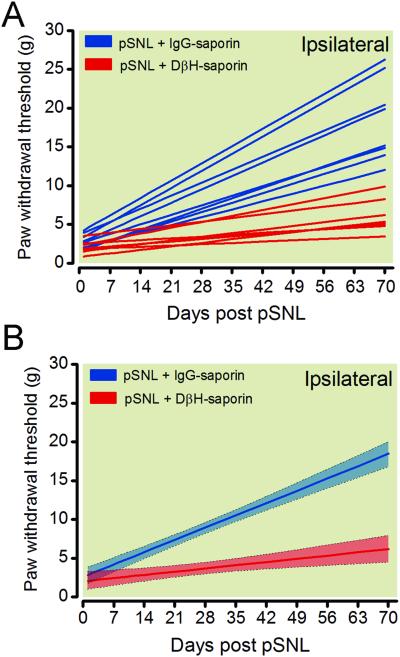
Growth curve analysis was successfully applied to mechanical withdrawal thresholds in the ipsilateral paw of DβH-saporin and IgG saporin pSNL rats to obtain individual (fig. 3A) and group trajectories (fig. 3B) of recovery. There was a statistically significant variance in the random effects for the intercept (p < 0.0001) and the slope (p < 0.0001) indicating that individuals varied substantially in their change processes and that further modeling of these parameters was warranted (table 1). The group trajectory of anti-DβH-saporin treated rats was significantly reduced compared to IgG saporin treated rats. The group modeled trajectories had non-overlapping 95% confidence intervals beginning 8 days postoperatively and the modeled trajectory of DβH-saporin treated rats had a significantly lower predicted slope (table 1) indicating a slower or delayed recovery period. The predicted intercepts were not significantly different between groups (table 1).
Figure 3.
Modeled trajectories of recovery from mechanical hypersensitivity after pSNL in rats treated with DβH-saporin or control IgG saporin 14 days prior to surgery. Longitudinal behavioral measurements of mechanical paw withdrawal thresholds from the ipsilateral paw of individual rats modeled using growth curve analysis were best fit to a linear function described by an intercept (modeled withdrawal threshold immediately following pSNL surgery) and slope (linear rate of change in withdrawal threshold after pSNL surgery) (A). Group trajectories depict the mean fit for all the animals within each treatment group with 95% confidence intervals indicated by shading (B). DβH = dopamine β hydroxylase; IgG = immunoglobulin G; pSNL = partial spinal nerve ligation
Table 1.
Growth Curve Modeling of Ipsilateral Mechanical Hypersensitivity in Rats with pSNL
| Predictor | Parameter | Estimate | (Lower Bound, Upper Bound) | p |
|---|---|---|---|---|
| Entire population | Intercept | 2.8432 | (1.7691, 3.1972) | <0.0001 |
| Entire population | Slope | 0.2265 | (0.1935, 0.2595) | <0.0001 |
| Group | Intercept DβH-saporin IgG-saporin |
−0.060 REF |
(−2.3358, 0.8086) | 0.3398 |
| Group | Slope DβH-saporin IgG-saporin |
−0.141 REF |
(−0.2156,−0.1190) | <0.0001 |
Comparison of growth curve parameters for pSNL rats treated with DβH-saporin or IgG-saporin 14 days prior to pSNL surgery. Growth curve analysis of paw withdrawal thresholds (Day 1-70) best fit a linear model giving rise to an intercept (hypersensitivity at time 0) and slope (linear rate of change in pain measure).
DβH = dopamine β hydroxylase; IgG = immunoglobulin G; pSNL= partial spinal nerve ligation; REF = reference value.
Disrupting spinal noradrenergic fibers prior to surgery enhances spinal glial activation following partial spinal nerve ligation
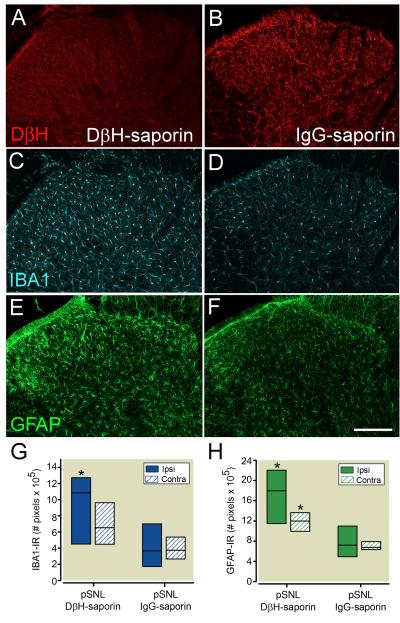
Spinal cord tissue was collected 70 days following pSNL in rats treated with DβH-saporin (n = 7) or IgG saporin (n=8) for immunohistochemical analysis to verify depletion of noradrenergic fibers and for examination of spinal glial activation (fig. 4). All rats administered spinal DβH-saporin had complete loss of noradrenergic fibers at the lumbar level of the spinal cord. DβH-IR was uniformly distributed thought the dorsal spinal cord of IgG-saporin treated rats 70 days after pSNL (fig. 4A, B). We observed a significant increase in density of IBA1-IR the ipsilateral spinal cord of DβH-saporin treated rats 70 days after pSNL compared to IgG saporin treated pSNL rats (fig. 4C, D, G; P < 0.05). Spinal cords from DβH-saporin treated rats also had increased GFAP-IR in the ipsilateral (P < 0.05) and contralateral (P < 0.05) dorsal horn compared to pSNL IgG-saporin treated rats (fig. 4E, F, H). Increases in IBA1-IR and GFAP-IR appeared to be more prominent in superficial laminae of pSNL DβH-saporin treated rats. Similar to other studies,10,27 we didn’t observe differences in GFAP-R or IBA1-IR in norepinephrine depleted rats that did not undergo surgery (data not shown).
Figure 4.
Representative images of noradrenergic innervation and glial activation in ipsilateral dorsal spinal cord 70 days following partial spinal nerve ligation (pSNL) in rats treated with spinal DβH-saporin (A, C, E) or IgG-saporin (B, D, E) 14 days prior to surgery. A, B: Long term depletion of spinal noradrenergic fibers is evident in the dorsal spinal cord of DβH-saporin treated rats based on loss of DβH-immunoreactivity (IR). IBA1-IR (C, D) and GFAP-IR (E,F) were increased in the superficial dorsal horn of nerve injured rats with depletion of noradrenergic fibers. Lower panels depict quantification of spinal cord IBA1 (G) and GFAP-IR (H) levels. Values represent median ± 25th and 75th percentile with n= 7 for DβH-saporin and n=8 for IgG-saporin group. Data were analyzed with Mann Whitney rank sum test. * P< 0.05 within side comparison to pSNL + IgG-saporin value. Scale bar in F = 200 μm. DβH = dopamine β hydroxylase; IgG = immunoglobulin G; contra = contralateral; ipsi = ipsilateral, IBA1 = ionized calcium binding adapter molecule 1; GFAP = glial fibrillary acidic protein; IR = immunoreactivity
Disrupting spinal noradrenergic fibers after resolution of mechanical hypersensitivity following pSNL reinstates postoperative mechanical hypersensitivity
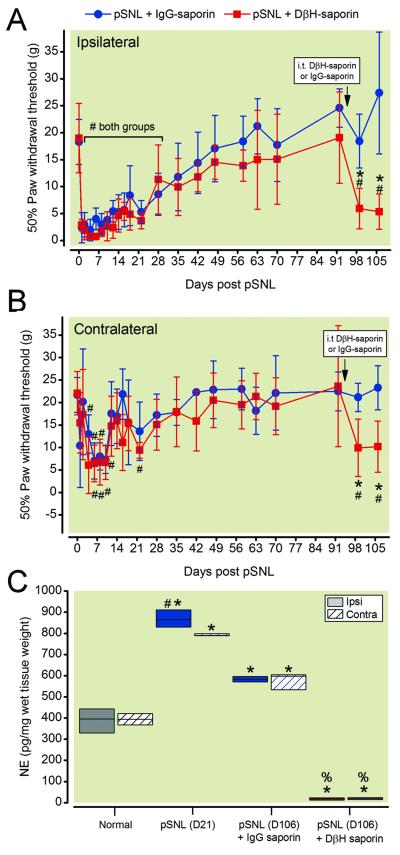
In order to test the hypothesis that endogenous spinal norepinephrine tonically maintains resolution of mechanical hypersensitivity, we depleted noradrenergic fibers after pSNL induced hypersensitivity resolved. We delivered DβH-saporin or IgG saporin 91 days after pSNL (approximately 7 weeks after resolution of mechanical hypersensitivity) and assessed mechanical hypersensitivity one and two weeks later. Two-way RM ANOVA showed a significant group × time interaction for the ipsilateral paw withdrawal thresholds (F22, 220 = 5.162; p < 0.001) and for the contralateral paw withdrawal thresholds (F22, 220 = 3.377; p < 0.001). Spinal injection of DβH saporin reinstated ipsilateral (fig. 5A) and contralateral (fig. 5B) mechanical hypersensitivity in pSNL rats 7 and 14 days after treatment (fig. 5A, B, p < 0.001).
Figure 5.
Spinal norepinephrine tonically inhibits mechanical hypersensitivity after partial spinal nerve ligation (pSNL). Rats received intrathecal treatment with DβH-saporin or IgG-saporin 91 days after pSNL surgery (approximately 7 weeks after mechanical hypersensitivity resolved). Mechanical hypersensitivity was reinstated in the ipsilateral (A) and contralateral (B) paw of DβH-saporin treated rats. Two-way repeated measures ANOVA with Bonferroni multiple comparisons. * p < 0.002 for within timepoint comparison to pSNL + IgG saporin values or # p < 0.003 for within treatment group comparisons to Pre-surgery baseline value. Values represent mean ± SD with n=6 per group. In the same groups of rats, norepinephrine content was significantly reduced in the dorsal spinal cord of DβH-saporin treated pSNL rats compared to IgG saporin treated pSNL rats (C). Norepinephrine content examined in a separate group of normal and pSNL rats showed increased content three weeks postoperatively that persisted through fifteen weeks. Kruskal-Wallis one-way analysis of variance on ranks using pairwise multiple comparison procedures (Student –Newman-Keuls method) or Mann Whitney Rank Sum test * p< 0.05 vs. normal. % p < 0.05 vs. pSNL (D106) +IgG-saporin. # p< 0.05 vs. contra within group comparison. Values represent median ± 25th and 75th percentile with n=6 per group. DβH = dopamine β hydroxylase; IgG = immunoglobulin G; contra = contralateral; ipsi = ipsilateral; i.t. = intrathecal; NE = norepinephrine
We verified depletion of spinal norepinephrine by assessing norepinephrine content in the ipsilateral and contralateral dorsal spinal cord of pSNL rats two weeks after treatment (106 days after pSNL). Norepinephrine content in the spinal cord of DβH-saporin treated pSNL rats was significantly lower compared to IgG-saporin treated pSNL rats (fig. 5C). We compared norepinephrine content at this late stage of recovery to separate group of rats without surgery and at earlier stage of recovery. Norepinephrine content was significantly increased three weeks until at least fifteen weeks post-pSNL compared to normal rats (fig. 5C).
Correlation between CPM and recovery from hypersensitivity after pSNL
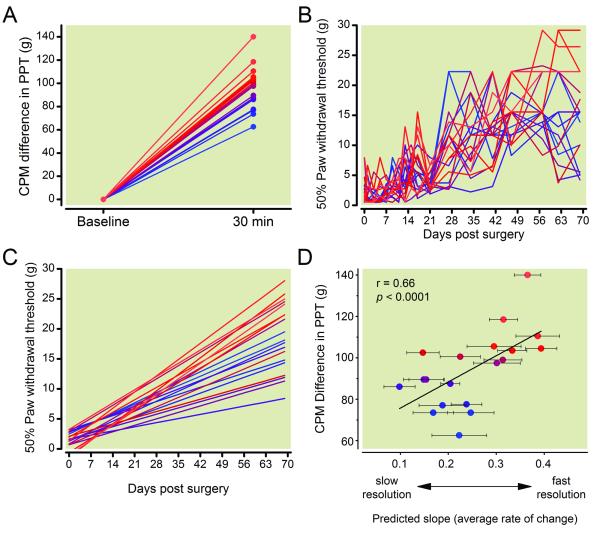
We examined the relationship between the magnitude of CPM and time course of postoperative resolution of hypersensitivity in nineteen rats. Rats were tested preoperatively for CPM to assess degree of endogenous analgesia. At 30 min postforepaw capsaicin injection, there was considerable interanimal variability in strength of CPM response, with a difference from baseline ranging from 62.5 −140 grams (fig. 6A).
Figure 6.
Preoperative conditioned pain modulation (CPM) correlated with timecourse of postoperative resolution of mechanical hypersensitivity after partial L5 spinal nerve ligation (pSNL). A, Twenty four hours prior to pSNL surgery CPM was tested in 19 individual rats. Rats produced a variable increase in hindpaw pressure thresholds 30 minutes following intraplantar administration of capsaicin (150 μg/ 50 μl) into the forepaw. Values for individual rats are displayed as a gradient with lower CPM in blue and greater CPM in red. B, Paw withdrawal thresholds to mechanical stimulation were assessed for ten weeks following pSNL surgery and individual daily withdrawal thresholds were recorded. C, Individual repeated daily withdrawal thresholds were analyzed using growth curve analysis to better model the change in mechanical withdrawal thresholds over time. D, The slope of trajectory of withdrawal thresholds correlated to the preoperative difference in CPM as rats with lower endogenous analgesia or CPM had slower resolution of mechanical hypersensitivity. Error bars in D indicate variability in predicted slope over time within individual rats. PPT = paw pressure threshold
We conducted pSNL surgery on these same rats and measured paw withdrawal thresholds in the ipsilateral paw longitudinally until 70 days postsurgery. Individual rat’s actual longitudinal behavioral data (fig. 6B) were modeled following surgery using growth curve analysis to describe trajectories of recovery from hypersensitivity (fig. 6C). The mean intercept for the 19 rats was 1.46 g, with a standard error of 0.31 g. The mean slope was 0.2497 g/day, with a standard error of 0.0202 g/day. There was a significant correlation between the individual animal’s strength of preoperative CPM and its slope of recovery (fig. 6D, r = 0.660, p = 0.006) as rats with lower endogenous analgesia had slower resolution of mechanical hypersensitivity. We did not observe a significant correlation between the intercept (modeled withdrawal threshold immediately following pSNL surgery) and slope of recovery (r = −0.18, p = 0.07) suggesting that greater initial hypersensitivity was not predictive of slower recovery.
Preoperative CPM testing including injection of forepaw capsaicin did not impact development or resolution of hypersensitivity in the hindpaws of pSNL rats as the slopes of recovery were not statistically different compared to separate cohorts of control pSNL rats that did not undergo preoperative CPM testing. Mean slope 0.25 ± 0.02 g/day for rats that had preoperative CPM testing versus 0.23 ± 0.03 g/day for rats without preoperative CPM testing (p = 0.52).
DISCUSSION
Better understanding of mechanisms of CPSP would guide preventive strategies, and the current study provides a new approach for such translation, including novel tools to test hypotheses to distinguish causation from association and mechanisms by which individual factors affect recovery. Our data show that rats, like humans, demonstrate an individual association between the strength of CPM preoperatively and speed of recovery from pain or hypersensitivity after surgery. The results support a causal link for this association, reflected in engagement of descending noradrenergic signaling, and suggest that this increased signaling is important to time course of recovery and maintenance of the recovered state.
Mechanisms of CPM in rodents
CPM has been studied in rats using a variety of conditioning stimuli (intradermal capsaicin or formalin, electrical stimulation) and test stimuli (heat, mechanical pressure). 19,20,30 We used a form of CPM developed in rats called noxious stimulation induced analgesia (NSIA).19,20 Previous studies of NSIA have focused primarily on supraspinal opioid and dopaminergic mechanisms19,31. In the current study, we extend the proposed role of spinal noradrenergic activity in CPM 30 to NSIA by showing that forepaw capsaicin induced a 2-fold increase in norepinephrine release in the lumbar spinal cord, consistent with the known effect of noxious stimulation to induce spinal norepinephrine release 7,32 and by showing that NSIA is partially blocked by intrathecal injection of the α2-adrenergic antagonist idazoxan and by ablation of descending noradrenergic innervation of the cord. The partial effect of disrupting noradrenergic signaling suggests that other descending controls are likely involved, including opioidergic, serotonergic or oxytocinergic systems.33,34,35Since idazoxan antagonizes imidazoline receptors, which can inhibit hypersensitivity in rat models of inflammatory, neuropathic pain and incisional pain36,37, we cannot exclude a contribution of spinal imidazoline receptors in this model of CPM.
Role of descending noradrenergic signaling in resolution of postoperative hypersensitivity and glial plasticity
Pharmacological blockade,9,11 genetic disruption38 and toxin-mediated ablation of descending noradrenergic projections10,11,27,39 have all been applied to understand the contribution of spinal noradrenergic systems in the degree of hypersensitivity induced by nerve injury. In our study, using a surgical model with resolution of hypersensitivity over 5-10 weeks, preoperative depletion of spinal noradrenergic fibers delayed recovery, with separation from control animals within 1 week of the surgery. Others have shown that ablation of spinal noradrenergic fibers with DβH-saporin increased the severity of mechanical hypersensitivity for 4 months in the ipsilateral paw following chronic constriction injury10 and that spinal blockade of α2 adrenergic receptors following tibial nerve transection hastened the onset of ipsilateral mechanical hypersensitivity and unmasked contralateral mechanical hypersensitivity9. Once mechanical hypersensitivity was established α2 adrenergic receptor blockade did not further increase ipsilateral hypersensitivity, suggesting that the noradrenergic system spatially restricts and temporally delays the onset of neuropathic pain9 but may be insufficient to completely block ipsilateral sensitization. In contrast to our results with spinal noradrenergic fiber ablation, other data suggest that supraspinally projecting noradrenergic neurons in the LC may facilitate neuropathic hypersensitivity 11. LC projections are topographically organized, with ventral LC and subcoeruleus neurons preferentially innervating the dorsal spinal cord and dorsal LC core neurons predominantly innervating the cortex, amygdala, and hippocampus.38,40,41 In accord with this, selective activation of ventral neurons in the LC, results in antinociception, whereas stimulation of more dorsal core regions of the LC results in pronociception. 42 Therefore, the behavioral effects of ablative or pharmacological strategies to disrupt the noradrenergic system on mechanical hypersensitivity are dependent on the location of ablation and the time of testing which may partially explain previous discrepant results.
In addition to evoked responses to exogenous stimuli, we show that disrupting spinal noradrenergic innervation after surgery affects behavior potentially indicative of spontaneous pain. As such, intrathecal anti-DβH-saporin pretreatment hastened the shifting of weight away from the hindpaw ipsilateral to injury, although it did not affect the time course of recovery. The shorter time course of this non-evoked measure of pain compared to hypersensitivity is consistent with other studies in neuropathic pain models.43 We speculate that this shorter time course of recovery in weight bearing compared to evoked responses in pSNL rats may be due in part to the development of contralateral hypersensitivity.
Spinal glial activation likely contributes to central sensitization and mechanical hypersensitivity after surgical nerve injury.44,45 We show that a reduction in spinal norepinephrine results in long term increases in spinal glial activation following peripheral nerve injury, similar to previous studies in our lab that examined earlier time points.27 Consistent with our findings, noradrenergic receptor agonists have been shown to inhibit glial activation in vivo in rodent neuropathic pain models46,47 and in vitro in spinal microglia48 and astrocyte cultures49. Enhanced glial activation after injury likely results from increased primary afferent release of stimulating factors (e.g., glutamate, substance P) due to a loss of presynaptic α2A inhibition50 or due to a reduced inhibitory or antiinflammatory influence of norepinephrine through direct effects on α2 or β adrenergic receptors expressed on spinal glia.48,49,51 Future studies are needed to determine if the enhanced glial activation in NE depleted rats is causally related to the delayed recovery of hypersensitivity.
Role of descending noradrenergic signaling after recovery: A new homeostasis
Behavioral recovery from sensory sensitization may reflect a new balance of increased inhibitory and excitatory influences. Opioid systems have been most studied in this regard, and recovery from hypersensitivity after high dose opioid agonist exposure52 or inflammation can be reversed by naloxone, although naloxone is without effect on sensitivity in normal animals.17 We observed a reinstatement of ipsilateral and contralateral mechanical hypersensitivity when DβH-saporin was delivered to rats that recovered from hypersensitivity after pSNL providing evidence that the spinal noradrenergic system is involved in returning withdrawal threshold to baseline after surgery. This is consistent with the observation that mechanical hypersensitivity in the ipsilateral paw is unmasked by delivery of spinal α2 adrenergic receptor antagonists to rats that fail to develop allodynia from a surgical injury.9,53,54 The mechanisms by which spinal noradrenergic tone is increased in a sustained manner after injury are unclear. We previously observed anatomic plasticity, with upregulation of noradrenergic innervation (DβH-IR) within the spinal cord following chronic sciatic nerve constriction55 and complete L5/6 SNL28 although others report a decrease in density of spinal DβH positive fibers in the ipsilateral spinal cord following tibial nerve transection.9 Additionally, basal spinal norepinephrine release and spinal norepinephrine content are also increased following complete L5/6 SNL injury.56 In the current study, we extend our previous observations to a less severe model of nerve injury that resolves in 5 −10 weeks. We observed increased spinal norepinephrine content as early as 21 days until greater than 3 months after pSNL, time points that correspond to the time course of resolution of mechanical hypersensitivity. Based on the effects of postsurgery DβH-saporin, this ongoing increased noradrenergic tone may suppress latent neuropathic hypersensitivity in the current model.
Method development for studying individual differences linking CPM to CPSP
Individual differences and variability in response to CPM and nerve injury in animal models of neuropathic pain may depend on strain, species, environment, and type of surgical injury.57 We observed considerable interanimal variability in strength of CPM as well as in the rate of recovery of mechanical hypersensitivity likely due to a combination of genetic and environmental factors as we use a genetically heterogeneous outbred strain of rats. We posit that some of this variability is due to differences in the ability to engage descending noradrenergic inhibitory pathways. Inter- and intrastrain differences in the engagement of descending inhibition have been reported following peripheral nerve injury in rats 53. Similarly, strains of mice differ in stress-induced analgesia and endogenous opioid mediated inhibition.58-60 Thus, individual differences in biological responses to stress (induced by CPM or surgery) or differential engagement of other endogenous inhibitory circuits 58 may also contribute to the observed variability.
Clinically, the association between CPM and CPSP has relied on definition of CPSP as pain being present or absent at an arbitrary time after surgery. Since recovery from pain occurs for years postoperatively61,62 this dichotomous approach at a single time fails to accurately depict the recovery process. In the current study, we chose to define recovery from hypersensitivity in a nondichotomous manner using growth curve modeling,29 as we and others are applying to define time course of recovery from pain after surgery in humans.63,64 Collectively, these studies suggest that targeting therapies that augment descending inhibition (e.g., norepinephrine reuptake inhibitors) to patients with reduced preoperative CPM may decrease the severity and incidence of CPSP in those most at risk. Less efficient CPM in patients with chronic diabetic neuropathy predicted analgesic efficacy of duloxetine65 and tapentodol66 in recent studies, however similar studies involving relatively pain free patients prior to surgery have not been conducted.
In summary, the ability to engage descending inhibition during acute nociception correlates with time course of recovery from hypersensitivity following surgery in rodents, mirroring the correlation in humans. These data suggest that descending noradrenergic pathways are important to acute inhibition, and that their sustained activation is important to the recovery process following neuropathic injury.
Acknowledgments
Acknowledgements: This work was supported from grant R01-GM099863 (to CMP), grant R01-DA27690 (to KH) and grant R37-GM44805 (to JCE) from National Institutes of Health (Bethesda, Maryland).
Footnotes
Portions of this research were previously presented at Neuroscience 2013 on November 9-13 2013 in San Diego, California.
Competing Interests: The authors declare no competing interest related to the topic of this article.
REFERENCES
- 1.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 2.Althaus A, Hinrichs-Rocker A, Chapman R, Arranz Becker O, Lefering R, Simanski C, Weber F, Moser KH, Joppich R, Trojan S, Gutzeit N, Neugebauer E. Development of a risk index for the prediction of chronic post-surgical pain. Eur J Pain. 2012;16:901–10. doi: 10.1002/j.1532-2149.2011.00090.x. [DOI] [PubMed] [Google Scholar]
- 3.McGreevy K, Bottros MM, Raja SN. Preventing chronic pain following acute pain: Risk factors, preventive strategies, and their efficacy. Eur J Pain Suppl. 2011;5:365–72. doi: 10.1016/j.eujps.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diatchenko L, Nackley AG, Tchivileva IE, Shabalina SA, Maixner W. Genetic architecture of human pain perception. Trends Genet. 2007;23:605–13. doi: 10.1016/j.tig.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–8. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Wilder-Smith OH, Schreyer T, Scheffer GJ, Arendt-Nielsen L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: A pilot study. J Pain Palliat Care Pharmacother. 2011;24:119–28. doi: 10.3109/15360281003706069. [DOI] [PubMed] [Google Scholar]
- 7.Tyce GM, Yaksh TL. Monoamine release from cat spinal cord by somatic stimuli: An intrinsic modulatory system. J Physiol. 1981;314:513–29. doi: 10.1113/jphysiol.1981.sp013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Hughes SW, Hickey L, Hulse RP, Lumb BM, Pickering AE. Endogenous analgesic action of the pontospinal noradrenergic system spatially restricts and temporally delays the progression of neuropathic pain following tibial nerve injury. Pain. 2013;154:1680–90. doi: 10.1016/j.pain.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jasmin L, Boudah A, Ohara PT. Long-term effects of decreased noradrenergic central nervous system innervation on pain behavior and opioid antinociception. J Comp Neurol. 2003;460:38–55. doi: 10.1002/cne.10633. [DOI] [PubMed] [Google Scholar]
- 11.Brightwell JJ, Taylor BK. Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain. Neuroscience. 2009;160:174–85. doi: 10.1016/j.neuroscience.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 13.Sterling P, Eyer J. In: Allostasis: A new paradigm to expalin arousal pathology, Handbook of Life Stress, Cognition and Health. Fisher S, Reason J, editors. John Wiley & Sons; New York: 1988. pp. 629–64. [Google Scholar]
- 14.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–5. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asiedu MN, Tillu DV, Melemedjian OK, Shy A, Sanoja R, Bodell B, Ghosh S, Porreca F, Price TJ. Spinal protein kinase M zeta underlies the maintenance mechanism of persistent nociceptive sensitization. J Neurosci. 2011;31:6646–53. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Heijnen CJ, van Velthoven CT, Willemen HL, Ishikawa Y, Zhang X, Sood AK, Vroon A, Eijkelkamp N, Kavelaars A. Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Invest. 2013;123:5023–34. doi: 10.1172/JCI66241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK. Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science. 2013;341:1394–9. doi: 10.1126/science.1239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solway B, Bose SC, Corder G, Donahue RR, Taylor BK. Tonic inhibition of chronic pain by neuropeptide Y. Proc Natl Acad Sci U S A. 2011;108:7224–9. doi: 10.1073/pnas.1017719108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gear RW, Aley KO, Levine JD. Pain-induced analgesia mediated by mesolimbic reward circuits. J Neurosci. 1999;19:7175–81. doi: 10.1523/JNEUROSCI.19-16-07175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari LF, Gear RW, Levine JD. Attenuation of activity in an endogenous analgesia circuit by ongoing pain in the rat. J Neurosci. 2010;30:13699–706. doi: 10.1523/JNEUROSCI.2867-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan Y, Yuan F, Carteret AF, Raja SN. A partial L5 spinal nerve ligation induces a limited prolongation of mechanical allodynia in rats: An efficient model for studying mechanisms of neuropathic pain. Neurosci Lett. 2011;471:43–7. doi: 10.1016/j.neulet.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 23.Wrenn CC, Picklo MJ, Lappi DA, Robertson D, Wiley RG. Central noradrenergic lesioning using anti-DBH-saporin: Anatomical findings. Brain Res. 1996;740:175–84. doi: 10.1016/s0006-8993(96)00855-4. [DOI] [PubMed] [Google Scholar]
- 24.Wiley RG, Kline IR. Neuronal lesioning with axonally transported toxins. J Neurosci Methods. 2000;103:73–82. doi: 10.1016/s0165-0270(00)00297-1. [DOI] [PubMed] [Google Scholar]
- 25.Barthelemy I, Martineau D, Ong M, Matsunami R, Ling N, Benatti L, Cavallaro U, Soria M, Lappi DA. The expression of saporin, a ribosome-inactivating protein from the plant Saponaria officinalis, in Escherichia coli. J Biol Chem. 1993;268:6541–8. [PubMed] [Google Scholar]
- 26.Martin WJ, Gupta NK, Loo CM, Rohde DS, Basbaum AI. Differential effects of neurotoxic destruction of descending noradrenergic pathways on acute and persistent nociceptive processing. Pain. 1999;80:57–65. doi: 10.1016/s0304-3959(98)00194-8. [DOI] [PubMed] [Google Scholar]
- 27.Hayashida K, Peters CM, Gutierrez S, Eisenach JC. Depletion of endogenous noradrenaline does not prevent spinal cord plasticity following peripheral nerve injury. J Pain. 2012;13:49–57. doi: 10.1016/j.jpain.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashida K, Clayton BA, Johnson JE, Eisenach JC. Brain derived nerve growth factor induces spinal noradrenergic fiber sprouting and enhances clonidine analgesia following nerve injury in rats. Pain. 2008;136:348–55. doi: 10.1016/j.pain.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aschenbrenner CA, Houle TT, Gutierrez S, Eisenach JC. Modeling individual recovery after peripheral nerve injury in rats and the effects of parturition. Anesthesiology. 2014;121:1056–67. doi: 10.1097/ALN.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen YR, Wang CC, Yeh GC, Hsu SF, Huang YJ, Li YL, Sun WZ. DNIC-mediated analgesia produced by a supramaximal electrical or a high-dose formalin conditioning stimulus: Roles of opioid and alpha2-adrenergic receptors. J Biomed Sci. 2010;17:19. doi: 10.1186/1423-0127-17-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt BL, Tambeli CH, Levine JD, Gear RW. Adaptations in nucleus accumbens circuitry during opioid withdrawal associated with persistence of noxious stimulus-induced antinociception in the rat. J Pain. 2003;4:141–7. doi: 10.1054/jpai.2003.12. [DOI] [PubMed] [Google Scholar]
- 32.Eisenach JC, Detweiler DJ, Tong C, D'Angelo R, Hood DD. Cerebrospinal fluid norepinephrine and acetylcholine concentrations during acute pain. Anesth Analg. 1996;82:621–6. doi: 10.1097/00000539-199603000-00034. [DOI] [PubMed] [Google Scholar]
- 33.Le Bars D, Bourgoin S, Clot AM, Hamon M, Cesselin F. Noxious mechanical stimuli increase the release of Met-enkephalin-like material heterosegmentally in the rat spinal cord. Brain Res. 1987;402:188–92. doi: 10.1016/0006-8993(87)91066-3. [DOI] [PubMed] [Google Scholar]
- 34.Le Bars D, Chitour D, Kraus E, Dickenson AH, Besson JM. Effect of naloxone upon diffuse noxious inhibitory controls (DNIC) in the rat. Brain Res. 1981;204:387–402. doi: 10.1016/0006-8993(81)90597-7. [DOI] [PubMed] [Google Scholar]
- 35.Tambeli CH, Quang P, Levine JD, Gear RW. Contribution of spinal inhibitory receptors in heterosegmental antinociception induced by noxious stimulation. Eur J Neurosci. 2003;18:2999–3006. doi: 10.1111/j.1460-9568.2003.03031.x. [DOI] [PubMed] [Google Scholar]
- 36.Lanza M, Ferrari F, Menghetti I, Tremolada D, Caselli G. Modulation of imidazoline I2 binding sites by CR4056 relieves postoperative hyperalgesia in male and female rats. Br J Pharmacol. 2014;171:3693–701. doi: 10.1111/bph.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li JX, Thorn DA, Qiu Y, Peng BW, Zhang Y. Antihyperalgesic effects of imidazoline I(2) receptor ligands in rat models of inflammatory and neuropathic pain. Br J Pharmacol. 2014;171:1580–90. doi: 10.1111/bph.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howorth PW, Thornton SR, O'Brien V, Smith WD, Nikiforova N, Teschemacher AG, Pickering AE. Retrograde viral vector-mediated inhibition of pontospinal noradrenergic neurons causes hyperalgesia in rats. J Neurosci. 2009;29:12855–64. doi: 10.1523/JNEUROSCI.1699-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Conklin D, Ma W, Zhu X, Eisenach JC. Spinal noradrenergic activation mediates allodynia reduction from an allosteric adenosine modulator in a rat model of neuropathic pain. Pain. 2002;97:117–25. doi: 10.1016/s0304-3959(02)00011-8. [DOI] [PubMed] [Google Scholar]
- 40.Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: Topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- 41.Bruinstroop E, Cano G, Vanderhorst VG, Cavalcante JC, Wirth J, Sena-Esteves M, Saper CB. Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats. J Comp Neurol. 2012;520:1985–2001. doi: 10.1002/cne.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hickey L, Li Y, Fyson SJ, Watson TC, Perrins R, Hewinson J, Teschemacher AG, Furue H, Lumb BM, Pickering AE. Optoactivation of locus ceruleus neurons evokes bidirectional changes in thermal nociception in rats. J Neurosci. 2014;34:4148–60. doi: 10.1523/JNEUROSCI.4835-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ewan EE, Martin TJ. Differential suppression of intracranial self-stimulation, food-maintained operant responding, and open field activity by paw incision and spinal nerve ligation in rats. Anesth Analg. 2014;118:854–62. doi: 10.1213/ANE.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 44.Ji RR, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–62. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Li SS, Zhang WS, Ji D, Zhou YL, Li H, Yang JL, Xiong YC, Zhang YQ, Xu H. Involvement of spinal microglia and interleukin-18 in the anti-nociceptive effect of dexmedetomidine in rats subjected to CCI. Neurosci Lett. 2014;560:21–5. doi: 10.1016/j.neulet.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Xu B, Zhang WS, Yang JL, Lu N, Deng XM, Xu H, Zhang YQ. Evidence for suppression of spinal glial activation by dexmedetomidine in a rat model of monoarthritis. Clin Exp Pharmacol Physiol. 2010;37:e158–66. doi: 10.1111/j.1440-1681.2010.05426.x. [DOI] [PubMed] [Google Scholar]
- 48.Morioka N, Tanabe H, Inoue A, Dohi T, Nakata Y. Noradrenaline reduces the ATP-stimulated phosphorylation of p38 MAP kinase via beta-adrenergic receptors-cAMP-protein kinase A-dependent mechanism in cultured rat spinal microglia. Neurochem Int. 2009;55:226–34. doi: 10.1016/j.neuint.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Morioka N, Abe H, Araki R, Matsumoto N, Zhang FF, Nakamura Y, Hisaoka-Nakashima K, Nakata Y. A beta1/2 adrenergic receptor-sensitive intracellular signaling pathway modulates CCL2 production in cultured spinal astrocytes. J Cell Physiol. 2014;229:323–32. doi: 10.1002/jcp.24452. [DOI] [PubMed] [Google Scholar]
- 50.Bourgoin S, Pohl M, Mauborgne A, Benoliel JJ, Collin E, Hamon M, Cesselin F. Monoaminergic control of the release of calcitonin gene-related peptide- and substance P-like materials from rat spinal cord slices. Neuropharmacology. 1993;32:633–40. doi: 10.1016/0028-3908(93)90076-f. [DOI] [PubMed] [Google Scholar]
- 51.Mori K, Ozaki E, Zhang B, Yang L, Yokoyama A, Takeda I, Maeda N, Sakanaka M, Tanaka J. Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology. 2002;43:1026–34. doi: 10.1016/s0028-3908(02)00211-3. [DOI] [PubMed] [Google Scholar]
- 52.Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: Preventive effect of ketamine. Anesthesiology. 2000;92:465–72. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 53.De Felice M, Sanoja R, Wang R, Vera-Portocarrero L, Oyarzo J, King T, Ossipov MH, Vanderah TW, Lai J, Dussor GO, Fields HL, Price TJ, Porreca F. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain. 2011;152:2701–9. doi: 10.1016/j.pain.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu M, Kontinen VK, Kalso E. Endogenous noradrenergic tone controls symptoms of allodynia in the spinal nerve ligation model of neuropathic pain. Eur J Pharmacol. 1999;366:41–5. doi: 10.1016/s0014-2999(98)00910-8. [DOI] [PubMed] [Google Scholar]
- 55.Ma W, Eisenach JC. Chronic constriction injury of sciatic nerve induces the up-regulation of descending inhibitory noradrenergic innervation to the lumbar dorsal horn of mice. Brain Res. 2003;970:110–8. doi: 10.1016/s0006-8993(03)02293-5. [DOI] [PubMed] [Google Scholar]
- 56.Nakajima K, Obata H, Iriuchijima N, Saito S. An increase in spinal cord noradrenaline is a major contributor to the antihyperalgesic effect of antidepressants after peripheral nerve injury in the rat. Pain. 2012;153:990–7. doi: 10.1016/j.pain.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 57.Mogil JS. Animal models of pain: Progress and challenges. Nat Rev Neurosci. 2009;10:283–94. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 58.Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc Natl Acad Sci U S A. 1999;96:7744–51. doi: 10.1073/pnas.96.14.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marek P, Panocka I, Sadowski B. Selective breeding of mice for high and low swim analgesia: Differential effect on discrete forms of footshock analgesia. Pain. 1987;29:393–8. doi: 10.1016/0304-3959(87)90054-6. [DOI] [PubMed] [Google Scholar]
- 60.Marek P, Yirmiya R, Liebeskind JC. Strain differences in the magnitude of swimming-induced analgesia in mice correlate with brain opiate receptor concentration. Brain Res. 1988;447:188–90. doi: 10.1016/0006-8993(88)90984-5. [DOI] [PubMed] [Google Scholar]
- 61.Johansen A, Romundstad L, Nielsen CS, Schirmer H, Stubhaug A. Persistent postsurgical pain in a general population: Prevalence and predictors in the Tromso study. Pain. 2012;153:1390–6. doi: 10.1016/j.pain.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 62.Kaasa T, Romundstad L, Roald H, Skolleborg K, Stubhaug A. Hyperesthesia one year after breast augmentation surgery increases the odds for persisting pain at four years. Scand J Pain. 2010;1:75–81. doi: 10.1016/j.sjpain.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 63.Chapman CR, Davis J, Donaldson GW, Naylor J, Winchester D. Postoperative pain trajectories in chronic pain patients undergoing surgery: The effects of chronic opioid pharmacotherapy on acute pain. J Pain. 2011;12:1240–6. doi: 10.1016/j.jpain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Chapman CR, Donaldson GW, Davis JJ, Bradshaw DH. Improving individual measurement of postoperative pain: The pain trajectory. J Pain. 2011;12:257–62. doi: 10.1016/j.jpain.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153:1193–8. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 66.Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth. 2014;113:148–56. doi: 10.1093/bja/aeu056. [DOI] [PubMed] [Google Scholar]