Abstract
Background
Recent studies in rodents suggest that repeated and prolonged anesthetic exposure at early stages of development leads to cognitive and behavioral impairments later in life. However, the underlying mechanism remains unknown. In this study, we tested whether exposure to general anesthesia during early development will disrupt the maturation of synaptic circuits and compromise learning-related synaptic plasticity later in life.
Methods
Mice received ketamine/xylazine (20/3 mg/kg) anesthesia for one or three times, starting at either early [postnatal day 14 (P14)] or late (P21) stages of development (n=105). Control mice received saline injections (n=34). At P30, mice were subjected to rotarod motor training and fear conditioning. Motor learning-induced synaptic remodeling was examined in vivo by repeatedly imaging fluorescently-labeled postsynaptic dendritic spines in the primary motor cortex before and after training using two-photon microscopy.
Results
Three exposures to ketamine/xylazine anesthesia between P14–18 impair the animals’ motor learning and learning-dependent dendritic spine plasticity [new spine formation, 8.4 ± 1.3% (mean ± SD) versus 13.4 ± 1.8%, P = 0.002] without affecting fear memory and cell apoptosis. One exposure at P14 or three exposures between P21–25 has no effects on the animals’ motor learning or spine plasticity. Finally, enriched motor experience ameliorates anesthesia-induced motor learning impairment and synaptic deficits.
Conclusion
Our study demonstrates that repeated exposures to ketamine/xylazine during early development impair motor learning and learning-dependent dendritic spine plasticity later in life. The reduction in synaptic structural plasticity may underlie anesthesia-induced behavioral impairment.
Introduction
In recent years, there has been a growing concern about the safety of anesthetics on the developing brain. Emerging clinical evidence suggests that receiving multiple anesthetic exposures early in life may be a significant risk factor for the children to develop learning disabilities, attention-deficit and hyperactivity disorders.1–4 In line with these findings, animal studies have shown that early exposure to clinically used general anesthetics causes long-term learning and cognitive impairment in rodents and non-human primates.5–14
It has been shown that prolonged exposure to general anesthetics during the first postnatal week leads to neuronal apoptosis in rodents.5–11,15 In rhesus monkeys, exposure to ketamine at postnatal days (P) 5–6, also causes significant neuronal cell death.16,17 These studies suggest that anesthesia-induced neuronal apoptosis in the developing brain could be an important contributing factor to behavioral and cognitive impairment later in life. However, because anesthesia-induced neuronal apoptosis seems to be restricted to neonatal stages of the development (before P10),18 it remains unclear whether anesthesia, when administered beyond the window of vulnerability to apoptosis (after P10), can also result in behavioral impairments through mechanisms unrelated to cell death.
Many lines of evidence indicate that experience-induced synaptic plasticity is important for learning and memory formation.19–21 It has been shown that motor skill learning and fear extinction induce rapid formation of dendritic spines, the postsynaptic sites of excitatory synapses, in the motor and frontal associative cortices, respectively.22–26 Furthermore, the degree of persistent new spines induced by learning strongly correlates with the performance improvement after training.22–24,26 These findings raise the possibility that learning-dependent synaptic plasticity may be impaired in mice receiving multiple anesthesia early during development and that such deficits may underlie behavioral and cognitive deficits later in life.
To test this hypothesis, we examined synaptic structural plasticity during motor skill learning in the young adult mice with or without anesthesia during different stages of development. This was achieved by longitudinally following the same dendritic segments in the motor cortex of living mice with a transcranial two-photon imaging technique.27 Ketamine is a widely used agent for pediatric procedural sedation. In rodent models, ketamine/xylazine (KX) combination is the most widely used anesthetics and is therefore chosen to induce anesthesia in this study. Our results show that three KX (20/3 mg/kg) exposures at early (P14–18) but not late stages (P21–25) of brain development impair the animals’ motor learning ability later in life without inducing neuronal apoptosis. Motor learning-associated dendritic spine remodeling is significantly reduced in young adult mice after repeated exposure to KX anesthesia at P14–18. In addition, we found that enriched motor experience following exposure to KX anesthesia enhances motor learning-induced spine remodeling and improves animals’ behavioral performance later in life.
Materials and Methods
Experimental animals and anesthetic treatment
The animal protocol was approved by Institutional Animal Care and Use Committee at New York University Medical Center (New York, NY). Mice expressing yellow fluorescent protein (YFP) in layer 5 pyramidal neurons (Thy1-YFP H-line) were purchased from the Jackson Laboratory (Bar Harbor, ME) and group-housed in the Skirball animal facilities. A total of 143 mice were randomly assigned to different treatment groups as listed in table 1. Both male and female mice were used and no mice were lost to attrition during the experiment. The group size was determined based on our previous studies using the same methodology.22,28 There were two single-exposure groups and two multiple-exposure groups. The single-exposure groups received one intraperitoneal injection of KX (20/3 mg/kg) either on P14 or on P21. The multiple-exposure groups received one injection of KX (20/3 mg/kg) every 2 days for a total of 3 injections, which took place either on P14, 16 and 18 or on P21, 23 and 25. Control group received saline injections. We found that one injection of KX (20/3 mg/kg) produces a light surgical level of anesthesia. About 20% of our mice showed reaction to physical stimulation 30 min after injection and 80% of mice showed voluntary movement within 1 h. During anesthesia, a heating pad was used to maintain the animal’s body temperature at approximately 37°C. In a separate group of animals, blood gas analysis was performed with handheld i-STAT system (Abbott Point of Care, Princeton, NJ). In agreement with our previous observations,28 we confirmed that 1 h of KX anesthesia didn’t induce significant alteration in blood gas values including pH and partial pressure of oxygen and carbon dioxide. In addition, a group of P7 mice were exposed to a higher dose of KX (40/4 mg/kg) for 6 h, and used as positive controls for assessment of apoptosis by immunohistochemistry.
Table 1.
Experimental groups
| Groups | Treatments before P30 (KX: 20/3 mg/kg) | Number of mice treated | Experiments (P30 except for immunohistochemistry) |
|---|---|---|---|
| KX (P14) | 1 KX injection on P14 | 10 (5 male, 5 female) | Rotarod (n=10) |
| KX (P21) | 1 KX injection on P21 | 10 (6 male, 4 female) | Rotarod (n=10) |
| KX (P14,16,18) | 3 KX injections between P14–P18 | 33 (18 male, 15 female) | Rotarod (n=10) |
| Fear conditioning (n=10) | |||
| Immunohistochemistry (n=4) | |||
| In vivo imaging with no training (n=4) | |||
| In vivo imaging with rotarod training (n=5) | |||
| KX (P21,23,25) | 3 KX injections between P21–P25 | 34 (18 male, 16 female) | Rotarod (n=10) |
| Fear conditioning (n=10) | |||
| Immunohistochemistry (n=4) | |||
| In vivo imaging with no training (n=5) | |||
| In vivo imaging with rotarod training (n=5) | |||
| Control | Saline | 34 (20 male, 14 female) | Rotarod (n=10) |
| Fear conditioning (n=10) | |||
| Immunohistochemistry (n=4) | |||
| In vivo imaging with no training (n=5) | |||
| In vivo imaging with rotarod training (n=5) | |||
| KX (P14,16,18) +EE | 3 KX injections between P14–P18; EE from P18–P28 | 18 (7 male, 11 female) | Rotarod (n=10) |
| In vivo imaging with no training (n=4) | |||
| In vivo imaging with rotarod training (n=4) | |||
| Positive control for cleaved Caspase-3 staining | P7; 6-h anesthesia maintained by 40/4 mg/kg KX | 4 (2 male, 2 female) | Immunohistochemistry (n=4) |
EE = enriched environment; KX = ketamine/xylazine.
Rotarod training
Animals were subjected to rotarod training for 2 days using a protocol described previously.22 An EZRod system with a test chamber (Omnitech Electronics, Columbus, OH) was used to perform the rotarod training. After animals were placed on the motorized rod in the chamber, the rotation speed of the rod increased gradually from 0 to 100 r. p. m. over the course of 3 min. The time latency and rotation speed were recorded when the animal was unable to keep up with the increasing speed and fell. Rotarod training/testing was performed in one 30 min session (20 trials per session) per day. Performance was measured as the average speed that the animals achieved during the 20 trials. Both control and experimental groups were trained daily from P30 to 32 to assess motor skill acquisition, and further tested on P37 to examine motor skill retention.
Cued and contextual fear conditioning
Fear conditioning was performed in a FreezeFrame fear conditioning system (Coulbourn Instruments, Whitehall, PA). Mice were placed in a cleaned chamber for 2 min before they were presented with a auditory cue (a 400-Hz, 80-dB tone) for 30 s. A mild foot shock (0.5-mA) was administered during the last 2 s of the tone presentation and co-terminated with the tone. A total of three trials were repeated with a 60–210 s intertrial interval on the training day. The next day, contextual fear memory was tested by returning mice to the same chamber for 5 min without applying the shock. Contextual fear memory was measured by the percent of time that the animals spent on freezing in response to representation of the context. Auditory-cued fear memory was tested by placing mice to a different chamber and presenting the auditory cue. Fear memory was measured by the percent of time that the animals spent on freezing duirng the tone presentation.
Immunohistochemistry
Four groups of mice were used for immunohistochemistry studies (table 1). Mice were anesthetized with KX (100/15 mg/kg) and immediately perfused with 20 ml phosphate buffered saline (PBS). Brain tissue was removed and fixed for 1 h in 4% paraformaldehyde at 4°C. Tissue was rinsed three times with PBS, embedded in 2% agarose, and sectioned at 100 μm with a vibratome. Sections were permeabilized in 1% Titron X-100 in PBS for 3 h and blocked with 5% normal goat serum for 1 h. Sections were incubated overnight with primary antibodies against cleaved Caspase 3 (1:200). Sections were then washed three times with PBS/0.05% Tween-20, and then incubated with Alexa Fluor-conjugated goat anti-rabbit IgG secondary antibodies (1:500) in PBS for 2 h and again washed with washing buffer. The nuclei were stained with 0.5 μg/ml 4′,6′-diamidino-2-phenylindole for 5 min. Sections were washed as before and mounted for imaging. Confocal images were obtained on a Biorad Radiance 2000 confocal microscope (Hercules, CA). Investigators were blinded to experimental condition in the process of imaging and data analysis.
In vivo transcranial two-photon imaging and data analysis
The procedure for chronic imaging of neuronal structure in the living cortex has been described in our previous work.27,28 Briefly, surgical anesthesia in P30 mice was achieved using an intraperitoneal injection of KX (100/15 mg/kg). A midline scalp incision exposed the skull and the area to be imaged was identified based on stereotaxic coordinates for the primary motor cortex. The head was immobilized, and a cranial window was created by thinning a circular area of the skull (~200 μm in diameter) to approximately 20 μm in thickness by using a high-speed drill and a microsurgical blade. Upon completion of the skull thinning under a dissection microscope, the animal was placed under a multi-photon microscope while still under anesthesia. Image stacks of dendritic segments within a depth of 100 μm from the pial surface were collected using a 60× objective (NA 1.1) immersed in artificial cerebrospinal fluid. High-magnification (66.7 μm × 66.7 μm; 512 × 512 pixels; 0.75 μm step) imaging was used to obtain images suitable for spine analysis. After imaging, the scalp was sutured with 6–0 silk and the animal was returned to the home cage until the next viewing.
Analysis of spine plasticity was performed using the ImageJ software as described previously.22,29 Briefly, for each dendritic segment, filopodia were identified as long, thin protrusions without enlarged heads and the remaining protrusions were classified as spines. Images of the same dendritic segments identified from two views were compared. Spines were considered stable if they were present in both views, eliminated if they were present in the first view but not in the second view or newly formed if they were present in the second view but not in the first view. The elimination and formation rates were measured as the number of spines eliminated or formed in the second view divided by the number of spines existing in the first view. Investigators were blinded to experimental condition when performing data analysis.
Enriched Environment
A group of mice were housed in a special cage (36.5×20.7×14 cm) containing a running wheel from P18 to P28, where they have ad libitum access to food, water and a freely rotating running wheel. The running wheel was used to enrich the animals’ living environment and increase their voluntary exercise activity.
Statistical Analysis
All data were presented as means ± S.D. Group differences in behavioral performance, immunohistochemistry, dendritic spine density and the percentage of filopodia were determined using a one-way analysis of variance (ANOVA) followed by Neuman-Keuls multiple comparison post hoc test. In analysis of spine head diameters, comparisons of the means of each group were performed using a two-way ANOVA with repeated measures. A two-tailed unpaired Student t-test was used to determine the difference between KX- and saline-treated groups in the rates of dendritic spine formation and elimination. P < 0.05 was considered statistically significant. Prism software (GraphPad 6.0, La Jolla, CA) was used to analyze the data.
Results
Multiple exposures to KX during P14–18 cause motor learning deficits in mice at P30
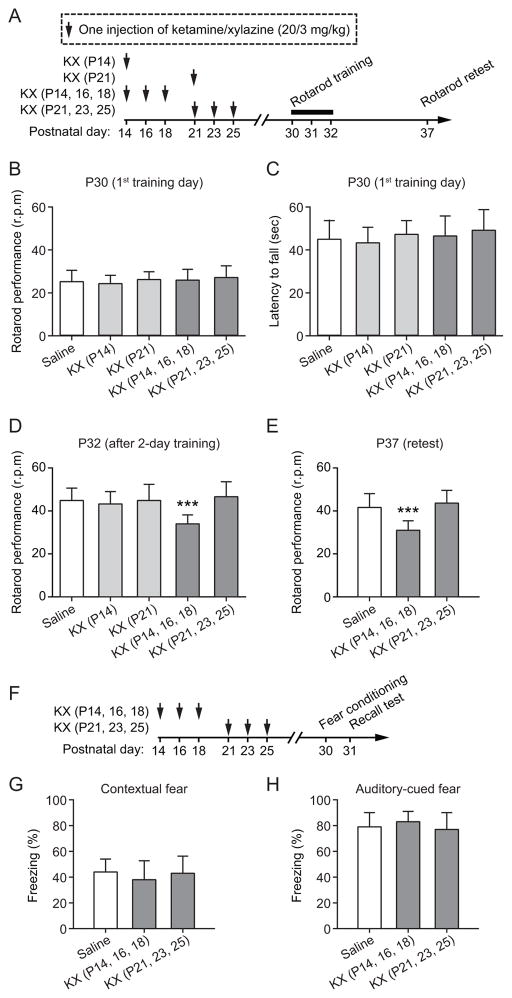
To investigate whether and when exposures to anesthesia during development cause learning impairment in later life, we treated mice with ketamine/xylazine (KX) (20/3 mg/kg) anesthesia for one or three times during the second or third week of postnatal life (fig. 1A) (table 1). Control mice received saline injections. From P30 to P32, all groups of mice were trained to run on a rotating rod. During this rotarod running task, the animals change their gait pattern to maintain their balance on the accelerating rod.30 On the first training day (P30), both saline- and KX-injected mice showed similar running performance [F (4, 45) = 0.629, P = 0.644, one-way ANOVA] (fig. 1B) and task participation [F (4, 45) = 0.654, P = 0.627, one-way ANOVA] (fig. 1C), suggesting that exposure to KX during early developmental stages has no significant effects on the animals’ basic motor function. After 2-day training, all mice improved their performance on the task at P32. One injection of KX on P14 or P21, or three injections of KX during P21–25 had no significant effect on the animals’ performance improvement after motor training as compared to saline-injected control mice (P = 0.690) (fig. 1D). Notably, we found that mice receiving three injections of KX during P14–18 showed significant reduction in performance improvement as compared to the control (P < 0.0001) (fig. 1D). When the mice were retested with the same task 5 days later (P37), the performance of mice exposed to KX at P14–18 remained significantly lower than saline controls (P < 0.0001) (fig. 1E). Together, these findings suggest that multiple exposures to KX during the early postnatal development lead to motor learning deficits later in life.
Fig. 1.
Impaired motor skill learning in 1-month-old mice with three ketamine/xylazine (KX) exposures at P14–18. (A) Experimental design. Animals received a single or three injections of KX starting at P14 or P21, and were subjected to rotarod training at P30–32 and testing at P37. (B-C) All groups of mice showed similar rotarod performance on the first training day. (D) After 2-day training, mice with three injections of KX at P14–18 showed significant lower performance than all other groups. (E) When retested on P37, mice with three injections of KX at P14–18 continually showed reduced performance than saline controls. (F) Animals received three injections of KX at P14–18 or P21–25, and were subjected to fear conditioning tests at P30. (G-H) Exposures to KX at P14–18 or P21–25 had no effects on the animals’ contextual fear memory (G) and auditory-cued fear memory (H). Comparison of means of each group was performed using a one-way ANOVA followed by Neuman-Keuls multiple comparison post hoc test. Data are presented as means ± S.D. ***P < 0.001.
To determine if multiple exposures to KX anesthesia affect behaviors other than motor learning, we performed contextual and auditory-cued fear conditioning tests (fig. 1F). We found that mice with three injections of KX at either P14–18 or P21–25 had similar freezing response as compared to control mice that received saline injections [Contextual fear: F (2, 27) = 0.702, P = 0.504; Auditory-cued fear: F (2, 27) = 0.874, P = 0.429, one-way ANOVA] (fig. 1G and H), indicating that neural circuits involved in fear learning may be resistant to the disruption caused by anesthesia at early stages. Taken together, these results indicate that KX anesthesia has long-lasting adverse effects on the animals’ behavioral performance on selected tasks, and the effects are age- and exposure frequency-dependent.
No increase of cell apoptosis after multiple exposures to KX starting at P14 or P21
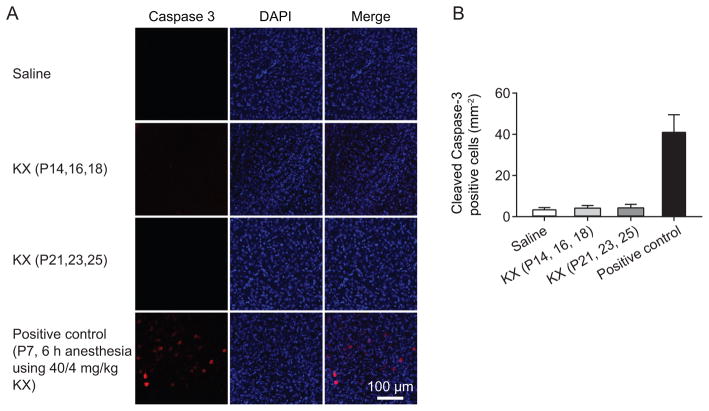
Previous studies have shown a significant increase in cell apoptosis after prolonged exposure to anesthesia during the first week of postnatal development.5–11,15 To test whether cell apoptosis might contribute to anesthesia-induced motor learning deficits, we examined whether the anesthesia regimen used in the present study might cause cell death in P14 and older mice. Cell apoptosis was assessed by the immunostaining of cleaved Caspase-3 on the day when mice received the last injection of KX (fig. 2A). We found that three injections of KX at either P14–18 or P21–25 had no significant effect on cell apoptosis (P = 0.334, one-way ANOVA) (fig. 2B). As a positive control for detecting anesthesia-induced cell death, we also performed a 6-h-long anesthesia in P7 mice using a higher dose of KX (40/4 mg/kg) (fig. 2A). Consistent with previous observations,31 this anesthesia condition increased cell apoptosis (P < 0.0001, one-way ANOVA followed by Neuman-Keuls post hoc test) (fig. 2B). These data indicate that repetitive exposures to low-dose KX have no significant effects on neuronal death in P14 and older mice.
Fig. 2.
No cell death is detected in the motor cortex after three injections of ketamine/xylazine (KX). (A) Cell apoptosis in the motor cortex was assessed by the immunostaining of cleaved Caspase-3 on the day when the mice received the last injection of KX (20/3 mg/kg). Nucleic acid was stained with 4′,6′-diamidino-2-phenylindole (DAPI). Saline injection was used as control group. A 6-h-long anesthesia maintained by a higher dose of KX (40/4 mg/kg) in P7 mice was used as a positive control group. Three injections of KX at either P14–18 or P21–25 had no significant effect on cell apoptosis. Scale bar, 100 μm. (B) Quantification of cleaved Caspase-3 positive cell number under various conditions. Comparison of means of each group was performed using a one-way ANOVA followed by Neuman-Keuls multiple comparison post hoc test. Data are presented as means ± S.D.
Multiple exposures to KX have no effects on dendritic spine density and size in adulthood
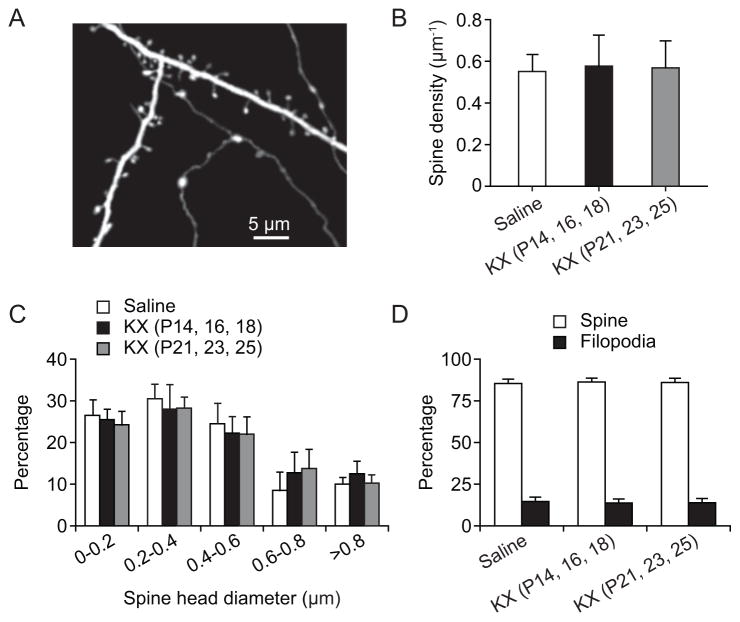
Previous study from fixed brain preparations has shown that ketamine anesthesia causes rapid and significant changes in dendritic spine density and size in various brain regions during early postnatal development.32 To determine whether repeated exposure to KX anesthesia may have long-lasting effects on dendritic spine development, we measured the density and size of dendritic spines in P30 mice that received three injections of KX during either P14–18 or P21–25 (fig. 3). Transgenic mice expressing cytoplasmic YFP in a subpopulation of layer 5 pyramidal neurons were used in this study33 (fig. 3A). At P30, dendritic spine density on apical tuft dendrites was 0.58 ± 0.15 μm−1 (500 spines, 5 mice) and 0.57 ± 0.13 μm−1 (500 spines, 5 mice), respectively, in the primary motor cortex of mice that received three injections of KX at P14–18 or P21–25. There was no significant difference in spine density between KX- and saline-treated mice (0.55 ± 0.08 spines μm−1, 500 spines, 5 mice; P = 0.956, one-way ANOVA) (fig. 3B). In addition, analysis of spine head diameters revealed no significant difference between KX-treated mice and the control (P > 0.9999, two-way ANOVA with repeated measures) (fig. 3C). The percentage of dendritic filopodia, the precursors of dendritic spines, was also not different among all groups (P = 0.879, one-way ANOVA) (fig. 3D). These results indicate that repeated exposure to low-dose KX after P14 does not lead to significant alterations in the density and size of synaptic connections later in life.
Fig. 3.
No change in the density and size of dendritic spines in P30 mice after early ketamine/xylazine (KX) injections. (A) In vivo transcranial two-photon imaging of dendritic branches in the motor cortex of P30 mice. Scale bar, 5 μm. (B) Density of dendritic spines on the apical tuft dendrites of layer 5 pyramidal neurons from saline- and KX-injected mice. There was no significant difference between saline- and KX-injected groups. Comparison of means of each group was performed using a one-way ANOVA. (C) Frequency distribution histogram of spine head diameter shows that the KX treatment had no significant effect on spine head size. Comparison of means of each group was performed using a two-way ANOVA with repeated measures. (D) Percentage of dendritic filopodia was similar in mice with or without early KX injections. Comparison of means of each group was performed using a one-way ANOVA. Data are presented as means ± S.D.
Multiple exposures to KX after P14 have no effects on baseline dynamics of dendritic spines at P30
Next, we asked whether baseline dynamics of dendritic spines were altered in mice that received three exposures to KX anesthesia and housed under the standard laboratory housing condition. We repeatedly imaged apical dendritic branches and followed the formation and elimination of dendritic spines in the primary motor cortex using transcranial two-photon microscopy (fig. 4A-B). Consistent with previous studies,22,24 we found that a small percentage of dendritic spines were formed and eliminated over 2 days in 1-month-old mice (fig. 4C-D). From P30 to P32, 5.7 ± 0.7% dendritic spines were formed and 6.4 ± 0.5% were eliminated in control mice (768 spines, n = 5 mice). In mice that received 3 injections of KX at P14–18 or P21–25, spine turnover over 2 days in the motor cortex was comparable to that in saline-treated controls (P = 0.632 and P = 0.248) (fig. 4C-D). Spine formation and elimination rates over 2 days were 6.3 ± 0.3% and 7.0 ± 0.4%, respectively, in mice injected with KX during P14–18 (617 spines, n = 4 mice), and 6.0 ± 1.4% and 7.1 ± 0.9% in mice injected during P21–25 (775 spines, n = 5 mice). These results indicate that the baseline dynamics of dendritic spines are not altered after repeated exposure to KX anesthesia.
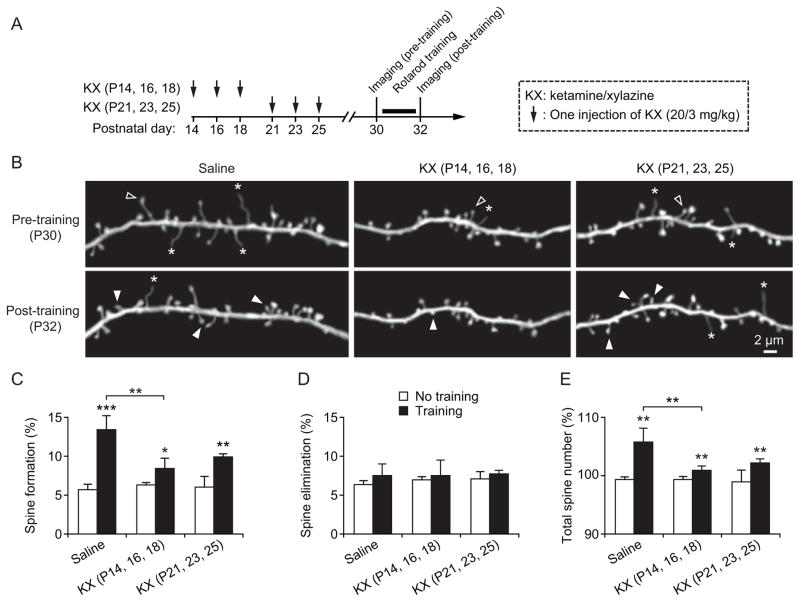
Fig. 4.
Reduction of motor learning-induced spine formation after multiple exposures to ketamine/xylazine (KX) at P14–18. (A) Experimental design. (B) In vivo time-lapse imaging of the same dendritic segments over 2 days (pre- and post-training) in the primary motor cortex of 1-month-old animals that received saline or KX injections at various ages. Filled and empty arrowheads indicate dendritic spines that were formed and eliminated between the two views. Asterisks indicate dendritic filopodia. Scale bar, 2 μm. (C) Percentage of dendritic spines formed over 2 days in saline- and KX-treated mice. All groups showed significant increase in spine formation after rotarod training. Motor learning-induced increase in spine formation was significantly lower in mice that received three injections of KX at P14–18 as compared to saline-injected mice. (D) Percentage of spines eliminated over 2 days in saline- and KX-treated mice. There was no significant difference between saline- and KX-treated groups. (E) The total number of spines increased over 2-day motor training in all groups. Motor learning earning-induced increase in total spine number was significantly reduced in mice with three KX injections during P14–18 as compared with saline-injected mice. Comparisons of means of KX-treated group relative to saline-treated group were carried out using two-tailed unpaired Student t-tests. Data are presented as means ± S.D. *P < 0.05. **P < 0.01. ***P < 0.001.
Motor learning-induced spine formation is reduced in mice receiving multiple exposures of KX at P14–18
Previous studies indicate that motor skill learning induces rapid formation of new dendritic spines.22–25 Moreover, the extent of dendritic spine remodeling strongly correlates with behavioral improvement after learning, suggesting that the remodeling of synaptic connections has an important role in learning and memory formation.22,24 Because multiple exposures to KX anesthesia during early development results in motor learning deficits later in life (fig. 1), it is possible that learning-dependent remodeling of synaptic connections is reduced in animals that have been exposed to general anesthetics during early development.
To test this hypothesis, we examined motor learning-dependent spine remodeling in P30 mice that have received three KX injections at either P14–18 or P21–25 (fig. 4). All mice were trained on the rotarod for 2 days starting at P30. Dendritic spines in the primary motor cortex were imaged before and after rotarod learning to determine learning-induced spine formation and elimination in different groups (fig. 4A). Consistent with our previous findings,22,25 we found that in saline-injected control mice, rotarod training over two days significantly increased the formation of new spines in the motor cortex (13.4 ± 1.8%; 687 spines, 5 mice; P = 0.0003; Student t-test) (fig. 4B–C). Motor training also induced significant increases in spine formation in KX-injected mice as compared to mice with no training (P = 0.02 for P14–18 injection group; P = 0.001 for P21–25 injection group) (fig. 4C). However, the degree of learning-induced spine formation was significantly lower in mice with three KX injections at P14–18 (8.4 ± 1.3%; 760 spines, 5 mice) as compared to that in control mice (P = 0.002) (fig. 4C). Because 2-day training had no significant effects on dendritic spine elimination in both saline and KX-injected mice (P = 0.965) (fig. 4D), motor learning-induced increase in total spine number was reduced in mice that received KX injections at P14–18 as compared to saline controls (P = 0.007) (fig. 4E). Taken together, these results indicate that motor learning-induced spine remodeling is compromised in young adult mice that have received repetitive KX anesthesia during early developmental stages.
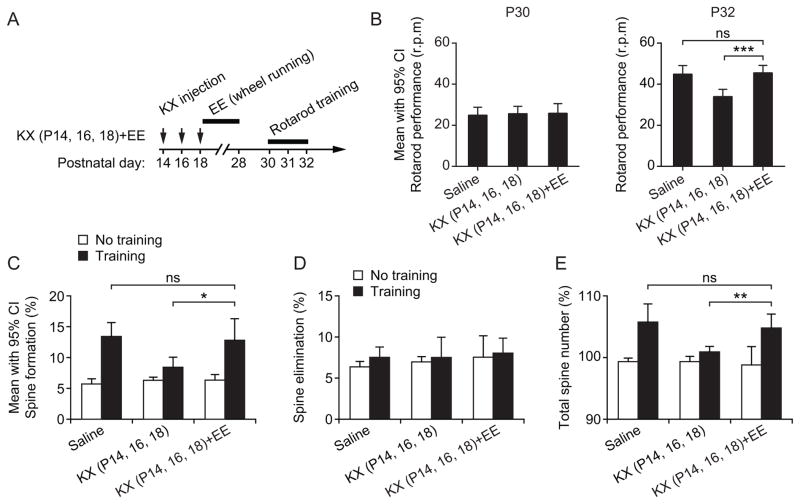
Enriched motor experience attenuates KX anesthesia-induced deficits in motor learning and learning-dependent spine remodeling
Previous studies have shown that enriched environment (EE) can accelerate synapse development and promote dendritic spine plasticity in the cortex.22,34 To test whether EE is beneficial for learning-induced spine remodeling and motor performance in mice following anesthetic exposure, we placed mice in a special cage where they have access to a freely rotating wheel for ten days (from P18 to P28). Specifically, we asked whether wheel-running activity in early postnatal life can enhance both learning-induced spine remodeling and rotarod performance in KX-treated mice to the levels comparable to those in saline-treated controls.
After receiving three injections of KX from P14 to P18, mice were housed in the cage where mice could run on a rotating wheel voluntarily over 10 days. We then tested their performance on the rotarod task from P30 to P32 (fig. 5A). As shown in fig. 5B, all groups of mice showed similar rotarod performance on the first day of training [F (2, 27) = 0.082, P = 0.921, one-way ANOVA]. After 2-day training, the rotarod performance in mice with wheel-running activity after KX injections was comparable to that in saline-treated controls (P = 0.784, one-way ANOVA) and significantly better than mice with KX treatments alone (P < 0.0001, one-way ANOVA followed by Neuman-Keuls post hoc test) (fig. 5B). Furthermore, we found that wheel running significantly increased rotarod learning-induced spine formation over 2 days in KX-injected mice (12.8 ± 1.1%; 579 spines, 4 mice; P = 0.019) (fig. 5C). No significant difference in spine elimination (P = 0.503) (fig. 5D) and total spine number (P = 0.478) (fig. 5E) was found between control mice and mice that were housed in EE after early KX exposure. Thus, enriched motor experience effectively increased both learning-induced spine remodeling and motor performance in mice with early exposure to KX anesthesia.
Fig. 5.
Enriched motor experience ameliorates motor learning and synaptic deficits in mice receiving multiple ketamine/xylazine (KX) exposures. (A) Experimental design. Mice received three injections of KX during P14–18 and were then housed in enriched environment (EE) where they had access to a freely rotating wheel for ten days. Motor performance on the rotarod task was examined from P30 to P32. (B) Rotarod performance in saline- and KX-treated mice. Rotarod performance was similar in all three groups in the first training session at P30. After 2-day training, rotarod performance was significantly higher in the KX group with voluntary running activity than without. Comparisons were performed using a one-way ANOVA followed by Neuman-Keuls multiple comparison post hoc test. (C) Percentage of newly formed dendritic spines from P30 to P32. EE following KX exposure ameliorated anesthesia-induced deficits in spine formation after motor training. Comparisons were carried out using a two-tailed unpaired Student t-test. (D) Percentage of spines eliminated from P30 to P32. There was no significant difference in spine elimination among all groups of animals. Comparisons were performed using a two-tailed unpaired Student t-test. (E) Motor learning-induced increase in total spine number was comparable between saline-injected control mice and mice housed in EE after early KX exposure. Comparisons were carried out using a two-tailed unpaired Student t-test. Data are presented as mean with 95% confidence intervals (CI). *P < 0.05. **P < 0.01. ***P < 0.001.
Discussion
Recent studies in rodents and monkeys suggest that exposure to anesthesia during early postnatal development results in learning and behavioral abnormalities later in life. However, the mechanisms underlying anesthesia-induced learning impairments remain unclear. In this study, we report that three exposures to KX (20/3 mg/kg) anesthesia in the second week (P14–18), but not the third week (P21–25) of postnatal development, cause motor learning deficit in mice. We find that cell apoptosis, dendritic spine density and size are not altered in KX-exposed mice. However, motor training-induced formation of new spines is significantly reduced. Enriched motor experience for 10 days following the initial KX exposure rescues motor learning and synaptic deficits in adulthood. Together, our results reveal that multiple exposures to anesthesia in developing brain can cause long-lasting impairment of synaptic plasticity, which could be reversed with appropriate behavioral intervention.
Ketamine is a dissociative anesthetic and primarily blocks N-methyl-d-aspartic acid (NMDA)-receptors.35 In rodents, ketamine is frequently used together with the α2-adrenoreceptor agonist xylazine to enhance sedative effects and decrease the ketamine dose. Notably, although a single exposure to KX anesthesia at P14 has no long-lasting effects on the animals’ motor and fear learning, three rounds of KX anesthesia, which is spaced every other day between P14–18, produce motor learning deficit in young adult mice. One or three KX anesthesias during P21–25 have no significant effects on the animals’ behavioral performance at one month of age. These results imply that anesthesia, when administered during early postnatal age for multiple times, have accumulative impacts on the developing brain and lead to behavioral impairment later in the life. These results are consistent with a recent study reporting that anesthesia with 3% sevoflurane for 2 h in P6 mice doesn’t induce cognitive impairment, while anesthesia with 3% sevoflurane for 2 h daily for 3 days induce cognitive impairment detected at one month of age.14
Previous studies have linked anesthesia-induced cell death to animals’ behavioral impairment later in life.5–7 Several anesthetic agents that act as NMDA receptor antagonists and/or γ-aminobutyric acid type A receptor agonists can induce extensive neuronal apoptosis in the developing brain in an age-dependent manner.5–7,15,16,18 The window of vulnerability to the neurotoxicity of ketamine is restricted to late fetal and early postnatal ages (before P10 in rodents).18 Consistent with these studies, our results show that exposure to KX anesthesia after P14 has no significant effect on cell apoptosis. The fact that these mice show motor learning impairment at P30 suggests that apoptotic cell death is unlikely the major pathological event underlying anesthesia-induced cognitive impairment. Instead, abnormal development and plasticity of synaptic connections may play an important role in anesthesia-induced behavioral deficits.
Exposure to general anesthetics during the peak stage of synaptogenesis has been shown to cause rapid and substantial alterations in the number of dendritic spines within hours.32,36–38 Five hours of anesthesia with midazolam, propofol or ketamine caused a substantial increase (up to 2-fold) in the density of dendritic spines in the mouse somatosensory cortex and hippocampus at P15 and P20.32 A substantial increase (~30–50%) in dendritic spine density of layer five pyramidal cells was observed in rat medial prefrontal cortex within 2 h after exposure to isoflurane, sevoflurane, desflurane or propofol at P15 or P20.36,38 In the present study, dendritic spine density was examined at P30, 1–2 weeks after initial exposure to anesthesia at P14 or P21. We didn’t find significant changes in spine density or size in the primary motor cortex of mice that received three injections of KX at either P14–18 or P21–25 as compared to saline-treated controls. Furthermore, the percentage of dendritic filopodia, the precursors of dendritic spines, is also comparable between mice with and without early KX treatments. These observations indicate that multiple exposures to KX anesthesia after P14 have no long-lasting effects on dendritic spine number and morphology in mouse motor cortex. It has been shown that propofol anesthesia-induced modification in dendritic spine density in the rat medial prefrontal cortex persists into adulthood.38 Therefore, the effect of anesthesia on spine density likely depends on the type of anesthetic drugs and brain regions.
Consistent with previous studies,22,24,25 we found that in both saline- and KX-treated mice, rotarod training over two days increases the rate of dendritic spine formation in the primary motor cortex. However, the degree of learning-induced new spines was substantially lower in mice with multiple sessions of KX anesthesia at P14–18. We also observed lower rates of spine formation after training in mice that received KX anesthesias at P21–25, although the difference is not significant. The reduction of learning-dependent spine dynamics in adulthood indicates that multiple exposures to KX anesthesia during early development have a long-lasting detrimental impact on neural circuits. Recent in vivo imaging studies have shown that the acquisition of a new motor skill is accompanied by new spine formation in the primary motor cortex,22–25 and the persistence of motor learning-induced new spines correlates with the retention of the motor skill,22–24 The reduced synaptic plasticity in response to rotarod motor learning might underlie the behavioral deficit in ketamine-treated mice.
The mechanisms by which anesthesia impairs learning-dependent synaptic plasticity remain to be determined. An important feature of the nervous system is that neuronal activity profoundly affects patterns of neuronal connectivity during development. Ketamine depresses neuronal activity and reduces calcium influx into neurons primarily through blockade of NMDA receptors.39–41 NMDA receptor mediated calcium influx is critical for neuronal differentiation, migration and synaptogenesis.42 Therefore, it is possible that ketamine-induced NMDA receptor inactivation could significantly reduce activity-mediated Ca2+ influx into neurons, which in turn would affect synapse formation and plasticity.
It is well acknowledged that environmental stimulation has an important role in neural circuit formation and function.43–46 Recent studies have shown that mice reared in the enriched environment for the first two weeks of postnatal development show accelaterated synapse maturation, including increased levels of NMDA and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors and glutamatergic synaptic transmission as indicated by the increased amplitude of miniature and spontaneous excitatory postsynaptic currents.34 It has been reported that EE alleviates sevoflurane anesthesia-induced behaviorial deficits in fear conditioning and water maze tests.14,47 We found that enriched motor experience in early life can ameliorate KX anesthesia-induced decrease in dendritic spine plasticity associated with motor learning in the motor cortex. With the recovery of synaptic deficits in KX-treated mice, motor learning deficits are also rescued. Our finding suggests that promoting synapse development after general anesthesia by EE is benefical in preventing the long-lasting impact of general anesthetics on cortical function. It is important to point out that the present study focused on the effects of EE on motor learning and motor learning-dependent synaptic plasticity in the motor cortex after early KX exposure. In the future, it will be of great interest to examine whether the effects of EE on synapse plasticity could be generalized to other brain regions and for other types of anesthetics.
It is important to point out several limitations in this study. First, surgical procedure for in vivo transcranial two-photon imaging may cause local inflammation and affect the dynamics of dendritic spines under the cranial window. Although our previous study has shown that carefully performed skull thinning does not activate microglia, the innate immune cells in the brain,27,48 precaution must be taken to avoid over thinning or cracking the skull during the surgery. Second, we only examined the apoptosis and dendritic spine plasticity in the primary motor cortex of KX-treated mice. In addition to the motor cortex, motor skill learning requires multiple brain regions, including the cerebellum, thalamus, and basal ganglia. Future studies on synaptic plasticity in these regions will be required to obtain a more comprehensive understanding of the effects of anesthetics on neuronal circuit development. Third, similar to most animal studies assessing the developmental toxicity of anesthetic drugs, caution is warranted in evaluating the clinical relevance of our findings with mouse models as the timeline of brain development is substantially different between human and mouse. Finally, it is important to note that we didn’t investigate the effects of ketamine exposure alone in the present study because doses of ketamine as high as 40 mg/kg are insufficient to produce anesthesia in young mice.31 To avoid neurotoxicity associated with high dose of ketamine, we chose to use KX mixture (20/3 mg/kg) which reliably induces anesthesia in young mice and provides immobilization for about 1 h. Because KX combination is not used clinically, future studies are required to distinguish the contribution of ketamine in KX anesthesia-induced synaptic and motor learning impairments.
In summary, we have found that repeated exposure to KX anesthesia during the period of rapid synaptogenesis can cause behavioral deficits without triggering cell death. Following early anesthetic exposure, cortical circuits are less responsive to the modulation by motor learning experience in adulthood, suggesting that the reduction in learning-dependent synaptic plasticity is an important cortical defect underlying anesthesia-induced cognitive impairments. Finally, enriched experience in early life is benefical in mitigating the adverse effects of KX anesthesia on brain function.
Acknowledgments
Supported by R01 GM107469 from the National Institutes of Health, Bethesda, Maryland; Whitehall Foundation research grant, Palm Beach, Florida (to Dr. Yang); National Natural Science Foundation (grant no. 81001129), Beijing, China; Guangdong Natural Science Foundation (grant no. 10451051501004726), Guangdong, China; Medical Scientific Research Foundation of Guangdong Province (grant no. B2010170), Guangdong, China (to Dr. Huang).
The authors thank Thomas Blanck, M.D., Ph.D. (Department of Anesthesiology, New York University Medical Center, New York, New York) and members in Yang lab for their critical comments on the manuscript.
Footnotes
Competing interests: The authors declare no competing interests.
A part of this work was presented at the annual meeting of American Society of Anesthesiologists, October 10–12, 2013, San Francisco, California.
References
- 1.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–61. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanic K, Welch TL, Olson MD, Hanson AC, Schroeder DR, Wilder RT, Warner DO. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–9. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ing C, Dimaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJ, Li G, Sun LS. Long-term Differences in Language and Cognitive Function After Childhood Exposure to Anesthesia. Pediatrics. 2012;130:e476–85. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 5.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–36. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 7.Bercker S, Bert B, Bittigau P, Felderhoff-Muser U, Buhrer C, Ikonomidou C, Weise M, Kaisers UX, Kerner T. Neurodegeneration in newborn rats following propofol and sevoflurane anesthesia. Neurotox Res. 2009;16:140–7. doi: 10.1007/s12640-009-9063-8. [DOI] [PubMed] [Google Scholar]
- 8.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 9.Yuede CM, Wozniak DF, Creeley CE, Taylor GT, Olney JW, Farber NB. Behavioral consequences of NMDA antagonist-induced neuroapoptosis in the infant mouse brain. PLoS One. 2010;5:e11374. doi: 10.1371/journal.pone.0011374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodama M, Satoh Y, Otsubo Y, Araki Y, Yonamine R, Masui K, Kazama T. Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011;115:979–91. doi: 10.1097/ALN.0b013e318234228b. [DOI] [PubMed] [Google Scholar]
- 11.Fredriksson A, Archer T, Alm H, Gordh T, Eriksson P. Neurofunctional deficits and potentiated apoptosis by neonatal NMDA antagonist administration. Behav Brain Res. 2004;153:367–76. doi: 10.1016/j.bbr.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Jr, Wang C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–30. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viberg H, Ponten E, Eriksson P, Gordh T, Fredriksson A. Neonatal ketamine exposure results in changes in biochemical substrates of neuronal growth and synaptogenesis, and alters adult behavior irreversibly. Toxicology. 2008;249:153–9. doi: 10.1016/j.tox.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–15. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–27. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 16.Slikker W, Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–58. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 17.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Martin LD, Dissen GA, Creeley CE, Olney JW. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–84. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 19.Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 20.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–78. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 21.Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–82. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- 22.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–4. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–9. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang G, Lai CS, Cichon J, Ma L, Li W, Gan WB. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344:1173–8. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- 27.Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010;5:201–8. doi: 10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang G, Chang PC, Bekker A, Blanck TJ, Gan WB. Transient effects of anesthetics on dendritic spines and filopodia in the living mouse cortex. Anesthesiology. 2011;115:718–26. doi: 10.1097/ALN.0b013e318229a660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–6. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 30.Buitrago MM, Schulz JB, Dichgans J, Luft AR. Short and long-term motor skill learning in an accelerated rotarod training paradigm. Neurobiol Learn Mem. 2004;81:211–6. doi: 10.1016/j.nlm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146:189–97. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Roo M, Klauser P, Briner A, Nikonenko I, Mendez P, Dayer A, Kiss JZ, Muller D, Vutskits L. Anesthetics rapidly promote synaptogenesis during a critical period of brain development. PLoS One. 2009;4:e7043. doi: 10.1371/journal.pone.0007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 34.He S, Ma J, Liu N, Yu X. Early enriched environment promotes neonatal GABAergic neurotransmission and accelerates synapse maturation. J Neurosci. 2010;30:7910–6. doi: 10.1523/JNEUROSCI.6375-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: Evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1992;260:1209–13. [PubMed] [Google Scholar]
- 36.Briner A, De Roo M, Dayer A, Muller D, Habre W, Vutskits L. Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology. 2010;112:546–56. doi: 10.1097/ALN.0b013e3181cd7942. [DOI] [PubMed] [Google Scholar]
- 37.Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–25. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L. Developmental Stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology. 2011;115:282–93. doi: 10.1097/ALN.0b013e318221fbbd. [DOI] [PubMed] [Google Scholar]
- 39.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–14. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 40.Yamakura T, Bertaccini E, Trudell JR, Harris RA. Anesthetics and ion channels: Molecular models and sites of action. Annu Rev Pharmacol Toxicol. 2001;41:23–51. doi: 10.1146/annurev.pharmtox.41.1.23. [DOI] [PubMed] [Google Scholar]
- 41.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 42.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 43.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 44.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–8. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 45.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 46.Sale A, Berardi N, Maffei L. Enrich the environment to empower the brain. Trends Neurosci. 2009;32:233–9. doi: 10.1016/j.tins.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Shih J, May LD, Gonzalez HE, Lee EW, Alvi RS, Sall JW, Rau V, Bickler PE, Lalchandani GR, Yusupova M, Woodward E, Kang H, Wilk AJ, Carlston CM, Mendoza MV, Guggenheim JN, Schaefer M, Rowe AM, Stratmann G. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology. 2012;116:586–602. doi: 10.1097/ALN.0b013e318247564d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci. 2007;10:549–51. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]