Abstract
Background
Intracoronary (IC) delivery of cardiosphere-derived cells (CDCs) has been demonstrated to be safe and effective in porcine and human chronic myocardial infarction (MI). However, IC delivery of CDCs after reperfusion in acute MI has never been assessed in a clinically-relevant large animal model. We tested CDCs as adjunctive therapy to reperfusion in a porcine model of MI.
Methods and Results
First, escalating doses (5, 7.5 and 10 million [M] cells) of allogeneic CDCs (allo-CDCs) were administered IC 30 minutes after reperfusion. Forty-eight hours later, left ventriculography (LVG) was performed and animals sacrificed to measure area at risk (AAR), infarct size (IS) and/ or microvascular obstruction (MVO). Secondly, identical endpoints were measured in a pivotal study of mini-pigs (n=14) that received 8.5-9M allo-CDCs, placebo solution or sham. Multiple indicators of cardioprotection were observed with 7.5M and 10M allo-CDCs, but not 5M CDCs, relative to control. In the pivotal study, IS, MVO, cardiomyocyte apoptosis and adverse LV remodeling were all smaller in the CDC group than in sham or placebo groups. In addition, serum troponin I level at 24 hours was lower after CDC infusion than that in the placebo or sham groups, consistent with the histologically-demonstrated reduction in IS.
Conclusions
IC delivery of allo-CDCs is safe, feasible and effective in cardioprotection, reducing IS, preventing MVO and attenuating adverse acute remodeling. This novel cardioprotective effect, which we call “cellular postconditioning”, differs from previous strategies to reduce IS in that it works even when initiated with significant delay after reflow.
Keywords: allogeneic transplantation, cardiosphere-derived cells, cardioprotective effect, microvascular obstruction
Percutaneous coronary intervention (PCI) has become standard therapy worldwide for patients with acute myocardial infarction (AMI), not only reducing short-term adverse cardiac events but also improving long-term clinical outcome.1 Nevertheless, many AMI patients progress to HF even with best current therapy. Adverse left ventricular (LV) remodeling after AMI is a precursor to the development of overt HF and is an important predictor of mortality.2, 3 Prominent among the factors that underlie adverse remodeling is microvascular obstruction (MVO) after reperfusion,4, 5 a state of myocardial tissue hypoperfusion despite patent epicardial coronary arteries. Severe MVO may lead to “no-reflow”, which is an independent predictor of adverse clinical outcome after AMI5, 6, 7. Numerous strategies have been tested to reduce infarct size (IS), but, once reflow has occurred, nothing appears to work. All successful pharmacologic strategies must be applied prior to reperfusion.8 Ischemic postconditioning (created by cyclical intracoronary balloon inflations) requires immediate manipulation of flow at the time of reperfusion, with loss of benefit if there is any significant delay.9, 10
Cell therapy has the potential to revise the paradigm, but little is known about the utility and/or risks of intracoronary cell administration soon after (i.e., within 30 min of) reperfusion. No clinical data are available; most cell therapy clinical trials have infused cells 1-14 days post-AMI. By that time, cardiomyocytes at risk are already dead11, so that there is limited potential (if any) for myocardial salvage. Given the delays intrinsic to autologous tissue harvesting and cell processing, applications in the acute reperfusion phase will require allogeneic (off-the-shelf donor-derived) products. Preclinical studies of acutely-administered allogeneic mesenchymal stem cells (MSCs)12, 13, 14 or their precursors15 have yielded variable results. Although heart-derived stem cells have been tested in both large animals and humans in chronic ischemic settings,16, 17 the only studies in an acute ischemia/reperfusion model were in rats,18, 19 where structural and functional outcomes were improved dramatically by the intracoronary infusion of cardiosphere-derived cells (CDCs) 20 min post-AMI. However, the 3-week endpoint in those studies made it impossible to separate cardioprotection from regeneration.20
We sought to determine whether early infusion of allogeneic CDCs during reperfusion leads to myocardial protection in a clinically-relevant large-animal model. To avoid possible ischemic postconditioning due to balloon inflations, and to mimic clinical reality, the initiation of therapy was delayed until 30 min post-reperfusion. We used a 48-hour endpoint in order to assess acute cardioprotection without the confounding effects of tissue regeneration, which may contribute to the final outcome of longer-term studies.20
Methods
For detailed methods please see the Online Supplement. All animal studies were performed with approval from the Institutional Animal Care and Use Committee of the Cedars-Sinai Health System. Three separate experimental protocols were performed, as depicted schematically in Figure 1 and in Supplementary Figure 1A. A total of 15 Yucatan mini-pigs were studied in a dose-escalation study (Figure 1A); 14 completed the pivotal study (Figure 1B); and 6 pigs were dedicated to the measurement of cell engraftment (Supplementary Figure 1B). One Sinclair mini-pig (#0111) served as the donor for allogeneic heart-derived cells.
Figure 1. Study protocols.
Dose-escalation study (A) and pivotal study (B). CDCs: cardiosphere-derived cells, LVG: left ventriculography, AMI: acute myocardial infarction, TnI: troponin I.
Cell culture
Allogeneic CDCs were grown from a freshly-explanted heart obtained from one male Sinclair mini-pig.
Myocardial infarct creation and CDC infusion
AMIs were created in adult Yucatan mini-pigs by inflation of an angioplasty balloon (TREK® OTW 2-3mm, Abbott Vascular, Santa Clara, CA) in the mid-left anterior descending artery (LAD) (distal to the 1st diagonal branch) for 1.5 h. Two studies were performed contemporaneously, involving a total of 39 minipigs: a dose-escalation study, and a pivotal study. Because of acute reperfusion-related mortality (n=4) or technical reasons detailed in the Results (n=6), 10 animals failed to complete the protocol.
Fifteen mini-pigs completed the dose-escalation study. Allogeneic CDCs (5, 7.5 and 10M cells; n=5 pigs per dose) were formulated, frozen and count-verified just prior to intracoronary infusion, which was performed 30 minutes post-reperfusion via an over-the-wire balloon catheter, placed in the mid-LAD. From the dose-escalation cell counts, post-thaw cell recoveries were 69.6 ± 1.5% and cell viability 90.7 ± 0.4% (n=7). The CDCs were administered in 3 equally-divided cycles of balloon inflation separated by 3 min of deflation (Figure 1A).16
Fourteen mini-pigs completed the pivotal study and were randomized to receive CDCs (n=4, with the cell infusion procedure as in the dose-escalation study), placebo (n=5) or sham (n=5; balloon placement in the LAD but without inflations, to exclude possible confounding effects related to ischemic post-conditioning). The latter two groups, which had similar distributions and medians, were statistically indistinguishable (p=0.11 to 0.59 in all of histological and functional indicators) and thus were pooled as controls for the dosing study. The pivotal study was designed to mimic a clinical situation in which preformulated bags of CDCs containing a nominal dose of 12.5M CDCs were frozen and thawed for administration, without cell counting prior to infusion; based on the post-thaw cell recovery data in the dose-escalation study, actual delivered total cell dosage was estimated at ∼8.7 M.
Left ventriculography
To measure global LV function and volumes, left ventriculography (LVG) was performed before infusion and prior to sacrifice on day 2 using a pigtail catheter inserted retrogradely into the LV, with imaging in the 40° left anterior oblique projection (30 frames per second) using non-ionic contrast (27∼30 ml/ 3 sec). Left ventricular volumes were indexed to body surface area (BSA), calculated as per the formula (BSA= 0.121 * BW kg ^ 0.575).21
Safety evaluation (coronary flow and arrhythmia)
Coronary flow was evaluated utilizing Thrombolysis In Myocardial Infarction (TIMI) and corrected TIMI frame count grading systems. ECGs were recorded before induction of AMI, before CDC/vehicle infusion, immediately after CDC/vehicle infusion, 1 hour after CDC/vehicle infusion (only in pivotal study) and prior to sacrifice to monitor for arrhythmias. In addition, ECGs were continuously monitored during intracoronary infusion and 1 hour post-intervention for arrhythmias.
Histopathological evaluation
Two days after CDC or vehicle infusion, mini-pigs underwent median sternotomy and direct injection into the left atrium of dyes (Gentian Violet and Thioflavin T) to assess area at risk (AAR) and MVO. Then, mini-pigs were euthanized and the hearts explanted and sectioned into 1 cm thick short-axis slices to measure AAR, infarct size (IS) and MVO. Each slice was imaged digitally; IS and MVO areas were determined by manual tracing by a researcher blinded to treatment allocation. The fraction of the AAR within the total area was equated with its weight percent of the total weight of LV. MVO and IS were expressed as the weight percent of AAR.
Hemorrhage in the heart was investigated by hematoxylin and eosin staining of myocardial samples (fixed in 10% formalin, paraffin-embedded) obtained from the infarct, border and remote myocardium; analysis was performed by an experienced cardiac pathologist (DL) blinded to treatment allocation with an arbitrary scoring system (graded as none, mild, moderate or severe utilizing 0, 1, 2 and 3, respectively). To measure apoptotic cardiomyocytes, 8 μm sections from myocardial samples (fixed in 10% formalin, paraffin-embedded) obtained from the infarct, border and remote zones underwent immunostaining for TUNEL (Roche 12156792910), α-sarcomeric actinin (ab9465 Abcam) and wheat-germ agglutinin (Invitrogen) (to visualize cell borders). Vascular density was quantified in pigs sacrificed 48 hours post reperfusion. A total of 3 to 5 sections obtained from the border zones were evaluated per heart with immunostaining for Isolectin (Invitrogen I21411) and α-smooth muscle actin (ab5694 abcam). Alexa Fluor-conjugated secondary antibodies (Molecular Probes) were used and counterstained for DAPI (Molecular probes). Sections were imaged using a confocal laser scan microscope (Leica Microsystems) and images were processed by Leica Application Suite software. The border zone was defined as the region at the edges of the scar (comprising areas of both viable and scarred myocardium).
Blood examination
In the pivotal study, blood draws were performed 24 hours after CDC or vehicle infusion and prior to sacrifice (48 hours) to measure serum troponin I (TnI) as a marker of cardiac injury.
Engraftment study
Engraftment was measured (see Supplemental Figure 1A) as described.22 Luciferase-labeled CDCs were delivered via intracoronary infusion (n=6) 15 minutes post-reperfusion. Animals were sacrificed 15 minutes (n=3) or 48 hours (n=3) later and tissues were taken for luciferase measurement.
Statistical Analysis
Pooled data are expressed as box plots. Mann-Whitney U test was used for comparisons between 2 independent groups. Multiple groups were compared using Kruskal-Wallis test with Bonferroni post-hoc testing. Dynamite plot data are expressed as mean ± standard error of the mean. p < 0.05 was considered statistically significant.
Results
Adverse events and mortality
In the dose-escalation study (Supplementary Table 1), 1 animal out of the 18 (6%) died due to cardiogenic shock immediately after LVG post-reperfusion. Two pigs were excluded due to technical failure or incomplete dye study. There was no further mortality (0%), nor were any adverse events noted during the infusion procedure in either group. In the pivotal study, 3/21 animals (14%) died during creation of MI due to ventricular arrhythmia; 3 pigs were excluded due to technical failure of MI creation or incomplete dye study. One pig was excluded due to incidentally-discovered hypertrophic cardiomyopathy at necropsy. Thus, a total of 14 pigs completed the pivotal study. There was no further mortality within the protocol (0%), nor were there any other adverse events (Supplementary Table 2).
Dose-Escalation Study
Functional and histological benefits of CDC infusion with optimal dose
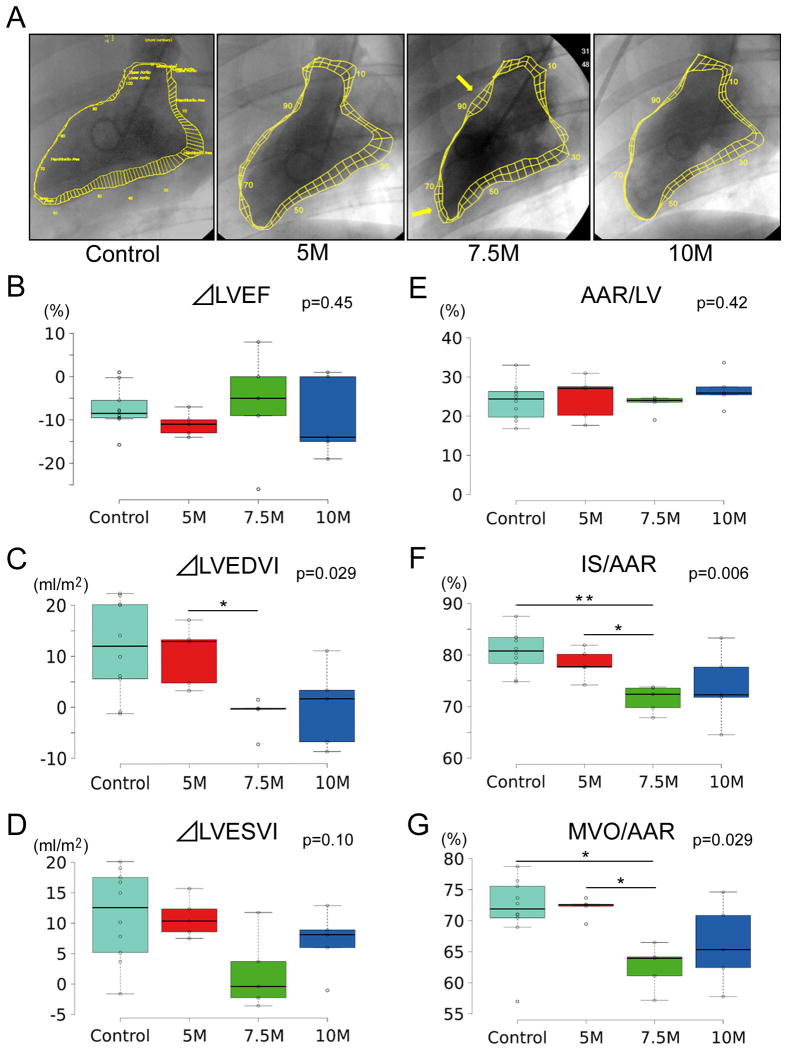
To assess differences of treatment effects with escalating doses of allogeneic CDCs, we measured cardiac function and volumes by LVG. Representative ventriculograms 48 hours post-CDC treatment show amelioration of LV anterior wall motion compared with control, especially in the 7.5M pig (yellow arrows, Fig. 2A). Although the changes in the ejection fraction from baseline to 48 hours post CDC infusion were not significantly different among groups (Fig. 2B), mini-pigs in control and 5M groups underwent progressive LV dilatation (both at end-diastole [Fig. 2C] and end–systole [Fig. 2D]); the change in LVEDVI was significantly attenuated in the pigs that received 7.5M (Fig. 2C). In addition, we evaluated AAR, IS and MVO by dye study 48 hrs post-infusion. Although AAR in the 4 groups was comparable, IS and MVO (as a % of AAR) were smaller (7.5M) or strongly tended to be smaller (10M) than in the control or 5M groups (Fig. 2 E, F and G).
Figure 2. Comparison of different doses of CDCs for assessment of functional and histological efficacy.
Representative left ventriculograms at each dose of CDCs (A). Arrow indicates preserved wall motion in the border zone 48 hours post-intervention. Yellow lines indicate a contour of end-diastolic and -systolic left ventricular endocardial margins. Pooled change in LVEF (B), LVEDVI (C) and LVESVI (D) between baseline and two days post CDCs infusion with escalating dosage. Comparison of area at risk (E), infarct size (F), and MVO (as % of AAR) 48 hours post-infusion.
LVEDVI: left ventricular end-diastolic volume index; LVESVI: left ventricular end-systolic volume index. p value is the result of Kruskal-Wallis test., *: p<0.05 and **: p<0.01 between two groups.
Pathological assessment and cytoprotective effect of CDCs with optimal dosage
The extent of intramyocardial hemorrhage was indistinguishable in all groups (Supplementary Fig.2A, B). In contrast, CDC infusion blunted apoptosis as revealed by TUNEL staining (Fig 3A). The number of TUNEL-positive cardiomyocytes in the border zone was lower in all CDC groups than in control (p=0.002; Fig. 3B, left panel), a pattern repeated, albeit with less significance (p=0,052), in the ischemic zone (Fig 3B, right panel).
Figure 3. Optimized doses of CDCs attenuate apoptosis.
Comparison of apoptosis of cardiomyocytes assessed by TUNEL staining (A) and quantitative evaluation of TUNEL-positive cell number in border and infarct zones (B). WGA: wheat-germ agglutinin; α-SA; α-sarcomeric actinin. p value means the result of Kruskal-Wallis test., *; p<0.05 between two groups.
As for coronary flow evaluation, absolute corrected TIMI frame counts (cTFC) post-infusion were different in the 4 groups (p<0.05, Supplementary Table 1), with significant pairwise differences between control and 10M, but no differences among control, 5M or 7.5M groups (Supplementary Fig. 3).
Overall, although there is a tendency for 10M to be worse than 7.5M, both 7.5M and 10M appear to be better than control or 5M in terms of functional, histological and safety concern. We conclude that 7.5-10M is the optimal therapeutic dosage range.
Pivotal Study
Having established a target dosage range of 7.5-10M CDCs, we performed a pivotal preclinical study with animals randomized to placebo, sham or CDC groups. The CDC group mimicked the clinical situation in which a preformulated bag is acutely thawed for infusion at the site of reperfusion. It is clinically-unrealistic to perform cell counting prior to product release in the AMI setting, so that infusion bags filled with 12.5M CDCs were used, without onsite cell counting and recognizing that some of the cell product would be retained in the dead space of the bags and tubing. From the cell count findings in the dose-escalation study, actual delivered cell dosage was estimated at ∼8.7M, well within the desired therapeutic range of 7.5-10M.
Histological efficacy of intracoronary infusion of CDCs as adjunctive therapy to reperfusion
To assess the acute benefit of allogeneic CDCs, we evaluated histological efficacy of CDCs by dye study at 48 hours. Fig. 4A shows representative slices stained with gentian violet to delineate AAR, and TTC (2,3,5-triphenyl tetrazolium chloride) to quantify IS.23 The infarcted zone (white/ yellow) within the AAR (brick-red) is visibly smaller than that of sham or placebo. The AAR/LV (as a % of the LV) was similar among all three groups, indicating comparable degrees of initial injury (Fig. 4B). IS (as a % of AAR) was smaller in the CDC group (59.7%, p<0.05 vs sham and placebo) compared with sham (81.0%) or placebo (80.3%; Fig. 4C). In addition, IS (as a % of the LV) was also smaller in the CDC group (12.5%, p<0.05 vs sham and placebo) than in sham (19.9%) or placebo (18.5%) groups (Fig. 4D).
Figure 4. Allogeneic CDCs attenuate infarct and no-reflow size.
Representative transverse cardiac slices stained with Gentian violet and TTC (A). Area at risk (as % of LV) (B), infarct size (as % of area at risk) (C) and infarct size (as % of LV) (D) at 48 hours post-intervention. Representative transverse cardiac slices stained with Thioflavin-T under ultraviolet light (E). No-reflow area (as % of area at risk) 48 hours post-intervention (F). AAR: area at risk; IS: infarct size; TTC: 2,3,5-triphenyl tetrazolium chloride; NR: no-reflow. p value is the result of Kruskal-Wallis test., *: p<0.05between two groups.
We also evaluated MVO in the pivotal study. Fig. 4E shows ultraviolet epifluorescent images of representative slices stained with thioflavin T; here, the MVO area appears dark as it has not taken up the infused thioflavin and thus is non-fluorescent. MVO area is distinctly smaller in the CDC-treated pig than in sham or placebo, as verified by the pooled data in Fig. 4F.
Functional efficacy in the acute phase of intracoronary infusion of allogeneic CDCs
We assessed the acute treatment effects of allogeneic CDCs on LV function and volumes by LVG. Although the change of ejection fraction (Fig. 5A) between baseline and 48 hours post-intervention was similar in all groups, mini-pigs in sham and placebo groups underwent progressive LV dilatation (Fig. 5B,C), which was attenuated in CDC-treated pigs.
Figure 5. Functional efficacy and safety assessment after intracoronary infusion of allogeneic CDCs.
Comparison of changes in LV ejection fraction (A), end-diastolic volume index (B) and end-systolic volume index (C) in sham, placebo and CDCs groups. D: Serum troponin I levels at 24 and 48 hrs post-intervention LVEF: left ventricular ejection fraction; LVEDVI: left ventricular end-diastolic volume index; LVESVI: left ventricular end-systolic volume index. p value is the result of Kruskal-Wallis test (0.03 at 24 hrs and 0.24 at 48 hrs). *: p<0.05 between two groups.
Safety evaluation of intracoronary infusion of CDCs
Consistent with the structural evidence of reduced myocardial injury (Fig. 4), CDC treatment decreased serum troponin I level measured 24 hours post-MI (Fig. 5D). These data support the conclusion that CDCs exert a cardioprotective effect, even when cell administration is delayed to 30 min after reperfusion.
Pathological assessment and cytoprotective effect in pivotal study
The extent of intramyocardial hemorrhage was comparable in the three experimental groups (Supplementary Fig. 4A, B). We also quantified apoptosis in the three groups by staining border and infarct zone samples for TUNEL (Fig 6A). Consistent with the results of the dose escalation study, the percentages of TUNEL-positive myocytes in the border (49.5%) was lower in CDC-treated hearts than in sham (68.8%) or placebo (64.3%) (Fig 6B, upper panel). Here we additionally see unequivocal benefit in the infarct zone (Fig 6B, lower panel).
Figure 6. Allogeneic CDCs decreased apoptosis of cardiomyocytes assessed by TUNEL staining.
Comparison of apoptosis of cardiomyocytes assessed by TUNEL staining (A) and quantitative evaluation of TUNEL-positive cell number in border and infarct zones (B). WGA: wheat-germ agglutinin; α-SA: α-sarcomeric actinin. p value is the result of Kruskal-Wallis test., *: p<0.05 between two groups.
Measurement of CDC engraftment by luciferase
Cardiac engraftment of CDCs decreased from 39.9±9.6% (n=3) at 15 minutes to 3.7±1.6% (n=3) at 48 hours. There was a significant difference in engraftment at the two time points (Supplementary Figure 1B).
Assessment of vascularization
We analyzed vessel density in border zone samples by immunostaining in dose-escalation and pivotal studies (Supplementary Fig 5A, B). Quantification of vessel density did not reveal any significant differences among groups.
Discussion
Early and successful myocardial reperfusion with PCI is the most effective strategy for AMI.24, 25 However, reperfusion itself has the potential to aggravate injury, which may in part explain why the incidence of HF after AMI approaches 25%.26 Here we have demonstrated a novel benefit of infused CDCs administered 30 min after reperfusion: such treatment decreased IS and MVO in pigs with AMI. Our findings are notable for two reasons: first, CDCs work despite having been administered relatively late after reperfusion. No other cardioprotective modality has successfully reduced IS without pretreatment (ischemic or pharmacologic preconditioning) and/or immediate intervention upon reopening the affected artery (ischemic postconditioning). Secondly, our study design, with 48-hour structural and functional endpoints, guarantees that we are studying acute cardioprotection; otherwise it is impossible to exclude a partial or dominant contribution from longer-term regenerative effects of the cells, which are evident only weeks after treatment.20 Given the novelty of the phenomenon described here, we call it “cellular postconditioning”.
The influence of post-conditioning on reperfusion injury
Ischemic “post-conditioning”, described by Zhao et al.27, is a cardioprotective phenomenon which can be recruited by applying intermittent cycles of ischemia immediately after reperfusion. There is some inconsistency in the literature as to how long a delay after reperfusion can be imposed while retaining the benefits of ischemic postconditioning, with most studies claiming rapid evanescence (<10 min) 9, 10, but one showing that delays of up to 30 min may be possible.28 To see whether ischemic postconditioning might have influenced our own results, we compared the placebo group (where three cycles of stop-flow ischemia were applied without cell infusion) to the sham group, which simply had a catheter placed in the LAD without intermittent balloon inflations. Results in the two groups were indistinguishable, ruling out a contributory role for ischemic postconditioning in our protocol. Thus, the cardioprotective effects of CDCs infused at 30 min are distinct from those of ischemic postconditioning. Not only is the trigger different (cells vs ischemia), but also the window of treatment opportunity is longer. Such a delay is consistent with clinical reality: when a patient presents with AMI, the immediate focus is on prompt recanalization of the occluded vessel. Only after patency has been re-established and a stent deployed will the typical clinician consider adjunctive therapy; the allowance of 30 min to initiate infusion accomodates decision to treat, product thawing and technical preparation for administration.
The feasibility and safety of intracoronary infusion of CDCs in AMI
In addition to the considerations above, translation will be facilitated by the fact that we used standard clinical equipment and intracoronary infusion for cell transplantation. Intracoronary infusion can be performed in a minimally-invasive manner, and has been safely used in numerous preclinical studies and clinical trials17, 18, 22, 29, 30 Some previous large-animal studies have questioned the safety of intracoronary infusion of stem cells post-MI, with decreased coronary flow and elevation of cardiac enzymes attributed to microvascular plugging.12, 13, 14, 31 Houtgraaf et al. had more favorable results after careful attention to cell dosage, size and infusion rate.15 In terms of cell dosage, we have validated the safety of 12.5M intracoronary CDCs in pigs with chronic ischemic cardiomyopathy, and 25M CDCs administered to post-MI patients in the CADUCEUS trial, without complications such as microembolization.18, 22 Cell dosage must be carefully validated in the acute phase of reperfusion post-MI, given the presence of infarct-related microvascular injury.32 As for cell size, although it is reported that the average diameter of mesenchymal stem cells (MSC) is 30-50 μm33, CDCs are ∼20μm in diameter.22 In addition, previous studies demonstrated micro-infarction or slow-flow phenomena at high infusion rates and/or high cell doses (e.g., 50M cells at a rate of 1.5M cells/min).12, 14 On the other hand, Houtgraaf et al. found that slow infusion of mesenchymal progenitor cells (MPCs; 0.5M cells/min) enabled intracoronary infusion of 50M cells without compromise of coronary flow.15 Here, we infused relatively low cell numbers at rates of 0.8-1.6M/min following reperfusion; 7.5M-10M CDCs at rates of 1.25M-1.6M cells/min were well-tolerated and therapeutically active, in general agreement with Houtgraaf et al.15 Our studies differ in that we delayed cell infusion until 30 min after reflow (Houtgraaf et al.15 began infusion at 15 min of reflow) and in the timing of IS quantification: we focused on 48-hour endpoints, while Houtgraaf et al.15 quantified IS only at 8 weeks, at which time longer-term regenerative effects may cloud the evaluation of cardioprotection.20
Potential mechanism of benefit in CDC-treated hearts
In the vast majority of experimental studies, the number of differentiated myocytes derived from transplanted stem cells is too small to account for the observed improvements in cardiac function.34 Thus, the prevailing concept of stem cell efficacy has shifted towards the ‘paracrine hypothesis’, according to which the transplanted cells are proposed to produce soluble factors that are beneficial to the infarcted heart.35 Indeed, skeletal myoblasts,36 bone-marrow (BM) derived cells37 and cardiac-derived cells38 produce and secrete a broad variety of cytokines. In small comparative studies, CDCs outperformed MSCs in vitro39 and in vivo.40 In a head-to-head comparison of four different cell types (CDCs, BM-derived mononuclear cells, BM-derived MSCs and adipose-derived MSCs) in the same animal model in the same laboratory, CDCs emerged as superior in terms of paracrine factor secretion, angiogenesis, cardiomyogenic differentiation, ischemic tissue preservation, anti-remodeling effects and functional benefit post-MI.41
Potential cardioprotective effects of paracrine factors include anti-apoptotic effects on resident myocytes, upregulation of angiogenesis, modulation of inflammatory processes resulting in better infarct healing, improvements of cardiac metabolism and contractility, promotion of cardiomyocyte cell cycle re-entry, and induction of secondary humoral effects in the host tissue.42, 43 Here, we observed histological benefits including reduction of IS, MVO and apoptotic cardiomyocytes only 48 hours post cell transplantation, which is consistent with paracrine effects but not differentiation of transplanted cells into cardiomyocytes or vessels, as demonstrated in Supplementary Figures 1 and 5. Beyond these considerations, however, the present study provides no insight into the detailed mechanisms of benefit of cellular postconditioning.
Limitations
This study has several limitations. First, this model is one of iatrogenic AMI caused by balloon occlusion. The pathophysiological situation differs from acute coronary syndrome with regard to influence of micro-embolization derived from atherosclerotic plaque or thrombus. Also, the well-defined duration of ischemia here differs from the clinical situation, which is much more heterogeneous. Second, we infused allogeneic CDCs in this study, but did not look for a potential immune response given the short-term nature of the endpoints. However, allogeneic heart-derived cells have been transplanted without eliciting deleterious immune reactions in previous reports.16, 44, 45 Although “off-the-shelf” allogeneic products are clearly necessary for the infusion of cells immediately after PCI, we need to test the safety concerns of allogeneic cells in longer-term experiments.
Conclusions
We demonstrate a cardioprotective effect of CDCs after reperfusion utilizing a large animal model and strategies that are compatible with standard clinical practice. These results motivate not only potential clinical exploration of acutely-administered CDCs in AMI, but also the further exploration of long-term efficacy and mechanism of benefit.
Supplementary Material
Acknowledgments
We thank Adrian Glenn, Hao Zeng, Miguel Huerta, Claudia Anchante, Julie Avalos, and Stephen Taylor for their excellent technical and surgical support, Nina Duong for blood processing, and Jackelyn Valle and Weixin Liu for cell transfection and tissue processing. H.K. was supported in part by a fellowship from the Astellas Foundation for Research on Metabolic Disorders. K.C. is supported by American Heart Association 12BGIA12040477.
Sources of Funding: This work was partially supported by a grant to Capricor from NIH (HL103356). General laboratory support was provided by the California Institute for Regenerative Medicine and the Cedars-Sinai Board of Governors Heart Stem Cell Center.
Footnotes
Kanazawa et al: Cellular Post-Conditioning of Allogeneic CDCs
Disclosures: EM and LM own equity in Capricor, Inc. LM and MK are employed by Capricor. KM is a consultant for Capricor, Inc. Other authors have no relationships with industry.
References
- 1.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 3.John Sutton M, Pfeffer MA, Plappert T, et al. SAVE Investigators. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. Circulation. 1994;89:68–75. doi: 10.1161/01.cir.89.1.68. [DOI] [PubMed] [Google Scholar]
- 4.Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, Higashino Y, Fujii K, Minamino T. Clinical implications of the ‘no-reflow’ phenomenon: a predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–228. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- 5.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008;117:3152–3156. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- 7.Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, Matsui H, Toki Y, Ito T, Hayakawa T. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. 2000;36:1202–1209. doi: 10.1016/s0735-1097(00)00865-2. [DOI] [PubMed] [Google Scholar]
- 8.Kloner RA. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ Res. 2013;113:451–463. doi: 10.1161/CIRCRESAHA.112.300627. [DOI] [PubMed] [Google Scholar]
- 9.Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 11.Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med. 2011;208:549–560. doi: 10.1084/jem.20101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–784. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 14.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 15.Houtgraaf JH, de Jong R, Kazemi K, de Groot D, van der Spoel TI, Arslan F, Hoefer I, Pasterkamp G, Itescu S, Zijlstra F, Geleijnse ML, Serruys PW, Duckers HJ. Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circ Res. 2013;113:153–166. doi: 10.1161/CIRCRESAHA.112.300730. [DOI] [PubMed] [Google Scholar]
- 16.Malliaras K, Smith RR, Kanazawa H, Yee K, Seinfeld J, Tseliou E, Dawkins JF, Kreke M, Cheng K, Luthringer D, Ho CS, Blusztajn A, Valle I, Chowdhury S, Makkar RR, Dharmakumar R, Li D, Marbán L, Marbán E. Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation. 2013;128:2764–2775. doi: 10.1161/CIRCULATIONAHA.113.002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng K, Malliaras K, Li TS, Sun B, Houde C, Galang G, Matsushita N, Marbán E. Magnetic enhancement of cell retention, engraftment and functional benefit after intracoronary delivery of cardiac-derived stem cells in a rat model of ischemia/reperfusion. Cell Transplantation. 2012;21:1121–1135. doi: 10.3727/096368911X627381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marbán E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swindle MM. Swine in the Laboratory: Surgery, Anesthesia, Imaging and Experimental Techniques. 2nd. Boca Raton, FL: CRC Press; 2007. [Google Scholar]
- 22.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marbán E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, Corday E, Ganz W. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101:593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- 24.Brodie BR, Stuckey TD, Wall TC, Kissling G, Hansen CJ, Muncy DB, Weintraub RA, Kelly TA. Importance of time to reperfusion for 30-day and late survival and recovery of left ventricular function after primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 1998;32:1312–1319. doi: 10.1016/s0735-1097(98)00395-7. [DOI] [PubMed] [Google Scholar]
- 25.De Luca G, Suryapranata H, Zijlstra F, van't Hof AW, Hoorntje JC, Gosselink AT, Dambrink JH, de Boer MJ ZWOLLE Myocardial Infarction Study Group. Symptom-onset-to-balloon time and mortality in patients with acute myocardial infarction treated by primary angioplasty. J Am Coll Cardiol. 2003;42:991–997. doi: 10.1016/s0735-1097(03)00919-7. [DOI] [PubMed] [Google Scholar]
- 26.Yellon Derek M, DSc, Hausenloy Derek J., PhD N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 27.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 28.Roubille F, Franck-Miclo A, Covinhes A, Lafont C, Cransac F, Combes S, Vincent A, Fontanaud P, Sportouch-Dukhan C, Redt-Clouet C, Nargeot J, Piot C, Barrère-Lemaire S. Delayed postconditioning in the mouse heart in vivo. Circulation. 2011;124:1330–1336. doi: 10.1161/CIRCULATIONAHA.111.031864. [DOI] [PubMed] [Google Scholar]
- 29.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomized controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 30.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 31.Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, Nam KI, Cho JG, Kang PM, Park JC. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. 1999;43:860–878. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- 33.Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, Wang W, Yerebakan C, Li W, Gaebel R, Li RK, Vollmar B, Steinhoff G, Ma N. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77:370–376. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korf-Klingebiel M, Kempf T, Sauer T, Brinkmann E, Fischer P, Meyer GP, Ganser A, Drexler H, Wollert KC. Bone marrow cells are a rich source of growth factors and cytokines: implications for cell therapy trials after myocardial infarction. Eur Heart J. 2008;29:2851–2858. doi: 10.1093/eurheartj/ehn456. [DOI] [PubMed] [Google Scholar]
- 37.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 38.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marbán E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koninckx R, Daniëls A, Windmolders S, Carlotti F, Mees U, Steels P, Rummens JL, Hendrikx M, Hensen K. Mesenchymal stem cells or cardiac progenitors for cardiac repair? A comparative study. Cell Mol Life Sci. 2011;68:2141–2156. doi: 10.1007/s00018-010-0560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takehara N, Tsutsumi Y, Tateishi K, Ogata T, Tanaka H, Ueyama T, Takahashi T, Takamatsu T, Fukushima M, Komeda M, Yamagishi M, Yaku H, Tabata Y, Matsubara H, Oh H. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J Am Coll Cardiol. 2008;52:1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 41.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marbán L, Marbán E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-Ilzarbe M, Agbulut O, Pelacho B, Ciorba C, San Jose-Eneriz E, Desnos M, Hagège AA, Aranda P, Andreu EJ, Menasché P, Prósper F. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur J Heart Fail. 2008;10:1065–1072. doi: 10.1016/j.ejheart.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Tseliou E, Pollan S, Malliaras K, Terrovitis J, Sun B, Galang G, Marbán L, Luthringer D, Marbán E. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. J Am Coll Cardiol. 2013;61:1108–1119. doi: 10.1016/j.jacc.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 45.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marbán L, Marbán E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.