Abstract
Background and Purpose
Hypokinesia and Bradykinesia as movement deficits of Parkinson disease (PD) are thought to be mediated both by basal ganglia dysfunction as well as a loss of muscle mass and strength commensurate with aging and decreased levels of physical activity. For these reasons, we sought to utilize resistance training as a means to increase muscle force and minimize hypokinesia and bradykinesia in PD and examine the effects of exercise and medication on Body Structure and Function [muscle force production and muscle cross-sectional area], Activity [mobility], and Participation [Health Status]) outcomes.
Methods
Forty-two participants were enrolled in a 12-week randomized controlled trial that compared two active exercise interventions; a (standard care control group [Active Control] and an experimental group that underwent Resistance Exercise via Negative Eccentric Work [RENEW]).
Results
Participants in both groups improved in muscle force production and mobility as a result of exercise and medication (p < 0.02). There were no significant interaction or between group differences and no significant changes in muscle cross sectional area or health status were observed. Effect sizes for exercise and medication combined exceeded the effect sizes of either intervention in isolation.
Discussion and Conclusions
Taken together, these results point to the complementary effects of exercise and medication on the Body structure and Function and Activity outcomes but little effect on Participation outcomes. Video Abstract available for more insights from the authors (see Supplemental Digital Content 1).
Introduction
Hypokinesia and Bradykinesia as movement deficits of Parkinson disease (PD) are defined as decreased amplitude and speed of movement respectively. They are thought to be mediated both by basal ganglia dysfunction as well as a loss of muscle mass and strength commensurate with aging and decreased levels of physical activity.1,2 The combination of central nervous system (CNS) dysfunction and skeletal muscular factors lead to a positive feedback loop of inactivity. This contributes to progressive deficits in muscle force production and increased difficulties with movement amplitude and speed.3 Given that skeletal muscle is the final effector of movement commands from the CNS, increasing muscle force is a logical target for exercise interventions designed to minimize both hypokinesia and bradykinesia.4,5
Even when participating in an exercise program, persons with PD will demonstrate lower amplitude and velocity movements unless purposely compelled to move at a higher intensity.6 For this reason, high intensity exercise, in particular, high intensity resistance exercise is currently advocated as an important component of management of PD.7 While a variety of resistance training protocols have been used in previous studies, we have focused on eccentric resistance training. The rationale for the use of eccentric training is the coupling of high muscular force with low energetic cost.8
Regardless of the type of resistance exercise utilized, such an intervention will not occur in isolation. Virtually all persons with moderate PD will be treated with dopamine replacement medications. No exercise studies have examined the combined effects of high intensity resistance exercise and dopamine replacement on measures of muscle force or mobility. In addition, few lower extremity resistance exercise studies have compared high intensity resistance training to other interventions using stringent randomized clinical trial (RCT) methodology, including blinding of assessors and intention to treat analyses.7
Based on this background, the purpose of this study was to examine the effects of high intensity exercise and medication on a spectrum of outcomes following a 12-week exercise intervention. In order to determine whether high intensity resistance training affects disability, our outcomes encompassed the 3 domains of the World Health Organization’s International Classification of Function, Disability, and Health (ICF) model (Body Structure and Function [muscle force production; muscle cross-sectional area], Activity [mobility], and Participation [health status]) outcomes.9 The primary outcome measure was muscle force production. The secondary outcome measures reflected other aspects of Body Structure and Function, Activity, and Participation (PD motor severity, dynamic stability during gait, gait endurance, and health status). We hypothesized that exercise would improve outcomes but that a high intensity eccentric resistance exercise program (Resistance Exercise using Negative Eccentric Work [RENEW]) group would improve to a greater degree than an Active Control group. In addition, we hypothesized that effect sizes (ES) reflecting exercise and medication together would exceed those produced by exercise or medication alone.
Methods
Participants
Persons with PD in our community comprised the accessible population for recruitment for the RCT. Inclusion criteria were: age over 40 years, a neurologist confirmed diagnosis of idiopathic PD,10 independently ambulatory with gait hypokinesia / bradykinesia (decreased step length / gait speed), and taking dopamine replacement medication (Carbidopa / Levo-dopa). General exclusion criteria were previous surgical PD management, uncontrolled motor fluctuations, or other medical conditions that affected cognition, mobility, or balance. Participants that met the general inclusion and exclusion criteria but were tremor predominant, based on observation and Unified Parkinson Disease Rating Scale (UPDRS) scores, were included in the trial but excluded from the MRI testing of their muscle size. This was done because of the tremor induced movement artifact in the MRI scans.
Study Design
Participants were enrolled in a 12-week RCT that compared two active exercise interventions: a standard care control group [Active Control] and an experimental group that performed RENEW. The a priori power calculation was based on effect sizes from muscle force outcomes from previous studies and indicated that we should recruit 40–45 individuals for the overall trial with an expected attrition of 25%.11 All physical performance measurements were performed when the participants were OFF dopamine replacement medication initially (12 hours after their last dose) and then repeated in the ON medication state (1–1.5 hours after medication intake).12
Primary Outcome
Quadriceps force production was determined via a maximum voluntary isometric contraction (MVIC) on a KinCom dynamometer (Chattanooga Inc.,Hixon, TN). Isometric testing was chosen to be a conservative assessment of force production that differed from the training paradigms and due to the fact that previous research has supported the reliability, validity, and sensitivity to change of this measure.13 In both OFF and ON medication conditions, participants were stabilized by chest and thigh straps and seated with their knees fixed at 60 degrees of flexion with their arms folded across their chest. Participants practiced two submaximal contractions (50 and 75%) and practiced one maximal contraction trial. After a 2-minute rest period, three separate maximal contractions were performed with each held for 5 seconds and 3-minute rests between trials. The average force produced on three trials was used as the dependent variable.
Secondary Outcomes
An additional Body Structure and Function measure was thigh muscle cross sectional area (CSA). Thigh muscle CSA was determined using magnetic resonance imaging (MRI) scans of both thighs. A scout scan was used to identify the superior and inferior boundaries of the femur. Bilateral thighs were then imaged to generate eleven axial T1 weighted images using an image matrix of 512×512 and a slice thickness of 1cm representing the middle 1/3 of each thigh. These images were used to determine average CSA (cm2) of lean tissue using custom written image analysis software (MatLab; Mathworks, Natick, MA). This technique has demonstrated high levels of intrarater reliability, test-retest reliability and concurrent validity.11 A trained examiner, blinded to time point of scan and slice location performed all measurements. Body Function was additionally quantified using the UPDRS motor subsection.14 The motor subsection consists of 14-items with each item rated on a 5-point (0–4) ordinal scale, for a total score of 108 with higher scores indicating more severe impairment. A physical therapist who was blinded to group assignment and had undergone standardized UPDRS training performed the measurements. For the purposes of muscle force and muscle size measures, the lower extremities were labeled as more affected and less affected by patient report and confirmed via UPDRS ratings.
The Activity domain was quantified using 2 tests of mobility. Dynamic stability during gait was quantified using the Functional Gait Assessment (FGA). The FGA is a reliable and valid 10-item standardized test for assessing stability during various walking tasks.15 Items are scored using a 4-point ordinal scale with the total score ranging from 0 to 30. Higher scores indicate better performance. Gait endurance was quantified using the Six Minute Walk test (6MW). The 6MW’s test-retest reliability is high, ranging from 0.94–0.96, in older populations with various co-morbid conditions.16 The Participation domain was quantified using the Parkinson’s Disease Questionnaire (PDQ-39). The psychometric properties of the PDQ-39 have been established in community dwelling persons with PD.17
Procedures
Potential participants were screened to determine eligibility. Following screening, eligible volunteers signed an institution-approved consent form. In order to minimize the effects of fatigue, testing took place over three non-consecutive days in one week. On Testing Day 1, demographic, OFF and ON PD status measures (duration of disease, motor signs, more affected extremities, medication regimen, UPDRS score, Hoehn and Yahr rating), and mobility tests (FGA, 6MW) were gathered. On Testing Day 2, OFF and ON medication muscle force was measured. A tester blinded to group assignment collected muscle force production on both the more affected and less affected lower extremities. During the time period between OFF medication and ON medication testing, participants completed the PDQ-39. Non-tremor predominate participants underwent MRI scans of the thighs two days after strength testing.
Following pre-intervention testing, participants were randomly assigned to one of the two groups (Active Control or RENEW). Randomization assignments were generated via a randomization program and were sealed in envelopes prior to initiation of the study. Upon completion of all pre-intervention testing, the principal investigator opened an envelope and assigned each participant to his/her group. After twelve weeks, all post-intervention measures were repeated within 3 days after the completion of training. In order to preserve blinding, training took place when testing staff were not scheduled in the clinic and participants were counseled not to reveal their group assignment to the testers at their post intervention testing.
Intervention
All exercise was supervised and took approximately 60 minutes per session to complete. Heart rate, blood pressure, and rating of perceived exertion (RPE) were recorded before, during, and after exercise. Throughout the 12 weeks of training, a target RPE was 13 (somewhat hard)on the 20 point RPE scale was sustained.18 The Active Control group completed exercises targeted at improving general strength and fitness.19,20 In contrast, the RENEW group completed a similar program with the only difference being the inclusion of 15 minutes of RENEW training targeted at bilateral lower extremity extensor musculature. Both groups were progressed in workload in order to sustain a RPE of 13. Due to the metabolic efficiency of eccentric muscle contractions, participants in the RENEW group were able to achieve high intensity muscular work while not being limited by their concentric strength or any cardiorespiratory limitations.21 Details of both interventions are provided in the appendix.
Data Analysis
Data were analyzed with SPSS version 20.0 (IBM). Descriptive statistics were calculated for all variables. Prior to statistical analysis, data were screened to determine if it met the assumptions of planned parametric analyses. Missing values were replaced using intent to treat analysis by carrying forward the previous testing value. More affected and less affected limb measures for muscle force and muscle CSA were subjected to bivariate correlations. If these variables were highly correlated (>0.80), one variable of the pair was selected for further analysis.
Quadriceps muscle force production, UPDRS, FGA, and 6MW were subjected to separate 2 (group [RENEW. Active Control])×2 (medication state [ON meds, OFF meds])×2 (exercise training state [pre-exercise, post-exercise]) repeated-measures analyses of variance (ANOVA). Because muscle CSA and PDQ-39 did not vary based on medication status, there variables were subjected to separate 2 (group)×2 (training state) repeated measures ANOVA. Post hoc analyses (Tukey’s HSD tests) were performed as needed. The initial level of significance was set at alpha < 0.05 and was adjusted using a Bonforroni correction within each category of variables.
In addition, in order to compare the combined effect of exercise training state and medication state to the effects of each intervention alone, probability of superiority ES22 comparisons were calculated on muscle force, UPDRS, FGA and 6MW. Probability of superiority (also called the Common Language Effect Size) represents the percentage of times that a randomly sampled participant in the higher performing group will have a higher mean than a randomly sampled participant in the lower performing group. The intent of this measure is to clarify the relationships of the sample distributions being compared.22 Comparisons between pre-exercise OFF medication performance to post-exercise ON medication performance were calculated to represent the combined effects of exercise training state and medication state. Comparisons between pre-exercise OFF medication to pre-exercise ON medication were calculated to represent a medication state ES, while comparisons between pre–exercise OFF medication to post-exercise OFF medication were calculated to represent an exercise training state ES.
Results
Participant Characteristics
Forty-one individuals consented to participate and were randomized to the RENEW group (n = 20) or the Active Control group (n = 21). (Table 1) The interval estimators for all demographic variables for each group substantially overlapped. The flow of participants through the trial is summarized in Figure 1. Participants that completed the trial attended greater than 85% of their scheduled exercise sessions and none of these participants altered their medication regimen during the study.
Table 1.
Participant Demographics
| RENEW (n=20) | Control (n=21) | |
|---|---|---|
| Age (years) | 66.00 (14.78) (59.09–72.91) |
70.71 (9.19) (66.53–74.90) |
| Sex (males/females) | 11/9 | 14/7 |
| Years since Diagnosis (years) | 8.00 (4.48) (5.72–10.28) |
5.70 (4.23) (3.72–7.68) |
| LEDD (mg) | 547.08 (277.74) (320.62–723.55) |
557.14 (290.87) (389.20–725.09) |
| Disease Severity | ||
|
Hoehn and Yahr ON Score Median (min-max) |
2.0 (2–4) | 2.0 (1–4) |
|
Hoehn and Yahr OFF Score Median (min-max) |
3.0 (2–4) | 2.5 (2–4) |
All values = mean (standard deviation) / 95% confidence interval unless otherwise indicated.
LEDD = Levo-dopa equivalent daily dose
Figure 1.
CONSORT diagram illustrating the flow of participants through the trial.
Body Structure and Function
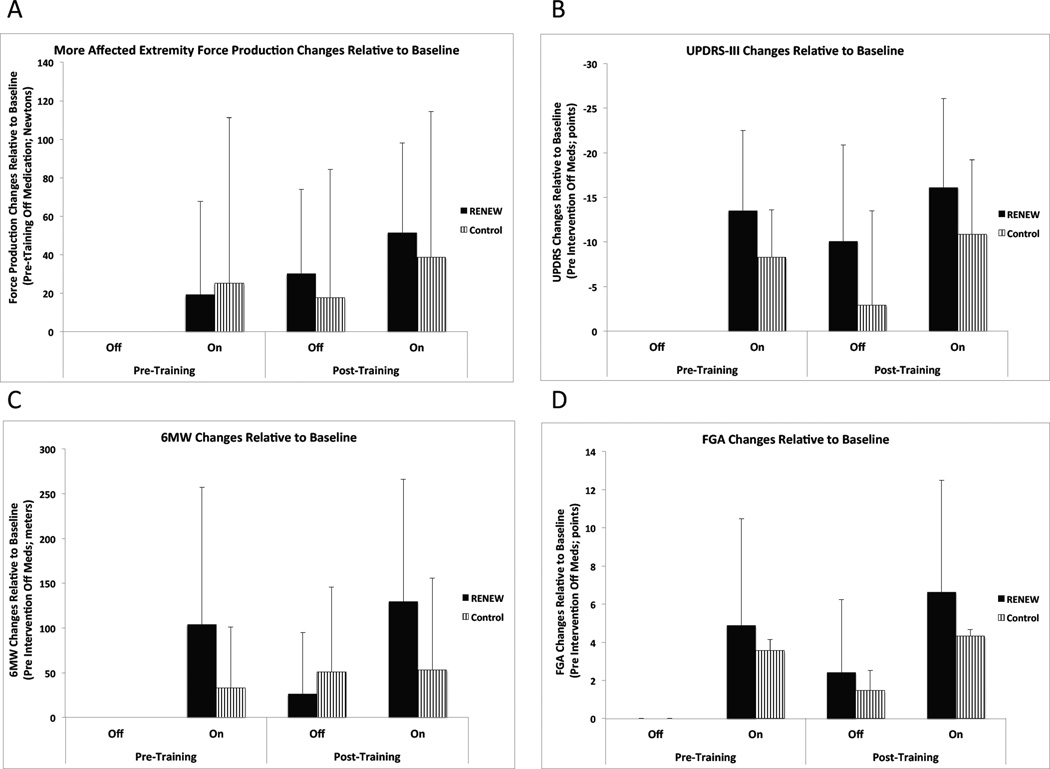
Because muscle force production for the more affected and less affected extremities were highly correlated (r >0.85), only the more affected extremity results are reported. No interaction or between group main effects were significant (p > 0.05). There were significant main effects for exercise training state and medication state on muscle force production (p < 0.02). Post-exercise muscle force exceeded pre-exercise muscle force production. While ON medication, participants’ muscle force exceeded their OFF medication muscle force production. (Table 2, Figure 2) The within group exercise training state and medication state ES for the RENEW group were relatively equivalent to that of the Active Control group. For both groups, the probability of superiority of the combined effect of exercise training state and medication state exceeded that of either intervention alone. (Table 3)
Table 2.
Body structure / Function and Activity Outcomes
| Pre-Intervention Mean (SD) |
Post-Intervention Mean (SD) |
Within Group Exercise Mean Difference/95% CI |
Within Group Medication Mean Difference/95% CI |
|||
|---|---|---|---|---|---|---|
| Variable | On | Off | On | Off | ||
| Average Force (N) | ||||||
| RENEW | 283.20 (126.83) | 263.88 (125.03) | 315.32 (122.59)* | 294.12 (123.43) | 31.18/(16.63–45.72) | 20.26/(1.03–39.48) |
| Active Control | 305.53 (109.52) | 280.38 (106.61) | 319.15 (114.80)* | 298.00 (111.91) | 15.62/(−3.71–34.94) | 23.15/(0.77–47.07) |
| Muscle CSA (cm2) | ||||||
| RENEW | 105.73 (25.76) | N/A | 107.03 (25.69) | N/A | 1.30/(−0.48–3.07) | N/A |
| Active Control | 119.66 (27.00) | N/A | 120.54(28.73) | N/A | 0.88/(−1.74–3.48) | N/A |
|
UPDRS Motor Score |
||||||
| RENEW | 15.05 (7.78) | 28.08 (7.89) | 12.70 (9.30)* | 23.80 (12.03) | −3.31./( −3.85–0.79) | −12.08/(−15.98- −9.28) |
| Active Control | 15.43 (8.62) | 22.14 (10.32) | 13.14 (9.88)* | 19.38 (13.61) | −2.53/(−1.46–2.01) | −6.47/(−10.61- −5.34) |
| FGA (points / 30) | ||||||
| RENEW | 22.10 (6.77) | 17.40 (7.35) | 23.75 (7.00)* | 19.75 (8.39) | 2.00/(0.75- 3.24) | 4.35/(1.77- 6.92) |
| Active Control | 21.14 (8.27) | 17.57 (7.27) | 21.90 (7.89)* | 19.05 (7.41) | 1.13/(0.10–2.13) | 3.21/(1.21–5.22) |
| 6 min walk (m) | ||||||
| RENEW | 558.07 (182.49) | 454.06 (181.74) | 583.70 (181.61)* | 480.46 (180.92)# | 26.01 /(5.92–46.11) | 103.63/38.36- 168.69) |
| Active Control | 485.99 (158.95) | 452.85 (144.01) | 506.09 (192.41)* | 503.90 (174.03)# | 35.58/(−1.98–72.99) | 17.67/(11.61–46.94) |
= significant time and medication main effects (p< 0.02);
= significant group×meds interaction effect
95% CI = 95% Confidence Interval, CSA = Cross sectional area, UPDRS = Unified Parkinson Disease Rating Scale, FGA = Functional Gait Assessment
Positive values indicate improvement in all variables except the UPDRS motor score where negative scores indicate reduced disease severity.
Figure 2.
Outcomes for domains of disablement. a) muscle force of more affected extremity, b) UPDRS motor subsection, c) 6MW, d) FGA. Note: Values for figures are derived from the average change from Pre-exercise OFF medication values for each participant, not the average measures provided in table 2.
Table 3.
Effect size comparisons
| Variable | Combined Exercise Training State and Medication State Effect (Pre-Exercise OFF Meds to Post- Exercise ON Meds Comparison) |
Exercise Training State Effect (Pre-Exercise OFF meds to Post- Exercise OFF meds Comparison) |
Medication State Effect (Pre-Exercise OFF Meds to Pre- Exercise ON Meds Comparison) |
|---|---|---|---|
| Average Force | |||
|
RENEW Group Probability of Superiority |
61% | 57% | 54% |
|
Active Control Group Probability of Superiority |
59% | 55% | 57% |
| UPDRS Motor Score | |||
|
RENEW Group Probability of Superiority |
91% | 62% | 89% |
|
Active Control Group Probability of Superiority |
74% | 56% | 69% |
| FGA | |||
|
RENEW Group Probability of Superiority |
74% | 58% | 68% |
|
Active Control Group Probability of Superiority |
66% | 56% | 63% |
| 6 min walk | |||
|
RENEW Group Probability of Superiority |
70% | 54% | 66% |
|
Active Control Group Probability of Superiority |
59% | 59% | 56% |
RENEW = Resistance Exercise via Negative Eccentric Work, UPDRS = Unified Parkinson Disease Rating Scale, FGA = Functional Gait Assessment
Muscle CSA values for the more affected and less affected extremities were highly correlated therefore only the more affected extremity results are reported. Only 26 participants (14 from RENEW, 12 from Active Control) were analyzed. Eight participants were not scanned due to concerns regarding tremor artifact while an additional 7 participants were excluded due to motion artifacts in either the pre or post intervention scans. There were no significant interaction effects or main effects on CSA for exercise or group (p > 0.05). (Table 2)
No significant interaction effects or between group main effect for the UPDRS motor score were noted (p > 0.05). There were significant main effects for exercise training state and medication state on UPDRS scores (p < 0.02). Post-exercise UPDRS scores were less than pre test scores while ON medication scores were less than OFF medication. (Table 2, Figure 2) For the UPDRS, the within group exercise training state ES for the RENEW group and the Active Control group were relatively equivalent. The within group Medication state ES for the RENEW group exceeded that of the Active Control group. For both groups, the probability of superiority of the combined effect of exercise training state and medication state exceeded that of either intervention alone. (Table 3)
Activity
No significant interaction effects or a between group main effect were noted (p > 0.05) for the FGA. There were significant main effects for exercise training state and medication on the FGA. Post-exercise FGA performance exceeded pre-exercise performance, while ON medication FGA performance exceeded OFF medication performance (Table 2, Figure 2). For the FGA, the within group exercise training state ES for the RENEW group exceeded those in the Active Control group. The within group medication state ES for the RENEW group and the Active Control group were relatively equivalent. For both groups, the probability of superiority of the combined effect of exercise training state and medication exceeded that of either intervention alone. (Table 3)
While the 3-way interaction effect was not significant, there was a significant medication×group interaction effect for the 6MW. Post hoc testing revealed that the ON medication 6MW distance in the RENEW group was significantly greater than OFF medication performance in the RENEW group and both ON and OFF medication performance in the Active Control group. In addition, there were significant main effects for exercise training state and medication on the 6MW test (p < 0.02). Post-exercise walk distance exceeded pre-exercise walk distance, while ON medication walk distance exceeded OFF medication distance. No between group main effect was noted (p > 0.05) (Table 2, Figure 2). For the 6MW, the within group exercise training state ES for the RENEW group exceeded those in the Active Control group. The within group medication state ES for the RENEW group exceeded that of the Active Control group. For both groups, the probability of superiority of the combined effect of exercise training state and medication state exceeded that of either intervention alone. (Table 3)
Participation
There were no interaction effects or main effects for group or exercise training state on the PDQ-39 Single Index score or subscores. Post-exercise results for each group were relatively equivalent to the pre-exercise results as indicated by within group ES being close to zero for all aspects of the PDQ-39 (p < 0.05).
Discussion
Regardless of the presence or absence of neurologic disease, skeletal muscle translates the movement commands from the central nervous system into the forces necessary for movement. In a disorder such as PD, resistance exercise interventions have been proposed as a means to increase muscle force and therefore minimize hypokinesia and bradykinesia.4,5 Following this line of reasoning,, we sought to examine the effects of high intensity eccentric exercise and medication on a spectrum of outcomes that encompassed the 3 domains of the World Health Organization’s ICF model .9 As hypothesized, exercise and medication resulted in improvements in muscle force and mobility. In addition, the combined effect of exercise and medication exceeded the ES of either of the interventions alone. Contrary to our hypotheses, there was no consistent effect of exercise on muscle CSA or PDQ-39, and there were no significant between group effects for any outcomes.
Equivalent Effects of Exercise Types
The lack of a group effect may indicate that the presence of exercise was more important than the type of exercise. The use of efficacious interventions within the active control group may have allowed Active Control participants to experience similar training related gains to the RENEW group. While we controlled for exertion of both groups via RPE, the muscular work between the two groups may have been equivalent. Regardless, the net effect was a minimization of the between group effect sizes. Interestingly, the within group exercise training state effect sizes observed here were similar to those reported in a recent meta-analysis of exercise effects in PD.23 A potential design feature in exercise trials of persons with PD that may limit between group differences are limitations in the dosage of the training intervention. For example, in their study of progressive resistance exercise, the baseline to 6 months results of Corcos et al24 demonstrated significant time effects but not between group differences. Although Corcos et al did not show between group differences at 6 months, continued intervention over 24 months revealed significantly better outcomes for the resistance training group in terms of disease severity, upper extremity force production, and OFF medication movement speed.24 An additional example of this effect is seen by comparing the current studies results to our previous study of resistance training in PD.11,25 In that study, between group differences were observed when participants trained 3 times per week as opposed to the 2 times per week training used here. Certainly, to clearly capture the full extent of the efficacy and effectiveness of resistance training interventions in PD, future studies must consider adequate volume of training (frequency, intensity, duration), as well as the use of inactive control groups.
Exercise and Medication Exert Complementary Effects
The combined effects of exercise and dopamine replacement appeared to be complementary, as indicated by improvements in muscle force, UPDRS motor scores, and mobility (6MW and FGA) when comparing post-exercise ON medication performance to pre-exercise OFF medication testing. In all cases, the ES favored the combined effect of resistance exercise training and dopamine replacement medication over the isolated effect of either intervention (Table 3). Although some express concerns about dopamine resistant motor symptoms and the side effects of dopamine replacement medications,26 our results point strongly to the benefits of medications in terms of muscle force, motor severity, and gait related mobility.
Muscle Force Production Remains Adaptable
The improvements in force production seen in the absence of CSA changes agree with resistance training physiology studies that demonstrate alterations in neural recruitment as a contributor to force production increases.27 While using anatomical measures rather than neurophysiologic measures, our results are consistent with findings from studies of electrical stimulation burst superimposition28 that point to neural recruitment deficits in persons with PD relative to age matched controls.
Our findings suggest that persons with moderate PD retain neuromuscular adaptability as indicated by their improved muscle quality (force output per cross sectional area).29 Our results are consistent with previous studies that have demonstrated that muscle quality may be improved in neurologically healthy older adults as a result of progressive resistance exercise.30 To our knowledge, combined use of neural recruitment and anatomic methods have not been utilized in studies of persons with PD.
Unequal Responsiveness of ICF Domains to Exercise Effects
Despite improvements in Body Structure and Function and Activity measures, Participation as measured by the PDQ-39 was unresponsive to exercise effects in this study. Currently, there are conflicting reports in the literature regarding exercise effects on health status in persons with PD. Previously, we and others have reported improvements in PDQ-39 in response to resistance, Tai Chi, or Dance training.25,31 However more recently, Schenkman and colleagues reported no change in PDQ-39 scores despite improvements on measures of aerobic fitness.20 In addition, the most recent Cochrane review of exercise in PD supports the efficacy of exercise in measures of mobility and disease severity but not as measured by Participation scales such as the PDQ-39.23 At the very least, these results suggest that exercise, when delivered in a rigidly defined confines of a clinical trial may not be sufficient to reliably impact the health status of persons with PD.
Limitations and Conclusions
Given the limitations of this study, the results should be interpreted with caution. First, although this was the largest study to date of eccentric muscle training in PD, the sample size was relatively small. In addition, the inclusion of an Active Control group resulted in a minimization of any between group effects. Future research should utilize larger, more varied samples, maximize between group effects, and include outcomes across the disability domains.
Despite these limitations, twelve weeks of exercise, regardless of the type, produced muscle force and mobility improvements. In addition, the combined effects of exercise and dopamine replacement appeared to be complementary. Such results emphasize the need for optimization of pharmacologic and rehabilitative care to minimize disability in persons with PD.
Supplementary Material
List of Supplemental Digital Content
Supplemental Digital Content 1: Video Abstract JNPT-D-14-00027.mov
ACKNOWLEDGMENTS
This study was supported in part by the NIH / NCMRR (grant #: 1 R15 HD056478-01)
Appendix
| Exercise (2x/wk for 12 weeks) | Duration |
|---|---|
| Warm up exercises: Stationary bicycling or treadmill training following American College of Sports Medicine Guidelines. [ACSM]20,37 | 15 min |
| Flexibility Training: Axial mobility exercises19 | 5 min |
| Balance Training: Static base of support (BOS) stability training and dynamic activities requiring control of the center of mass within a moving (BOS).36 | 10 min |
| Upper extremity Concentric Resistance Training: Focus on Upper extremity extensors based on potential for differential impairment of extensors over flexors.34,35 | 5 min |
| Active Control Group: Lower extremity Concentric Ergometer Training (NuStep)33 RENEW Experimental Group: Lower extremity Eccentric Ergometer Training.11,32 |
15 min |
Footnotes
Portions of this data has been presented in abstract form at the following conferences: 2012 International Movement Disorders Congress, Dublin Ireland; 2012 International Society for Posture and Gait Research, Trondheim, Norway.
References
- 1.Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain. 2001 Nov;124(Pt 11):2131–2146. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- 2.Morris ME. Movement disorders in people with Parkinson disease: a model for physical therapy. Physical therapy. 2000 Jun;80(6):578–597. [PubMed] [Google Scholar]
- 3.Speelman AD, van de Warrenburg BP, van Nimwegen M, Petzinger GM, Munneke M, Bloem BR. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol. 2011 Sep;7(9):528–534. doi: 10.1038/nrneurol.2011.107. [DOI] [PubMed] [Google Scholar]
- 4.Allen NE, Canning CG, Sherrington C, Fung VS. Bradykinesia, muscle weakness and reduced muscle power in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2009 Jul 15;24(9):1344–1351. doi: 10.1002/mds.22609. [DOI] [PubMed] [Google Scholar]
- 5.Allen NE, Sherrington C, Canning CG, Fung VS. Reduced muscle power is associated with slower walking velocity and falls in people with Parkinson's disease. Parkinsonism & related disorders. 2010 May;16(4):261–264. doi: 10.1016/j.parkreldis.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson's disease patients. Neurorehabilitation and neural repair. 2009 Jul-Aug;23(6):600–608. doi: 10.1177/1545968308328726. [DOI] [PubMed] [Google Scholar]
- 7.Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson's disease: a meta-analysis of the effect of exercise and motor training. Mov Disord. 2011 Aug 1;26(9):1605–1615. doi: 10.1002/mds.23790. [DOI] [PubMed] [Google Scholar]
- 8.LaStayo PC, Pierotti DJ, Pifer J, Hoppeler H, Lindstedt SL. Eccentric ergometry: increases in locomotor muscle size and strength at low training intensities. Am J Physiol Regul Integr Comp Physiol. 2000 May;278(5):R1282–R1288. doi: 10.1152/ajpregu.2000.278.5.R1282. [DOI] [PubMed] [Google Scholar]
- 9.Jette AM. Toward a common language for function, disability, and health. Physical therapy. 2006 May;86(5):726–734. [PubMed] [Google Scholar]
- 10.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992 Mar;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dibble LE, Hale TF, Marcus RL, Droge J, Gerber JP, LaStayo PC. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson's disease. Mov Disord. 2006 Sep;21(9):1444–1452. doi: 10.1002/mds.20997. [DOI] [PubMed] [Google Scholar]
- 12.Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson's disease (CAPSIT-PD) Mov Disord. 1999 Jul;14(4):572–584. doi: 10.1002/1531-8257(199907)14:4<572::aid-mds1005>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Hunter S, White M, Thompson M. Techniques to evaluate elderly human muscle function: a physiological basis. J Gerontol A Biol Sci Med Sci. 1998 May;53(3):B204–B216. doi: 10.1093/gerona/53a.3.b204. [DOI] [PubMed] [Google Scholar]
- 14.Goetz C, Poewe W, Rascol O, Sampaio C, Stebbins GT. The Unified Parkinson's Disease Rating Scale (UPDRS): Status and Recommendations. Movement Disorders. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 15.Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, Internal Consistency, and Validity of Data Obtained With the Functional Gait Assessment. Physical therapy. 2004;84(10):906–918. [PubMed] [Google Scholar]
- 16.Brusse KJ, Zimdars S, Zalewski KR, Steffen TM. Testing functional performance in people with Parkinson disease. Physical therapy. 2005 Feb;85(2):134–141. [PubMed] [Google Scholar]
- 17.Fitzpatrick R, Jenkinson C, Peto V, Hyman N, Greenhall R. Desirable properties for instruments assessing quality of life: evidence from the PDQ-39. J Neurol Neurosurg Psychiatry. 1997 Jan;62(1):104. doi: 10.1136/jnnp.62.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dishman RK. Prescribing exercise intensity for healthy adults using perceived exertion. Med Sci Sports Exerc. 1994 Sep;26(9):1087–1094. [PubMed] [Google Scholar]
- 19.Schenkman M, Cutson TM, Kuchibhatla M, et al. Exercise to improve spinal flexibility and function for people with Parkinson's disease: a randomized, controlled trial. J Am Geriatr Soc. 1998 Oct;46(10):1207–1216. doi: 10.1111/j.1532-5415.1998.tb04535.x. [DOI] [PubMed] [Google Scholar]
- 20.Schenkman M, Hall DA, Baron AE, Schwartz RS, Mettler P, Kohrt WM. Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial. Physical therapy. 2012 Nov;92(11):1395–1410. doi: 10.2522/ptj.20110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lastayo P, Marcus RL, Dibble L, Frajacomo F, Lindstedt SL. Eccentric Exercise in Rehabilitation: Safety, Feasibility and Application. Journal of applied physiology. 2013 Jul 3; doi: 10.1152/japplphysiol.00008.2013. [DOI] [PubMed] [Google Scholar]
- 22.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. Journal of experimental psychology. General. 2012 Feb;141(1):2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 23.Tomlinson CL, Patel S, Meek C, et al. Physiotherapy intervention in Parkinson's disease: systematic review and meta-analysis. BMJ. 2012;345:e5004. doi: 10.1136/bmj.e5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corcos DM, Robichaud JA, David FJ, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2013 Mar 27; doi: 10.1002/mds.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dibble LE, Hale TF, Marcus RL, Gerber JP, LaStayo PC. High intensity eccentric resistance training decreases bradykinesia and improves Quality Of Life in persons with Parkinson's disease: a preliminary study. Parkinsonism & related disorders. 2009 Dec;15(10):752–757. doi: 10.1016/j.parkreldis.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA : the journal of the American Medical Association. 2014 Apr 23–30;311(16):1670–1683. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 27.Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006;36(2):133–149. doi: 10.2165/00007256-200636020-00004. [DOI] [PubMed] [Google Scholar]
- 28.Stevens-Lapsley J, Kluger BM, Schenkman M. Quadriceps muscle weakness, activation deficits, and fatigue with Parkinson disease. Neurorehabil Neural Repair. 2012 Jun;26(5):533–541. doi: 10.1177/1545968311425925. [DOI] [PubMed] [Google Scholar]
- 29.Hairi NN, Cumming RG, Naganathan V, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010 Nov;58(11):2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 30.Ivey FM, Tracy BL, Lemmer JT, et al. Effects of strength training and detraining on muscle quality: age and gender comparisons. J Gerontol A Biol Sci Med Sci. 2000 Mar;55(3):B152–B157. doi: 10.1093/gerona/55.3.b152. discussion B158-159. [DOI] [PubMed] [Google Scholar]
- 31.Hackney ME, Earhart GM. Health-related quality of life and alternative forms of exercise in Parkinson disease. Parkinsonism Relat Disord. 2009 Nov;15(9):644–648. doi: 10.1016/j.parkreldis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.David FJ, Rafferty MR, Robichaud JA, et al. Progressive resistance exercise and Parkinson's disease: a review of potential mechanisms. Parkinson's disease. 2012;2012:124527. doi: 10.1155/2012/124527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridgel AL, Peacock CA, Fickes EJ, Kim CH. Active-assisted cycling improves tremor and bradykinesia in Parkinson's disease. Archives of physical medicine and rehabilitation. 2012 Nov;93(11):2049–2054. doi: 10.1016/j.apmr.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Hass CJ, Collins MA, Juncos JL. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: a randomized trial. Neurorehabil Neural Repair. 2007 Mar-Apr;21(2):107–115. doi: 10.1177/1545968306293449. [DOI] [PubMed] [Google Scholar]
- 35.Robichaud JA, Pfann KD, Vaillancourt DE, Comella CL, Corcos DM. Force control and disease severity in Parkinson's disease. Mov Disord. 2005 Apr;20(4):441–450. doi: 10.1002/mds.20350. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL. An exercise intervention to prevent falls in people with Parkinson's disease: a pragmatic randomised controlled trial. J Neurol Neurosurg Psychiatry. 2011 Nov;82(11):1232–1238. doi: 10.1136/jnnp-2011-300919. [DOI] [PubMed] [Google Scholar]
- 37.Roitman JL, LaFontaine T. The Exercise Professional's Guide to Optimizing Health : Strategies for Preventing and Reducing Chronic Disease. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of Supplemental Digital Content
Supplemental Digital Content 1: Video Abstract JNPT-D-14-00027.mov