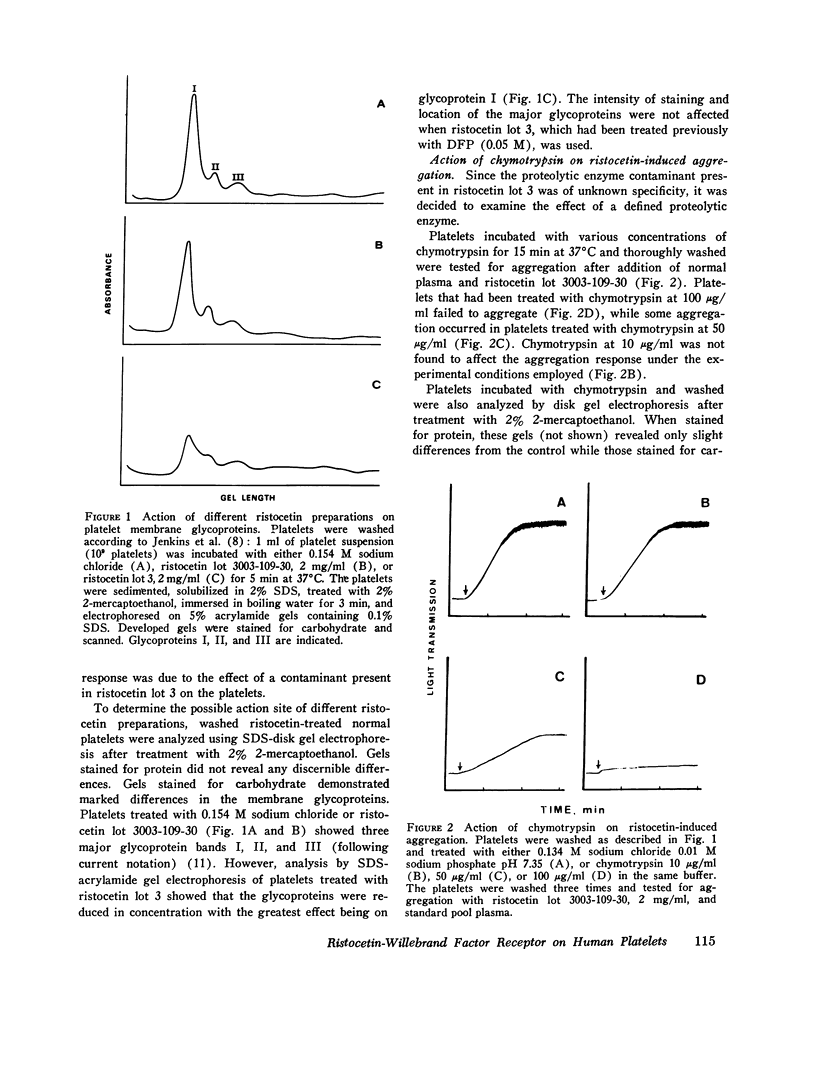
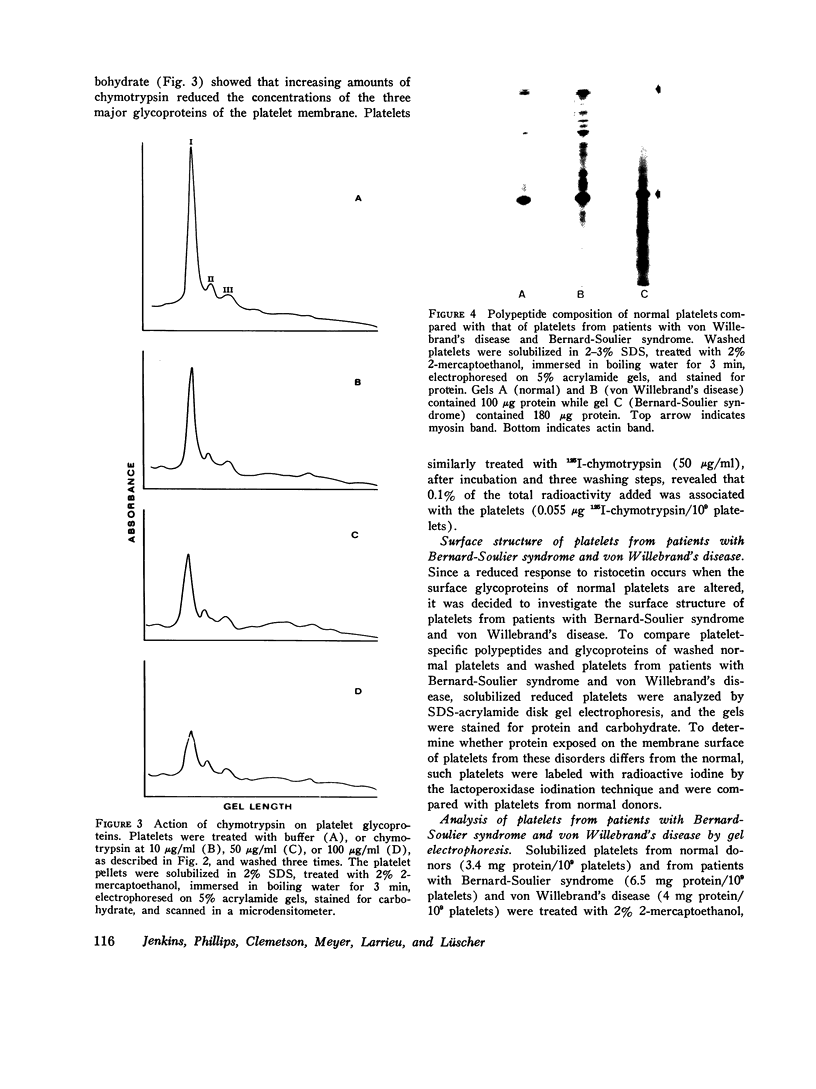
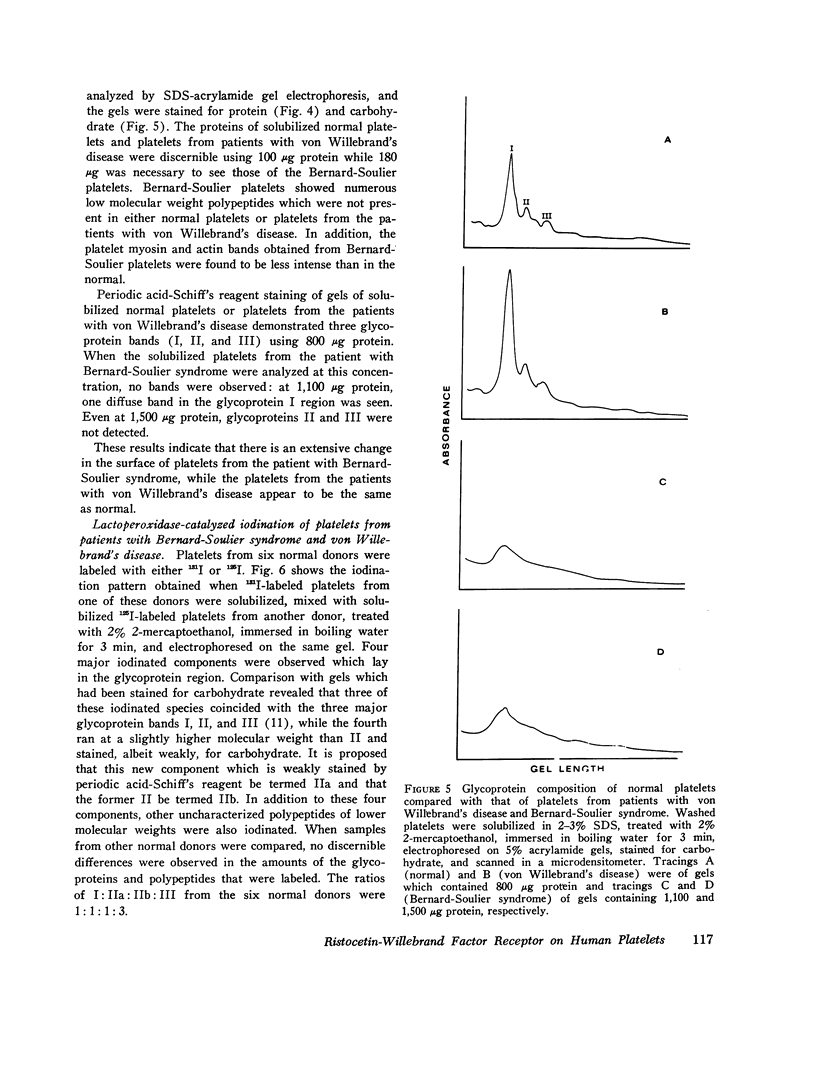
Abstract
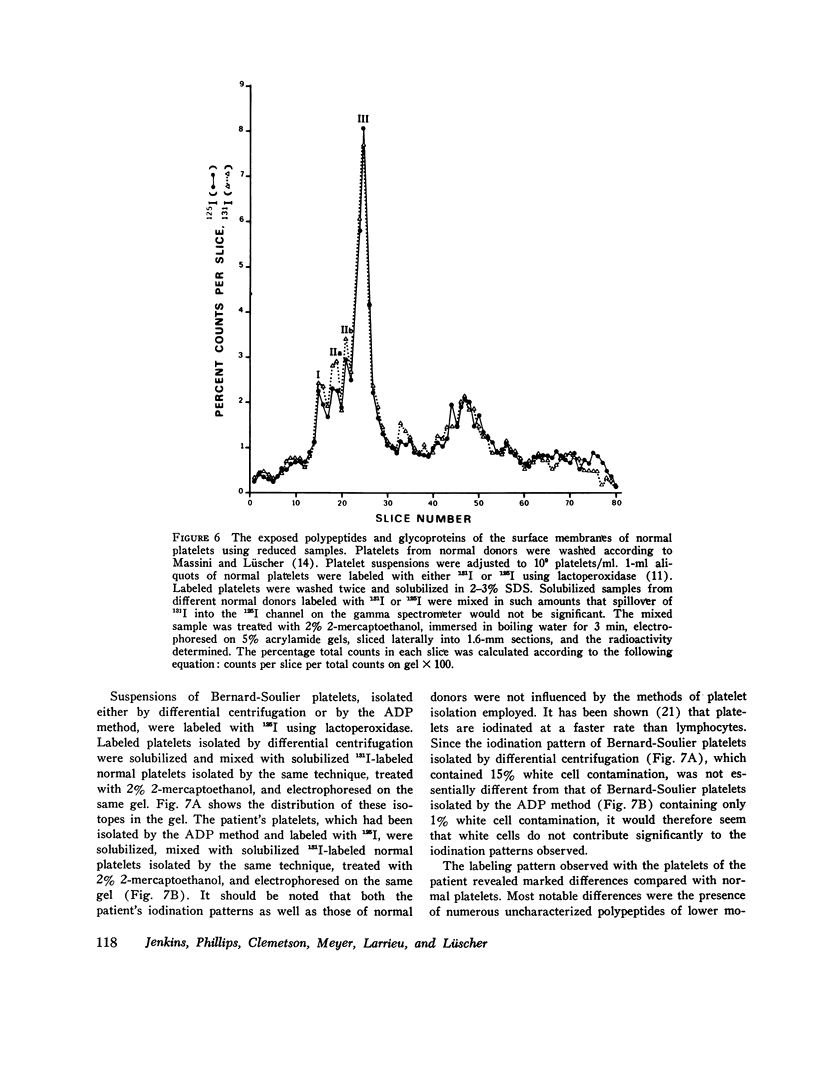
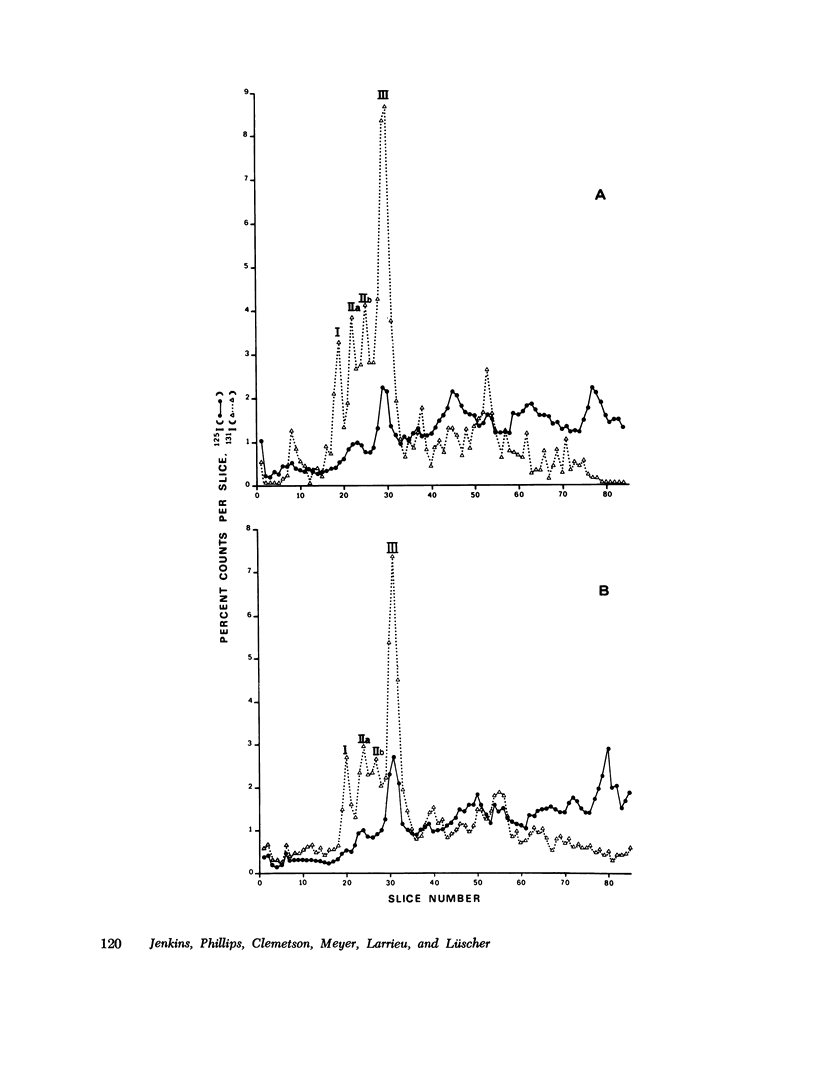
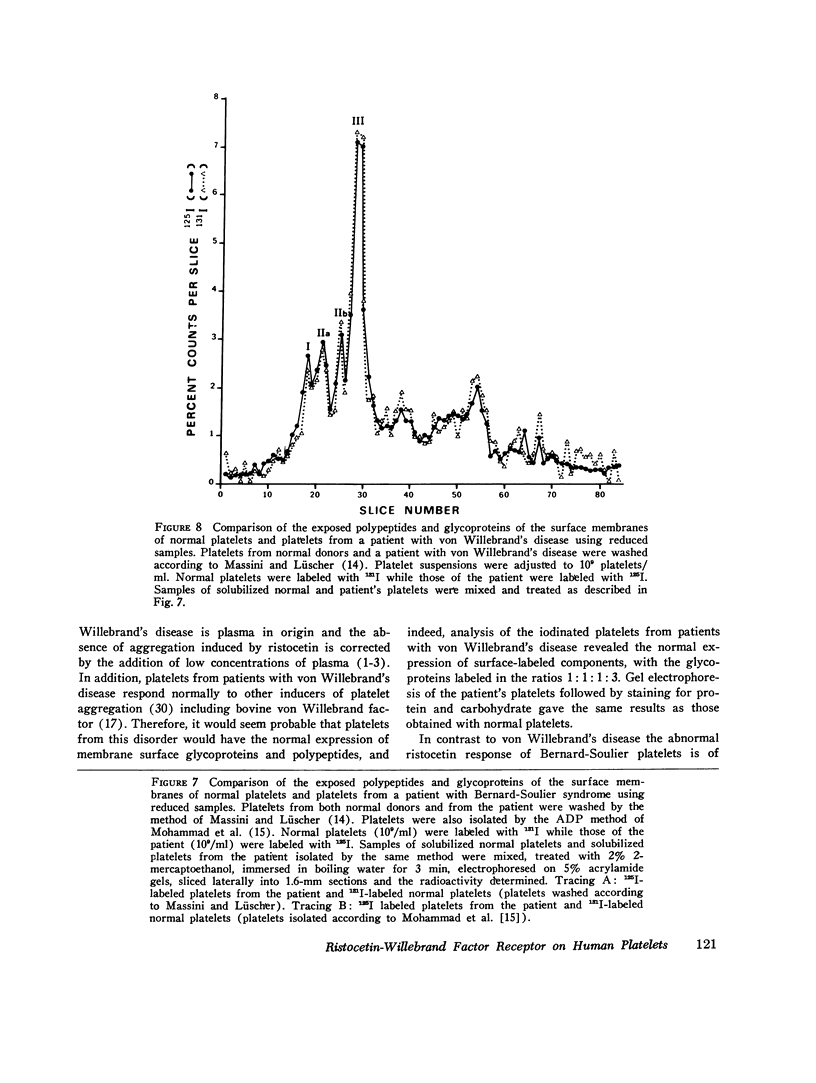
The antibiotic ristocetin only aggregates platelets in the presence of plasma von Willebrand factor. Platelets from patients with Bernard-Soulier syndrome do not aggregate upon addition of ristocetin although, in contrast to von Willebrand's disease, plasma levels of factor VIII complex (factor VIII clotting activity, von Willebrand factor activity, and von Willebrand antigen) are normal. The membrane surface of normal platelets was modified and compared to the surface of platelets from a patient with Bernard-Soulier syndrome in an attempt to identify the receptor involved in von Willebrand factor-ristocetin-induced aggregation. After the incubation of washed normal platelets with a preparation of ristocetin previously shown to contain a proteolytic contaminant, the aggregation response is significantly decreased on addition or normal plasma. Analaysis by gel electrophoresis of such platelets when stained for carbohydrate revealed a decrease in the relative amounts of membrane glycopro-eins. Chymotrypsin-treated normal platelets had less membrane glycoproteins in addition to giving a reduced aggregation response in ristocetin-induced aggregation. Staining of gels for protein and carbohydrate indicated that there was an extensive change in the surface of Bernard-Soulier platelets, whereas those from patients with von Willebrand's disease appeared the same as normal. Platelets from patients were labeled by the lactoperoxidase iodination technique. Not only was the relative intensity of staining of platelet-specific proteins and glycoproteins changed in Bernard-Soulier platelets, but the iodination of the glycoproteins on the membrane surface relative to other membrane constituents was lower. In contrast, platelets from patients with von Willebrand's disease showed a normal exposure of membrane components. These data suggest therefore that membrane glycoproteins may play a functional role in ristocetin-induced aggregation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALAGILLE D., JOSSO F., BINET J. L., BLIN M. L. LA DYSTROPHIE THROMBOCYTAIRE H'EMORRAGIPARE. DISCUSSION NOSOLOGIQUE. Nouv Rev Fr Hematol. 1964 Nov-Dec;4:755–790. [PubMed] [Google Scholar]
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. I. METHODS. J Clin Invest. 1964 May;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain J. P., Cooper H. A., Wagner R. H., Brinkhous K. M. Platelets fixed with paraformaldehyde: a new reagent for assay of von Willebrand factor and platelet aggregating factor. J Lab Clin Med. 1975 Feb;85(2):318–328. [PubMed] [Google Scholar]
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Beroza M., Muschik G. M., Gentry C. R. Small proportion of opposite geometric isomer increases potency of synthetic pheromone of oriental fruit moth. Nat New Biol. 1973 Aug 1;244(135):149–150. doi: 10.1038/newbio244149a0. [DOI] [PubMed] [Google Scholar]
- Davey M. G., Lüscher E. F. Actions of thrombin and other coagulant and proteolytic enzymes on blood platelets. Nature. 1967 Dec 2;216(5118):857–858. doi: 10.1038/216857a0. [DOI] [PubMed] [Google Scholar]
- Gates R. E., Phillips D. R., Morrison M. The distinguishing characteristics of the plasma membrane are its exposed proteins. Biochem J. 1975 May;147(2):373–376. doi: 10.1042/bj1470373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. H., Jamieson G. A. The effects of various lectins on platelet aggregation and release. Biochim Biophys Acta. 1974 Apr 29;345(2):231–242. doi: 10.1016/0005-2736(74)90261-2. [DOI] [PubMed] [Google Scholar]
- Gröttum K. A., Solum N. O. Congenital thrombocytopenia with giant platelets: a defect in the platelet membrane. Br J Haematol. 1969 Mar;16(3):277–290. doi: 10.1111/j.1365-2141.1969.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Howard M. A., Firkin B. G. Ristocetin--a new tool in the investigation of platelet aggregation. Thromb Diath Haemorrh. 1971 Oct 31;26(2):362–369. [PubMed] [Google Scholar]
- Howard M. A., Hutton R. A., Hardisty R. M. Hereditary giant platelet syndrome: a disorder of a new aspect of platelet function. Br Med J. 1973 Jun 9;2(5866):586–588. doi: 10.1136/bmj.2.5866.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber C. T., Morrison M. Heterogeneity of the outer membrane of mitochondria. Biochemistry. 1973 Oct 9;12(21):4274–4282. doi: 10.1021/bi00745a036. [DOI] [PubMed] [Google Scholar]
- Jenkins C. S., Meyer D., Dreyfus M. D., Larrieu M. J. Willebrand factor and ristocetin. I. Mechanism of rustocetin-induced platelet aggregation. Br J Haematol. 1974 Dec;28(4):561–578. doi: 10.1111/j.1365-2141.1974.tb06675.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORRISON M., HULTQUIST D. E. LACTOPEROXIDASE. II. ISOLATION. J Biol Chem. 1963 Aug;238:2843–2849. [PubMed] [Google Scholar]
- Majerus P. W., Brodie G. N. The binding of phytohemagglutinins to human platelet plasma membranes. J Biol Chem. 1972 Jul 10;247(13):4253–4257. [PubMed] [Google Scholar]
- Massini P., Lüscher E. F. Some effects of ionophores for divalent cations on blood platelets. Comparison with the effects of thrombin. Biochim Biophys Acta. 1974 Nov 4;372(1):109–121. doi: 10.1016/0304-4165(74)90077-4. [DOI] [PubMed] [Google Scholar]
- Meyer D., Jenkins C. S., Dreyfus M. D., Fressinaud E., Larrieu M. J. Willebrand factor and ristocetin. II. Relationship between Willebrand factor, Willebrand antigen and factor VIII activity. Br J Haematol. 1974 Dec;28(4):579–599. doi: 10.1111/j.1365-2141.1974.tb06676.x. [DOI] [PubMed] [Google Scholar]
- Meyer D., Jenkins C. S., Dreyfus M., Larrieu M. J. Letter: Experimental model for von Willebrand's disease. Nature. 1973 Jun 1;243(5405):293–294. doi: 10.1038/243293a0. [DOI] [PubMed] [Google Scholar]
- Mohammad S. F., Reddick R. L., Mason R. G. Characterization of human platelets separated from blood by ADP-induced aggregation. Am J Pathol. 1975 Apr;79(1):81–94. [PMC free article] [PubMed] [Google Scholar]
- Nachman R. L., Ferris B. Studies on the proteins of human platelet membranes. J Biol Chem. 1972 Jul 25;247(14):4468–4475. [PubMed] [Google Scholar]
- Nicolson G. L. Temperature-dependent mobility of concanavalin A sites on tumour cell surfaces. Nat New Biol. 1973 Jun 13;243(128):218–220. doi: 10.1038/newbio243218a0. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. An abnormal platelet glycoprotein pattern in three cases of Glanzmann's thrombasthenia. Br J Haematol. 1974 Oct;28(2):253–260. doi: 10.1111/j.1365-2141.1974.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. Specific roles for platelet surface glycoproteins in platelet function. Nature. 1975 Jun 26;255(5511):720–722. doi: 10.1038/255720a0. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Thrombin substrates and the proteolytic site of thrombin action on human-platelet plasma membranes. Biochim Biophys Acta. 1974 Jun 13;352(2):218–227. doi: 10.1016/0005-2736(74)90213-2. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Thrombin-induced alterations in the surface structure of the human platelet plasma membrane. Ser Haematol. 1973;6(3):292–310. [PubMed] [Google Scholar]
- Phillips D. R. Effect of trypsin on the exposed polypeptides and glycoproteins in the human platelet membrane. Biochemistry. 1972 Nov 21;11(24):4582–4588. doi: 10.1021/bi00774a025. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jenkins C. S., Lüscher E. F., Larrieu M. Molecular differences of exposed surface proteins on thrombasthenic platelet plasma membranes. Nature. 1975 Oct 16;257(5527):599–600. doi: 10.1038/257599a0. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp T. B., Weiss H. J., Baumgartner H. R. Decreased adhesion of platelets to subendothelium in von Willebrand's disease. J Lab Clin Med. 1974 Feb;83(2):296–300. [PubMed] [Google Scholar]
- Walsh P. N., Mills D. C., Pareti F. I., Stewart G. J., Macfarlane D. E., Johnson M. M., Egan J. J. Hereditary giant platelet syndrome. Absence of collagen-induced coagulant activity and deficiency of factor-XI binding to platelets. Br J Haematol. 1975 Apr;29(4):639–655. doi: 10.1111/j.1365-2141.1975.tb02750.x. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Rogers J., Brand H. Defective ristocetin-induced platelet aggregation in von Willebrand's disease and its correction by factor VIII. J Clin Invest. 1973 Nov;52(11):2697–2707. doi: 10.1172/JCI107464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. J., Tschopp T. B., Baumgartner H. R., Sussman I. I., Johnson M. M., Egan J. J. Decreased adhesion of giant (Bernard-Soulier) platelets to subendothelium. Further implications on the role of the von Willebrand factor in hemostasis. Am J Med. 1974 Dec;57(6):920–925. doi: 10.1016/0002-9343(74)90170-3. [DOI] [PubMed] [Google Scholar]
- Weiss H. J. Von Willebrand's disease--diagnostic criteria. Blood. 1968 Oct;32(4):668–679. [PubMed] [Google Scholar]