Abstract
The objective of this study was to identify frequency and predictors of gaps in care in a longitudinal cohort of HIV-infected patients in urban New England. We conducted a retrospective cohort study in Providence, RI of 581 newly diagnosed HIV-patients >18 entering into care from 2004-2010 and followed their care through the end of 2011. The outcome of interest was gaps in care, defined as an interruption of medical care for > 6 months. Time to the first gap was characterized using Kaplan-Meier (KM) curves. Anderson-Gill proportional hazards (AGPH) model was used to identify the risk factors of recurrent gaps in care. During the study period, 368 patients (63%) experienced at least 1 gap in care, 178 (30%) had ≥ 2 gaps, 84 (14.5%) had ≥ 3 gaps, and 21 (3.6%) died;77% of gaps were followed by a re-linkage with care The KM curves estimate that one quarter of patients (95%CI=22-29%) would experience ≥ 1 gap in care by year one; nearly one-half (CI=45-54%) by year two; and 90% (CI=93-96%) by year eight;. A prior gap was a strong predictor (HR=2.36; CI=2.16-2.58) of subsequent gaps; other predictors included age <25 (HR=1.29; CI=1.04-1.60), and no prescription of ART in first year of care (HR=1.23; CI=1.01-1.50). The results of this study suggest that a significant proportion of newly diagnosed HIV-infected patients will experience multiple gaps in care and yet re-engagement is possible. Interventions should focus on both prevention of gaps as well as re-engaging those lost to follow-up.
Keywords: HIV, retention in care, treatment adherence, gaps
Background
Over the past 15 years, potent, well-tolerated antiretroviral therapy (ART) has transformed HIV into a manageable chronic illness in the United States (Bhaskaran et al., 2008). As with other chronic diseases, favorable outcomes are dependent on adequate access to and consistent engagement with medical care (Swendeman, Ingram, & Rotheram-Borus, 2009). HIV infected individuals who are poorly adherent to HIV medical care are less likely to receive ART, have lower adherence to ART, increased virological failure, and poorer health outcomes (Giordano et al., 2007; Mugavero et al., 2009). Maintaining continuity in HIV care is a significant public health issue as adherence to ART markedly decreases HIV transmission (Cohen et al., 2011). The U.S. Centers for Disease Control and Prevention (CDC) estimates that almost half of all HIV-infected individuals in the U.S. are not in regular care, and less than one-third have an undetectable viral load (“Vital signs: HIV prevention through care and treatment--United States,” 2011). The significance of this issue is highlighted by the emphasis placed on maximizing retention in care in the U.S. National HIV/AIDS Strategy and by new guidelines from the Department of Health and Human Services advocating universal access to ART as part of a treatment as prevention approach to impacting the HIV epidemic (DHHS, 2013).
Improving retention with HIV care requires a detailed understanding of the longitudinal course of engagement with outpatient care. The transition process from HIV diagnosis to retention comprises several steps from diagnosis to full engagement with HIV treatment, and the treatment cascade is not unidirectional (Malitz & Eldred, 2007). Thus, identifying those most at risk for loss to follow-up (LTFU), and importantly, multiple gaps in care, can considerably impact the development of interventions at clinic and community levels.
Studies conducted in a wide variety of settings in the US have identified individual predictors of poor retention in HIV care including younger age, minority status, poverty, substance use, and incarceration (Catz, McClure, Jones, & Brantley, 1999; Giordano et al., 2003; Kerr et al., 2005; Olatosi, Probst, Stoskopf, Martin, & Duffus, 2009). Yet, most of these studies were based on cross-sectional or fixed panel (e.g. every 6 months) data and did not account for the fact that retention is a dynamic process and clinical visits for care are irregular in time. A better understanding of these factors and their impact on the time-dependent risk of disengagement in HIV care is necessary. We therefore studied engagement with care, gaps in care, and re-engagement over an eight-year period at the care site where the majority of patients in Rhode Island receive their HIV care. An advantage of this care setting is that there is less geographic mobility in this population than in others in the US (U.S. Census Bureau, 2011). Using retrospective data from a center database, we want to address two scientific questions: 1) What were the rates of having gap(s) in HIV care and re-engagements? and 2) What factors predicted gaps in HIV care?
Methods
Participants
The Miriam Hospital Immunology Center is an urban clinic that provides care for 1,600 HIV infected patients in Rhode Island. In this analysis, we considered all patients presenting for care within one year of their HIV diagnosis and registered into the Immunology Center Database (ICDB) between January 1, 2004-December 31, 2010 (N = 581), and included their follow-up visit data until December 31, 2011. The ICDB is a relational database created in 2003 on a Structured Query Language server maintained by the Lifespan/Miriam Hospital Information Systems department. It contains socio-demographics, detailed clinic visits, and medical data. All study procedures were approved by The Miriam Hospital Institutional Review Board.
Variables
Multiple measures of retention in HIV care have been proposed including missed visits, gaps in care, visit constancy, and the HRSA HIV/AIDS Bureau annual measure (at least 2 kept visits separated by ≥ 90 days), with no single measure outperforming others in relationship to clinical outcomes (Mugavero, Westfall, et al., 2012). Given our primary interest in identifying individuals with inconsistent engagement with longitudinal HIV care, we focused our analysis on characterizing patients who would experience at least one gap in care, defined as an interruption of HIV medical care for >6 months, and contextualizing the factors that may inform those gaps.
Covariates included gender, age, race, ethnicity, education level, birth origin (U.S. versus other), clinic registration year, HIV risk category, CD4+ T cell count and AIDS diagnosis at presentation to care, prescription of ART within 1 year of care entry, and history of: mental illness, substance use, incarceration, and housing instability. Age, race, education, registration date, HIV risk category and CD4 count were coded as categorical variables; all other variables were coded as binary variables. Gender, age, race, ethnicity, education level, birth origin, and history of mental illness, substance use, housing, were based on self-report at initial intake.
The primary outcome is time to recurrent gaps in care. HIV Plasma viral load (PVL) and experience of prior gap(s) in HIV medical care were included in the regression analyses as time-dependent variables.
Analyses
The characteristics of study population were summarized by tabulating their baseline (at registration) demographic, clinical, and behavioral covariates.
Time to the primary endpoint was measured from registration date to the occurrence(s) of having a gap (>6 months) between medical visits. The primary endpoint can be right-censored (i.e. only known to occur later than the last observation time) due to reasons such as “moving out of the state”, “transfer to other HIV clinic”, “incarceration”, or the end of study period (December 31, 2011). Death is first considered as a censoring event, not as a primary endpoint. We also tested models in which death was considered as one of the primary endpoints, i.e. as a terminal loss-to-follow-up. This approach reflects that both gaps and deaths are undesired endpoints, and of clinical interest.
In order to account for the variable length of follow up of patients, we used time-to-event (survival) analysis methods. Distribution of time to the first gap was graphically summarized using the Kaplan-Meier survival curves. The Anderson-Gill proportional hazards (AGPH) model was used to quantify the effect of risk factors on retention in HIV care (Andersen & Gill, 1982). Univariable AGPH regressions were used to examine the predictive value of each factor individually. Important factors (p-value <0.10 in univariable analyses) were then included in a multivariable AGPH model.
Early engagement in HIV care within one year of diagnosis has been shown to be predictive of clinical outcomes and is a particular focus of nationwide outreach campaigns (Mugavero, Amico, et al., 2012; Tripathi, Youmans, Gibson, & Duffus, 2011). As an exploratory analysis, we further conducted a cross-sectional analysis restricting the follow-up period to the first year of entry to care (Table 3). We compared the baseline demographic and health characteristics between those patients who had a gap in care in their first year and those who did not, using Pearson's Chi-square test or Fisher's exact test (if any cell of the contingency table has a count of ≤5) (Agresti, 2002).
Table 3. Cox regression analysis of predictors of Gaps in Care (Death as a censoring event).
| Univariable Cox Model | Multivariable Cox Model | ||||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) |
P value | Hazard Ratio (95% CI) |
P value | ||
| Gender | Female (REF) | ||||
| Male | 1.10 (0.90--1.33) | 0.34 | --- | --- | |
| Age (years) | > 45 (REF) | ||||
| 35-45 | 1.23 (0.95--1.59) | 0.11 | 1.05 (0.88--1.24) | 0.61 | |
| 25-35 | 1.48 (1.14--1.92) | <.01 | 1.14 (0.95--1.37) | 0.16 | |
| < 25 | 2.29 (1.73--3.03) | <.01 | 1.35 (1.09--1.68) | <0.01 | |
| Registration Yr | 2004 (REF) | ||||
| 2005 | 0.73 (0.53--1.00) | 0.05 | 1.11 (0.88--1.40) | 0.36 | |
| 2006 | 0.80 (0.61--1.05) | 0.10 | 1.01 (0.83--1.22) | 0.96 | |
| 2007 | 0.84 (0.61--1.16) | 0.30 | 1.02 (0.80--1.31) | 0.85 | |
| 2008 | 0.91 (0.66--1.25) | 0.56 | 0.99 (0.77--1.27) | 0.95 | |
| 2009 | 0.99 (0.74--1.33) | 0.96 | 1.14 (0.91--1.41) | 0.26 | |
| 2010 | 0.70 (0.43--1.13) | 0.14 | 0.93 (0.65--1.35) | 0.72 | |
| Hispanic | Yes (REF) | ||||
| No | 1.39 (1.09--1.76) | 0.01 | 0.99 (0.85--1.17) | 0.94 | |
| Race | White (REF) | ||||
| Black | 1.18 (0.98--1.43) | 0.08 | 1.10 (0.94--1.28) | 0.25 | |
| Others | 1.12 (0.75--1.66) | 0.58 | 1.38 (1.04--1.82) | 0.03 | |
| Education | < 12 years (REF) | ||||
| High school grad | 0.76 (0.58--0.98) | 0.04 | 0.96 (0.80--1.15) | 0.67 | |
| Some college | 1.12 (0.90--1.41) | 0.32 | 1.04 (0.87--1.26) | 0.66 | |
| College grad | 0.96 (0.73--1.26) | 0.78 | 0.98 (0.79--1.21) | 0.87 | |
| Origin in US | No (REF) | ||||
| Yes | 1.14 (0.95--1.37) | 0.16 | --- | --- | |
| AIDS status | Yes (REF) | ||||
| No | 1.47 (1.23--1.76) | <.01 | 1.03 (0.85--1.26) | 0.74 | |
| Substance abuse | No (REF) | ||||
| Yes | 1.23 (1.02--1.47) | 0.03 | 0.95 (0.82--1.11) | 0.54 | |
| Homeless | No (REF) | ||||
| Yes | 1.29 (1.05--1.58) | 0.02 | 1.03 (0.87--1.21) | 0.75 | |
| Incarceration | No (REF) | ||||
| Yes | 1.62 (1.18--2.23) | <.01 | 1.31 (0.98--1.75) | 0.07 | |
| Psychiatric Illness | No (REF) | ||||
| Yes | 1.02 (0.84--1.25) | 0.84 | --- | --- | |
| CD4 (/mL) | < 200 (REF) | ||||
| 200-500 | 1.44 (1.14--1.82) | <.01 | 0.97 (0.79--1.20) | 0.80 | |
| >500 | 1.71 (1.34--2.18) | <.01 | 1.02 (0.80--1.31) | 0.88 | |
| ART in Year 1 | Yes (REF) | ||||
| No | 1.42 (1.18--1.71) | <.01 | 1.21 (1.01--1.58) | 0.05 | |
| MSM | No (REF) | ||||
| Yes | 1.06 (0.88--1.27) | 0.54 | --- | --- | |
| IDU | No (REF) | ||||
| Yes | 1.07 (0.66--1.74) | 0.79 | --- | --- | |
| PVL* | Undetectable (RFE) | ||||
| Detectable | 1.47 (1.24--1.74) | <.01 | 1.01 (0.86--1.20) | 0.87 | |
| Unavailable | 9.51 (3.88--23.3) | <.01 | 1.67 (0.20--14.2) | 0.64 | |
| Prior Gap | No (REF) | ||||
| Yes | 2.49 (2.26--2.74) | <.01 | 2.41 (2.20--2.64) | 0.00 | |
A level of significance of <0.05 was used throughout this paper. All analyses were carried out using Stata (Version 12.1, College Station, TX 77845).
Results
Participant Characteristics
Demographic and health characteristics of the 581 individuals are summarized in Table 1. The study samples are comparable to the overall clinic population at The Miriam Hospital Immunology Center (Lifespan/Tufts/Brown Center for AIDS Research, 2013). The majority were male (71%), white (62%), non-Hispanic (76%), U.S. born (58%), and had a high-school or higher education (64%). The median age at entry to care was 38 years (IQR = 35-45), 46% were classified as AIDS at presentation, with 60% of all patients prescribed ART during the first year of entry into care. Risk factors for HIV included men who have sex with men (MSM, 47%), unprotected heterosexual sex (47%), and intravenous drug use (4%).
Table 1.
Baseline demographic, clinical, and behavioral characteristics of patients at entry to care.
| N = 581 | No. | % | |
|---|---|---|---|
| Gender | Female | 169 | 29 |
| Male | 412 | 71 | |
| Age at entry to care* | <25 | 65 | 11 |
| 25-35 | 144 | 25 | |
| 35-45 | 206 | 36 | |
| > 45 | 166 | 29 | |
| Ethnicity | Hispanic | 139 | 24 |
| Non-Hispanic | 442 | 76 | |
| Race | White | 361 | 62 |
| Black | 184 | 32 | |
| Others | 35 | 6 | |
| NA | 1 | 0 | |
| Education* | < High School | 184 | 32 |
| High school graduate | 118 | 20 | |
| Some college | 136 | 23 | |
| College Graduate | 118 | 20 | |
| NA | 25 | 4 | |
| Origin of Birth | Foreign Born | 243 | 42 |
| US Born | 338 | 58 | |
| Year of Entry to Care | 2004 | 110 | 19 |
| 2005 | 61 | 11 | |
| 2006 | 82 | 14 | |
| 2007 | 72 | 12 | |
| 2008 | 87 | 15 | |
| 2009 | 99 | 17 | |
| 2010 | 70 | 12 | |
| HIV Risk Factor | MSM | 262 | 45 |
| IVDU | 12 | 2 | |
| MSM/IVDU | 9 | 2 | |
| Heterosexual | 272 | 47 | |
| Other/Unknown | 26 | 4 | |
| MSM | No | 309 | 53 |
| Yes | 272 | 47 | |
| History of IV Drug Use | No | 560 | 96 |
| Yes | 21 | 4 | |
| HIV status at entry | HIV | 316 | 54 |
| AIDS | 265 | 46 | |
| CD4 count* | < 200 | 179 | 31 |
| 200-500 | 221 | 38 | |
| > 500 | 176 | 30 | |
| NA | 5 | 1 | |
| ART status in Year 1 | No | 207 | 36 |
| Yes | 348 | 60 | |
| NA | 26 | 4 | |
| Substance Abuse | Ever | 246 | 42 |
| Never | 325 | 56 | |
| NA | 10 | 2 | |
| Smoker | Ever | 227 | 39 |
| Never | 335 | 58 | |
| NA | 19 | 3 | |
| Homeless* | Yes | 125 | 22 |
| No | 452 | 78 | |
| NA | 4 | 1 | |
| Incarceration | Ever | 40 | 7 |
| Never | 521 | 90 | |
| NA | 20 | 3 | |
| Psychiatric illness | Ever | 172 | 30 |
| Never | 389 | 67 | |
| NA | 20 | 3 | |
Percentages may not add up to 100 due to rounding.
In this cohort, 368 patients (63%) experienced at least one gap of > 6 months in care, 178 (30%) had ≥ 2 gaps, 84 (14.5%) had ≥ 3 gaps in care, and 21 (3.6%) died over their follow-up period (Table 2). Seventy-seven percent (77%) of gaps were followed by a subsequent visit in the clinic while the rest (23%) were considered completely LTFU. Among those experiencing at least one gap, the length of gap had a median of 325 days and an interquartile range (IQR) of 231∼366 days. Of note, these estimates do not account for differential follow-up times of the study population.
Table 2. Frequency and Quantity of Gaps in Care >6 months.
| Number of Gaps >6 months | 0 | 1 | 2 | 3 | 4 | >4 | (death) |
| Number of patients | 213 | 190 | 94 | 43 | 23 | 18 | 21 |
| % | 0.37 | 0.33 | 0.16 | 0.074 | 0.04 | 0.031 | 0.036 |
Analyses of Gaps in Care
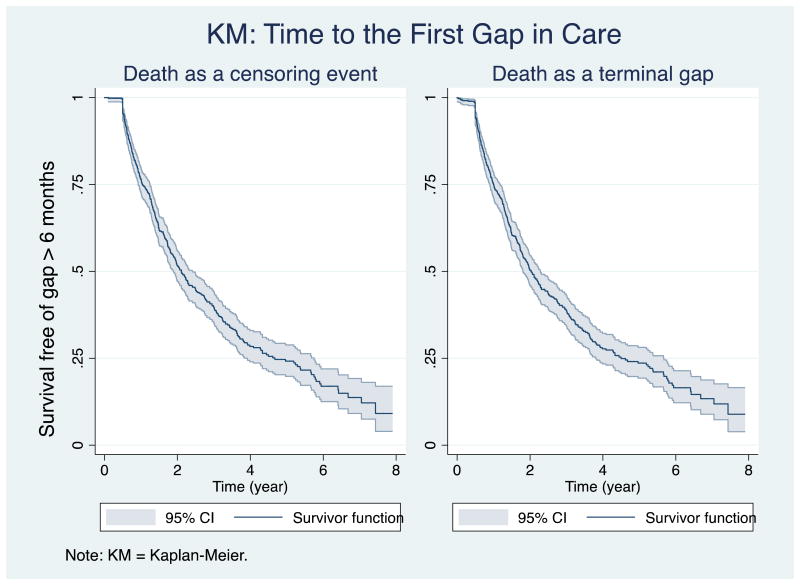
The Kaplan-Meier curve (Figure 1, left panel) looking at time to the first gap estimates that of all patients, 24% (95% Confidence Interval (CI) = 20-27%) experienced the first gap (in other words, at least one gap) in the first year of care; nearly one-half (48%, 95% CI=44-53%) by year two; and 90% (95% CI=83-96%) by their eighth year of follow-up, if death is not counted as a gap. When including death as a primary endpoint of interest, the curve (Figure 1, right panel) is almost identical.
Figure 1.
Treating death as a censoring event, the univariable AGPH regression analyses (Table 3, left panel) show that age <35 (HR=1.48-2.29), non-hispanic ethnicity (HR=1.39; CI=1.09-1.767), non-AIDS at presentation (HR=1.47; CI=1.23-1.76), substance use (HR=1.23; CI=1.02-1.47), homelessness (HR=1.29; CI=1.05-1.58), history of incarceration (HR=1.62; CI=1.18-2.23), CD4 count > 200 (HR=1.44-1.71), no ART prescription during the first year of care (HR=1.42; CI=1.18-1.71), last PVL detectable or unavailable (HR=1.47-9.51), and having a prior gap (HR=2.49; CI=2.26-2.74) were associated with a higher risk of experiencing gaps in care. Registration in Year 2005 (HR=0.73; CI=0.53 -1.0) and ≥ high school education (HR=0.76; CI=0.58-0.98) were associated with a decreased likelihood of experiencing a gap in care. Univariable AGPH analyses treating death as an outcome yield mostly the same results (not shown).
Multivariable AGPH analysis using death as a censoring event (Table 3, right panel) shows that age younger than 25 (HR=1.35; CI=1.09-1.68), not prescribed ART during the first year of care (HR=1.21; CI=1.01-1.58), and a prior gap (HR=2.41; CI=2.20-2.64) were independently associated with a higher risk of experiencing gaps in care. Analyses using death as an outcome were similar (not shown).
Given the relatively high rate of gaps in care in year 1, we performed a cross sectional analysis of the first-year follow-up (Table 4). Younger age (<35), non AIDS presentation, ever-smoking, never having psychiatric illness, high CD4 count, and not on ART in the first year were associated with having a gap in care in the first year. The comparisons were not adjusted for death, incarcerations, or transfer of care.
Table 4. Comparison of Subjects with Gap in Year One vs No Gap in Year One.
| Having a gap in care in Year 1? | p-value | Death | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| No. | % | No. | % | No. | % | ||
| Gender | |||||||
| Female | 135 | 30% | 33 | 26% | 0.33 | 1 | 0.5 |
| Male | 315 | 70% | 96 | 74% | 1 | 0.5 | |
| Age at entry to care | |||||||
| less than 25 | 46 | 10% | 19 | 15% | 0.04 | 0 | 0 |
| 25-35 | 104 | 23% | 40 | 31% | 0 | 0 | |
| 35-45 | 162 | 36% | 44 | 34% | 0 | 0 | |
| greater than 45 | 138 | 31% | 26 | 20% | 2 | 1 | |
| Hispanic | |||||||
| Yes | 111 | 25% | 28 | 22% | 0.49 | 0 | 0 |
| No | 339 | 75% | 101 | 78% | 2 | 1 | |
| Race | |||||||
| White | 285 | 63% | 74 | 57% | 0.41 a | 2 | 1 |
| Black | 140 | 31% | 44 | 34% | 0 | 0 | |
| Others | 24 | 5% | 11 | 9% | 0 | 0 | |
| NA | 1 | 0% | 0 | 0% | 0 | 0 | |
| Education | |||||||
| less than 12 yrs | 144 | 32% | 40 | 31% | 0.86 a | 0 | 0 |
| High school graduate | 88 | 20% | 30 | 23% | 0 | 0 | |
| Some college | 103 | 23% | 31 | 24% | 2 | 1 | |
| College Graduate | 95 | 21% | 23 | 18% | 0 | 0 | |
| NA | 20 | 4% | 5 | 4% | 0 | 0 | |
| US Born | |||||||
| No | 190 | 42% | 53 | 41% | 0.82 | 0 | 0 |
| Yes | 260 | 58% | 76 | 59% | 2 | 1 | |
| Year of Entry to Care | |||||||
| 2004 | 82 | 18% | 27 | 21% | 0.65 | 1 | 0.5 |
| 2005 | 49 | 11% | 11 | 9% | 1 | 0.5 | |
| 2006 | 65 | 14% | 17 | 13% | 0 | 0 | |
| 2007 | 53 | 12% | 19 | 15% | 0 | 0 | |
| 2008 | 73 | 16% | 14 | 11% | 0 | 0 | |
| 2009 | 74 | 16% | 25 | 19% | 0 | 0 | |
| 2010 | 54 | 12% | 16 | 12% | 0 | 0 | |
| MSM | |||||||
| No | 240 | 53% | 68 | 53% | 0.90 | 1 | 0.5 |
| Yes | 210 | 47% | 61 | 47% | 1 | 0.5 | |
| IDU | |||||||
| No | 434 | 96% | 124 | 96% | 0.79 a | 2 | 1 |
| Yes | 16 | 4% | 5 | 4% | 0 | 0 | |
| HIV status at entry | |||||||
| HIV | 231 | 51% | 85 | 66% | <.01 | 0 | 0 |
| AIDS | 219 | 49% | 44 | 34% | 2 | 1 | |
| CD4 count | |||||||
| < 200 | 150 | 33% | 27 | 21% | <.01 a | 2 | 1 |
| 200-500 | 179 | 40% | 42 | 33% | 0 | 0 | |
| > 500 | 120 | 27% | 56 | 43% | 0 | 0 | |
| NA | 1 | 0% | 4 | 3% | 0 | 0 | |
| ART status in Year 1 | |||||||
| No | 147 | 33% | 59 | 46% | <.01 | 1 | 0.5 |
| Yes | 293 | 65% | 54 | 42% | 1 | 0.5 | |
| NA | 10 | 2% | 16 | 12% | 0 | 0 | |
| Incarceration | |||||||
| Ever | 29 | 6% | 11 | 9% | 0.58 a | 0 | 0 |
| Never | 404 | 90% | 115 | 89% | 2 | 1 | |
| NA | 17 | 4% | 3 | 2% | 0 | 0 | |
| Psychiatric illness | |||||||
| Ever | 139 | 31% | 32 | 25% | 0.04 a | 1 | 0.5 |
| Never | 292 | 65% | 96 | 74% | 1 | 0.5 | |
| NA | 19 | 4% | 1 | 1% | 0 | 0 | |
| Total | 450 | 129 | 2 | ||||
Note: All p-values are calculated using Pearson's Chi-square tests or Fisher's Exact tests (indexed by the superscript ‘a’).
Discussion
This study has two main findings. First, gaps in care are frequent among HIV-infected patients. In our study period, 63% of patients experienced at least one gap in care and had they all been followed up for eight years, it is estimated that 90% of patients would experience one or more gaps. Second, in multivariable models younger age, not being on ART in the first year of follow-up, and previous gaps were the only predictors of gaps in care.
Our findings on rates of gaps in care contrasts with a recent study evaluating longitudinal retention patterns in patients entering into the North American AIDS Cohort Collaboration (NA-ACCORD) from 2000-2008 where 75% of individuals were consistently retained in HIV care (Rebeiro et al., 2013). However, NA-ACCORD only included individuals who completed at least two clinical visits in the prior 12 months, thus potentially excluding individuals who disengage with care within the first year. A strength of our study is that we included new HIV diagnoses at the time of entry into medical care from 2004-2010. Approximately 24% of our patients experienced their first gap in care within the first year, and those who experienced one gap were more likely to experience subsequent gaps. Early engagement in HIV care within one year of diagnosis has been shown to be predictive of clinical outcomes and is a particular focus of nationwide outreach campaigns (Mugavero, Amico, et al., 2012; Tripathi et al., 2011). Also, NA-ACCORD defined LTFU as no laboratory data in ≥12 months whereas we used the definition of no clinic visit >6months. Laboratory results may not accurately reflect actual clinic visits (Mugavero, Davila, Nevin, & Giordano, 2010). This may explain the lower rates of continuous engagement in our study compared to the NA-ACCORD study.
The survival analysis evaluating time to first gap in care (Figure 1) displays a steep initial drop within the first 2 years of care that continues to decline over the entire follow-up period. This pattern suggests that over time, LTFU is virtually certain in the longitudinal care of HIV infected patients. However, patients may return to care after LTFU. Our data shows that re-engagement is possible with the majority (77%) of gaps followed by a subsequent re-linkage to care, even with a median gap of almost 1 year. A recent study in New York testing an active public health outreach effort for patients LTFU for >9 months successfully re-linked over one-half of all patients (Udeagu, Webster, Bocour, Michel, & Shepard, 2013). Consequently, the development of retention interventions should focus both on preventing LTFU in the first place, but also on re-engaging patients once it occurs.
Our multivariable models indicated that younger age, lack of prescription of ART in year one, and a history of prior gap are predictors of gaps in care; younger age and lack of ART prescription were also associated with a gap in the first year of care. Younger age has been associated with poor retention in HIV care in prior studies, likely reflective of more recent, asymptomatic infections (Catz et al., 1999; Olatosi et al., 2009). In this cohort, 40% of patients were not prescribed ART in the first year. While this finding seems to support current recommendations to offer ART to all HIV-infected individuals, this may reflect a selection bias in that individuals who stayed in care in their first year were more likely to have the opportunity to be prescribed ART. Having experienced a gap in care was also highly predictive of subsequent gaps in care both on univariable and on multivariable analyses. In another longitudinal cohort of >17,000 HIV-infected patients in the U.S., 59% of patients engaged in HIV care had a >6 month gap between outpatient visits, with 28% having one or more gaps >12 months (Yehia et al., 2012). Considering that almost 1 in 4 patients in this cohort experienced a gap within their first year of entry into care, focusing resources on the first year of entry in care could potentially impact future engagement and thus longitudinal outcomes in the highest risk patients. Moreover, individuals re-engaging after experiencing gaps in care will likely benefit from enhanced services that can address their individual reasons for lack of follow-up in order to prevent these gaps from occurring again.
Certain characteristics associated with inconsistent engagement in other studies, including prior incarceration, homelessness, substance use, and higher CD4 counts while significant on the univariable model were not significant in the multivariable model. The incarceration rate in our cohort is low which may explain this discrepancy on the multivariable model. All three variables may also be underreported as they are self-reported measures collected at the time of entry to care and are not necessarily reflective of ongoing or developing problems with these issues. Collinearity between the variables of homelessness and incarceration, and incarceration and substance use may also explain this finding. Likewise, CD4 counts were strongly associated with ART status in the first year of care.
Our study also did not find an association between inconsistent engagement in HIV care with having a detectable HIV PVL as described in other studies (Mugavero, Amico, et al., 2012). This may be explained by the fact that patients who were nonadherent to treatment were also not having lab work performed.
Our finding of few predictors of gaps in care may indicate that particular subgroups are not more likely to be lost to care in our clinic, suggesting fewer disparities in care at the Immunology Center. It also suggests that quality improvement approaches should target the broad population of patients entering care rather than developing interventions targeting subgroups. The finding that the risk of gaps in care this cohort is high (63% experienced at least one gap in care) also supports this approach.
There are several limitations to this study. As mentioned previously, certain variables such as incarceration, homelessness and substance use are based on self-report at the time of entry in care; a better assessment of the ongoing role of this factors in longitudinal retention would more accurately describe their impact. Additionally, this is a single site study with a limited sample size (N=581). Both the population and local context that impact retention in care may not be generalizable to other populations. However, forming meaningful, cost-effective interventions addressing an issue as complex as retention requires acknowledging the influence of multiple individual and local environmental factors; a detailed synthesis of these determinants is a critical component of this process. As this study included data from a single clinic, we are assuming that patients were not receiving HIV care elsewhere. It is possible that patients may switch to a different provider in the same locality, move elsewhere even for a temporary period where they are engaged with care, or become incarcerated or institutionalized but continue to have access to HIV medication and treatment. In this case, access to laboratory results reported to a central entity such as a state health department could supplement available clinical data as a proxy for out-of-network care in analysis purposes, with the knowledge that laboratory results may not necessarily equate with clinical care.
While gaps in care were not associated with mortality, investigation of this association was limited due to the overall low number of deaths in this cohort. Additionally, our data quantifying frequency of gaps in care (Table 2) do not account for differential follow-up times of the study population, and hence provide underestimates of actual gap rates.
To address the HIV epidemic in the country, the 2010 U.S. National HIV/AIDS Strategy proposed as one of its key measures establishing “a seamless system to immediately link people to continuous and coordinated quality care when they are diagnosed with HIV.” Our study found that gaps in care are frequent and likely over the course of time, and yet re-engagement is also possible. Therefore, equal emphasis must be placed on the prevention of initial gaps in care as well as on reengaging those LTFU. Few predictors of gaps in care in a setting with high risk of gaps suggests quality improvement interventions may need to be broadly applicable rather than focusing on particular subgroups. Clinic-level evaluations to understand the elements contributing to inconsistent engagement with care are necessary step prior to the implementation of interventions to achieve this goal.
Acknowledgments
This research was supported by the Lifespan/Tufts/Brown Center for AIDS Research (P30AI042853), and by supporting grants from the National Institute of Mental Health (A.I.R 1K23MH100955; I.B.W. 2K24MH092242; C.Z 5K24MH070769).
Contributor Information
Aadia I. Rana, Email: arana@lifespan.org, Department of Medicine Alpert Medical School of Brown University, The Miriam Hospital, Providence, RI 02906.
Tao Liu, Email: tliu@stat.brown.edu, Department of Biostatistics, Center for Statistical Sciences, Brown University School of Public Health, Providence, RI 02912.
Fizza S. Gillani, Email: fgillani@lifespan.org, Department of Medicine, Alpert Medical School of Brown University, The Miriam Hospital, Providence, RI 02906.
Rebecca Reece, Email: rreece@lifespan.org, Department of Medicine, Alpert Medical School of Brown University, The Miriam Hospital, Providence, RI 02906.
Erna M. Kojic, Email: ekojic@lifespan.org, Department of Medicine, Alpert Medical School of Brown University, The Miriam Hospital, Providence, RI 02906.
Caron Zlotnick, Email: czlotnick@butler.org, Department of Human Behavior and Psychology, Alpert Medical School of Brown University/University of Cape Town, Providence, RI 02906.
Ira B. Wilson, Email: ira_wilson@brown.edu, Department of Health Services, Policy, and Practice, Brown University School of Public Health, Providence, RI 02912.
References
- Agresti A. Categorical Data Analysis. Hoboken, NJ: Johns Wiley & Sons; 2002. [Google Scholar]
- Andersen PK, Gill RD. Cox's Regression Model for Counting Processes: A Large Sample Study. The Annals of Statistics. 1982;10(4):1100–1120. doi: 10.1214/aos/1176345976. [DOI] [Google Scholar]
- Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, Porter K. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. Jama. 2008;300(1):51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- Catz SL, McClure JB, Jones GN, Brantley PJ. Predictors of outpatient medical appointment attendance among persons with HIV. AIDS Care. 1999;11(3):361–373. doi: 10.1080/09540129947983. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Fleming TR. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS. Guidelines for the use of antiretorival agents in HIV-1 infected adults and adolescents. 2013 Retrieved from http://aidsinfor.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
- Giordano TP, Gifford AL, White AC, Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, Morgan RO. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- Giordano TP, White AC, Jr, Sajja P, Graviss EA, Arduino RC, Adu-Oppong A, Visnegarwala F. Factors associated with the use of highly active antiretroviral therapy in patients newly entering care in an urban clinic. J Acquir Immune Defic Syndr. 2003;32(4):399–405. doi: 10.1097/00126334-200304010-00009. [DOI] [PubMed] [Google Scholar]
- Kerr T, Marshall A, Walsh J, Palepu A, Tyndall M, Montaner J, Wood E. Determinants of HAART discontinuation among injection drug users. AIDS Care. 2005;17(5):539–549. doi: 10.1080/09540120412331319778. [DOI] [PubMed] [Google Scholar]
- Lifespan/Tufts/Brown Center for AIDS Research. The Miriam Hospital Immunology Center Database (ICDB) Annual Data Report, 2012. 2013;VIII [Google Scholar]
- Malitz FE, Eldred L. Evolution of the special projects of national significance prevention with HIV-infected persons seen in primary care settings initiative. AIDS Behav. 2007;11(5 Suppl):S1–5. doi: 10.1007/s10461-007-9252-5. [DOI] [PubMed] [Google Scholar]
- Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JH, Saag MS. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2012;59(1):86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010;24(10):607–613. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, Allison JJ. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Westfall AO, Zinski A, Davila J, Drainoni ML, Gardner LI, Giordano TP. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61(5):574–580. doi: 10.1097/QAI.0b013e318273762f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatosi BA, Probst JC, Stoskopf CH, Martin AB, Duffus WA. Patterns of engagement in care by HIV-infected adults: South Carolina, 2004-2006. Aids. 2009;23(6):725–730. doi: 10.1097/QAD.0b013e328326f546. [DOI] [PubMed] [Google Scholar]
- Rebeiro P, Althoff KN, Buchacz K, Gill J, Horberg M, Krentz H, Gange SJ. Retention among North American HIV-infected persons in clinical care, 2000-2008. J Acquir Immune Defic Syndr. 2013;62(3):356–362. doi: 10.1097/QAI.0b013e31827f578a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendeman D, Ingram BL, Rotheram-Borus MJ. Common elements in self-management of HIV and other chronic illnesses: an integrative framework. AIDS Care. 2009;21(10):1321–1334. doi: 10.1080/09540120902803158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Youmans E, Gibson JJ, Duffus WA. The impact of retention in early HIV medical care on viro-immunological parameters and survival: a statewide study. AIDS Res Hum Retroviruses. 2011;27(7):751–758. doi: 10.1089/aid.2010.0268. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. Geographical Mobility: 2008 to 2009. 2011 Retrieved from http://www.census.gov/prod/2011pubs/p20-565.pdf.
- Udeagu CC, Webster TR, Bocour A, Michel P, Shepard CW. Lost or just not following up: public health effort to re-engage HIV-infected persons lost to follow-up into HIV medical care. Aids. 2013;27(14):2271–2279. doi: 10.1097/QAD.0b013e328362fdde. [DOI] [PubMed] [Google Scholar]
- Vital signs: HIV prevention through care and treatment--United States. MMWR Morb Mortal Wkly Rep. 2011;60(47):1618–1623. [PubMed] [Google Scholar]
- Yehia BR, Fleishman JA, Metlay JP, Korthuis PT, Agwu AL, Berry SA, Gebo KA. Comparing different measures of retention in outpatient HIV care. Aids. 2012;26(9):1131–1139. doi: 10.1097/QAD.0b013e3283528afa. [DOI] [PMC free article] [PubMed] [Google Scholar]