Abstract
Epidemiological studies of humans and experimental studies with mouse models suggest that sunburn resulting from exposure to excessive UV light and damage to DNA confers an increased risk for melanoma and non-melanoma skin cancer. Previous reports have shown that both nucleotide excision repair, which is the sole pathway in humans for removing UV photoproducts, and DNA replication, are regulated by the circadian clock in mouse skin. Furthermore, the timing of UV exposure during the circadian cycle has been shown to affect skin carcinogenesis in mice. Because sunburn and skin cancer are causally related, we investigated UV-induced sunburn apoptosis and erythema in mouse skin as a function of circadian time. Interestingly, we observed that sunburn apoptosis, inflammatory cytokine induction, and erythema were maximal following an acute early morning exposure to UV and minimal following an afternoon exposure. Early morning exposure to UV also produced maximal activation of Atr-mediated DNA damage checkpoint signaling including activation of the tumor suppressor p53, which is known to control the process of sunburn apoptosis. To our knowledge these data provide the first evidence that the circadian clock plays an important role in the erythemal response in UV-irradiated skin. The early morning is when DNA repair is at a minimum, thus the acute responses likely are associated with unrepaired DNA damage. The prior report that mice are more susceptible to skin cancer induction following chronic irradiation in the AM, when p53 levels are maximally induced, is discussed in terms of the mutational inactivation of p53 during chronic irradiation.
Introduction
Excessive exposure to solar ultraviolet radiation (UVR) has a variety of adverse effects on the skin, such as aging, sunburn and the induction of melanoma and non-melanoma skin cancers (Geller et al., 2012; Sorrentino et al., 2014; Ziegler et al., 1994). The sunburn process is an early inflammatory response to UV exposure that is regulated by the tumor suppressor protein p53 and follows molecular and cellular responses that include DNA repair, DNA damage checkpoint signaling, and apoptosis. Evidence from both epidemiological studies on humans (Whiteman et al., 2001) and experimental studies with mouse melanoma models (Kannan et al., 2003; Noonan et al., 2001) suggest that sunburn poses a significant risk of developing malignant melanoma, which is the deadliest form of skin cancer. Moreover, due to changes in lifestyle and the environment, the incidence of sunburn-associated malignant melanoma has been steadily increasing (Fisher and James, 2010), and novel approaches are needed to address this problem.
Organisms from bacteria to humans possess circadian clocks, which are molecular time-keeping systems that maintain daily rhythms in physiological and biochemical processes with a periodicity of ~24 hrs (Lowrey and Takahashi, 2004; Partch et al., 2014; Reppert and Weaver, 2001). At the molecular level, circadian oscillation in higher organisms is generated by a transcription-translation feedback loop in which the heterodimeric transcription factor CLOCK/BMAL1 drives the expression of many clock-controlled target genes, including the Cryptochrome (Cry1/2) and Period (Per1/2) genes, by binding to E-box elements in their promoters. A negative feedback loop is formed when CRY/PER protein complexes shuttle into the nucleus, inhibit CLOCK/BMAL1-mediated transactivation, and thereby inhibit their own transcription (Reppert and Weaver, 2001; Sahar and Sassone-Corsi, 2009; Vitaterna et al., 1999). Loss of CRY/PER by proteolytic degradation re-initiates transactivation by CLOCK/BMAL1 (Antoch and Kondratov, 2010; Lowrey and Takahashi, 2004). Consequently, the genes and pathways that are controlled by the molecular clock have a periodicity of ~24 hrs. The expression of as many as 10% of mammalian genes is regulated by the circadian clock (Akhtar et al., 2002; Hughes et al., 2009), and most of the circadian gene expression patterns are tissue specific (Partch et al., 2014).
Among the proteins that are regulated by the clock is XPA (Xeroderma Pigmentosum complementation group A), which is one of the six core factors that are required for removing UV photoproducts from DNA. Patients with the disease XP exhibit solar sensitivity and a greatly increased risk of skin cancer (Kraemer et al., 1987). In mice, the levels of XPA RNA and protein have been found to be rhythmic in brain (Kang et al., 2009), liver (Kang et al., 2010), and more recently, skin (Gaddameedhi et al., 2011), which is known to harbor a functional circadian clock that regulates gene expression (Sporl et al., 2012; Tanioka et al., 2009). Oscillation of XPA levels was found to parallel the oscillation of repair activity in these tissues (Gaddameedhi et al., 2011; Kang et al., 2009; Kang et al., 2011). In addition to excision repair oscillation, using a circadian clock-deficient genetic mouse model, we and others have shown that DNA replication activity is circadian in nature in proliferating tissues such as the intestine and epidermis (Gaddameedhi et al., 2011; Geyfman et al., 2012). This circadian rhythmicity of DNA replication is anti-phase to that of DNA repair. Interestingly, mice were found to be more sensitive to the development of skin cancer when chronically irradiated in the early morning (when repair activity was low and DNA synthesis was high) than when chronically irradiated in the late afternoon (when repair activity was high and DNA synthesis was low).
Melanomas and basal cell carcinomas in humans are commonly associated with episodes of sunburn early in life. Sunburn is initiated by excessive acute ultraviolet radiation-induced DNA damage and is followed by p53-dependent apoptosis and inflammation (Ziegler et al., 1994). The importance of DNA damage and repair to the induction of sunburn is indicated by the greatly enhanced apoptotic and inflammatory responses to UVR seen in XPA-knockout mice (Brash et al., 2001; Katiyar et al., 2011; Miyauchi-Hashimoto et al., 1996; Okamoto et al., 1999). In fact, apoptosis and sunburn may be considered to be readouts of the level of DNA damage that is produced by UVR. Sunburn-associated apoptosis is mediated by the tumor suppressor p53 (Ziegler et al., 1994) and is considered to be a protective mechanism by which potentially neoplastic cells are removed from the skin. Though sunburn apoptosis and skin cancer are both produced by DNA damage and involve p53, there are important distinctions that can be made regarding how p53 impacts these processes. Apoptosis and sunburn are acute responses mediated by p53 regulation of gene expression, while carcinogenesis results from chronic exposures during which p53 and its protective pro-apoptotic functions are commonly lost by mutational inactivation.
Because the levels of repair and replication are under the control of the circadian clock in the skin and likely contribute to many biological responses to UVR, we examined how the circadian clock influences the erythemal response to UVR in mouse skin. We found elevated erythemal responses following UV exposures in the early morning in comparison to exposures in the late afternoon. This enhanced response appears to be due not only to circadian control of repair and replication but also to circadian clock regulation of p53.
Results
Circadian control of sunburn erythema
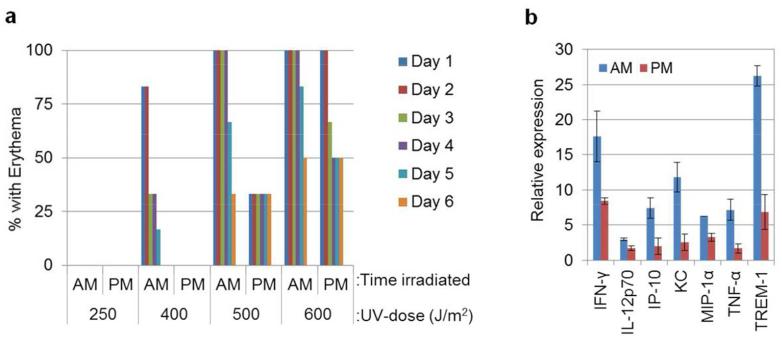
Evidence that skin carcinogenesis is influenced by the timing of UVR exposure during the circadian cycle (Gaddameedhi et al., 2011), combined with the well-known association between sunburn and skin cancer (Geller et al., 2012; Noonan et al., 2001; Whiteman et al., 2001), indicated that the development of sunburn may also be controlled by the circadian clock. To investigate this hypothesis, SKH-1 hairless mice were immobilized in a restraining tube containing four holes (0.5 cm diameter) in the top, and each mouse was irradiated with four different doses of UVR on the dorsal aspect. This arrangement resulted in the production of discreet circular areas of erythema, depending on the dose. An “AM group” of mice (n=6) was irradiated at ZT21, and a “PM group” (n=6) was irradiated at ZT09. In conventional terms, with lights on at 7AM, ZT21 corresponds to 4AM and ZT9 corresponds to 4PM. Each mouse was scored for erythema daily for six days following UVR. The results are shown graphically in Figure 1A. The extent of the erythemal response was greater in the AM group than the PM group, particularly following doses of 400 and 500 J/m2 of UVR. The experiment was repeated with a second set of mice and the same trend was observed (Supplemental Figure 1). Thus, the sensitivity of mice towards sunburn is controlled by the time of day of UV exposure, and this sensitivity as a function of circadian time parallels the sensitivity of mice towards the development of UVR-induced skin cancer (Gaddameedhi et al., 2011), such that the greatest responses occur following early AM exposures.
Figure 1.
Effect of time of day on UV-induced erythema and inflammatory cytokine induction in mouse skin. (A) Effect of circadian time on erythema in wild-type SKH-1 hairless mice. SKH-1 hairless mice kept under LD12:12 cycle were irradiated either at ZT21 (4AM) or at ZT09 (4PM) with lights off at ZT12. Mice were immobilized in a restraining tube during irradiations. Four holes (each 5 mm in diameter) in the top of the tube enabled the dorsal aspect of each mouse to be irradiated with four different doses. Erythema was scored each day up to 6 days post-UVR (n= 6 mice AM and 6 mice PM). (B) SKH-1 mice were irradiated with 500 J/m2 of UV at either ZT21 (4AM) or ZT9 (4PM), and whole skin was collected 12 hrs after UVR. Protein lysates were probed with a mouse cytokine antibody array, and the expression levels of cytokine proteins were plotted by irradiation time of the day (p < 0.05 for all 7 cytokines shown in the bar graph, n = 2 mice for each time point and error bars= SD). Relative expression is as described in the Supplemental Figure 2 legend.
Effect of time-of-day on cytokine induction following UVR
The erythemal response proceeds through the production of pro-inflammatory cytokines by neutrophils and macrophages in the skin (Faustin and Reed, 2008) as a result of UV damage. We utilized a mouse cytokine antibody array to detect cytokines in extracts of either unirradiated whole mouse skin or skin harvested 12 hrs following irradiation with 500 J/m2 of UVR, which elicits erythema. This array contains 40 different antibodies, and protein/cytokine expression patterns can be determined in a single assay at the protein level. Many of the proteins detected with this array (Supplemental Figure 2A and B) were clearly induced by UV and tended to be more abundant following morning exposure compared to evening exposure. Seven proteins were induced to a significantly (p < 0.05) greater extent following a morning exposure compared to an evening exposure (Figure 1B). Among these, IFN-γ, TNF-α, IL-12p70, and MIP-1α are known to be pro-inflammatory cytokines, whereas IP-10 and KC are chemokines that play essential roles in the initiation and/or promotion of inflammation. Most interestingly, both TNF-α and IFN-γ are known to be involved in UVR-induced erythema and melanoma promotion in mice (Murakawa et al., 2006; Zaidi et al., 2011). In addition, it was recently reported that TNF-α levels are influenced by the circadian rhythm in mouse macrophages (Keller et al., 2009). TREM-1, which is also more highly induced by UV in the morning, is a receptor that is found on immune cells. TREM-1 is involved in antigen detection, secretion of inflammatory mediators, and increased acute inflammatory response and is upregulated during the inflammatory response (Bouchon et al., 2001). Several additional cytokines appeared to be more highly induced in the morning (Supplemental Figure 2C) but were of borderline statistical significance. These included IL-2, IL-4, IL-7, IL-13 and MIG. Overall, we find that the inflammatory cytokine response parallels the erythemal response and supports the physiological data that show a role of the time of day of UV exposure in the erythemal response.
Circadian regulation of sunburn apoptosis
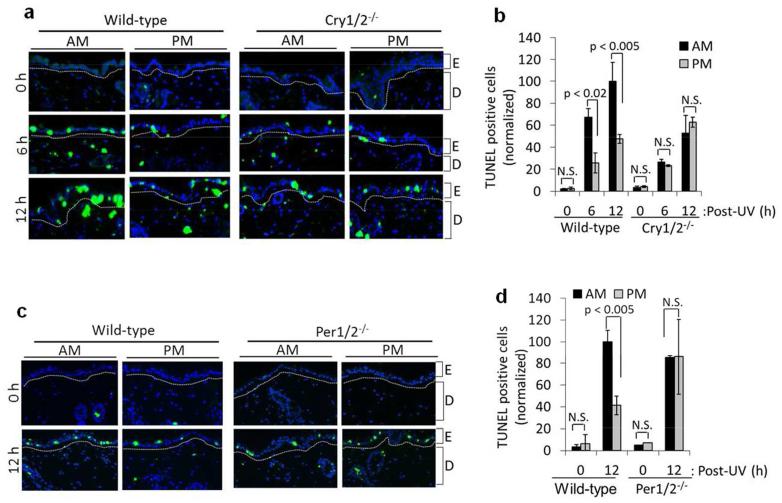
At the cellular level, erythemogenic doses of UVR are associated with apoptosis (also called “sunburn apoptosis”) (Ziegler et al., 1994). To determine the effect of the circadian clock on sunburn apoptosis, we irradiated C57BL/6 mice with UVR either in the early morning or the late afternoon and then harvested mouse skin at 0, 6, and 12 hrs after UVR. Apoptosis was measured using the fluorometric TUNEL assay (TdT-mediated dUTP Nick-End Labeling) in which fragmented DNA from apoptotic cells is end-labeled with fluorophore. Figure 2 shows a greater apoptotic response in the AM group compared to the PM group. To determine if sunburn apoptosis is controlled by the circadian clock, we used mice in which the clock was disrupted by mutating essential clock genes. In both Cry1, Cry2-double knockout mice (Cry1/2−/− in Figure 2A, B) and Per1, Per2 double knockout mice (Per1/2−/− in Figure 2C, D), the effect of the time of the day on sunburn apoptosis was abolished. The elevated apoptosis in wild-type mice irradiated in the AM was found to be correlated with reduced repair of UV-induced DNA photoproducts (Gaddameedhi et al., 2011). Although it has been reported that p53 contributes to excision repair and UV survival in human cells (Ferguson and Oh, 2005; Ford and Hanawalt, 1997), mouse p53 protein doesn’t seems to have a role in excision repair or UV survival (Ishizaki et al., 1994), which could be due to the fact that the mouse XPE gene, which encodes the DDB2 protein, doesn’t have a p53 recognition domain (Tan and Chu, 2002). Therefore, circadian oscillation of p53 in mouse skin may not have a significant role in regulating excision repair capacity.
Figure 2.
Circadian regulation of UV-induced sunburn apoptosis in mouse skin. (A) Representative immunofluorescence images of TUNEL assay for the detection of apoptotic cells in the skin of UV-irradiated mice with the C57BL/6 background. Mice were maintained in an LD12:12 cycle and shaved 1 day before irradiation. Both wild-type and Cry1/2−/−mice were irradiated with UV (4000 J/m2) either at ZT21 (4AM) or ZT09 (4PM), and skin tissues were collected at 0, 6, and 12 hr post-UV for analysis with TUNEL assays. TUNEL-positive cells are green and nuclei are stained blue with DAPI. E, epidermis; D, dermis. (B) TUNEL-positive (apoptotic) cells in wild-type and Cry1/2−/− mouse epidermis as a result of UVR at ZT21 (4AM) and ZT09 (4PM). Positive cells were calculated as the percentage of the total number of DAPI-positive cells. All of the values were then normalized to 100 relative to the 12 hr, AM wild-type response sample (which actually had a value of 20% TUNEL-positive cells). (C) Representative immunofluorescence images of TUNEL assay from wild-type and Per1/2−/− mouse skin. Samples were processed as described in B. (D) Quantification of C. AM wild-type response sample contained 10% TUNEL-positive cells but was normalized to 100 for quantification. n=2 or 3 mice at each time point. Error bars represent means ± standard deviation (SD). NS= Not Significant.
In addition to the C57BL/6 mouse strain, we also observed a circadian effect of sunburn apoptosis in SKH-1 hairless mice by classical H&E staining (Supplemental Figure 3). Thus, enhanced apoptosis parallels the enhanced sunburn and reduced repair that is observed following exposure in the AM.
Effect of time-of-day of UV exposure on p53 function
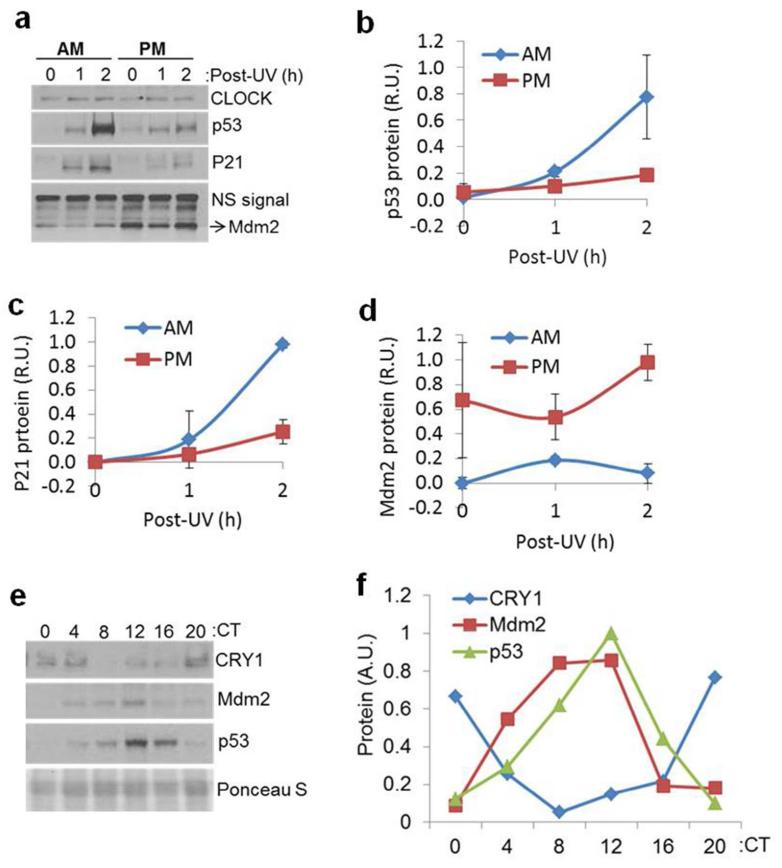
Both sunburn and the apoptotic response are known to be controlled by the tumor suppressor p53 (Ziegler et al., 1994), and the absolute protein level, phosphorylation status, and activity of p53 are known to be induced by DNA damage. We therefore first investigated p53 protein levels following UVR as a function of circadian time. Skin was harvested from SKH-1 mice at 0, 1, and 2 hrs following irradiation and analyzed for p53 content by immunoblotting. The results (Figure 3A and B) show that p53 accumulated to a greater extent in mice irradiated in the AM in comparison to mice irradiated in the PM. The cell cycle regulatory protein P21, which is directly controlled at the level of transcription by p53, also showed a stronger induction following exposure in the AM than following exposure in the PM (Figure 3A and C). Together, these results demonstrate that both p53 protein levels and activity following UVR are controlled by the circadian clock.
Figure 3.
(A) p53 stabilization and activity following UVR is time-of-day dependent in mouse skin. SKH-1 hairless mice kept under LD12:12 cycle were irradiated with 300 J/m2 of UV at either ZT21 (4AM) or ZT09 (4PM) and sacrificed at the indicated time points. Protein lysates were then prepared for immunoblotting. The non-specific signal from an unknown protein that cross-reacts with anti-Mdm2 antibody (NS) was used as an internal control. (B-D) Quantitative analysis of p53, P21, and Mdm2 protein levels in the mouse skin, respectively, from two independent experiments. Error bars represent means ± standard deviations (SD) (n=2 mice at each time point). (E) Circadian oscillation of p53 and Mdm2 proteins in mouse skin. C57BL/6 mice kept under a DD12:12 cycle for 24 hrs were sacrificed at the indicated circadian times and the levels of the indicated proteins were determined from whole skin by immuno-blotting. Note the robust oscillation of the CRY1, p53 and Mdm2proteins. Ponceau Stain was used as a loading control. N=2 mice at each time point. (F) Quantitative analysis of CRY1, p53 and Mdm2 protein levels in the mouse skin, respectively, from E and another experiment (not shown). R.U: relative unit.
Circadian influence on basal p53 and Mdm2 protein expression
The p53 protein is regulated by Mdm2, which ubiquitinates p53 to promote its degradation by the proteosome. We noticed that higher Mdm2 protein levels in the PM samples (Figure 3A and D) were correlated with a lower induction of p53 protein by UV (Figure 3A and B), which indicated that circadian control of Mdm2 protein may contribute to the p53 response. In an effort to better understand the circadian regulation of p53, we examined the expression of p53 and Mdm2 in unirradiated mouse skin as a function of circadian time. We found that both proteins were rhythmically expressed in both C57BL/6 and SKH-1 mice (Figure 3E-F and Supplemental Figure 4). In contrast, p53 and Mdm2 mRNA levels appeared to be non-rhythmic (Supplemental Figure 5). Furthermore, the mRNA levels of p53 were not induced by UVR (Supplemental Figure 5C). The results in Figure 3E and F show that p53 and Mdm2protein oscillations are in phase with one another. When the levels of both proteins are low in the early AM, interactions between the two would be expected to be minimal, which could result in less ubiquitination and degradation of p53 and hence higher levels of p53 and a more robust p53-dependent responses, including apoptosis. The diminished capacity of Mdm2 to target p53 for degradation in the AM is therefore seen as a likely contributor to the extreme elevation in p53 levels following UVR in the AM (Figure 3A and B). UV induction in the AM propels the level of p53 from its nadir of circadian expression to greatly exceed its peak circadian expression level, which is near the detection limit at the zero irradiation time point in Figure 3A. Though additional factors, including p19ARF and HAUSP have been reported to impact Mdm2and p53 function (Khoronenkova et al., 2012; Li et al., 2004; Zhang et al., 1998), neither of these proteins displayed a circadian pattern of expression (data not shown). We therefore conclude that low levels of Mdm2 in the morning and high levels in the afternoon may contribute to the different effects of UVR on p53 protein induction and activity at these time points.
Circadian influence on the UV-induced, Atr-mediated DNA damage checkpoint response
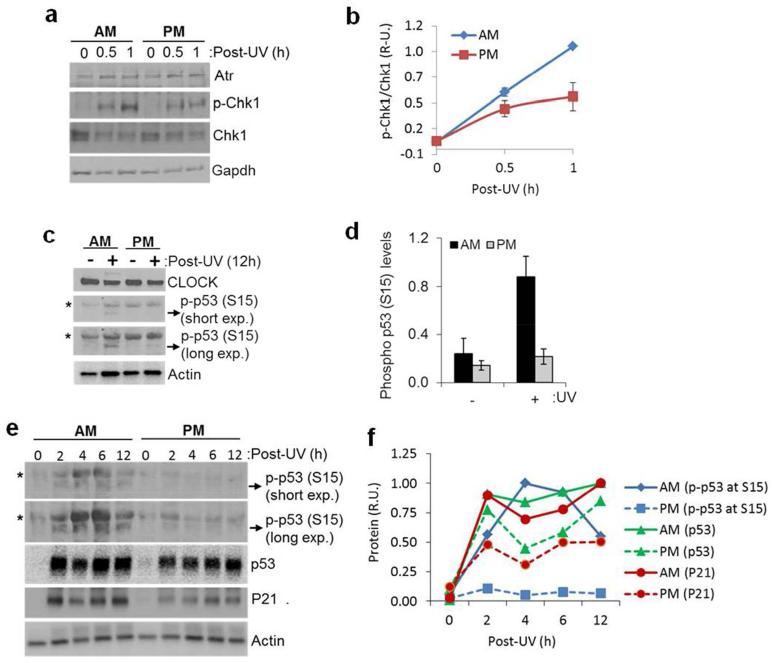
The lower levels of UV photoproduct repair and the higher levels of DNA synthesis that are observed in the AM (Gaddameedhi et al., 2011) are expected to lead to increased stalling of DNA polymerases at UV photoproducts, which is known to be a potent trigger of Atr kinase-mediated DNA damage response signaling (Sancar et al., 2004; Zeman and Cimprich, 2014). In response to DNA damage-induced replication stress, Atr phosphorylates numerous substrates, including the cell cycle checkpoint kinase Chk1 and the tumor suppressor protein p53. As shown in Figure 4A and B, more phosphorylation of the Atr substrate Chk1 was observed following an AM exposure than following a PM exposure. This result is interesting in that to our knowledge it is the first data demonstrating a circadian variation of the Atr-dependent DNA damage checkpoint response in whole animals.
Figure 4.
(A) Chk1 phosphorylation following UVR is time-of-day dependent in mouse skin. C57BL/6 mice kept under LD12:12 cycle were irradiated with 1000 J/m2 of UV at either ZT 21 (4 AM) or ZT 09 (4 PM) and sacrificed at the indicated times and the whole ear was collected and immediately snap frozen to prepare lysates for western blot. Gapdh levels were used as an internal control. Note: The reason we chose ear tissue instead of the dorsal skin tissue was that, in our hands we consistently have noticed that ear tissue has a cleaner total Chk1 signal compared with the dorsal skin tissue, nonetheless, we have found consistently elevated p-Chk1 signal in the AM time point compared with the PM time point of dorsal skin tissue of the mouse (data not shown). (B) Quantitative analysis of p-Chk1 (Ser-345)/Chk1 ratios in the mouse ear from two independent experiments. Error bars represent means ± standard deviations (SD). (n=2 mice at each time point). (C) p53 phosphorylation at Ser15 following UVR is time-of-day dependent in mouse skin. SKH-1 hairless mice kept under LD12:12 cycle were irradiated with 500 J/m2 of UV at either ZT21 (4AM) or ZT09 (4PM) and sacrificed 12-hrs later, and then whole skin was collected and immediately snap frozen. Protein lysates were then prepared for western blot. Actin was used as an internal control. (D) Quantitative analysis of phospho-p53 protein levels (p-p53 band) in the mouse skin 12 hrs after UVR, respectively, from two independent experiments. Error bars represent means ± standard deviations (SD) (n=2 mice at each time point). (E) Kinetics of p53 phosphorylation at Ser15 following 500J/m2 of UVR in mouse as a function of the time-of-day of UV exposure. Mice were irradiated either at ZT21 or ZT09, and ensuing levels of phospho-p53 (Ser15), p53 and P21 were analyzed by immunoblot. The band identified as phospho-p53 (p-p53) in A and C co-migrates with the positive control phospho-p53 from UV-treated cells (mouse embryonic fibroblasts, not shown). The band indicated with the asterisk in the skin extracts was not observed in UV-treated MEFs and hence was not quantified (F) Quantitative analysis of phospho-p53 (Ser15) protein levels (p-p53 band) in the mouse skin from E. The circadian effect on p53 levels is larger following a lower dose of UV, as seen in Figure 3 A and B. The dose used in this figure is most suitable to detect phospho-p53.
We next examined the phosphorylation status of p53, which in response to DNA damage becomes phosphorylated on Ser15. This phosphorylation event stabilizes p53 by disrupting its interactions with Mdm2 (Lakin and Jackson, 1999; Shieh et al., 1997). We therefore tested whether p53 phosphorylation in mouse skin following UVR was influenced by the circadian time. As shown in Figure 4C-F, the phosphorylation of p53 at Ser15 was detectable as early as 2 hrs after UVR and was consistently higher (3-5 fold) in the AM-treated group than in the PM-treated group up to 12 hrs following UVR.
Together, these results demonstrate that UVR induces a more robust DNA damage signaling response in the early AM, which includes enhanced phosphorylation of p53 on a residue (Ser15) involved in stabilizing the protein by preventing its degradation by Mdm2. Thus, by controlling both the expression level of Mdm2 (Figure 3) and the phosphorylation of p53 (Figure 4 or 5), the circadian clock can regulate p53 activity in sunburn apoptosis through two independent but related mechanisms.
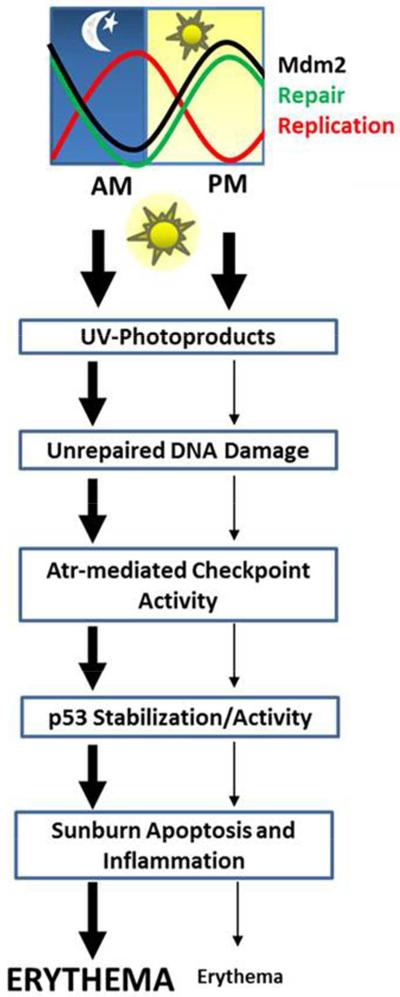
Figure 5.
Proposed model for the role of the circadian clock in sunburn inflammation. Low levels of repair and high levels of DNA replication in the AM lead to unrepaired UV photoproducts in DNA that cause replication stress and to enhanced DNA damage checkpoint signaling compared to in the PM. The enhanced Atr-mediated checkpoint signaling in the AM coupled with reduced levels of Mdm2 lead to greater phosphorylation, stabilization, and activity of p53, which leads to more apoptosis and sunburn following UV exposure in the AM.
Discussion
In summary, our results show that the circadian clock plays a significant role in the development of erythema following UVR exposure. Because UV photoproducts in DNA are repaired less efficiently in the early AM (Gaddameedhi et al., 2011), the increased level of DNA synthesis during this time period (Gaddameedhi et al., 2011; Geyfman et al., 2012) leads to an increase in DNA replication stress that is associated with the stalling of DNA polymerases at unrepaired damage (Figure 4A) and to a more robust induction of p53 (Figures 3 and 4). Furthermore, reduced amounts of Mdm2 protein in the early AM (Figure 3A and E) may abrogate the major proteolytic pathway for p53 and contribute to higher level of p53 activity in the skin following UVR exposure (Figures 3A, 4C). The end result of this circadian regulation of p53 is the promotion of sunburn apoptosis (Figure 2) and erythema (Figure 1). A schematic summarizing this response is shown in Figure 5.
Based upon its function as a tumor suppressor, the enhanced level and activity of p53 following AM exposure to UVR that we find in this study would be expected to provide protection from skin cancer. However, we previously reported that UVR in the early morning produced more skin cancers compared to afternoon exposure (due to low repair and high replication in the morning) (Gaddameedhi et al., 2011). This apparent inconsistency may be explained by the fact that skin cancer cells commonly possess inactivating mutations in the p53 gene (Brash et al., 1991). Mutations in p53 have been found to be common even in ‘normal’, non-cancerous skin cells following exposure to UVR (Ziegler et al., 1994). The skin cancer study employed three times per week exposures for six months, and loss of p53 activity and skin cancer protection early in the study would make cells more vulnerable to carcinogenesis by UVR later in the study (Hill et al., 1999; Zhang et al., 2005). In contrast, our experiments into the erythemal response reported here utilized single UV exposures in which case p53 was assumed to be wild-type.
It should also be noted that mice are nocturnal and humans are diurnal and therefore the circadian clock outputs of the two organisms exhibit opposite phases (Sporl et al., 2012). Based on this general argument we predict that humans may be less prone to sunburn in the morning hours and more prone in the later afternoon. However, before public health recommendations can be made regarding preferred time of day for human exposure to sunlight or other sources of UVR, sunburn measurements must be made in human skin as a function of time of day of UV exposure.
Materials and Methods
UV treatment of mice
All animal procedures were approved by the University of North Carolina IACUC. Male outbred SKH-1 (8-12 weeks old) mice were obtained from Charles River Laboratories (Wilmington, MA). Wild-type mice and Per1 Per2 double knockout (Per1/2−/−) mice (Bae et al., 2001) of C57BL/6 genetic background were obtained from Jackson Laboratories. Cry1/2−/− mice with C57BL/6 genetic background were generated in our lab (Vitaterna et al., 1999). The mice were maintained on an LD 12:12 schedule (a daily cycle of 12 hrs of light and 12 hrs of dark) for 3 weeks before UV treatment. The time of day is expressed in several ways: One is the standard chronobiology terminology where ZT0 (Zeitgeber Time 0) is lights on and ZT12 is lights off for animals under LD 12:12 cycles. After mice are switched to constant darkness for at least 24 hrs, CT is used in place of ZT. To relate ZT time to time expression in common practice (standard time, ST), we also indicate time by AM and PM designation whereby ZT=0 (light on) is taken as 7 AM and ZT12 (light off) is expressed as 7 PM. Mice were irradiated in a relatively small chamber (18” high and 12” deep and 22” long) which was purchased from Plastic Design, Inc. The unfiltered lamp (Cat#21476-010), located at the top of the chamber above the mice, was from UVP (Upland, CA); it emits principally 290-350 nm light with a peak emission at 312 nm. The lid of the mouse cage was removed and the cage was placed into the chamber for irradiation. The UV dose rate was determined using a UVX radiometer (UVP Inc).
For apoptotic assays and protein expression studies, C57BL/6 wild-type, Per1/2−/− and Cry1/2−/− mice were treated with a single dose of UV at a rate of 15 J/m2/sec as indicated in the figure legends. Hair removal was done 24 hrs before UV irradiation by anesthetizing the mice with isofluorane and then shaving the hair with a clipper completely from an area (about 2 cm in diameter) of the dorsal aspect. Shaved mice were active while irradiated from above. A wide mesh screen located approximately 6 cm above the mice prevented vertical movement during irradiation. Most of the mice were singly housed and when multiply housed, there was no evidence of wounds caused by fighting.
Measurement of erythema
The circadian time effect on erythema was measured as described previously (Cole et al., 1983). Briefly, SKH-1 hairless mice kept under LD12:12 cycle were irradiated one time with UV light either at ZT21 (4AM) or at ZT09 (4PM) with lights off at ZT12. Mice were immobilized in a restraining tube during irradiations. Four holes (each 5 mm in diameter) in the top of the tube enabled the dorsal aspect of each mouse to be irradiated with four different doses. With the restraining tube and shielding, the only sites irradiated were the four circular patches of skin on the dorsal aspect. Erythema was scored each day up to 6 days post-UVR based on the physical appearance of redness on each circular, irradiated patch. Erythema was observed in both of the time points at a dose of 500 J/m2 which is equivalent to the exposure time of 33 seconds with our UV light setup.
Immunohistochemical (IHC) assays
For histologic analysis, whole skin tissues were fixed with 10% formalin for 24 hrs and then transferred to 70% ethanol for paraffin blocking and staining. The formalin-preserved tissue was sliced with a scalpel blade and arranged in a cassette containing foam biopsy pads and submitted to the UNC Animal Histopathology Core Facility for processing, embedding, sectioning, and Hematoxylin & Eosin (H&E) staining. For TUNEL assays to detect apoptotic cells, mouse skin was collected and formalin-fixed, and paraffin-embedded sections were analyzed with the DeadEnd Fluorometric TUNEL System (G-3250, Promega) to measure the fragmented DNA of apoptotic cells (Wang et al., 2011). To measure repair of UV-induced (6-4) photoproducts [(6-4) PPs], paraffin-embedded sections were stained with anti-(6-4) PP antibody (Cosmo Bio USA) as described previously (Gaddameedhi et al., 2010). Fluorescence images were captured using either a Leica inverted DMIRB fluorescence microscope at the UNC Michael Hooker Microscopy Facility or an Olympus IX81 inverted fluorescence microscope at the UNC Microscopy Services Laboratory. For each mouse, up to 25 fields were counted in a blinded manner.
Mouse cytokine measurements
Pro-inflammatory cytokine induction as a result of UVR was monitored using the Mouse Cytokine Array Panel A Array Kit (ARY006, R&D Systems) as recommended by the manufacturer. Briefly, SKH-1 mice were unirradiated (control) or were irradiated with 500 J/m2 of UV either at ZT 21 (4 AM) or ZT 9 (4 PM), and whole skin was collected 12 hrs after UVR and snap frozen. Protein lysates were prepared with tissue homogenization using a mortar and pestle in liquid nitrogen followed by suspending the powdered sample in 1× PBS with protease inhibitors and 1% Triton X-100. Clear protein lysates (250 μg) were then probed using the mouse cytokine antibody to monitor cytokine levels.
Immunoblotting
Protein lysates from mouse skin were harvested and analyzed by SDS-PAGE and western blotting as described previously (Gaddameedhi et al., 2012; Gaddameedhi et al., 2011). The following antibodies were used to detect the respective protein levels: CRY1 and CLOCK (Bethyl); Atr, Chk1 and Actin (Santa Cruz Biotechnology); P21, p-Chk1 (S345), Phospho-p53 (S15) and Gapdh (Cell Signaling Technology); p53 (Leica Microsystems); and Mdm2 (Provided by Dr. Yanping Zhang, Lineberger Comprehensive Cancer Center, UNC-Chapel Hill).
Total RNA isolation and real-time qPCR
Total RNA isolation from mouse skin, cDNA preparation, and qRT-PCR were performed as described previously (Gaddameedhi et al., 2012). The following primers were used for PCR (for p53 5′:TGC TCA CCC TGG CTA AAG TT and 3′:AGA GGT CTC GTC ACG CTC AT; for Cry1 5′:CGA TGG TGA ACC ATG CTG AG and 3′:GTA CTG ACT TTC CCA CCA AC; for Mdm2 5′:ATG TGC AAT ACC AAC ATC TCT GTG TC and 3′:GCT GAC TTA CAG CCA CTA AAT TTC; and for Actin 5′:GTT CCG ATG CCC TGA GGC TC and 3′:CAC TTG CGG TGC ACG ATG GA).
Statistical Analysis
Statistical significance was determined by using one-tailed Student’s t-tests for all comparisons, and t-values less than 0.05 were considered to be statistically significant.
Supplementary Material
Acknowledgments
We thank all members of the Sancar lab, Dr. Norman E. Sharpless, Lineberger Comprehensive Cancer Center (LCCC) at UNC Chapel Hill, and his lab personnel for advice. We thank Dr. Yanping Zhang (LCCC) for providing the Mdm2 antibody. This research was supported by NIGMS (GM32833) to AS and NIEHS (K99ES022640) to SG.
Footnotes
Conflict of Interest
None.
References
- Akhtar RA, Reddy AB, Maywood ES, et al. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–50. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- Antoch MP, Kondratov RV. Circadian proteins and genotoxic stress response. Circulation research. 2010;106:68–78. doi: 10.1161/CIRCRESAHA.109.207076. [DOI] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood ES, et al. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–36. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Bouchon A, Facchetti F, Weigand MA, et al. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–7. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- Brash DE, Rudolph JA, Simon JA, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991;88:10124–8. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash DE, Wikonkal NM, Remenyik E, et al. The DNA damage signal for Mdm2 regulation, Trp53 induction, and sunburn cell formation in vivo originates from actively transcribed genes. The Journal of investigative dermatology. 2001;117:1234–40. doi: 10.1046/j.0022-202x.2001.01554.x. [DOI] [PubMed] [Google Scholar]
- Cole CA, Davies RE, Forbes PD, et al. Comparison of action spectra for acute cutaneous responses to ultraviolet radiation: man and albino hairless mouse. Photochem Photobiol. 1983;37:623–31. doi: 10.1111/j.1751-1097.1983.tb04531.x. [DOI] [PubMed] [Google Scholar]
- Faustin B, Reed JC. Sunburned skin activates inflammasomes. Trends Cell Biol. 2008;18:4–8. doi: 10.1016/j.tcb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Ferguson BE, Oh DH. Proficient global nucleotide excision repair in human keratinocytes but not in fibroblasts deficient in p53. Cancer research. 2005;65:8723–9. doi: 10.1158/0008-5472.CAN-05-1457. [DOI] [PubMed] [Google Scholar]
- Fisher DE, James WD. Indoor tanning--science, behavior, and policy. The New England journal of medicine. 2010;363:901–3. doi: 10.1056/NEJMp1005999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Hanawalt PC. Expression of wild-type p53 is required for efficient global genomic nucleotide excision repair in UV-irradiated human fibroblasts. The Journal of biological chemistry. 1997;272:28073–80. doi: 10.1074/jbc.272.44.28073. [DOI] [PubMed] [Google Scholar]
- Gaddameedhi S, Kemp MG, Reardon JT, et al. Similar nucleotide excision repair capacity in melanocytes and melanoma cells. Cancer research. 2010;70:4922–30. doi: 10.1158/0008-5472.CAN-10-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddameedhi S, Reardon JT, Ye R, et al. Effect of circadian clock mutations on DNA damage response in mammalian cells. Cell Cycle. 2012;11:3481–91. doi: 10.4161/cc.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci U S A. 2011;108:18790–5. doi: 10.1073/pnas.1115249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AC, Balk SJ, Fisher DE. Stemming the tanning bed epidemic: time for action. J Natl Compr Canc Netw. 2012;10:1311–4. doi: 10.6004/jnccn.2012.0133. [DOI] [PubMed] [Google Scholar]
- Geyfman M, Kumar V, Liu Q, et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc Natl Acad Sci U S A. 2012;109:11758–63. doi: 10.1073/pnas.1209592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill LL, Ouhtit A, Loughlin SM, et al. Fas ligand: a sensor for DNA damage critical in skin cancer etiology. Science. 1999;285:898–900. doi: 10.1126/science.285.5429.898. [DOI] [PubMed] [Google Scholar]
- Hughes ME, DiTacchio L, Hayes KR, et al. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K, Ejima Y, Matsunaga T, et al. Increased UV-induced SCEs but normal repair of DNA damage in p53-deficient mouse cells. International journal of cancer Journal international du cancer. 1994;58:254–7. doi: 10.1002/ijc.2910580218. [DOI] [PubMed] [Google Scholar]
- Kang TH, Lindsey-Boltz LA, Reardon JT, et al. Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc Natl Acad Sci U S A. 2010;107:4890–5. doi: 10.1073/pnas.0915085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TH, Reardon JT, Kemp M, et al. Circadian oscillation of nucleotide excision repair in mammalian brain. Proc Natl Acad Sci U S A. 2009;106:2864–7. doi: 10.1073/pnas.0812638106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TH, Reardon JT, Sancar A. Regulation of nucleotide excision repair activity by transcriptional and post-transcriptional control of the XPA protein. Nucleic acids research. 2011;39:3176–87. doi: 10.1093/nar/gkq1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Sharpless NE, Xu J, et al. Components of the Rb pathway are critical targets of UV mutagenesis in a murine melanoma model. Proc Natl Acad Sci U S A. 2003;100:1221–5. doi: 10.1073/pnas.0336397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar SK, Mantena SK, Meeran SM. Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism. PloS one. 2011;6:e21410. doi: 10.1371/journal.pone.0021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–12. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoronenkova SV, Dianova II, Ternette N, et al. ATM-dependent downregulation of USP7/HAUSP by PPM1G activates p53 response to DNA damage. Mol Cell. 2012;45:801–13. doi: 10.1016/j.molcel.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987;123:241–50. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- Lakin ND, Jackson SP. Regulation of p53 in response to DNA damage. Oncogene. 1999;18:7644–55. doi: 10.1038/sj.onc.1203015. [DOI] [PubMed] [Google Scholar]
- Li M, Brooks CL, Kon N, et al. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol Cell. 2004;13:879–86. doi: 10.1016/s1097-2765(04)00157-1. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–41. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi-Hashimoto H, Tanaka K, Horio T. Enhanced inflammation and immunosuppression by ultraviolet radiation in xeroderma pigmentosum group A (XPA) model mice. The Journal of investigative dermatology. 1996;107:343–8. doi: 10.1111/1523-1747.ep12363295. [DOI] [PubMed] [Google Scholar]
- Murakawa M, Yamaoka K, Tanaka Y, et al. Involvement of tumor necrosis factor (TNF)-alpha in phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin edema in mice. Biochem Pharmacol. 2006;71:1331–6. doi: 10.1016/j.bcp.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Noonan FP, Recio JA, Takayama H, et al. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–2. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Mizuno K, Itoh T, et al. Evaluation of apoptotic cells induced by ultraviolet light B radiation in epidermal sheets stained by the TUNEL technique. The Journal of investigative dermatology. 1999;113:802–7. doi: 10.1046/j.1523-1747.1999.00757.x. [DOI] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–9. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–76. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9:886–96. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, et al. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- Shieh SY, Ikeda M, Taya Y, et al. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–34. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- Sorrentino JA, Krishnamurthy J, Tilley S, et al. p16INK4a reporter mice reveal age-promoting effects of environmental toxicants. J Clin Invest. 2014;124:169–73. doi: 10.1172/JCI70960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporl F, Korge S, Jurchott K, et al. Kruppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proc Natl Acad Sci U S A. 2012;109:10903–8. doi: 10.1073/pnas.1118641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T, Chu G. p53 Binds and activates the xeroderma pigmentosum DDB2 gene in humans but not mice. Molecular and cellular biology. 2002;22:3247–54. doi: 10.1128/MCB.22.10.3247-3254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanioka M, Yamada H, Doi M, et al. Molecular clocks in mouse skin. The Journal of investigative dermatology. 2009;129:1225–31. doi: 10.1038/jid.2008.345. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, Selby CP, Todo T, et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci U S A. 1999;96:12114–9. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Coleman DJ, Bajaj G, et al. RXRalpha ablation in epidermal keratinocytes enhances UVR-induced DNA damage, apoptosis, and proliferation of keratinocytes and melanocytes. The Journal of investigative dermatology. 2011;131:177–87. doi: 10.1038/jid.2010.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69–82. doi: 10.1023/a:1008980919928. [DOI] [PubMed] [Google Scholar]
- Zaidi MR, Davis S, Noonan FP, et al. Interferon-gamma links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469:548–53. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman MK, Cimprich KA. Causes and consequences of replication stress. Nat Cell Biol. 2014;16:2–9. doi: 10.1038/ncb2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Hanks AN, Boucher K, et al. UVB-induced apoptosis drives clonal expansion during skin tumor development. Carcinogenesis. 2005;26:249–57. doi: 10.1093/carcin/bgh300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–34. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Jonason AS, Leffell DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–6. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.