Abstract
Ischemic heart disease is the leading cause of morbidity and mortality throughout the world. Many clinical trials have suggested that lifestyle and pharmacologic interventions are effective in attenuating atherosclerotic disease progression and events development. However, an individualized approach with careful consideration to comprehensive vascular health is necessary to perform successful intervention strategies. Endothelial dysfunction plays a pivotal role in the early stage of atherosclerosis and is also associated with plaque progression and occurrence of atherosclerotic complications. The assessment of endothelial function provides us with important information about individual patient risk, progress and vulnerability of disease, and guidance of therapy. Thus, the application of endothelial function assessment might enable clinicians to innovate ideal individualized medicine. In this review, we summarize the current knowledge on the impact of pharmacological therapies for atherosclerotic cardiovascular disease on endothelial dysfunction, and argue for the utility of non-invasive assessment of endothelial function aiming at individualized medicine.
Keywords: endothelial function, cardiovascular disease, atherosclerosis, prognosis
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality all over the world. Atherosclerosis results from a complex interaction between genetic and environmental factors that causes the arterial wall to respond to inflammatory stimuli. It begins in childhood progressing over decades with a long subclinical phase and affects essentially all arterial beds.1 On occasion, atherosclerosis can cause sudden arterial occlusion from unstable lesions leading to acute clinical events. In order to reduce morbidity and mortality related to ASCVD, increased emphasis is being placed on early identification of at risk patients and their optimal treatment to stabilize, halt, or even modestly regress atherosclerosis.2 Owing to results from large randomized clinical trials, significant advancements have been made, over decades, to define effective treatment for ASCVD. However, there is a notable inter-individual heterogeneity in response to risk factors and cardiovascular (CV) drugs, affecting efficacy. Emerging paradigms that manage individual patients based on their comprehensive vascular health assessment have the potential to unveil novel mechanisms in disease pathogenesis.
Endothelial dysfunction is associated with unfavorable physiological vascular changes such as vasomotor tone alterations, thrombotic dysfunctions, smooth muscle cell proliferation and migration, as well as leukocyte adhesion, and plays a pivotal role in the initial development and progression of atherosclerotic plaque and occurrence of atherosclerotic complications.3, 4 Most CV risk factors have the potential to initiate endothelial cell injury causing endothelial dysfunction.5 Moreover endothelial function is not determined solely by the individual risk factor burden but rather, may be regarded as an integrated index of all atherogenic and atheroprotective factors present in an individual, including unknown factors and genetic predisposition (Figure 1).6 Increasing body of evidence suggests that improvement of endothelial function in response to therapy is associated with reduction in future events.7, 8 Therefore, assessment of endothelial function not only reflects ongoing CV risk but also success of therapy.
Figure 1. Risk factors of atherosclerosis and endothelial dysfunction.
Endothelial dysfunction is a consequence of the harmful effects of risk factors of atherosclerosis on the vessel wall, and may be an integrated index of all atherogenic and atheroprotective factors.
This review will present the current knowledge on the impact of therapeutic interventions, currently available and under development, on endothelial function. Clinical management strategies for ASCVD with endothelial function assessment might enable more accurate risk assessment guiding the indication of pharmacological therapy and more accurate evaluation of treatment efficacy guiding the selection or adjustment of a given pharmacological therapy. Thus, the introduction of endothelial function assessment into clinical practice will bring the development of more tailored medicine in both primary and secondary prevention settings.
2. Endothelial function assessment for individualized medicine
Common approaches to ASCVD risk assessment are based on identifying and quantifying the established risk factors for atherosclerotic diseases to estimate 10-year risk for ASCVD.9 This process represents a uniform, validated and robust method to identify individuals at high-risk for ASCVD. However, many individuals with coronary heart disease (CHD) have only one, or none, of the classic risk factors,10 and these risk factors overall are thought to account for only 50% of CHD,11 indicating the existence of non-traditional risk factors for atherosclerosis (i.e., mental stress, physical inactivity/fitness, genetic factor) (Figure 1). Thus, the current patient-specific approaches may have limitations that derive from the insufficiency of established risk factors to accurately identify individual risk or etiologic causes of atherosclerosis. Direct assessment of vascular damage by measuring endothelial function rather than risk factor estimation could be a reliable method to identify the functional significance of the risk factors. Precise detection of a risk profile will potentially allow both early identification of individuals susceptible to disease and discovery of potential targets for pharmacological or lifestyle intervention.
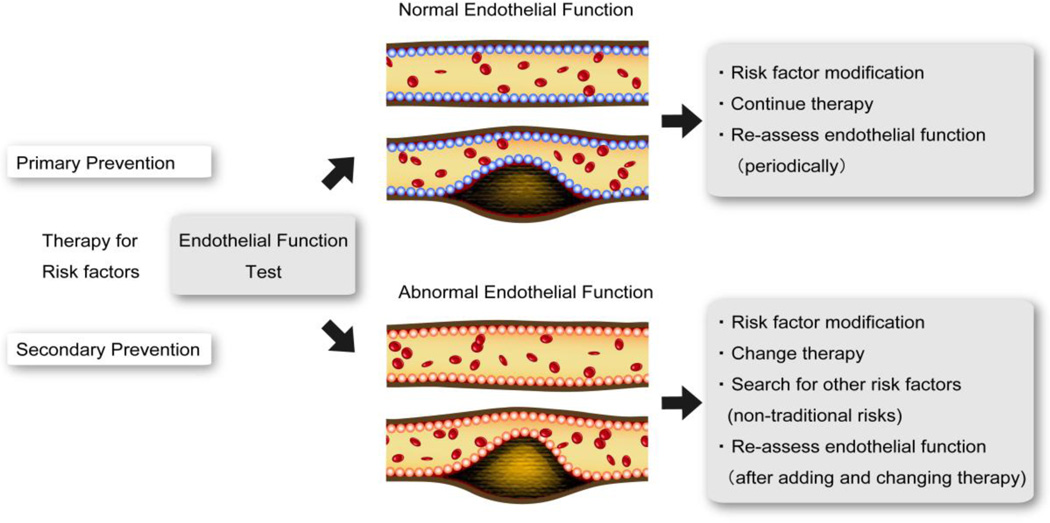
In addition to risk assessment, providing and adjusting optimal treatment in each individual is the goal of individualized medicine. In clinical practice, it is necessary for clinicians to translate scientific evidence from large clinical trials to the treatment of individual patients. Most clinical trials report relative risks or hazard ratios, which are obtained from treating a heterogeneous group of participants.12 In current practice, the same treatment is administered to a wide range of patients who are all assumed to be the “average” patient based on the single point estimate of treatment effect. However, the absolute treatment effect in each patient can largely be affected by individual characteristics. Endothelial function might be reversible at every phase of atherosclerosis, from initiation to atherothrombotic complication.13 Thus it can be a potentially useful clinical strategy, for both physicians and patients, to consider endothelial function in the assessment of atherosclerosis to prevent ASCVD and to determine the efficacy of current ongoing treatments (Figure 2). For example, if a patient had abnormal endothelial function even under optimal medical treatment for traditional risk factors, we need to consider changing therapy and searching other non-traditional risk factors in order to prevent CV events.
Figure 2. Strategy of endothelial function assessment for ASCVD prevention.
ASCVD: atherosclerotic cardiovascular disease.
3. Non-invasive assessment of peripheral endothelial function
Several invasive and noninvasive techniques have been developed for endothelial function testing into clinical practice. The features of commonly used methods to assess endothelial function are summarized in Table 1.14 Invasive assessment by catheterization is considered the reference standard for evaluating coronary endothelial function.14 Catheterization involves intra-arterial administration of endothelium-dependent substances (such as acetylcholine) that enhance release of endothelial nitric oxide (NO) and lead to measurable vasodilatation and increase in coronary blood flow in normal subjects but vasoconstriction and lack of increase in coronary blood flow in patients with endothelial dysfunction. The obvious disadvantage with such a method is that its invasive nature precludes widespread use in the population. Therefore, other non-invasive techniques have been developed based on the diffuse nature of endothelial dysfunction, most of which are based on the same principle of reactive hyperemia. The forearm flow mediated vasodilatation (FMD) is a non-invasive method to evaluate peripheral endothelial function, and its measures correlate well with coronary artery endothelial function by catheterization.15 Another major method based on the same principle to assess peripheral endothelial function is the reactive hyperemia-peripheral arterial tonometry (RH-PAT),16 whose response also correlates well with the presence of coronary artery endothelial dysfunction.17 Although, the majority of the non-invasive endothelial function tests use reactive hyperemia after occlusion as a trigger to detect endothelial dependent vasodilation, FMD represents conduit artery vasodilation, and RH-PAT represents microvessel vasodilation. Moreover, RH-PAT is adjusted for any changes that occur in the control arm, a distinction from the FMD method. Given its repeatability, non-invasive endothelial function assessment is useful in evaluating the clinical efficacy of traditional and new approaches for CV diseases.
Table 1.
The methods to assess endothelial function.
| CAG | Forearm perfusion technique |
FMD | RH-PAT | |
|---|---|---|---|---|
| Vascular beds | Coronary | Peripheral | Peripheral | Peripheral |
| Trigger | Infusion of endothelial dependent vasodilator | Infusion of endothelial dependent vasodilator | Reactive hyperemia | Reactive hyperemia |
| Measurement | Vessel diameter Blood flow | Plethysmogram | Vessel diameter | Plethysmogram |
| Non-invasive | − | − | + | + |
| Predictive for cardiovascular events | ++ | ++ | ++ | ++ |
| Reversible with interventions | + | + | + | + |
| Adjustment by control vessel | + | + | − | ++ |
| Operator independent | +/− | +/− | +/− | ++ |
| Easily operated | − | − | − | + |
| Not expensive | − | + | + | +/− |
CAG: coronary angiography, FMD: forearm flow mediated vasodilation, and RH-PAT: reactive hyperemia-peripheral arterial tonometry.
In the setting of established CHD, patients with endothelial dysfunction were reported to have a higher rate of adverse CV events,18 and improvement in peripheral endothelial function is associated with significant reduction in future CV events.7 A past report has documented that overall survival in patients with CHD is largely independent of the degree of coronary luminal stenosis.19 Matsuzawa et al. reported that even after adjustment for coronary plaque complexity, impaired peripheral endothelial function significantly predicted future CV events.20 The prognostic value of brachial FMD and RH-PAT for ASCVD events in both primary and secondary prevention has been demonstrated in several studies and meta-analyses.8, 21–25
5. The impact of pharmacologic interventions on endothelial function
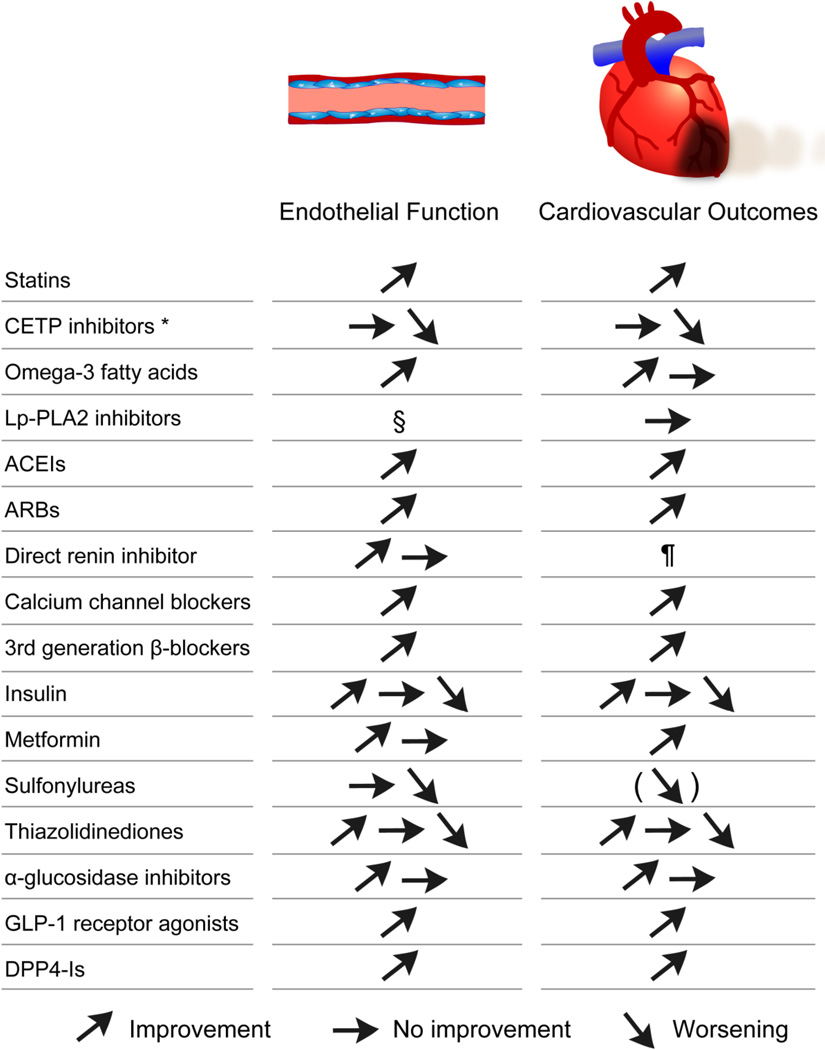
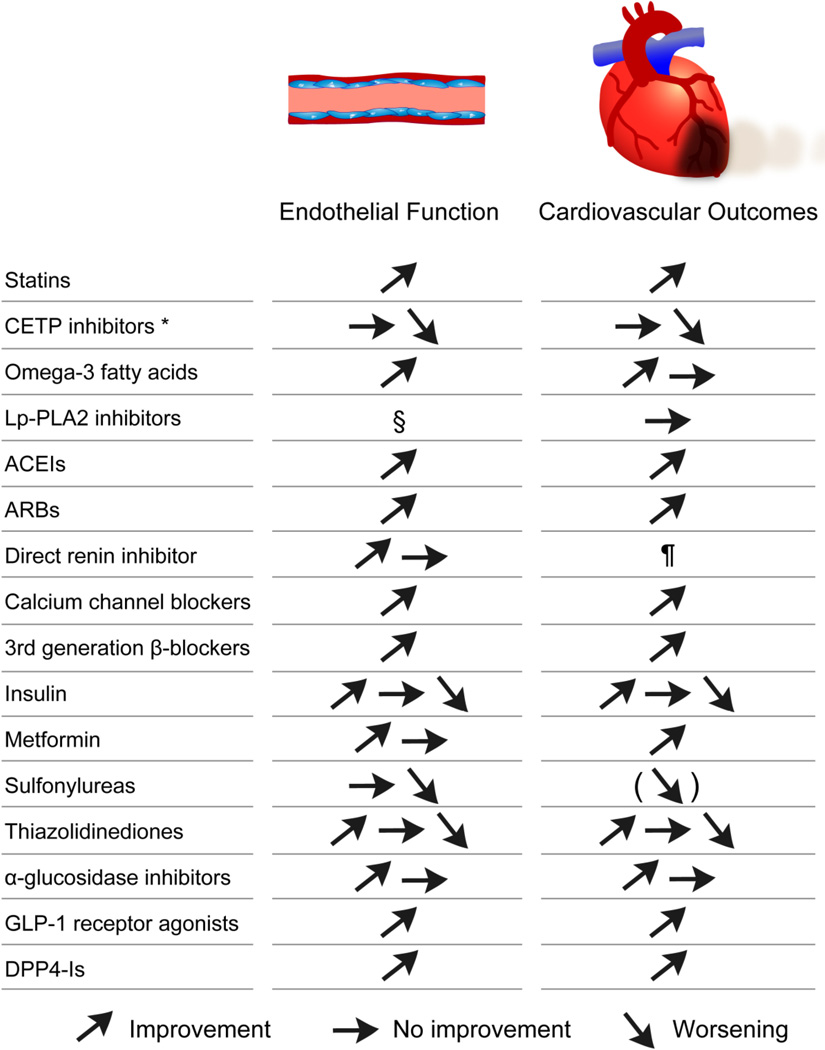
Effective management of atherosclerotic disease includes pharmacologic treatment of specific risk factors and lifestyle modifications, such as smoking cessation, weight loss, diet change, and exercise. Over the decades, pharmacological intervention in atherosclerotic diseases has advanced dramatically. Although, many clinical trials have suggested that many kinds of pharmacologic interventions are effective in preventing atherosclerotic disease progression, clinical management of atherosclerosis is quite difficult as there is no recognized method to prevent or improve the entire vascular bed. Thus, an individualized approach with comprehensive vascular health assessment by endothelial function test might be beneficial to provide a tailored treatment according to the specificities of atherosclerosis in a given patient. Here, we review the evidence of the impact of pharmacological treatment for atherosclerosis on endothelial function. Figure 3 shows a brief summary on the effects of each drug on endothelial function and CV outcomes.
Figure 3. Brief summary on the effects of each drug on endothelial function and cardiovascular outcomes.
* Torcetrapib and dalcetrapib.
§ Although there is no data on effect of Lp-PLA2 inhibitors on endothelial function, it was reported that Lp-PLA2 was not associated with endothelial dysfunction.50
¶ Clinical trials are ongoing to identify the role of direct renin inhibition for atherosclerotic diseases.
ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin II receptor blocker, CETP: cholesterol ester transfer protein, DPP4-I: dipeptidyl peptidase 4 inhibitor, GLP-1: glucagonlike peptide-1, and Lp-PLA2: lipoprotein-associated phospholipase A2.
5.1. Statins
The use of statins for ASCVD prevention is clearly supported by clinical evidence and is recommended by clinical guidelines.26 Statins reduce ASCVD events beyond their cholesterol-lowering effects and play an important role in the primary and secondary prevention of ASCVD in at-risk individuals.27 The beneficial effect of statins on coronary and peripheral endothelial function are attributed partly to their anti-inflammatory and anti-oxidant properties.27 A recent meta-analysis of 46 randomized clinical trials concluded that statin therapy is associated with a significant improvement in both coronary and peripheral endothelial function.28 Subgroup analyses revealed that this significant beneficial effect did not differ by diabetes or CHD.28 However, not all studies showed the beneficial effect of statins on endothelial function.29 It is noteworthy that considerable residual risk persists among statin-treated patients, with rates of CV events being approximately two-thirds to three-quarters that of placebo-treated patients in clinical trials.30, 31 It has been reported that even with maximal statin therapy, approximately 22% of patients with recent acute coronary syndrome (ACS) and 9% of patients with stable CHD proceeded to a second CV event at 2 years and 5 years of follow-up periods, respectively,32, 33 and therefore many patients are not completely protected by their current therapeutic regimens.
5.2. Cholesterol ester transfer protein inhibitors
Cholesterol ester transfer protein (CETP) is a plasma protein that facilitates the transport of cholesteryl esters from high-density lipoprotein (HDL) to apolipoprotein (Apo) B-containing lipoproteins, and is currently a target for increasing HDL-cholesterol, by inhibition of this transport between low-density lipoprotein (LDL) and HDL particles. Notwithstanding a significant increase in HDL-cholesterol, surprisingly an unexpected increase in CV events and mortality was observed in patients treated with torcetrapib, the CETP inhibitor, in the Investigation of Lipid Level Management to Understand Its Impact in Atherosclerotic Events (ILLUMINATE) study.34 It was suggested that the sustained and marked impairment of endothelial function may at least in part explain the increased mortality associated with torcetrapib treatment in the trial.35 Dalcetrapib did not improve endothelial function either in the Dal-VESSEL study.36 Subsequently, the phase III outcome trial for dalcetrapib, dal-OUTCOMES trial, was terminated at a prespecified interim analysis after a median follow-up of 31 months due to lack of efficacy on major CV outcomes.37 Anacetrapib and evacetrapib, highly potent CETP inhibitors, recently entered phase III outcome trials. In the phase III safety study (DEFINE), anacetrapib showed no torcetrapib-like adverse effects.38 The impact of anacetrapib and evacetrapib on endothelial function has not been investigated yet.
5.3. Omega-3 fatty acids
A number of randomized clinical trials have been designed specifically to provide a controlled evaluation of the effects of omega-3 fatty acids on CV events. Conflicting findings have been found on this issue,39 however, large scale prospective studies and meta-analyses have demonstrated that intake of omega-3 fatty acids has a beneficial impact on CV outcomes.40–42 Furthermore a recent meta-analysis of randomized controlled trials suggested that supplementation of omega-3 fatty acids may improve endothelial function.43 Although the mechanism underlying this protective effect has not been identified, reduced production of inflammatory cytokines might partly contribute.44
5.4. Lipoprotein-associated phospholipase A2
inhibitors Lipoprotein-associated phospholipase A2 (Lp-PLA2) is highly expressed in atherosclerotic lesions, in particular vulnerable plaques,45, 46 and has been shown to increase inflammation through producing arachidonic acid precursors from membrane glycerophospholipids,47 suggesting that this enzyme might be a potential therapeutic target. However the phase III Stabilization of Atherosclerotic Plaque By Initiation of Darapladib Therapy (STABILITY) trial involving 15,828 CHD patients reported that darapladib, a selective oral inhibitor of Lp-PLA2, did not significantly reduce the risk of the composite endpoint of CV death, myocardial infarction (MI), and stroke.48 Similarly, the randomized controlled trial of 13,026 patients with ACS, Darapladib-Thrombolysis in Myocardial Infarction (SOLID-TIMI 52) trial, demonstrated that the addition of darapladib to optimal medical therapy did not reduce the risk of major coronary events.49 The Multi-Ethnic Study of Atherosclerosis (MESA) which measured Lp-PLA2 and endothelial function in a total of 2809 participants,50 reported that Lp-PLA2 was not associated with endothelial dysfunction. So far, data on the impact of darapladib on endothelial function is lacking.
5.5. Renin-angiotensin system inhibitors
Angiotensin converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) are well established drugs to prevent CV events in heart failure patients and are recommended by the current guidelines.26 Also, ACEIs have been reported to reduce CV death, MI, and stroke in high-risk patients and stable CHD patients without a low ejection fraction or heart failure,51, 52 and are recommended in all patients with ASCVD.26 A randomised study, Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANSCEND) study, demonstrated that telmisartan modestly reduced the risk of CV death, MI, and stroke in patients intolerant to ACEIs. ACEIs and ARBs are involved in renin-angiotensin-aldosterone system (RAAS), and they have similar mechanisms of action. In addition to lowering blood pressure, ACEIs and ARBs possess direct CV protective effects. ACEIs improve endothelial function by reducing the production of angiotensin II by inhibiting angiotensin converting enzyme, a key enzyme affecting the transformation from angiotensin I to angiotensin II and increasing bradykinin production.53 Similarly, the mechanisms by which ARBs improve endothelial function are based on their ability to inhibit angiotensin II receptors.53 Although, studies on the effects of ACEIs and ARBs on endothelial dysfunction have yielded conflicting results, 2 meta-analyses of randomized controlled trials demonstrated that each of ACEIs and ARBs improved peripheral endothelial function and are superior to other anti-hypertensive drugs including calcium channel blockers (CCBs) and β adrenergic receptor blocking agents (βBs). Furthermore there was no significant difference between ACEIs and ARBs effect on endothelial function.54, 55
Aliskiren is a non-peptide renin inhibitor, which blocks RAAS at the first and rate-limiting step, reducing the circulating levels of angiotensin II. Thus, at least theoretically, aliskiren should inhibit RAAS more effectively than ACEIs and ARBs. A recent study of 30 uncontrolled hypertensive patients reported that endothelial function significantly improved 6 months after the addition of aliskiren.56 However, another study demonstrated that 4 months treatment with aliskiren decreased circulating endothelial progenitor cells, which are considered important contributors to vascular repair, compared to placebo.57 Evidence on the effect of aliskiren on endothelial function in humans is scarce, and the results are conflicting. Also, clinical efficacy of aliskiren on CV outcomes is still not clear. Ongoing clinical trials series, evaluating the effects of aliskiren on CV outcomes, will identify the role of direct renin inhibition as an alternative treatment for hypertension and other atherosclerotic diseases.
5.6. Calcium channel blockers
Because of increased adverse CV events associated with rapid release, the role of short acting CCBs in CHD treatment was previously limited.58 However, data from many subsequent large clinical trials confirmed the safety and efficacy of long-acting CCBs.59, 60 They are divided into dihydropyridines (DHP) and non-dihydropyridines (non-DHP). Although the mechanisms of CCBs action vary across classes, generally they improve the balance between myocardial oxygen supply and demand by inducing coronary artery dilation, systemic vasodilation, negative inotropic, and for non-DHPs negative chronotropic effect. In addition, CCBs have been reported to exert pleiotropic effects.61 Of particular importance, certain DHP-type CCBs have been shown to modify endothelial function by enhancing endothelial NO synthase activity resulting in increased NO production.62 Although not randomized, one study showed that lacidipine improved peripheral endothelial function as assessed by intra-brachial infusion of acetylcholine and bradykinin, in hypertensive subjects.63 The Evaluation of Nifedipine and Cerivastatin on Recovery of Coronary Endothelial Function (ENCORE)-1 and -2 trials showed that long-acting nifedipine consistently improved coronary endothelial function for up to 2 years in patients with stable CHD.64, 65 On the other hand, in a randomized double-blind study of apparently healthy young adults with a strong family history of premature CHD but no other identifiable risk factors, although amlodipine significantly improved peripheral endothelial function, its improvement did not significantly differ from the placebo group.66 To date no meta-analyses on the effects of CCBs on endothelial function have been reported. Further studies involving more patients and several classes of CCBs are needed to clarify these relationships.
5.7. β adrenergic receptor blocking agents
βBs are generally recommended for the treatment of patients with ACS or heart failure, because of their proven positive effects on life expectancy, risk of sudden cardiac death and left ventricular ejection fraction.26 βBs have emerged in 3 generations. The first and second generation βBs have no significant ancillary properties, with the former being nonselective and the latter selective for either β1 or β2 adrenergic receptors. However, a different effectiveness has been proposed for the third-generation βBs, such as nebivolol and carvedilol. Nebivolol causes vasodilation primarily through the release of endothelium-derived NO.67 Interestingly, infusion of nebivolol intra-arterially in the forearm of healthy subjects is associated with an increase in forearm blood flow, which can be prevented by NO synthesis inhibition.68 It is thought to be mediated through β3 receptor activation and by interaction with estrogen receptors.69 Carvedilol, a nonselective β-blocker with additional α1 adrenoceptor antagonist activity, also has been shown to elevate antioxidant effect and improve endothelial dysfunction.70 In a recent randomized study of patients with hypertension and diabetes mellitus, compared with metoprolol, carvedilol was able to improve endothelial function as assessed by FMD.71
5.8. Anti-diabetic drugs
Macrovascular disease is the major cause of morbidity and mortality in type 2 diabetes (T2D) and data suggests a consistent relationship between glycemic control and the frequency of diabetic complications.72 However, clinical trials to date, including the United Kingdom Prospective Diabetes Study (UKPDS)73 and The Diabetes Control and Complications Trial (DCCT)74, have not provided conclusive evidence that improved glycemic control reduces the risk for macrovascular diseases. Furthermore, recent landmark trials, including the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation (ADVANCE) trial, and Veterans Affairs Diabetes Trial (VADT), have raised new concerns about possible risks associated with intensive glycemic control.75–77 In accordance with these results, it was reported that brachial artery endothelial function was not influenced by reduction in hemoglobin A1c.78 A recent study using continuous glucose monitoring demonstrated that fluctuations in blood glucose levels play an important role in endothelial dysfunction in patients with T2D,79 suggesting that quality of blood glucose lowering including modification of blood glucose fluctuations is required in addition to lowering hemoglobin A1c.
5.8.1 Insulin therapy
Insulin therapy is well established as the most effective means of managing blood glucose levels in diabetes.73, 80 A past study reported that early introduction of insulin therapy may improve endothelial function in patients with type 1 diabetes.81 On the other hand, in patients with T2D, the role of insulin therapy in endothelial dysfunction is still controversial. Although the addition of isophane insulin glargine improved forearm vascular reactivity in patients treated with metformin alone,82 insulin may worsen endothelial dysfunction in subjects with impaired sensitivity of the phosphatidylinostol 3-kinase-dependent pathways.83 The controversy concerning the putative atherogenic effects of insulin may reflect differences in the way in which the two pathways (phosphatidylinositol kinase (PI-3K) pathway and mitogen-activated protein kinase (MAPK) pathway) are affected by insulin in the healthy and insulin-resistant endothelium.84 The lack of clarity surrounding this issue might also involve failure to separate the effects of insulin from those of pro-insulin, which is pro-atherogenic.
5.8.2 Metformin
Meta analyses, in which only studies prior to incretin-associated drugs were included, demonstrated that compared with other oral diabetes agents and placebo, metformin was moderately associated with a decreased risk of CV mortality.85 Thus metformin has been established as a first-line drug for the management of T2D. Metformin improves insulin sensitivity and glucose homeostasis and is demonstrated to activate 5’ adenosine monophosphate–activated protein kinase (AMPK) in tissues.86 AMPK system controls systemic energy balance and metabolism, and may be partly responsible for the health benefit of exercise.86 Interestingly, many studies in several patient groups, including patients with type 1 diabetes, have demonstrated that administration of metformin improves endothelial function,87–89 although, a few showed no significant beneficial effect.90
5.8.3 Sulfonylureas
Sulphonylurea use may elevate the risk of CV disease in patients with T2D,91 but there is insufficient evidence from randomized controlled trials to determine its clinical efficacy on CV outcomes.92 Through stimulating insulin secretion, sulfonylureas are believed to favor the development of hypoglycemia and weight gain, accelerate beta-cell apoptosis and beta-cell exhaustion and impair endothelial function, thereby increasing the risk for ischemic complications. Consistently, several studies have reported negative effects of glibenclamide on endothelial function, although a few showed improved endothelial function, e.g., gliclazide has anti-oxidant properties that might prevent endothelial dysfunction.86, 93 To date there is no robust clinical evidence, however, there might be differences among sulfonylureas regarding their effects on endothelial function.
5.8.4 Thiazolidinediones
A meta-analysis of 19 randomized trials with pioglitazone found a statistically significant reduction in the composite outcome of nonfatal MI, stroke, and all-cause mortality.94 Whereas, in 2007, a meta-analysis of 42 randomized controlled trials suggested that rosiglitazone increased the risk of MI and CV deaths.95 However, in 2009, the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes (RECORD) trial demonstrated no significant increased risk of CV events associated with rosiglitazone compared to metformin or sulfonylurea.96 Moreover, it was reported that both pioglitazone and rosiglitazone might increase the risk for congestive heart failure.97, 98 The peroxisome proliferator activated receptors γ, to which thiazolidinediones bind, are expressed in adipose tissue, pancreatic β-cells, endothelium and macrophages.99 Thiazolidinediones activate endothelial NO synthase,100 and might also have antioxidant properties thereby increasing NO bioavailability.101 Thus, it is suggested that thiazolidinediones have direct beneficial vascular effects in addition to secondary effects via the improvement in metabolic milieu. The salutary effects of thiazolidinediones on endothelium have been reported by several clinical studies,102–104 however, not all studies have come to the same conclusion.105 Furthermore, it is reported that rosiglitazone can reduce intracellular levels of the enzyme involved in tetrahydrobiopterin synthesis, and inhibit cytokine-induced NO synthesis.106 Thus, its effect on NO bioavailability and NO synthase function remains to be fully explored, and the issue of the independent effect of these drugs on endothelial function remains unsolved.
5.8.5 α-Glucosidase inhibitors
Repeated post-prandial hyperglycemia might have an important role in the development of atherosclerosis by suppressing vascular endothelial function.107 α-glucosidase inhibitors delay digestion of complex carbohydrates in the upper small bowel and subsequently retard absorption of glucose and ‘blunt’ postprandial hyperglycemia by inhibiting of α-glucosidases in the brush-border of the small intestine. Unlike some other blood glucose lowering agents, no adverse signals of potential CV risk have emerged in relation to α-glucosidase inhibitors use. On the contrary, significant beneficial CV outcome results have been reported by the landmark Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) trial.108 However other large randomised clinical trials, such as the Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus (HEART2D) trial and the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) trial, failed to show beneficial effect of α-glucosidase inhibitors on CV outcomes.109, 110 In agreement with these results from clinical trials, the reported effect of α-glucosidase inhibitors is also conflicting. Little evidence suggests that by diminishing postprandial hyperglycemia and thereby limiting glucotoxicity on the vessels acarbose improves endothelial function.111 However, some studies showed no significant beneficial effect of α-glucosidase inhibitors on endothelial function.112
5.8.6. Incretins GLP-1 and DPP4-I
In addition to the well-characterized actions of glucagonlike peptide-1(GLP-1) on glycemic controls, GLP-1 acts on endothelium and cardiac and vascular myocytes which express a functional GLP-1 receptor.113 Furthermore, GLP-1 receptor dependent and independent pathways have been proposed for the beneficial CV effects of GLP-1.113 Dipeptidyl peptidase 4 inhibitor (DPP4-I) maintains the plasma level of active GLP-1, and increases NO production with increased endothelial NO synthase phosphorylation.114 In recent years, incretin mimetics GLP-1 receptor agonists and DPP4-Is have received particular attention for their potential to positively impact CV outcomes. Although an increasing body of literature from preclinical and early phase clinical studies has indicated that both GLP-1 receptor agonists and DPP4-Is may exert glucose-independent beneficial effects on CV outcomes,115 the results from ongoing large-scale trials might provide valuable new insights about the impact of these incretin-based therapies on CV outcomes. Several clinical studies reported that endothelial function was improved by GLP-1 and DPP4-I,116–119 although their effect remains controversial, because most of these were non-randomized trials and included a small number of patients. Large-scale randomized studies are needed to further clarify the impact of GLP-1 and DPP4-I on endothelial function.
5.9. Other potential drugs and foods
Other than the above mentioned, to date, many other drugs and food products have been reported potentially to have beneficial effects on endothelial function. L-arginine, a semi-essential amino acid, acts as the substrate for endothelial NO synthase enzyme. Although several studies have reported the effect of oral L-arginine supplementation on endothelial function, the data in humans are varied, possibly because of small sample sizes and short durations.120 Tetrahydrobiopterin, an essential factor for endothelial NO synthase, has an important role in regulating endothelial NO synthase enzymatic activity, and it is demonstrated that acute administration of tetrahydrobiopterin improved endothelial function.121, 122 However, evidence of long term effects is lacking. Other potential agents include anti-oxidative vitamins including folic acid and vitamin C, flavonoids, dark chocolate, black tea, green tea, polyphenol-rich olive oil, and red wine. Although these may possess anti-athrogenic effects, the effects of these on endothelial function, atherosclerotic diseases progression and CV outcomes remain to be elucidated.
6. Future directions for research
Our knowledge on the mechanisms of atherosclerosis and CV diseases is still limited. There might be several unknown risk factors for atherosclerosis, and probably more effective interventions which are yet to be discovered. As reported in this review, the efficacy of drugs on endothelial function is mostly in line with results from large clinical trials; indicating that by using endothelial function assessment we can treat atherosclerosis itself, rather than risk factor control. Thus, endothelial function testing is a potential field for future research to evaluate the effect of different pharmacological and lifestyle interventions. Using pharmacological therapies, lifestyle modification, and other emerging approaches, therapy guided by individual endothelial function measurements might be feasible in CV practice. However, large scale randomized studies in this area are needed to answer the question of whether endothelial function-guided therapies will provide benefits in improving outcomes in patients with ASCVD risk factors and in patients with established ASCVD. Such further studies may usher us into a new era of individualized medicine in cardiology.
7. Conclusion
The concept of individualized medicine is currently applied in ASCVD management, in both primary and secondary prevention settings, although several aspects are still under investigation. Since the vascular endothelium plays a crucial role in the pathogenesis of atherosclerosis, assessment of endothelial function enables us to directly evaluate atherosclerosis instead of quantifying risk factors, some of which are yet unknown, and can provide us important information for individual patient risk assessment, prognosis determination, and guidance of therapy. It can also be applied to the field of pharmacology in developing new therapies and repositioning of existing drugs to treat new diseases. Thus, application of endothelial function assessment might contribute to innovate ideal individualized medicine. Although, most of the current pharmacological therapies for atherosclerosis have been demonstrated to improve endothelial function, our knowledge of the mechanisms involved in endothelial dysfunction is only the tip of the iceberg. Further research should be directed at shedding light on novel therapeutic targets and determining whether non-invasive endothelial function assessment can be useful to guide treatment and change outcomes. These will yield tremendous benefits in improving CV practice.
Acknowledgements
A.L. is supported by the National Institute of Health (NIH Grants HL-92954 and AG-31750), and the Mayo Foundation.
Abbreviations
- ACEI
Angiotensin converting enzyme inhibitor
- ACS
Acute coronary syndrome
- AMPK
5’ adenosine monophosphate–activated protein kinase
- Apo
Apolipoprotein
- ARB
Angiotensin II receptor blocker
- ASCVD
Atherosclerotic cardiovascular disease
- βB
β adrenergic receptor blocking agent
- CCB
Calcium channel blocker
- CETP
Cholesterol ester transfer protein
- CHD
Coronary heart disease
- CV
Cardiovascular
- DHP
Dihydropyridine
- DPP4-I
Dipeptidyl peptidase 4 inhibitor
- FMD
Flow mediated vasodilatation
- GLP-1
Glucagonlike peptide-1
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- Lp-PLA2
Lipoprotein-associated phospholipase A2
- MAPK
Mitogen-activated protein kinase
- MI
Myocardial infarction
- NO
Nitric oxide
- PI-3K
Phosphatidylinositol kinase
- RAAS
Renin-angiotensin-aldosterone system
- RH-PAT
Reactive hyperemia-peripheral arterial tonometry
- T2D
Type 2 diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of conflict of interest
Author A.L. declared consulting for Itamar Medical.
References
- 1.Libby P. Inflammation in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puri R, Nissen SE, Libby P, et al. C-reactive protein, but not low-density lipoprotein cholesterol levels, associate with coronary atheroma regression and cardiovascular events after maximally intensive statin therapy. Circulation. 2013;128(22):2395–2403. doi: 10.1161/CIRCULATIONAHA.113.004243. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Gossl M, Yoon MH, Choi BJ, et al. Accelerated coronary plaque progression and endothelial dysfunction: serial volumetric evaluation by IVUS. JACC Cardiovascular imaging. 2014;7(1):103–104. doi: 10.1016/j.jcmg.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117(19):2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arteriosclerosis, thrombosis, and vascular biology. 2003;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 7.Kitta Y, Obata JE, Nakamura T, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. Journal of the American College of Cardiology. 2009;53(4):323–330. doi: 10.1016/j.jacc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 8.Modena MG, Bonetti L, Coppi F, et al. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. Journal of the American College of Cardiology. 2002;40(3):505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 9.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013 doi: 10.1016/j.jacc.2013.11.005. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA : the journal of the American Medical Association. 2003;290(7):898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 11.Reriani MK, Flammer AJ, Jama A, et al. Novel Functional Risk Factors for the Prediction of Cardiovascular Events in Vulnerable Patients Following Acute Coronary Syndrome. Circulation Journal. 2012;76(4):778–783. doi: 10.1253/circj.cj-12-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA : the journal of the American Medical Association. 2007;298(10):1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 13.Pepine CJ. The effects of angiotensin-converting enzyme inhibition on endothelial dysfunction: potential role in myocardial ischemia. The American journal of cardiology. 1998;82(10A):23S–27S. doi: 10.1016/s0002-9149(98)00805-4. [DOI] [PubMed] [Google Scholar]
- 14.Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26(5):1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 16.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. American heart journal. 2003;146(1):168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 17.Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. Journal of the American College of Cardiology. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 18.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111(3):363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 19.Little WC, Downes TR, Applegate RJ. The underlying coronary lesion in myocardial infarction: implications for coronary angiography. Clinical cardiology. 1991;14(11):868–874. doi: 10.1002/clc.4960141103. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzawa Y, Sugiyama S, Sumida H, et al. Peripheral endothelial function and cardiovascular events in high-risk patients. Journal of the American Heart Association. 2013;2(6):e000426. doi: 10.1161/JAHA.113.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeboah J, Crouse JR, Hsu FC, et al. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115(18):2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 22.Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(6):502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. The international journal of cardiovascular imaging. 2010;26(6):631–640. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 24.Ras RT, Streppel MT, Draijer R, et al. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. International journal of cardiology. 2013;168(1):344–351. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Arora RC, Hiebert BM, et al. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. European heart journal cardiovascular Imaging. 2014 doi: 10.1093/ehjci/jet256. [DOI] [PubMed] [Google Scholar]
- 26.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 27.Bonetti PO, Lerman LO, Napoli C, et al. Statin effects beyond lipid lowering--are they clinically relevant? European heart journal. 2003;24(3):225–248. doi: 10.1016/s0195-668x(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 28.Reriani MK, Dunlay SM, Gupta B, et al. Effects of statins on coronary and peripheral endothelial function in humans: a systematic review and meta-analysis of randomized controlled trials. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2011;18(5):704–716. doi: 10.1177/1741826711398430. [DOI] [PubMed] [Google Scholar]
- 29.Vita JA, Yeung AC, Winniford M, et al. Effect of cholesterol-lowering therapy on coronary endothelial vasomotor function in patients with coronary artery disease. Circulation. 2000;102(8):846–851. doi: 10.1161/01.cir.102.8.846. [DOI] [PubMed] [Google Scholar]
- 30.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 31.Cholesterol Treatment Trialists C. Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. The New England journal of medicine. 2004;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 33.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. The New England journal of medicine. 2005;352(14):1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 34.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. The New England journal of medicine. 2007;357(21):2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 35.Simic B, Hermann M, Shaw SG, et al. Torcetrapib impairs endothelial function in hypertension. European heart journal. 2012;33(13):1615–1624. doi: 10.1093/eurheartj/ehr348. [DOI] [PubMed] [Google Scholar]
- 36.Luscher TF, Taddei S, Kaski JC, et al. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. European heart journal. 2012;33(7):857–865. doi: 10.1093/eurheartj/ehs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. The New England journal of medicine. 2012;367(22):2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 38.Cannon CP, Shah S, Dansky HM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. The New England journal of medicine. 2010;363(25):2406–2415. doi: 10.1056/NEJMoa1009744. [DOI] [PubMed] [Google Scholar]
- 39.Hooper L, Thompson RL, Harrison RA, et al. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. The Cochrane database of systematic reviews. 2004;(4):CD003177. doi: 10.1002/14651858.CD003177.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA : the journal of the American Medical Association. 2002;287(14):1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 41.He K, Song Y, Daviglus ML, et al. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109(22):2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 42.Casula M, Soranna D, Catapano AL, et al. Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: A meta-analysis of randomized, placebo controlled trials [corrected] Atherosclerosis Supplements. 2013;14(2):243–251. doi: 10.1016/S1567-5688(13)70005-9. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Liang X, Wang L, et al. Effect of omega-3 fatty acids supplementation on endothelial function: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221(2):536–543. doi: 10.1016/j.atherosclerosis.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Egert S, Stehle P. Impact of n-3 fatty acids on endothelial function: results from human interventions studies. Current opinion in clinical nutrition and metabolic care. 2011;14(2):121–131. doi: 10.1097/MCO.0b013e3283439622. [DOI] [PubMed] [Google Scholar]
- 45.Finn AV, Nakano M, Narula J, et al. Concept of vulnerable/unstable plaque. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(7):1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 46.Kolodgie FD, Burke AP, Skorija KS, et al. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(11):2523–2529. doi: 10.1161/01.ATV.0000244681.72738.bc. [DOI] [PubMed] [Google Scholar]
- 47.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochimica et biophysica acta. 2006;1761(11):1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Investigators S. White HD, Held C, et al. Darapladib for preventing ischemic events in stable coronary heart disease. The New England journal of medicine. 2014;370(18):1702–1711. doi: 10.1056/NEJMoa1315878. [DOI] [PubMed] [Google Scholar]
- 49.O'Donoghue ML, Braunwald E, White HD, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA : the journal of the American Medical Association. 2014;312(10):1006–1015. doi: 10.1001/jama.2014.11061. [DOI] [PubMed] [Google Scholar]
- 50.Garg PK, McClelland RL, Jenny NS, et al. Association of lipoprotein-associated phospholipase A(2) and endothelial function in the Multi-Ethnic Study of Atherosclerosis (MESA) Vascular medicine. 2011;16(4):247–252. doi: 10.1177/1358863X11411360. [DOI] [PubMed] [Google Scholar]
- 51.Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. The New England journal of medicine. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 52.Fox KM. Investigators EUtOrocewPiscAd: Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362(9386):782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe T, Barker TA, Berk BC. Angiotensin II and the endothelium: diverse signals and effects. Hypertension. 2005;45(2):163–169. doi: 10.1161/01.HYP.0000153321.13792.b9. [DOI] [PubMed] [Google Scholar]
- 54.Shahin Y, Khan JA, Samuel N, et al. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: a meta-analysis of randomised controlled trials. Atherosclerosis. 2011;216(1):7–16. doi: 10.1016/j.atherosclerosis.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 55.Li S, Wu Y, Yu G, et al. Angiotensin II Receptor Blockers Improve Peripheral Endothelial Function: A Meta-Analysis of Randomized Controlled Trials. Plos One. 2014;9(3) doi: 10.1371/journal.pone.0090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonadei I, Vizzardi E, D'Aloia A, et al. Role of aliskiren on arterial stiffness and endothelial function in patients with primary hypertension. Journal of clinical hypertension. 2014;16(3):202–206. doi: 10.1111/jch.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flammer AJ, Gossl M, Li J, et al. Renin inhibition with aliskiren lowers circulating endothelial progenitor cells in patients with early atherosclerosis. Journal of hypertension. 2013;31(3):632–635. doi: 10.1097/HJH.0b013e32835c6d2d. [DOI] [PubMed] [Google Scholar]
- 58.Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92(5):1326–1331. doi: 10.1161/01.cir.92.5.1326. [DOI] [PubMed] [Google Scholar]
- 59.Poole-Wilson PA, Lubsen J, Kirwan BA, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet. 2004;364(9437):849–857. doi: 10.1016/S0140-6736(04)16980-8. [DOI] [PubMed] [Google Scholar]
- 60.Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 61.Preston Mason R. Pleiotropic effects of calcium channel blockers. Current hypertension reports. 2012;14(4):293–303. doi: 10.1007/s11906-012-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang EH, Vanhoutte PM. Endothelial dysfunction: a strategic target in the treatment of hypertension? Pflugers Archiv : European journal of physiology. 2010;459(6):995–1004. doi: 10.1007/s00424-010-0786-4. [DOI] [PubMed] [Google Scholar]
- 63.Taddei S, Virdis A, Ghiadoni L, et al. Lacidipine restores endothelium-dependent vasodilation in essential hypertensive patients. Hypertension. 1997;30(6):1606–1612. doi: 10.1161/01.hyp.30.6.1606. [DOI] [PubMed] [Google Scholar]
- 64.Investigators E. Effect of nifedipine and cerivastatin on coronary endothelial function in patients with coronary artery disease: the ENCORE I Study (Evaluation of Nifedipine and Cerivastatin On Recovery of coronary Endothelial function) Circulation. 2003;107(3):422–428. doi: 10.1161/01.cir.0000046488.52939.bf. [DOI] [PubMed] [Google Scholar]
- 65.Luscher TF, Pieper M, Tendera M, et al. A randomized placebo-controlled study on the effect of nifedipine on coronary endothelial function and plaque formation in patients with coronary artery disease: the ENCORE II study. European heart journal. 2009;30(13):1590–1597. doi: 10.1093/eurheartj/ehp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clarkson P, Mullen MJ, Donald AE, et al. The effect of amlodipine on endothelial function in young adults with a strong family history of premature coronary artery disease: a randomised double blind study. Atherosclerosis. 2001;154(1):171–177. doi: 10.1016/s0021-9150(00)00455-x. [DOI] [PubMed] [Google Scholar]
- 67.Gao YS, Nagao T, Bond RA, et al. Nebivolol induces endothelium-dependent relaxations of canine coronary arteries. Journal of cardiovascular pharmacology. 1991;17(6):964–969. doi: 10.1097/00005344-199106000-00016. [DOI] [PubMed] [Google Scholar]
- 68.Cockcroft JR, Chowienczyk PJ, Brett SE, et al. Nebivolol vasodilates human forearm vasculature: evidence for an L-arginine/NO-dependent mechanism. The Journal of pharmacology and experimental therapeutics. 1995;274(3):1067–1071. [PubMed] [Google Scholar]
- 69.Broeders MA, Doevendans PA, Bekkers BC, et al. Nebivolol: a third-generation beta-blocker that augments vascular nitric oxide release: endothelial beta(2)-adrenergic receptor-mediated nitric oxide production. Circulation. 2000;102(6):677–684. doi: 10.1161/01.cir.102.6.677. [DOI] [PubMed] [Google Scholar]
- 70.Feuerstein GZ, Ruffolo RR., Jr Carvedilol, a novel multiple action antihypertensive agent with antioxidant activity and the potential for myocardial and vascular protection. European heart journal. 1995;16(Suppl F):38–42. doi: 10.1093/eurheartj/16.suppl_f.38. [DOI] [PubMed] [Google Scholar]
- 71.Bank AJ, Kelly AS, Thelen AM, et al. Effects of carvedilol versus metoprolol on endothelial function and oxidative stress in patients with type 2 diabetes mellitus. American journal of hypertension. 2007;20(7):777–783. doi: 10.1016/j.amjhyper.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 72.Harris MI. Noninsulin-dependent diabetes mellitus in black and white Americans. Diabetes/metabolism reviews. 1990;6(2):71–90. doi: 10.1002/dmr.5610060202. [DOI] [PubMed] [Google Scholar]
- 73.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 74.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. The New England journal of medicine. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 75.Action to Control Cardiovascular Risk in Diabetes Study G. Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. The New England journal of medicine. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Group AC, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. The New England journal of medicine. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 77.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. The New England journal of medicine. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 78.Reboussin DM, Goff DC, Jr, Lipkin EW, et al. The combination oral and nutritional treatment of late-onset diabetes mellitus (CONTROL DM) trial results. Diabetic medicine : a journal of the British Diabetic Association. 2004;21(10):1082–1089. doi: 10.1111/j.1464-5491.2004.01289.x. [DOI] [PubMed] [Google Scholar]
- 79.Torimoto K, Okada Y, Mori H, et al. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovascular diabetology. 2013;12:1. doi: 10.1186/1475-2840-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Franklin VL, Khan F, Kennedy G, et al. Intensive insulin therapy improves endothelial function and microvascular reactivity in young people with type 1 diabetes. Diabetologia. 2008;51(2):353–360. doi: 10.1007/s00125-007-0870-2. [DOI] [PubMed] [Google Scholar]
- 82.Vehkavaara S, Yki-Jarvinen H. 3.5 years of insulin therapy with insulin glargine improves in vivo endothelial function in type 2 diabetes. Arteriosclerosis, thrombosis, and vascular biology. 2004;24(2):325–330. doi: 10.1161/01.ATV.0000113817.48983.c5. [DOI] [PubMed] [Google Scholar]
- 83.Potenza MA, Gagliardi S, Nacci C, et al. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Current medicinal chemistry. 2009;16(1):94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 84.Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nature clinical practice Endocrinology & metabolism. 2007;3(1):46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 85.Selvin E, Bolen S, Yeh HC, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Archives of internal medicine. 2008;168(19):2070–2080. doi: 10.1001/archinte.168.19.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nathanson D, Nystrom T. Hypoglycemic pharmacological treatment of type 2 diabetes: targeting the endothelium. Molecular and cellular endocrinology. 2009;297(1–2):112–126. doi: 10.1016/j.mce.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 87.Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. Journal of the American College of Cardiology. 2001;37(5):1344–1350. doi: 10.1016/s0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 88.Pitocco D, Zaccardi F, Tarzia P, et al. Metformin improves endothelial function in type 1 diabetic subjects: a pilot, placebo-controlled randomized study. Diabetes, obesity & metabolism. 2013;15(5):427–431. doi: 10.1111/dom.12041. [DOI] [PubMed] [Google Scholar]
- 89.Vitale C, Mercuro G, Cornoldi A, et al. Metformin improves endothelial function in patients with metabolic syndrome. Journal of internal medicine. 2005;258(3):250–256. doi: 10.1111/j.1365-2796.2005.01531.x. [DOI] [PubMed] [Google Scholar]
- 90.Kelly AS, Bergenstal RM, Gonzalez-Campoy JM, et al. Effects of exenatide vs. metformin on endothelial function in obese patients with pre-diabetes: a randomized trial. Cardiovascular diabetology. 2012;11:64. doi: 10.1186/1475-2840-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Phung OJ, Schwartzman E, Allen RW, et al. Sulphonylureas and risk of cardiovascular disease: systematic review and meta-analysis. Diabetic medicine : a journal of the British Diabetic Association. 2013;30(10):1160–1171. doi: 10.1111/dme.12232. [DOI] [PubMed] [Google Scholar]
- 92.Hemmingsen B, Schroll JB, Lund SS, et al. Sulphonylurea monotherapy for patients with type 2 diabetes mellitus. The Cochrane database of systematic reviews. 2013;4 doi: 10.1002/14651858.CD009008.pub2. CD009008. [DOI] [PubMed] [Google Scholar]
- 93.Rakel A, Renier G, Roussin A, et al. Beneficial effects of gliclazide modified release compared with glibenclamide on endothelial activation and low-grade inflammation in patients with type 2 diabetes. Diabetes, obesity & metabolism. 2007;9(1):127–129. doi: 10.1111/j.1463-1326.2006.00571.x. [DOI] [PubMed] [Google Scholar]
- 94.Lincoff AM, Wolski K, Nicholls SJ, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA : the journal of the American Medical Association. 2007;298(10):1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 95.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England journal of medicine. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 96.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 97.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA : the journal of the American Medical Association. 2007;298(10):1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 98.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 99.Yki-Jarvinen H. Thiazolidinediones. The New England journal of medicine. 2004;351(11):1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 100.Ryan MJ, Didion SP, Mathur S, et al. PPAR(gamma) agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004;43(3):661–666. doi: 10.1161/01.HYP.0000116303.71408.c2. [DOI] [PubMed] [Google Scholar]
- 101.Hwang J, Kleinhenz DJ, Lassegue B, et al. Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. American journal of physiology Cell physiology. 2005;288(4):C899–C905. doi: 10.1152/ajpcell.00474.2004. [DOI] [PubMed] [Google Scholar]
- 102.Pistrosch F, Passauer J, Fischer S, et al. In type 2 diabetes, rosiglitazone therapy for insulin resistance ameliorates endothelial dysfunction independent of glucose control. Diabetes Care. 2004;27(2):484–490. doi: 10.2337/diacare.27.2.484. [DOI] [PubMed] [Google Scholar]
- 103.Martens FM, Visseren FL, de Koning EJ, et al. Short-term pioglitazone treatment improves vascular function irrespective of metabolic changes in patients with type-2 diabetes. Journal of cardiovascular pharmacology. 2005;46(6):773–778. doi: 10.1097/01.fjc.0000187176.13403.05. [DOI] [PubMed] [Google Scholar]
- 104.Campia U, Matuskey LA, Panza JA. Peroxisome proliferator-activated receptor-gamma activation with pioglitazone improves endothelium-dependent dilation in nondiabetic patients with major cardiovascular risk factors. Circulation. 2006;113(6):867–875. doi: 10.1161/CIRCULATIONAHA.105.549618. [DOI] [PubMed] [Google Scholar]
- 105.Sidhu JS, Cowan D, Kaski JC. Effects of rosiglitazone on endothelial function in men with coronary artery disease without diabetes mellitus. The American journal of cardiology. 2004;94(2):151–156. doi: 10.1016/j.amjcard.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 106.Linscheid P, Keller U, Blau N, et al. Diminished production of nitric oxide synthase cofactor tetrahydrobiopterin by rosiglitazone in adipocytes. Biochemical pharmacology. 2003;65(4):593–598. doi: 10.1016/s0006-2952(02)01562-9. [DOI] [PubMed] [Google Scholar]
- 107.Standl E, Schnell O, Ceriello A. Postprandial hyperglycemia and glycemic variability: should we care? Diabetes care. 2011;34(Suppl 2):S120–S127. doi: 10.2337/dc11-s206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 109.Raz I, Wilson PW, Strojek K, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes care. 2009;32(3):381–386. doi: 10.2337/dc08-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Group NS, Holman RR, Haffner SM, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. The New England journal of medicine. 2010;362(16):1463–1476. doi: 10.1056/NEJMoa1001122. [DOI] [PubMed] [Google Scholar]
- 111.Shimabukuro M, Higa N, Chinen I, et al. Effects of a single administration of acarbose on postprandial glucose excursion and endothelial dysfunction in type 2 diabetic patients: a randomized crossover study. The Journal of clinical endocrinology and metabolism. 2006;91(3):837–842. doi: 10.1210/jc.2005-1566. [DOI] [PubMed] [Google Scholar]
- 112.Pistrosch F, Schaper F, Passauer J, et al. Effects of the alpha glucosidase inhibitor acarbose on endothelial function after a mixed meal in newly diagnosed type 2 diabetes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2009;41(2):104–108. doi: 10.1055/s-0028-1103276. [DOI] [PubMed] [Google Scholar]
- 113.Ban K, Noyan-Ashraf MH, Hoefer J, et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117(18):2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 114.Matsubara J, Sugiyama S, Sugamura K, et al. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. Journal of the American College of Cardiology. 2012;59(3):265–276. doi: 10.1016/j.jacc.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 115.Advani A, Bugyei-Twum A, Connelly KA. Cardiovascular effects of incretins in diabetes. Canadian journal of diabetes. 2013;37(5):309–314. doi: 10.1016/j.jcjd.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 116.Tesauro M, Schinzari F, Adamo A, et al. Effects of GLP-1 on forearm vasodilator function and glucose disposal during hyperinsulinemia in the metabolic syndrome. Diabetes care. 2013;36(3):683–689. doi: 10.2337/dc12-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nystrom T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. American journal of physiology Endocrinology and metabolism. 2004;287(6):E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 118.van Poppel PC, Netea MG, Smits P, et al. Vildagliptin improves endothelium-dependent vasodilatation in type 2 diabetes. Diabetes care. 2011;34(9):2072–2077. doi: 10.2337/dc10-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matsubara J, Sugiyama S, Akiyama E, et al. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circulation journal : official journal of the Japanese Circulation Society. 2013;77(5):1337–1344. doi: 10.1253/circj.cj-12-1168. [DOI] [PubMed] [Google Scholar]
- 120.Preli RB, Klein KP, Herrington DM. Vascular effects of dietary L-arginine supplementation. Atherosclerosis. 2002;162(1):1–15. doi: 10.1016/s0021-9150(01)00717-1. [DOI] [PubMed] [Google Scholar]
- 121.Ueda S, Matsuoka H, Miyazaki H, et al. Tetrahydrobiopterin restores endothelial function in long-term smokers. Journal of the American College of Cardiology. 2000;35(1):71–75. doi: 10.1016/s0735-1097(99)00523-9. [DOI] [PubMed] [Google Scholar]
- 122.Heitzer T, Krohn K, Albers S, et al. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43(11):1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]