SUMMARY
Background
Emerging data suggest an important relationship between sleep and Alzheimer’s Disease (AD), but how poor sleep promotes the development of AD remains unclear.
Results
Here, using a Drosophila model of AD, we provide evidence suggesting that changes in neuronal excitability underlie the effects of sleep loss on AD pathogenesis. β-amyloid (Aβ) accumulation leads to reduced and fragmented sleep, while chronic sleep deprivation increases Aβ burden. Moreover, enhancing sleep reduces Aβ deposition. Increasing neuronal excitability phenocopies the effects of reducing sleep on Aβ, and decreasing neuronal activity blocks the elevated Aβ accumulation induced by sleep deprivation. At the single neuron level, we find that chronic sleep deprivation, as well as Aβ expression, enhances intrinsic neuronal excitability. Importantly, these data reveal that sleep loss exacerbates Aβ–induced hyperexcitability and suggest that defects in specific K+ currents underlie the hyperexcitability caused by sleep loss and Aβ expression. Finally, we show that feeding levetiracetam, an anti-epileptic medication, to Aβ-expressing flies suppresses neuronal excitability and significantly prolongs their lifespan.
Conclusions
Our findings directly link sleep loss to changes in neuronal excitability and Aβ accumulation and further suggest that neuronal hyperexcitability is an important mediator of Aβ toxicity. Taken together, these data provide a mechanistic framework for a positive feedback loop, whereby sleep loss and neuronal excitation accelerate the accumulation of Aβ, a key pathogenic step in the development of AD.
INTRODUCTION
Alzheimer’s disease (AD) is the most common cause of dementia worldwide, whose burden, both in terms of human suffering and health care costs, is expected to rise sharply in the next few decades [1]. β-amyloid peptides (Aβ), which are generated from sequential cleavage of amyloid precursor protein (APP), have been strongly implicated as having a key role in the pathogenesis of AD by substantial histopathologic, biochemical, and genetic data [2]. Thus, there is intense interest in identifying modifiable factors that modulate Aβ. Emerging evidence suggests potentially important links between sleep and AD [3]. It has long been appreciated that patients with AD have impaired sleep/wake cycles, with fragmented and reduced sleep at night [4–6]. Similarly, mouse models of AD have been shown to exhibit reduced sleep during their consolidated period [7, 8]. In humans, β-amyloid deposition, as inferred by a decrease in Aβ levels in cerebrospinal fluid, is associated with reduced sleep quality [9], and reduced sleep and poor quality sleep are associated with increased fibrillar Aβ burden in the brain [10].
Intriguingly, recent data also support a bidirectional relationship between sleep and amyloid--i.e., not only may Aβ accumulation impair sleep, but poor sleep may increase Aβ burden [3]. In humans, consolidated sleep attenuates the risk of developing AD conferred by the E4 allele of Apolipoprotein E (ApoE4, an important genetic polymorphism for AD) [11, 12]. Moreover, a prospective study revealed that markedly fragmented sleep, as measured by wrist actigraphy, increased the risk of developing AD by ~1.5-fold, compared to those with the least fragmented sleep [13]. Importantly, using mouse models of AD, Kang et al. (2009) demonstrated that chronic sleep deprivation led to an increase in Aβ burden in the brain.
What are the mechanisms by which sleep could modulate Aβ, and thus impact AD? A recent study has suggested that the “glymphatic system”--a system of perivascular cerebrospinal fluid (CSF) channels and glial processes that serves to remove metabolic waste from neurons in the brain--is more active during sleep than in wake [14]. In this study, the authors also showed that radioactively labelled Aβ injected into the cortex was cleared more efficiently during sleep, as compared to wakefulness. Another potential mechanism underlying the relationship between sleep and β-amyloid is an alteration in neuronal activity. Although the function(s) of sleep remain enigmatic, one proposed function of sleep is to downscale synaptic strength following the synaptic potentiation that occurs during wakefulness [15]. For example, in rodents, molecular and electrophysiological markers of synaptic strength are increased following wakefulness, as compared to following sleep [16]. Aβ accumulation also appears to be dependent on neuronal activity. For example, Aβ cleavage from APP is enhanced with increased neural activity [17–19].
The fruit fly Drosophila melanogaster has been shown to sleep [20–23], and also has been established as a model for AD [24, 25]. There are several fly AD models, and we focused on a model that uses direct expression of human Aβ42 coupled to a signal peptide. Aβ42-expressing flies have been shown to recapitulate several key features of AD, including Aβ deposition, age-dependent learning impairment, and neurodegeneration [26–28]. Here, using this model, we investigated the functional interactions between sleep, excitability, and Aβ. Our findings support a bidirectional relationship between sleep and β-amyloid and argue that increased neuronal excitability is a key mechanism underlying the effects of sleep on Aβ.
RESULTS
Aβ Expression Leads to Reduced and Fragmented Sleep
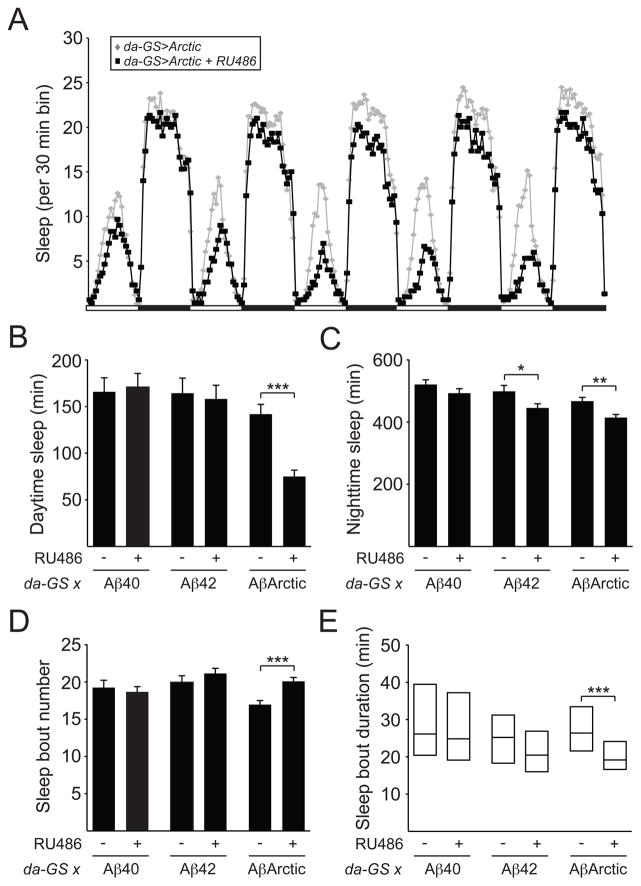
To investigate the effects of Aβ expression on sleep behavior in Drosophila, we used an established model of AD, whereby Aβ40, Aβ42, or AβArctic peptides are expressed pan-neuronally [28]. Aβ40 is less aggregate-prone than Aβ42, whereas AβArctic carries a disease-causing mutation that leads to enhanced aggregation [28, 29]. In order to bypass developmental effects, we induced expression of these Aβ peptides in all neurons during adulthood using daughterless-Geneswitch (da-GS) [30]. We examined daytime and nighttime sleep amount of da-GS>UAS-Aβ40, da-GS>UAS-Aβ42, and da- GS>UAS-AβArctic flies, and found that sleep amount was not significantly affected with overexpression of Aβ40 (Figures 1B and 1C). In contrast, nighttime sleep, but not daytime sleep, was significantly reduced with overexpression of Aβ42, while both daytime and nighttime sleep were significantly decreased with overexpression of AβArctic (Figures 1A–1C). These data suggest a “dose-dependent” relationship between Aβ aggregation and sleep amount. We next examined the sleep architecture of these flies and found no significant effect of expression of Aβ40 and Aβ42 on nighttime sleep bout number or duration. However, inducing AβArctic expression resulted in fragmentation of nighttime sleep, as evidenced by an increase in sleep bout number and reduction in sleep bout duration during the night (Figures 1D and 1E). Consistent with these findings, the sleep of AD patients has previously been reported to be fragmented at night [5, 6]. Together, these data suggest that, as is the case in humans and mice, Aβ expression in flies leads to reduced and fragmented sleep.
Figure 1. Induction of AβArctic expression reduces and fragments sleep.
(A) Sleep profile for da-GS>UAS-AβArctic flies fed 250 μM RU486 (black squares, n=92) or vehicle control (gray diamonds, n=77). Daytime sleep (B), nighttime sleep (C), nighttime sleep bout number (D), and nighttime sleep bout duration (E) for da-GS>Aβ40 fed RU486 (n=48) or vehicle (n=38), da-GS>Aβ42 fed RU486 (n=70) or vehicle (n=36), and da-GS>AβArctic fed RU486 or vehicle. Data in A are from the same flies as in B–E. In this and subsequent figures, error bars represent SEM. “*”, “**”, “***”, and “ns” denote P<0.05, P<0.01, P<0.001, and not significant, respectively.
Nighttime Sleep Deprivation Increases Amyloid Burden
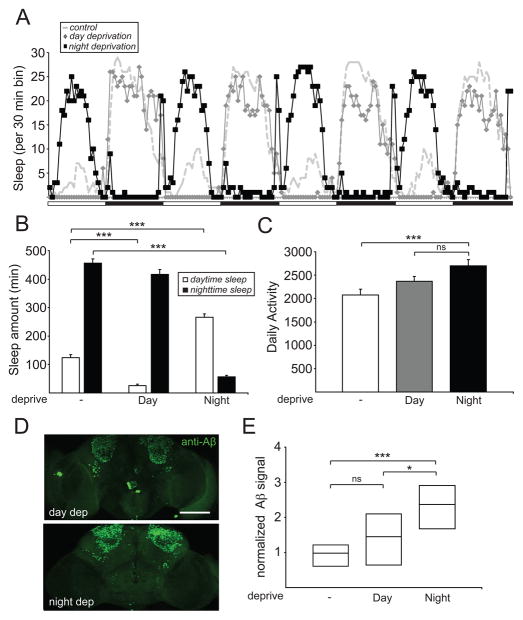
Previous work in mice has shown that chronic loss of sleep leads to an increase in Aβ burden, raising the intriguing possibility that poor sleep may promote the pathogenesis of AD [31]. We thus addressed whether this phenomenon was conserved in other animals such as fruit flies. To reduce the time needed to visualize Aβ deposits, we mainly focused on UAS-AβArctic [28]. In addition, because one of the core features of AD is memory loss [1] and the mushroom bodies (MB) in Drosophila play a critical role in learning and memory [32], we expressed AβArctic peptides in the Kenyon cells (KC) of the MB using OK107-Gal4. Using mechanical deprivation, we subjected OK107-Gal4>UAS-AβArctic flies to nighttime sleep deprivation for 1 week. As the use of chronic mechanical deprivation would increase locomotor activity and potentially result in “physical stress,” we also subjected flies to a similar deprivation paradigm during the daytime, to control for this potential confounder. As shown in Figures 2A and 2B, daytime sleep deprivation effectively reduced daytime sleep, but left nighttime sleep relatively intact, whereas nighttime sleep deprivation markedly reduced nighttime sleep and led to an increase in daytime sleep, likely reflecting “rebound sleep.” As expected, daily activity counts of OK107-Gal4>UAS-AβArctic subjected to nighttime sleep deprivation were significantly increased, compared to those of flies not subjected to mechanical deprivation. However, there was no significant increase in locomotor activity in these flies when compared to those undergoing daytime sleep deprivation (Figure 2C). To assess Aβ burden, we immunostained the brains of these flies using 6E10, a monoclonal antibody that detects an N-terminal epitope on the Aβ42 peptide. Strikingly, confocal imaging of whole-mount brains demonstrated a significant increase in Aβ signal in OK107-Gal4>UAS-AβArctic flies undergoing chronic nighttime sleep deprivation, compared to no deprivation or daytime sleep deprivation (Figures 2D and 2E). To reproduce these findings with a different MB-expressing driver, we examined MB247-LexA>LexAop-AβArctic flies [33] and obtained similar results (Figure S1A). We asked whether these findings were specific for the MB, and found that nighttime sleep deprivation significantly increased AβArctic accumulation in two other brain regions, the pars intercerebralis (using OK107-Gal4) and antennal lobes (using NP1227-Gal4) (Figure S1A). To examine whether these effects were specific to using UAS-AβArctic, we repeated these experiments using UAS-Aβ42. As shown in Figures S1C-E, the effects of nighttime and daytime sleep deprivation on sleep amount, daily activity, and Aβ burden were all recapitulated using OK107-Gal4>UAS-Aβ42 flies. We next assessed whether mechanical sleep deprivation would increase accumulation of an unrelated protein. As shown in Figure S1B, there was no increase in GFP signal when OK107-Gal4>UAS-GFP flies were subjected to nighttime sleep deprivation. Finally, we asked whether other manipulations that enhance cellular stress would affect AβArctic or GFP accumulation. Neither chronic exposure to 31°C or 1mM paraquat affected AβArctic or GFP burden in the MB KC (Figure S1F). Together, these data suggest that loss of nighttime sleep specifically leads to an increase in Aβ burden in a fly model of AD.
Figure 2. Mechanical sleep deprivation enhances Aβ burden.
(A) Sleep profile for OK107-Gal4>UAS-AβArctic flies undergoing no, daytime, or nighttime sleep deprivation from a representative experiment. White bars and black bars denote light and dark periods, respectively. Sleep amount (B) and daily activity (C) for OK107-Gal4>UAS-AβArctic flies, where “-”, “Day”, and “Night” denote no, daytime, and nighttime sleep deprivation, respectively. (D) Representative whole-mount brain confocal images for OK107-Gal4>UAS-AβArctic flies undergoing daytime (“day dep”) or nighttime (“night dep”) sleep deprivation, immunostained with anti-Aβ42 antibody (6E10). Maximum projection images are shown. (E) Normalized Aβ signal intensity in the MB KC from OK107-Gal4>UAS-AβArctic flies undergoing no (n=10), daytime (n=10), or nighttime (n=9) sleep deprivation. Aβ signal intensity is not normally distributed and is thus presented here and in subsequent figures as a simplified box plot with the median shown as the line inside the box, and the 75th and 25th percentiles shown as the top and bottom, respectively. Scale bar represents 100 μm.
Genetic Manipulation of Sleep Modulates Amyloid Burden
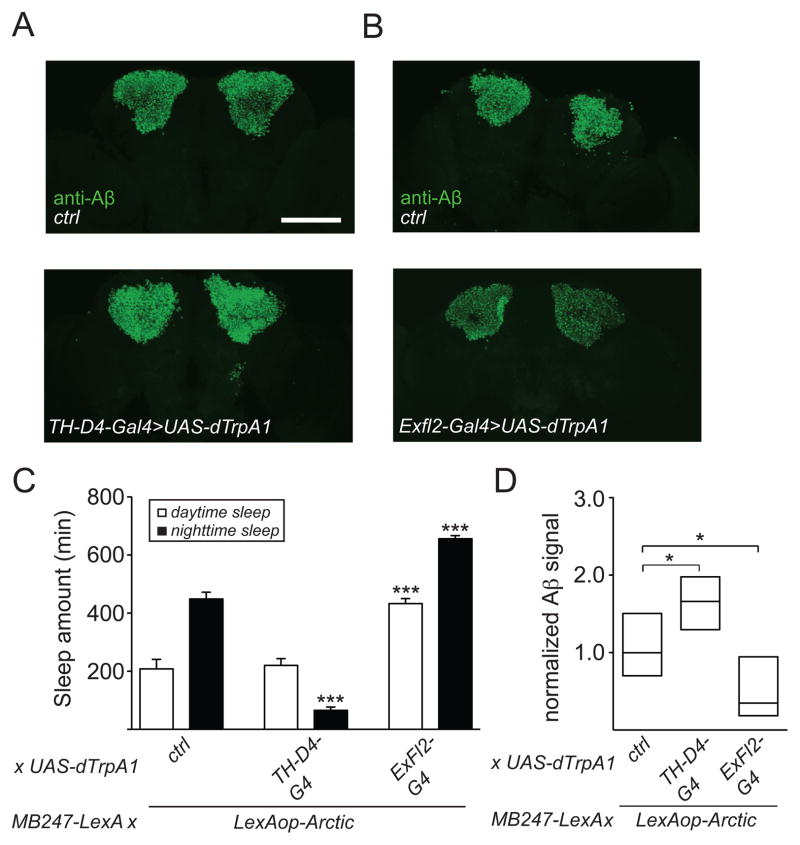
To further investigate how changes in sleep affect Aβ accumulation, we used genetic approaches to reduce or increase sleep, instead of mechanical deprivation. To do this, we used 2 binary expression systems: the Gal4/UAS system to manipulate neurons that regulate sleep and the LexA/LexAop system to express AβArctic in the MB. We previously demonstrated that activation of a subset of dopaminergic (DA) neurons using TH-D4-Gal4 significantly reduces sleep amount [34]. Thus, we used TH-D4-Gal4 to drive expression of dTrpA1, a heat-inducible cation channel [35] to activate DA neurons in flies expressing AβArctic in the MB (MB247-LexA>LexAop-AβArctic). As shown in Figure 3C, conditionally activating this subset of DA neurons resulted in a significant decrease in nighttime sleep, compared to controls. Similar to flies undergoing mechanical nighttime sleep deprivation, these flies exhibited a significant increase in Aβ accumulation, compared to controls (Figures 3A and 3D). It was previously shown that the ExFl2 fan-shaped body (FB) neurons promote sleep [36, 37], and we recently identified a restricted Gal4 driver (R72G06-Gal4) from the Rubin/Janelia Farm collection that contains these cells (data not shown). Thus, in order to address whether increasing sleep would cause the opposite phenotype, i.e. a decrease in Aβ burden, we generated R72G06-Gal4>UAS-dTrpA1, MB247-LexA>LexAop-AβArctic flies. As expected, conditional activation of FB neurons in these flies resulted in an increase in daytime and nighttime sleep (Figure 3C). Importantly, genetically increasing sleep in these flies decreased Aβ burden (Figures 3B and 3D). These data thus provide further evidence that sleep can modulate Aβ burden, and suggest that enhancing sleep can reduce Aβ pathology.
Figure 3. Genetic manipulation of sleep modulates Aβ levels.
(A and B) Representative maximum projection images of whole-mount brains immunostained with 6E10 from LexAop-AβArctic/+; UAS-dTrpA1/MB247-LexA (“ctrl,” top panels) and LexAop-AβArctic/+; UAS-dTrpA1/MB247-LexA, TH-D4-Gal4 (“TH-D4-Gal4>UAS-dTrpA1,” bottom panel), and LexAop-AβArctic/+; UAS-dTrpA1/R72G06-Gal4, MB247-LexA (“ExFl2-Gal4>UAS-dTrpA1,” bottom panel) flies. (C) Daytime and nighttime sleep amounts for LexAop-AβArctic/+; UAS-dTrpA1/MB247-LexA (n=24), LexAop-AβArctic/+; UAS-dTrpA1/MB247-LexA, TH-D4-Gal4 (n=24), and LexAop-AβArctic/+; UAS-dTrpA1/R72G06-Gal4, MB247-LexA (n=22). (D) Normalized Aβ signal intensity in the MB KC for the flies in (C), shown as a simplified box plot. dTrpA1 was chronically activated by subjecting flies to 29°C for 1 week. Scale bar represents 100 μm.
Manipulation of Neuronal Excitability Alters Aβ Accumulation
Previous work suggests that sleep deprivation increases neuronal excitability and synaptic transmission [15, 38]. We thus investigated whether alterations in neuronal excitability might underlie the changes in Aβ burden that we observe with manipulations of sleep. We first asked whether increasing neuronal excitability would result in an increase in Aβ accumulation. Expression of the bacterial sodium channel NaChBac [39] in the MB along with AβArctic (OK107-Gal4>UAS-NaChBac, UAS-AβArctic) resulted in a significant increase in Aβ signal in the MB (Figure S2B). However, this manipulation simultaneously reduced nighttime sleep in these flies (Figure S2A), making it difficult to disentangle whether activating these cells alone leads to an increase in Aβ burden. Therefore, to further assess this issue, we examined Aβ levels in the ExFl2 FB neurons in R72G06-Gal4>UAS-NaChBac, AβArctic flies. In flies expressing NaChBac and AβArctic simultaneously in these ExFl2 FB neurons, daytime sleep was increased compared to controls (Figure S2C), and a significant increase in Aβ burden was observed in the ExFl2 cells, compared to controls (Figure S2D). These data thus dissociate changes in sleep from changes in excitability and suggest that enhancing neuronal excitability acts downstream of changes in sleep to increase Aβ burden.
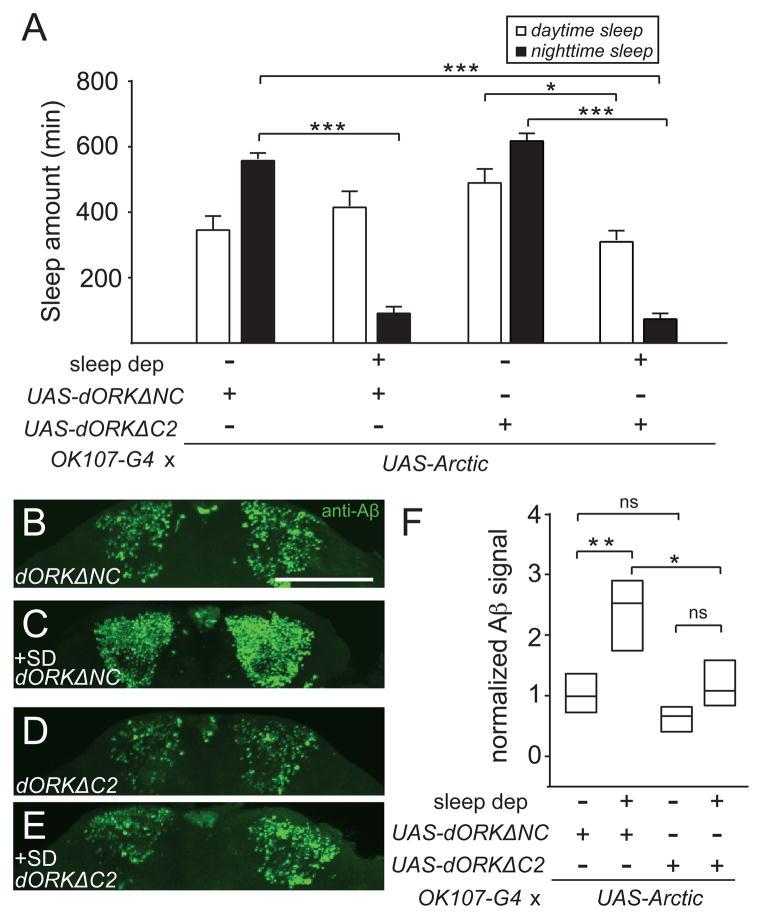
To further address whether changes in excitability act downstream of sleep in modulating Aβ, we asked whether reducing neuronal excitability could suppress the increase in Aβ signal seen with sleep deprivation. We used dORKΔC2, an outward rectifying potassium channel [40], to decrease excitability of MB KC neurons. As a control, we used the non-conducting version of this channel, dORKΔNC. Reducing neuronal excitability in MB KC cells led to a trend towards an increase in daytime sleep in animals not undergoing sleep deprivation, while a marked reduction in nighttime sleep was observed in all animals undergoing sleep deprivation (Figure 4A). As shown in Figures 4B and 4C, electrically inhibiting the MB KC cells essentially blocked the increase in Aβ burden caused by sleep deprivation. These data suggest that hyperexcitability is necessary for sleep deprivation-dependent increases in Aβ accumulation.
Figure 4. Inhibiting neuronal excitability suppresses Aβ accumulation induced by sleep loss.
Daytime and nighttime sleep amounts (A), representative maximum projection images of KC from whole-mount brains immunostained with 6E10 (B–E), and normalized Aβ signal intensity in the MB KC neurons (F) for OK107-Gal4>UAS-AβArctic, UAS-dORKΔNC without sleep deprivation (n=20) and with sleep deprivation (n=13) and OK107-Gal4>UAS-AβArctic, UAS-dORKΔC2 without sleep deprivation (n=17) and with sleep deprivation (n=13). Scale bar represents 100 μm.
Sleep Deprivation Increases Intrinsic Neuronal Excitability
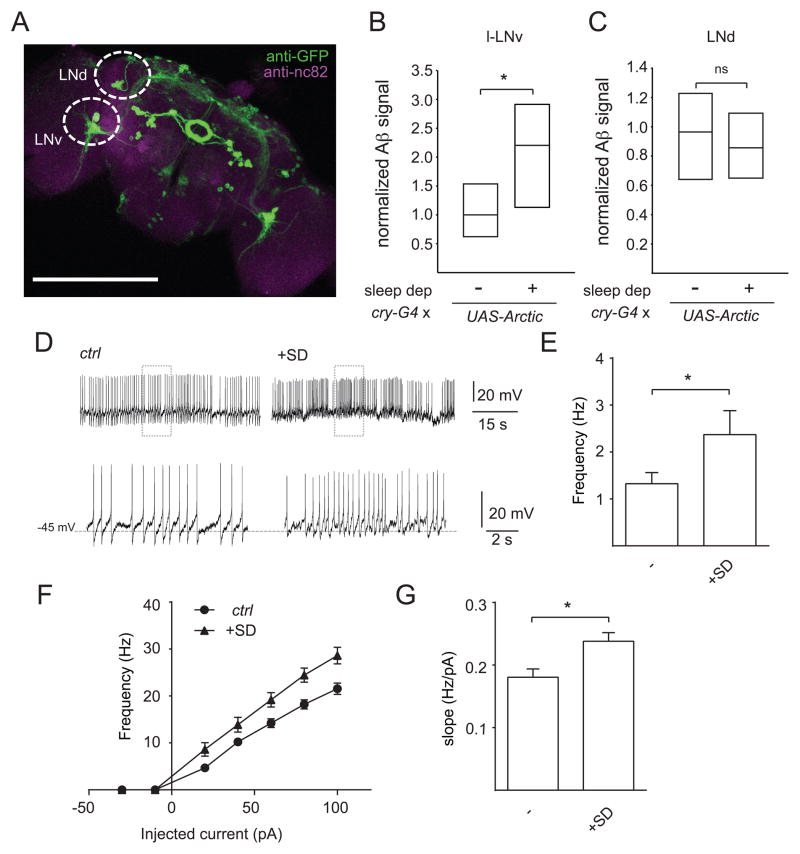
In order to investigate the functional relationship between sleep, neuronal excitability, and Aβ, we decided to examine different neuronal cell groups. The circadian network in Drosophila consists of ~150 neurons comprised of different cell groups with distinct patterns of neuronal activity [41, 42]. The lateral groups (the ventrolateral and dorsolateral) are particularly suitable for the identification and imaging of individual cells. In addition, the ventrolateral neurons can be readily accessed for whole-cell patch clamp recordings, and the large ventrolateral neurons (l-LNvs) in particular have been shown to play a role in sleep/wake regulation [43–48]. Thus, the l-LNvs can be used to examine the relationship between sleep, neuronal activity, and Aβ accumulation in a single cell type. We used the cry-Gal4 driver to manipulate the l-LNvs and the dorsolateral (LNd) groups (Figure 5A). We first expressed AβArctic in these cells and investigated whether chronic sleep deprivation increased Aβ burden. As shown in Figures 5B and 5C, nighttime sleep deprivation caused an increase in Aβ signal in l-LNv cells, compared to controls, but not in LNd cells (which may reflect higher baseline Aβ expression in those cells—data not shown).
Figure 5. Sleep deprivation increases intrinsic neuronal excitability.
(A) Maximum projection of a whole-mount brain immunostained with anti-GFP from cry-Gal4>UASCD8:: GFP. Normalized Aβ signal intensity in l-LNv (B) and LNd (C) cells for cry-Gal4>UAS-AβArctic with (n=24) or without sleep deprivation (n=21), shown as a simplified box plot. (D) Representative traces showing spontaneous AP firing of l-LNvs at ZT0-3 in cry-Gal4>UAS-CD8::GFP flies with or without sleep deprivation (SD). The bottom traces in (D) are expanded traces of the boxed regions in the top traces. Mean firing rate of spontaneous activity (E), mean frequency of spikes elicited in response to current injections with 300 ms stepping pulses at 20 pA increments, ranging from −30 pA to 100 pA (F), and f-I slope (G) of l-LNv neurons in control (cry-Gal4>UAS-CD8::GFP) animals with (n=12) or without (n=15) sleep deprivation. Recordings were performed in the presence of mecamylamine (50 μM) and picrotoxin (250 μM), in order to isolate these cells from most excitatory and inhibitory inputs. Scale bar represents 200 μm.
Our previous data suggest that changes in excitability act downstream of alterations in sleep to modulate Aβ levels. Indeed, previous studies have suggested that sleep “downscales” synaptic strength and that consequently, sleep deprivation leads to increased synaptic transmission [15]. Thus, we sought evidence that sleep deprivation could directly affect neuronal excitability. To address this question, we performed whole cell patch-clamp recordings of l-LNvs from animals with or without sleep deprivation and examined both the spontaneous action potential (AP) firing rate and evoked firing responses. As expected, nighttime sleep deprivation resulted in a significant decrease in nighttime sleep (Figure S3C). In order to isolate the l-LNv neurons from most excitatory and inhibitory inputs, we performed these recordings in the presence of mecamylamine (50 μM) and picrotoxin (250 μM). As shown in Figures 5D and 5E, the spontaneous AP firing rate of the l-LNv neurons was significantly increased (~1.8-fold) in flies subjected to sleep deprivation vs controls. Similar data were also obtained from measuring evoked responses from these neurons. Evoked AP firing rate was increased at all measured depolarizing currents (Figure 5F), and the frequency–current (f–I) slope was significantly increased in sleep-deprived animals, compared with controls (Figure 5G). We did not observe any significant change in resting membrane potential (RMP) in sleep-deprived animals vs controls (Figures S3A and S3B). Together, these data suggest that sleep deprivation enhances both spontaneous and evoked measures of intrinsic neuronal excitability.
Aβ-Dependent Hyperexcitability is Exacerbated by Sleep Deprivation
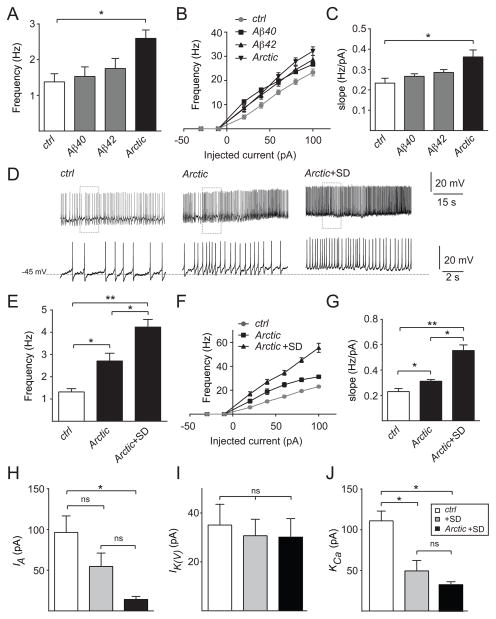
Next, we examined whether Aβ expression itself could also modulate neuronal excitability. Work in mammals has been conflicting, suggesting that Aβ can cause either a decrease or an increase in neuronal excitability [49–51]. To address this question in the Drosophila brain, we performed whole-cell patch clamp recordings on the l-LNv neurons in flies overexpressing Aβ40, Aβ42, or AβArctic using cry-Gal4. As shown in Figure 6A, expression of AβArctic, but not Aβ40 or Aβ42, led to a significant increase in spontaneous AP firing rate compared to controls. We next examined evoked parameters of excitability and found that overexpression of AβArctic, but not Aβ40 or Aβ42, resulted in a significant increase in intrinsic neuronal excitability. In particular, the frequency of AP firing was increased in response to a range of injected currents, and the f-I slope was similarly increased (Figures 6B and 6C). RMP was not significantly affected in any of these conditions (Figure S4A). Together, these data suggest that AβArctic expression on its own can enhance intrinsic neuronal excitability.
Figure 6. Sleep deprivation exacerbates Aβ-dependent neuronal hyperexcitability.
Mean firing rate of spontaneous activity (A), mean frequency of spikes elicited in response to current injections ranging from −30 pA to 100 pA (B), and f-I slope (C) of l-LNv neurons in control cry-Gal4>UAS-CD8::GFP (n=16), cry-Gal4>UAS-Aβ40, UAS-CD8::GFP (n=17), cry-Gal4>UAS-Aβ42, UAS-CD8::GFP (n=15), and cry-Gal4>UAS-AβArctic, UAS-CD8::GFP (n=18). (D) Representative traces showing AP firing of l-LNv neurons in control vs cry-Gal4>UAS-AβArctic, UAS-CD8::GFP flies +/− sleep deprivation (SD). Bottom traces in (D) are expanded traces of the boxed regions in the top traces. Mean firing rate of spontaneous activity (E), mean frequency of spike elicited in response to current injections ranging from −30 pA to 100 pA (F), and f-I slope (G) of l-LNv neurons in control (n=17) vs cry-Gal4>UAS-AβArctic, UAS-CD8::GFP with (n=15) or without sleep deprivation (n=19). IA (H), IK(V) (I), and KCa (J) current amplitude at the spike threshold (−30 mV) from l-LNvs for cry-Gal4>UAS-CD8::GFP with (n=5, 10, and 11, respectively) or without (n=5, 7, and 8, respectively) sleep deprivation and cry-Gal4>UAS-AβArctic, UAS-CD8::GFP with sleep deprivation (n=5, 6, and 4, respectively). Recordings were performed in the presence of mecamylamine (50 μM) and picrotoxin (250 μM), in order to isolate these cells from most excitatory and inhibitory inputs.
Given our previous finding that sleep deprivation alone could cause neuronal hyperexcitability, we next asked whether chronic sleep deprivation would further exacerbate AβArctic-induced hyperexcitability. We examined cry-Gal4>UAS-AβArctic flies subjected to chronic sleep deprivation (Figure S4C) and found that these flies exhibited a further increase in spontaneous and evoked intrinsic neuronal excitability, compared to non-sleep deprived cry-Gal4>UAS-AβArctic as well as controls (Figures 6D–6G). RMP was depolarized, further suggesting that excitability was increased in the presence of AβArctic with sleep deprivation (Figure S4B). In summary, these data suggest that sleep deprivation exacerbates the intrinsic neuronal hyperexcitability induced by Aβ expression.
Sleep Deprivation and Aβ Expression Lead to Impairment of Specific K+ Channel Currents
Changes in excitability could reflect alterations in a variety of ionic currents. Given that K+ channels in particular have been implicated in the regulation of sleep previously, we focused on changes to K+ currents [52–57]. To identify possible mechanisms for the hyperexcitability observed in l-LNv cells following chronic sleep deprivation (Figure S4D), we recorded steady state activation of three types of K+ currents: A-type K+ current (IA), sustained K+ current (IK(V)), and Ca2+-activated K+ currents (KCa) under voltage-clamp configuration. We found that over a range of membrane potentials, all three currents from sleep-deprived flies were significantly reduced compared with non-deprived control flies (Figures S5A–S5C). However, at a potential (−30 mV) near the spike threshold, only KCa currents showed a significant reduction compared to non-sleep deprived controls (Figures 6J and S5F). We next subjected cry-Gal4>UAS-AβArctic flies to sleep deprivation and found that, in addition to KCa currents, IA currents became markedly reduced at potentials near the spike threshold (Figures 6H and S5D). In sleep-deprived animals with or without AβArctic expression, there was no significant effect on IK(V) currents (Figures 6I and S5E). These data suggest that alterations in KCa and IA currents may play a role in the changes of excitability observed in l-LNv neurons under conditions of sleep deprivation and AβArctic expression.
Levetiracetam Prolongs the Lifespan of Aβ-expressing Animals
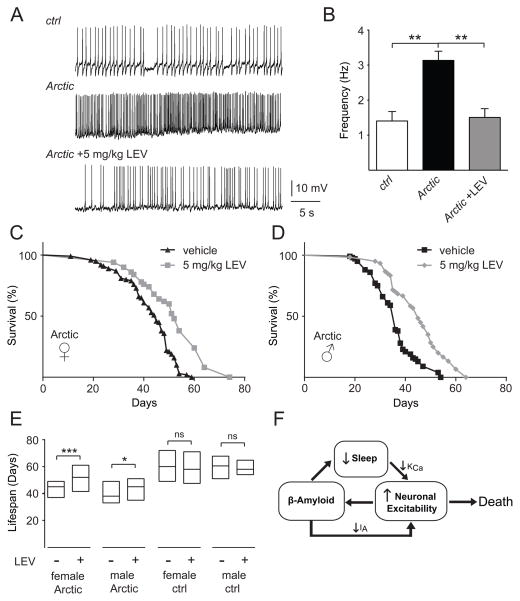
Our data point towards changes in neuronal excitability as a key mediator of the effects of sleep loss on Aβ deposition. Furthermore, Aβ expression itself can induce neuronal hyperexcitability. Interestingly, abnormal elevations in neuronal activity can be seen in a variety of “pre-Alzheimer” states, including mild cognitive impairment (MCI) and ApoE4 carrier status [58, 59]. Furthermore, recent studies in humans with MCI and an AD mouse model have suggested that reducing neuronal excitability using the anticonvulsant levetiracetam (LEV) improves performance in learning and memory tests [60, 61]. Therefore, we wished to test the functional relevance of the hyperexcitability observed in AβArctic-expressing flies by using LEV. We first examined whether feeding LEV to AβArctic-expressing flies would suppress the increased AP firing rate seen in their l-LNv neurons. As shown in Figures 7A and 7B, when cry-Gal4>UAS-AβArctic flies were chronically fed LEV (5 mg/kg) in their food, the increased AP firing rate of l-LNv neurons was reduced back to control levels. Neuronal activity can be associated with changes in neuronal structure [62], and the LNv neurons have been shown to exhibit experience- and sleep-dependent morphological changes [63, 64]. Therefore, we asked whether AβArctic expression coupled with sleep deprivation affected the synaptic morphology of these neurons and whether LEV might suppress this effect. The LNv neurons express the neuropeptide Pigment Dispersing Factor (PDF), and so we used anti-PDF to label the synaptic terminals of these cells. Sleep-deprived flies expressing AβArctic in their LNv neurons displayed a significant increase in PDF+ puncta in the optic lobes, and LEV treatment significantly inhibited this effect (Figure S6A). However, given that PDF is a releasable neuropeptide, we cannot rule out that these changes reflect alterations in the production or release of PDF. We next asked whether LEV treatment could inhibit the increase in Aβ burden in l-LNv neurons seen with sleep deprivation. As shown in Figure S6B, LEV treatment resulted in a trend towards suppression of the increased Aβ burden induced by sleep deprivation, although this effect was not statistically significant.
Figure 7. Levetiracetam suppresses neuronal firing and prolongs lifespan of Aβ Arctic-expressing flies.
(A) Representative traces showing spontaneous firing of l-LNv neurons in control (cry-Gal4>UASCD8:: GFP) vs cry-Gal4>UAS-AβArctic, UAS-CD8::GFP flies fed vehicle or 5 mg/kg levetiracetam (LEV). (B) Quantification of mean firing rates shown in (A) (n = 4 for control, n=5 for AβArctic, and n=8 for AβArctic + LEV). Survivorship curves of elav-Gal4>UAS-AβArctic female (C) and male (D) flies fed vehicle or 5 mg/kg LEV. (E) Lifespan extension of elav-Gal4>UAS-AβArctic female and male flies by LEV, where lifespan is displayed as a simplified box-plot. Data for the elav-Gal4>UAS-AβArctic (“Arctic”) flies shown here are the same as in (C) and (D) (n=98 for vehicle- and n=52 for LEV-fed females, and n=100 for vehicle- and n=60 for LEV-fed males). For elav-Gal4/+ (“ctrl”) flies, n=30 for vehicle- and LEV-fed females and males. (F) Model connecting sleep, neuronal excitability, and Aβ. Sleep loss leads to a reduction in Ca2+-dependent K+ currents, causing neuronal hyperexcitability. This enhanced excitability, in turn, results in increased Aβ accumulation. Aβ itself reduces sleep and further increases neuronal excitability via a decrease in voltage-gated K+ currents, generating a positive feedback loop whereby sleep loss and Aβ interact to substantially increase neuronal activity and Aβ burden. Increased neuronal excitability then contributes to reduced lifespan.
Finally, pan-neuronal expression of AβArctic peptide in flies has previously been shown to reduce lifespan [28], and so we asked whether suppressing neuronal hyperactivity by feeding these flies LEV would prolong their lifespan. We examined lifespan in elav-Gal4>UAS-AβArctic flies and found, as expected, that these flies exhibited a reduction in their median lifespan, when compared to elav-Gal4 controls (60 vs 45 days for females and 61 vs 38 days for males) (Figure 7E). Strikingly, when elav- Gal4>UAS-AβArctic flies were fed LEV (5 mg/kg), their median lifespan was significantly extended by ~16% and ~18% for females and males, respectively (Figures 7C–E). In contrast, LEV did not extend the lifespan of control elav-Gal4/+ female or male flies, suggesting that these effects are specific to AβArctic-expressing flies (Figures S6C and S6D). Together, these data strongly suggest that neuronal hyperexcitability is an important mediator of Aβ-induced toxicity.
DISCUSSION
Our study supports a bidirectional relationship between sleep and β-amyloid and points towards an intimate relationship between sleep, neuronal excitability, and Aβ. Loss of sleep leads to neuronal hyperexcitation, which in turn increases Aβ burden. Aβ expression both reduces sleep and further enhances neuronal excitability (Figure 7F). Furthermore, suppression of Aβ-induced neuronal hyperexcitability significantly prolongs lifespan, suggesting that abnormal neuronal activity is an important mediator of Aβ toxicity.
How are changes in sleep related to alterations in neuronal excitability? In animals ranging from flies to humans, sleep is associated with broad changes in patterns of electrical activity [65, 66]. Although the function of sleep remains controversial, one prominent hypothesis is that sleep functions to downscale synaptic strength [15]. Along these lines, prolonged wakefulness has been associated with an increase in evoked cortical local field potential amplitudes in rats [16] and transcranial magnetic stimulation (TMS) measures of cortical excitability in humans [67]. Our data support this hypothesis, as sleep deprivation in flies leads to hyperexcitability of l-LNv neurons. Another recent study found that the ExFl2 neurons in Drosophila also exhibited increased excitability with sleep deprivation, although in that case, it was suggested that this phenotype was related to the specific sleep-promoting function of those neurons [37].
There are a number of studies on the effects of APP/Aβ expression on neuronal activity in animal models of AD [49]. These studies, which have largely been conducted in mammalian systems, have been conflicting, possibly because different cell types and neuronal networks may behave differently in response to exposure to Aβ. Here, we have recorded from a single cell type (the l-LNvs) from intact fly brains, and found that Aβ expression markedly increases intrinsic neuronal excitability, and that this effect is exacerbated by sleep deprivation. Interestingly, patients with epilepsy exhibit elevated amounts of β-amyloid plaque in their brains [68]. Moreover, seizures are commonly seen in patients with early-onset AD carrying mutations in Presenilin 1 [69], and TMS studies have found increased excitability of primary motor cortex in patients with AD [70].
Our study suggests that neuronal hyperexcitability is an important and early contributor to the pathogenesis of AD. Changes in neuronal excitability in AD likely predate neurodegenerative changes [71]. For example, in young mice overexpressing APP, hippocampal neurons were found to be hyperactive, prior to the formation of β-amyloid plaques [50]. Furthermore, recent evidence suggests that increases in neuronal excitability may be deleterious for cognitive function. Indeed, patients with amnestic mild cognitive impairment (MCI) exhibit increased hippocampal activation by high-resolution fMRI, and reduction of this hippocampal activation with levetiracetam improved memory performance in these subjects [60]. Similar observations have been made in a mouse model of AD and in aged rats with cognitive impairment [61, 72]. We now provide evidence that reducing the neuronal activity of Aβ-expressing flies with levetiracetam prolongs their lifespan. Thus, taken together, these findings imply that early treatment of preclinical AD patients with antiepileptic medications may be beneficial in slowing the course of disease. Our findings reveal an important interaction between Aβ and sleep loss in modulating neuronal excitability and suggest that sleep loss, neuronal hyperexcitability, and Aβ accumulation form a positive feedback loop. As therapeutic interventions exist to manipulate sleep as well as neuronal excitability, these data suggest that targeting these pathways may be a fruitful approach towards slowing the progression or delaying the onset of this incurable disease.
Experimental Procedures
Details of experimental procedures are available in the online Supplemental Information.
Supplementary Material
Acknowledgments
We thank Drs. Tzumin Lee, Damian Crowther, Mark Stopfer, Paul Shaw, and the Bloomington Stock Center for fly stocks. We thank members of the Wu Lab, Tom Lloyd, and Marilyn Albert for helpful feedback. This work was funded by NINDS R01NS079584 (M.N.W.), a Burroughs-Wellcome Fund Career Award for Medical Scientists (M.N.W.), an Alzheimer’s Association New Investigator Research Grant (M.N.W.), and a Synaptic Plasticity and Cognitive Disorders Award from the Brain Science Institute at Johns Hopkins (A.S. and M.N.W.).
Footnotes
SUPPLEMENTAL INFORMATION includes 6 figures and Supplemental Experimental Procedures and can be found with this article online at xxxxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tarawneh R, Holtzman DM. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb Perspect Med. 2012;2:a006148. doi: 10.1101/cshperspect.a006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer’s disease. Cold Spring Harb Perspect Biol. 2011;3:a004457. doi: 10.1101/cshperspect.a004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moe KE, Vitiello MV, Larsen LH, Prinz PN. Sleep/wake patterns in Alzheimer’s disease: relationships with cognition and function. J Sleep Res. 1995;4:15–20. doi: 10.1111/j.1365-2869.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 5.Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, Gerber C. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging. 1982;3:361–370. doi: 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 6.Vitiello MV, Prinz PN, Williams DE, Frommlet MS, Ries RK. Sleep disturbances in patients with mild-stage Alzheimer’s disease. J Gerontol. 1990;45:M131–138. doi: 10.1093/geronj/45.4.m131. [DOI] [PubMed] [Google Scholar]
- 7.Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Platt B, Drever B, Koss D, Stoppelkamp S, Jyoti A, Plano A, Utan A, Merrick G, Ryan D, Melis V, et al. Abnormal cognition, sleep, EEG and brain metabolism in a novel knock-in Alzheimer mouse, PLB1. PLoS One. 2011;6:e27068. doi: 10.1371/journal.pone.0027068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, Holtzman DM. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E epsilon4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 2013;70:1544–1551. doi: 10.1001/jamaneurol.2013.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- 13.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep. 2013;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 17.Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 20.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 21.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 22.Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nat Rev Neurosci. 2009;10:549–560. doi: 10.1038/nrn2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sehgal A, Joiner W, Crocker A, Koh K, Sathyanarayanan S, Fang Y, Wu M, Williams JA, Zheng X. Molecular analysis of sleep: wake cycles in Drosophila. Cold Spring Harb Symp Quant Biol. 2007;72:557–564. doi: 10.1101/sqb.2007.72.018. [DOI] [PubMed] [Google Scholar]
- 24.Bonner JM, Boulianne GL. Drosophila as a model to study age-related neurodegenerative disorders: Alzheimer’s disease. Exp Gerontol. 2011;46:335–339. doi: 10.1016/j.exger.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Lu B, Vogel H. Drosophila models of neurodegenerative diseases. Annu Rev Pathol. 2009;4:315–342. doi: 10.1146/annurev.pathol.3.121806.151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iijima K, Liu HP, Chiang AS, Hearn SA, Konsolaki M, Zhong Y. Dissecting the pathological effects of human Abeta40 and Abeta42 in Drosophila: a potential model for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:6623–6628. doi: 10.1073/pnas.0400895101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finelli A, Kelkar A, Song HJ, Yang H, Konsolaki M. A model for studying Alzheimer’s Abeta42-induced toxicity in Drosophila melanogaster. Mol Cell Neurosci. 2004;26:365–375. doi: 10.1016/j.mcn.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Crowther DC, Kinghorn KJ, Miranda E, Page R, Curry JA, Duthie FA, Gubb DC, Lomas DA. Intraneuronal Abeta, non-amyloid aggregates and neurodegeneration in a Drosophila model of Alzheimer’s disease. Neuroscience. 2005;132:123–135. doi: 10.1016/j.neuroscience.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 29.Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001;4:887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 30.Tricoire H, Battisti V, Trannoy S, Lasbleiz C, Pret AM, Monnier V. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech Ageing Dev. 2009;130:547–552. doi: 10.1016/j.mad.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 33.Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Liu S, Kodama L, Driscoll MR, Wu MN. Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr Biol. 2012;22:2114–2123. doi: 10.1016/j.cub.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332:1571–1576. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donlea JM, Pimentel D, Miesenbock G. Neuronal machinery of sleep homeostasis in Drosophila. Neuron. 2014;81:860–872. doi: 10.1016/j.neuron.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scalise A, Desiato MT, Gigli GL, Romigi A, Tombini M, Marciani MG, Izzi F, Placidi F. Increasing cortical excitability: a possible explanation for the proconvulsant role of sleep deprivation. Sleep. 2006;29:1595–1598. doi: 10.1093/sleep/29.12.1595. [DOI] [PubMed] [Google Scholar]
- 39.Luan H, Lemon WC, Peabody NC, Pohl JB, Zelensky PK, Wang D, Nitabach MN, Holmes TC, White BH. Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila. J Neurosci. 2006;26:573–584. doi: 10.1523/JNEUROSCI.3916-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 41.Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 42.Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park D, Griffith LC. Electrophysiological and anatomical characterization of PDF-positive clock neurons in the intact adult Drosophila brain. J Neurophysiol. 2006;95:3955–3960. doi: 10.1152/jn.00117.2006. [DOI] [PubMed] [Google Scholar]
- 44.Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol. 2008;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao G, Nitabach MN. Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J Neurosci. 2008;28:6493–6501. doi: 10.1523/JNEUROSCI.1503-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung BY, Kilman VL, Keath JR, Pitman JL, Allada R. The GABA(A) receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr Biol. 2009;19:386–390. doi: 10.1016/j.cub.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu S, Lamaze A, Liu Q, Tabuchi M, Yang Y, Fowler M, Bharadwaj R, Zhang J, Bedont J, Blackshaw S, et al. WIDE AWAKE Mediates the Circadian Timing of Sleep Onset. Neuron. 2014;82:151–166. doi: 10.1016/j.neuron.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Randall AD, Witton J, Booth C, Hynes-Allen A, Brown JT. The functional neurophysiology of the amyloid precursor protein (APP) processing pathway. Neuropharmacology. 2010;59:243–267. doi: 10.1016/j.neuropharm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 50.Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B, Konnerth A. Critical role of soluble amyloid-beta for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2012;109:8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaczorowski CC, Sametsky E, Shah S, Vassar R, Disterhoft JF. Mechanisms underlying basal and learning-related intrinsic excitability in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2011;32:1452–1465. doi: 10.1016/j.neurobiolaging.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, Tononi G. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 53.Bushey D, Huber R, Tononi G, Cirelli C. Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. J Neurosci. 2007;27:5384–5393. doi: 10.1523/JNEUROSCI.0108-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;321:372–376. doi: 10.1126/science.1155942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu MN, Joiner WJ, Dean T, Yue Z, Smith CJ, Chen D, Hoshi T, Sehgal A, Koh K. SLEEPLESS, a Ly-6/neurotoxin family member, regulates the levels, localization and activity of Shaker. Nat Neurosci. 2010;13:69–75. doi: 10.1038/nn.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Espinosa F, Marks G, Heintz N, Joho RH. Increased motor drive and sleep loss in mice lacking Kv3-type potassium channels. Genes Brain Behav. 2004;3:90–100. doi: 10.1046/j.1601-183x.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 57.Douglas CL, Vyazovskiy V, Southard T, Chiu SY, Messing A, Tononi G, Cirelli C. Sleep in Kcna2 knockout mice. BMC Biol. 2007;5:42. doi: 10.1186/1741-7007-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, Devidze N, Ho K, Yu GQ, Palop JJ, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci U S A. 2012;109:E2895–2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 63.Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nitz DA, van Swinderen B, Tononi G, Greenspan RJ. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr Biol. 2002;12:1934–1940. doi: 10.1016/s0960-9822(02)01300-3. [DOI] [PubMed] [Google Scholar]
- 66.Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:d878–899. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- 67.Huber R, Maki H, Rosanova M, Casarotto S, Canali P, Casali AG, Tononi G, Massimini M. Human cortical excitability increases with time awake. Cereb Cortex. 2013;23:332–338. doi: 10.1093/cercor/bhs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mackenzie IR, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol. 1994;87:504–510. doi: 10.1007/BF00294177. [DOI] [PubMed] [Google Scholar]
- 69.Gomez-Tortosa E, Barquero S, Baron M, Gil-Neciga E, Castellanos F, Zurdo M, Manzano S, Munoz DG, Jimenez-Huete A, Rabano A, et al. Clinical-genetic correlations in familial Alzheimer’s disease caused by presenilin 1 mutations. J Alzheimers Dis. 2010;19:873–884. doi: 10.3233/JAD-2010-1292. [DOI] [PubMed] [Google Scholar]
- 70.Ferreri F, Pauri F, Pasqualetti P, Fini R, Dal Forno G, Rossini PM. Motor cortex excitability in Alzheimer’s disease: a transcranial magnetic stimulation study. Ann Neurol. 2003;53:102–108. doi: 10.1002/ana.10416. [DOI] [PubMed] [Google Scholar]
- 71.D’Amelio M, Rossini PM. Brain excitability and connectivity of neuronal assemblies in Alzheimer’s disease: from animal models to human findings. Prog Neurobiol. 2012;99:42–60. doi: 10.1016/j.pneurobio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.