Summary
Oral immunotherapy has had limited success in establishing tolerance in food allergy, reflecting failure to elicit an effective regulatory T (Treg) cell response. We show that disease-susceptible mice (Il4raF709) with enhanced IL-4 receptor (IL-4R) signaling exhibited STAT6-dependent impaired generation and function of mucosal allergen-specific Treg cells. This failure was associated with the acquisition by Treg cells of T helper 2 (Th2) cell-like phenotype, also found in peripheral blood allergen-specific Treg cells of food allergic children. Selective augmentation of IL-4R signaling in Treg cells induced their reprogramming into Th2-like cells and disease susceptibility, whereas Treg cell lineage-specific deletion of Il4 and Il13 was protective. IL-4R signaling impaired the capacity of Treg cells to suppress mast cell activation and expansion, which in turn drove Treg cell Th2 cell reprogramming. Interruption of Treg cell Th2 cell reprogramming may thus provide novel therapeutic strategies in food allergy.
Introduction
Food allergy is a major health problem in developed countries, where the prevalence reaches up to 6% in children and 3% in the adult population (Sicherer, 2011). Up to half of food allergic subjects are sensitized to multiple foods, reflecting a generalized, and fundamental breakdown in oral tolerance to foods (Liu et al., 2010; Sicherer, 2011). Oral immunotherapy (OIT) has had limited success in conferring long-term tolerance in food allergic subjects (Jones et al., 2014). Long-term tolerance, defined as persistent tolerance to the allergenic food for at least 6 months after withdrawal of maintenance OIT, has been achieved in only 13–28% of treated subjects (Moran et al., 2013; Sicherer, 2011). Hence, understanding how oral tolerance is subverted in food allergy is of critical importance in elucidating disease pathogenesis and in the design of rational therapeutic and preventive measures.
Oral tolerance to foods is an active immunological process that involves allergen-specific regulatory T (Treg) cells (Berin and Mayer, 2013; Liu et al., 2010; Sicherer, 2011). Genetic and immunological evidence supports a pivotal role for Treg cells in enforcing oral tolerance to foods (Chatila et al., 2000; Jones et al., 2014; Torgerson et al., 2007). In children who outgrow food allergy, tolerance is associated with the development of allergen-specific Treg cells (Karlsson et al., 2004).
Oral tolerance is dependent on the development of induced Treg (iTreg) cells from naïve conventional CD4+ T cells (CD4+ Tconv) upon their activation in the presence of TGF-β1 and CD103+ dendritic cells (DCs) in the gut (Apostolou and Boehmer, 2004; Haribhai et al., 2009; Mucida et al., 2005). iTreg cells regulate T helper 2 (Th2) cell responses at the mucosal surfaces (Curotto de Lafaille et al., 2008; Josefowicz et al., 2012). They are less stable and more plastic than thymic-derived natural Treg (nTreg) cells (Bilate and Lafaille, 2012; Schmitt et al., 2012). This plasticity is reflected at the epigenetic level: whereas the Foxp3 locus is stably hypomethylated in nTreg cells, it is weakly so in iTreg cells (Floess et al., 2007; Schmitt et al., 2012).
Notwithstanding the genetic and functional data linking Foxp3+ Treg cells to food allergy, the role of these cells in disease pathogenesis remains associative. In this report, we have made use of a murine model involving a gain of function IL-4Rα chain allele (Il4raF709) to dissect the role of Treg cells in food allergy (Burton et al., 2014; Mathias et al., 2011; Noval Rivas et al., 2013; Tachdjian et al., 2010). Our results reveal deficient formation and impaired function of allergen-specific Treg cells in food allergy, the latter a consequence of their Th2 cell reprogramming, that promote disease by disrupting a Treg cell-mast cell regulatory loop.
Results
Deficiency of allergen-specific Treg cells in food allergic Il4raF709 mice
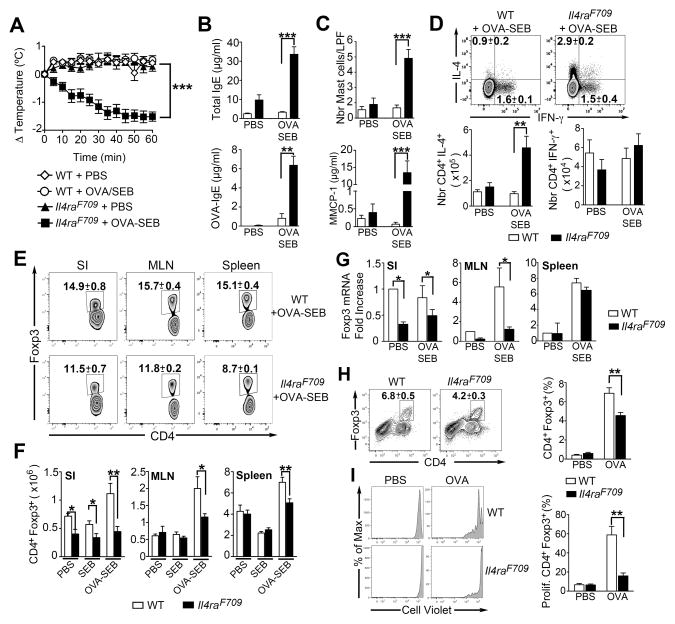
The interleukin-4 (IL-4) receptor (IL-4R) pathway has been implicated in pathogenesis of human food allergy. Increased allergen-induced IL-4 production has been associated with clinically active food allergy, and its decline with the emergence of oral tolerance (Sicherer et al., 2010; 2014). Both IL4RA and STAT6 polymorphisms have been associated with food allergen-specific IgE responses (Amoli et al., 2002; Brown et al., 2012). Accordingly, we employed in our studies mice carrying a mutation in the IL-4Rα (Il4raF709) chain that inactivates the receptor’s immunotyrosine inhibitory motif (ITIM), making them particularly prone to the development of oral allergic sensitization (Burton et al., 2014; Mathias et al., 2011; Noval Rivas et al., 2013; Tachdjian et al., 2010). Oral sensitization of Il4raF709, but not wild-type (WT), mice with ovalbumin (OVA) in combination with the adjuvant staphylococcal enterotoxin B (SEB) rendered them susceptible to robust anaphylaxis upon oral challenge with OVA. The food allergic response manifested by a marked drop in core body temperature upon OVA challenge, increased total and OVA-specific IgE, mast cell expansion and release of the mast cell granule protease 1 (MMCP-1) and intense Th2 cell skewing (Figures 1A–1D). Oral administration of SEB alone has no effect on the parameters of the anaphylactic response (Ganeshan et al., 2009). The differential susceptibility of Il4raF709 mice to oral sensitization and anaphylaxis was maintained when WT and Il4raF709 littermates were analyzed, indicating that it was primarily genotype-driven [(Noval Rivas et al., 2013) and data not shown]. The frequencies and numbers of Foxp3+ Treg cells in the spleens, mesenteric lymph nodes (MLN) and small intestinal (SI) lamina propria were decreased in OVA-SEB-sensitized Il4raF709 mice as compared to WT controls. This was especially so in the SI, where Treg cells were decreased even in PBS or SEB-treated Il4raF709 as compared to WT mice (Figures 1E and 1F). Furthermore, whereas Foxp3 mRNA expression in splenic tissues of PBS and OVA-SEB-sensitized WT and Il4raF709 mice was similar, it was significantly lower in the SI and the MLN of Il4raF709 mice (Figure 1G). Further analysis revealed that the Il4raF709 mice were particularly lacking in allergen-specific Treg cells. Incubation of MLN cells of OVA-SEB-sensitized mice with OVA323-338 peptide-pulsed DCs resulted in the increased proliferation of WT as compared to Il4raF709 Foxp3+ Treg cells (Figures 1H and 1I). These results revealed the presence of a deficient Treg cell response in food allergic Il4raF709 mice.
Figure 1. Deficiency of allergen-specific Treg cells in OVA-allergic Il4raF709 mice.
(A) Core body temperature changes in PBS or OVA-SEB-sensitized WT and Il4raF709 mice after oral OVA challenge. (B, C) Total and OVA-specific serum IgE concentrations (B), intestinal mast cell number per low power field (LPF) and serum MMCP-1 concentrations post anaphylaxis (C) in mouse groups shown in (A). (D) Flow cytometric analysis and enumeration of CD4+IL-4+ and CD4+IFN-γ+ T cells in the MLN of PBS or OVA-SEB-sensitized WT and Il4raF709 mice. (E, F) Frequency (E) and numbers (F) of CD4+Foxp3+ Treg cells in different tissues of PBS, SEB and OVA-SEB-sensitized WT and Il4raF709 mice. (G) Foxp3 mRNA in the SI, MLN and spleens of PBS or OVA-SEB-sensitized WT and Il4raF709 mice. (H) Flow cytometric analysis of CD4+Foxp3+ Treg cell frequencies in cultures of MLN cells isolated from OVA-SEB-sensitized WT and Il4raF709 mice and stimulated with OVA323-339 peptide-pulsed DCs. (I) Frequencies of proliferating cells in gated CD4+Foxp3+ Treg cells shown in (H) and identified by proliferative dye (CellTrace Violet) dilution. Results are representative of 3 independent experiments. *p<0.05; **p<0.01; ***p<0.001 by Student’s unpaired two tailed t test and 2-way ANOVA with post-test analysis. N=5–10 mice/group.
Impaired iTreg cell formation in food allergic Il4raF709 mice
Treg cells isolated from PBS and OVA-SEB-sensitized WT and Il4raF709 mice had similar profiles of key canonical markers, including Foxp3, CD25, and CTLA-4. Expression of ICOS and Helios was markedly increased in Treg cells of OVA-SEB-sensitized Il4raF709 mice, consistent with a heightened activation profile (Figure S1A) (Smigiel et al., 2014; Thornton et al., 2010). The same cells showed evidence of decreased proliferation, as revealed by Ki67 staining (Figures S1B and S1C). The Treg cell proliferative defect was further characterized using neuropillin-1 (Nrp1) as a marker to discriminate between nTreg and iTreg cells (Weiss et al., 2012). Results revealed that the decreased Treg cells proliferation in sensitized Il4raF709 mice affected the Nrp1lo Treg cell population, reflective of iTreg cells, but not the Nrp1hi population, reflective of nTreg cells (Figures S1D and S1E). The proliferative defect was compounded by increased Treg cells apoptosis, as detected by AnnexinV staining (Figures S1F and S1G). Analysis of the conserved non-coding region 2 (CNS2) of Foxp3 for epigenetic demethylation, which is normally more pronounced in nTreg cells, revealed its increase in mesenteric Treg cells of sensitized Il4raF709 mice, consistent with a selective decrease in iTreg cells (Figures S1H and S1I) (Floess et al., 2007; Haribhai et al., 2011; Schmitt et al., 2012). The apparent decrease in iTreg cells in allergen-sensitized Il4raF709 mice could not be ascribed to deficiency of factors critical for their generation in the SI, including TGF-β1 and the enzyme retinaldehyde dehydrogenase 2 (RALDH-2), as transcripts encoding both proteins were increased in the SI of sensitized Il4raF709 as compared to controls (Figure S1J).
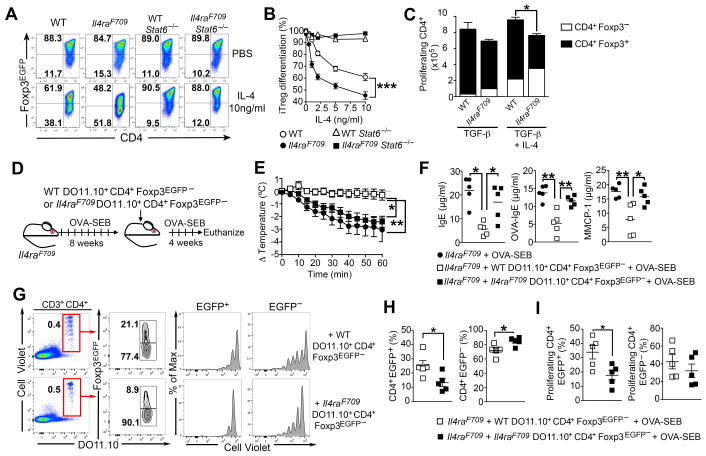
We hypothesized that decreased allergen-specific Treg cell responses in Il4raF709 mice reflected IL-4 signaling-mediated suppression of iTreg cell formation (Dardalhon et al., 2008; Veldhoen et al., 2008). Consistent with this hypothesis, in vitro differentiation of naïve CD4+CD62Lhi Il4raF709 T cells into iTreg cells was more sensitive to IL-4 inhibition as compared to WT controls (Figures 2A and 2B). The ability of IL-4 to disrupt naïve CD4+ Il4raF709 T cell differentiation into iTreg cells was abrogated in Il4raF709Stat6−/− double mutant T cells, indicating its STAT6-dependency (Figures 2A and 2B). IL-4 inhibition of Il4raF709 iTreg cell differentiation was not a dilutional artifact of excessive CD4+Foxp3− T cell proliferation as evidenced by enumeration of the respective cell population (Figure 2C).
Figure 2. Impaired allergen-specific iTreg cell formation in OVA-allergic Il4raF709 mice.
(A) TGF-β induction of iTreg cells from naïve CD4+ T cells isolated from WT, Il4raF709, Stat6−/− and Il4raF709Stat6−/− mice in the absence or presence of IL-4 (10 ng/ml). (B) Effect of different IL-4 concentrations on TGF-β induction of iTreg cells from naïve T cells of groups shown in (A). (C) Absolute numbers of proliferating WT and Il4raF709 CD4+Foxp3+ iTreg cells and CD4+Foxp3− effector cells. (D) Schema of the experimental design of in vivo iTreg cell induction studies. Naïve DO11.10+CD4+ Tconv cells from WT or Il4raF709 DO11.10Rag2−/−Foxp3EGFP mice were loaded with CellTrace Violet dye and injected into OVA-SEB-sensitized Il4raF709 recipients. (E) Core body temperature change following OVA challenge. (F) Total and OVA-specific serum IgE concentrations and MMCP-1 release post anaphylaxis. (G) Flow cytometric analysis of iTreg cell conversion of adoptively transferred DO11.10+CD4+ Tconv cells in MLN of recipient OVA-SEB-sensitized Il4raF709 mice. (H) Percentages of donor CD4+Foxp3EGFP+ (left panel) and CD4+Foxp3EGFP− (right panel) in the MLN of recipient mice of panel (G). (I) Frequencies of proliferating cells in gated CD4+Foxp3EGFP+ (left panel) and CD4+Foxp3EGFP− (right panel) from panel (G). Results are representative of 3 independent experiments. *p<0.05; **p<0.01; ***p<0.001 by Student’s unpaired two tailed t test and 2-way ANOVA with post-test analysis. N=5–10 mice/group.
Impaired allergen-specific iTreg cell formation in Il4raF709 mice was also observed in vivo by employing transgenic DO11.10Rag2−/−Foxp3EGFP mice, which express the DO11.10 TCR specific for OVA323-339 peptide on a RAG2-deficient genetic background. These mice normally carry naïve DO11.10+ CD4+ Foxp3−, but not Foxp3+, T cells. When fed with OVA, they develop OVA-specific iTreg cells that express the enhanced green fluorescent protein (EGFP) under control of Foxp3 (Figures S2A–S2C). Mice with the Il4raF709 allele developed only a third as many DO11.10+ iTreg cells when fed OVA as did mice with the WT Il4ra allele (Figures S2B and S2C). The Il4raF709 DO11.10+ iTreg cells had increased expression of IL-4 and the Th2 cell transcription factors GATA-3 and IRF-4, and decreased IFN-γ, consistent with a skewed Th2 cell-like phenotype (Figures S2D–S2F).
Impaired allergen-specific iTreg cell formation in Il4raF709 mice was further demonstrated in vivo in the context of an immunocompetent host. Naïve OVA-specific CD4+DO11.10+ Tconv cells derived from either WT or Il4raF709 DO11.10Rag2−/−Foxp3EGFP mice were loaded with a proliferation dye and transferred into OVA-SEB-sensitized Il4raF709 mice. Following cell transfer, the recipient mice were further sensitized with OVA-SEB then analyzed for susceptibility to anaphylaxis and for the presence of DO11.10+EGFP+ iTreg cells and DO11.10+EGFP− effector T (TEff) cells in their MLN (Figure 2E). OVA-SEB-sensitized Il4raF709 mice that received WT CD4+DO11.10+ Tconv cells were protected from anaphylaxis, whereas those that received Il4raF709 CD4+DO11.10+ T cells were not (Figures 2F and 2G). Transferred Il4raF709 DO11.10+CD4+ T cells were markedly less efficient in converting into EGFP+ iTreg cells, and those that converted proliferated less, as compared to WT DO11.10+CD4+ T cells (Figures 2H–2J). Altogether, these results established that iTreg cell induction is impaired in food allergic Il4raF709 mice.
Defective function of Il4raF709 Treg cells in suppressing food allergy
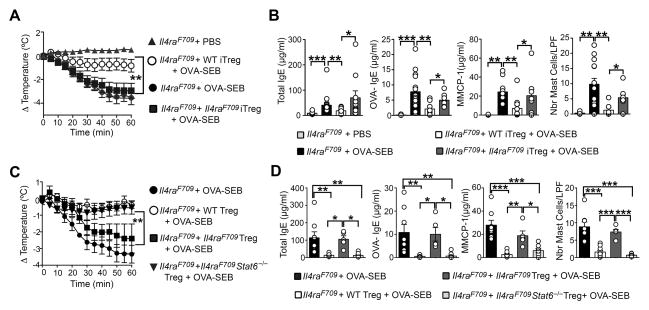
To establish whether allergen-specific Il4raF709 iTreg cells are functionally competent, we determined the capacity of OVA-specific iTreg cells to reverse established food allergy in OVA-SEB-sensitized Il4raF709 mice. WT- or Il4raF709-DO11.10+Foxp3EGFP+ iTreg cells were differentiated in vitro from naïve CD4+ T cells, further purified by cell sorting based on their Foxp3EGFP expression and given intravenously (2.5 × 106 cells/mouse) to sensitized Il4raF709 mice (Figure S3A). The recipient mice were further sensitized for 4 additional weeks and then orally challenged. Administration of a single dose of WT DO11.10+ iTreg cells suppressed the anaphylactic response of sensitized Il4raF709 mice challenged with OVA (Figure 3A). This suppression was associated with inhibition of total and OVA-specific IgE responses as well as mast cell expansion and activation, indicative of disease remission (Figure 3B). They also suppressed Th2 cell cytokine expression in the gut, including Il4, Il13 and Il9 (Figure S3B). In contrast Il4raF709 DO11.10+Foxp3EGFP iTreg cells failed to suppress anaphylaxis or to inhibit the aforementioned disease parameters (Figures 3A and 3B). They also failed to suppress Th2 cell cytokine expression in the SI of OVA-SEB-sensitized Il4raF709 mice. Transferred WT and Il4raF709 DO11.10+Foxp3EGFP iTreg cells were retrieved at similar numbers in the spleens and MLN of recipient mice, confirming that the Il4raF709 iTreg cells were functionally defective in suppressing disease (Figures S3C and S3D).
Figure 3. OVA-specific Il4raF709 Treg cells fail to suppress food allergy.
(A) Core body temperature changes following OVA challenge of OVA-SEB-sensitized Il4raF709 mice that had received in vitro generated WT- or Il4raF709 DO11.10+Foxp3EGFP iTreg, as described in Figure S3A. (B) Total and OVA-specific serum IgE concentrations, MMCP-1 release and small intestinal mast cell counts in mouse groups shown in panel (A). (C) Core body temperature changes following OVA challenge of OVA-SEB-sensitized Il4raF709 mice that were either left untreated or given either WT DO11.10+Foxp3EGFP+ Treg cells or Il4raF709DO11.10+Foxp3EGFP+ STAT6-sufficient or deficient Treg cells. (D) Total and OVA-specific serum IgE and serum MMCP-1 concentrations post anaphylaxis of the mouse groups from panel (C). N=5–17 mice per group, pooled from two different experiments. *p<0.05; **p<0.01; ***p<0.001, 1- and 2-way ANOVA with post-test analysis.
To determine whether defective suppression of oral allergic sensitization was also an attribute of in vivo–derived allergen-specific Il4raF709 Treg cells, isolated DO11.10+ Treg cells from RAG-sufficient WT and Il4raF709 DO11.10+Foxp3EGFP mice were employed in a Treg cell transfer model of enforced tolerance (Noval Rivas et al., 2013). WT but not Il4raF709 DO11.10+Foxp3EGFP Treg cells were found effective in preventing OVA-induced sensitization and anaphylaxis in Il4raF709 mice (Figures 3C and 3D). To determine whether Treg cell dysfunction in Il4raF709 mice resulted from excessive IL-4R/STAT6 signaling, we examined the capacity of Treg cells derived from STAT6-deficient WT and Il4raF709 DO11.10+Foxp3EGFP mice to suppress food allergy in sensitized Il4raF709 mice. Unlike STAT6-sufficient Il4raF709 Treg cells, STAT6-deficient Il4raF709 Treg cells were equivalent to their WT counterparts in suppressing sensitization and anaphylaxis (Figures 3C and 3D). All transferred DO11.10+ Treg cell populations were retrieved at similar frequencies and numbers in recipient mice, indicating that the failure of Il4raF709 DO11.10+Foxp3EGFP Treg cells to suppress food allergy reflected an intrinsic functional defect (Figures S3E and S3F).
We further explored the role of STAT6 signaling in disrupting Il4raF709 Treg cell function using an in vitro suppression assay of IgE-dependent mast cell activation by Treg cells. Treatment of IgE anti-DNP sensitized mast cells with DNP-BSA resulted in mast cell activation and degranulation, marked by the increased surface expression of the granule marker LAMP-1 (CD107a) (Burton et al., 2013; 2014; Grützkau et al., 2004; Leonard et al., 2012). Addition of WT iTreg cells suppressed LAMP-1 expression upon mast cell exposure to antigen (Figure S4). Suppression by Il4raF709 but not WT iTreg cells was sensitive to IL-4 addition, resulting in ineffective inhibition of LAMP-1 expression. Concurrent STAT6 deficiency corrected the deficit in Il4raF709 iTreg cell suppression of mast activation, as evidenced decreased LAMP-1 expression on activated mast cells (Figure S4). These findings confirmed that Il4raF709 Treg cells were functionally impaired in suppressing mast cell activation due to excessive STAT6 signaling.
Treg cell Th2 cell reprogramming is critical to oral allergic sensitization
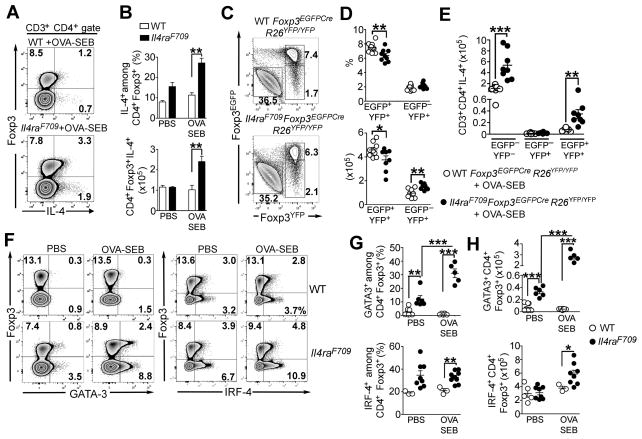
Consistent with the pivotal role of STAT6 in incapacitating oral tolerance induction by Il4raF709 Treg cells, a substantial fraction of Treg cells in OVA-SEB Il4raF709 mice underwent reprogramming into Th2 cell-like cells. MLN Treg cells of OVA-SEB-sensitized Il4raF709, but not WT, mice manifested increased expression of IL-4, all the while maintaining their Foxp3 expression (Figures 4A and 4B). To determine whether the Th2 cell-like Treg cells were a transitional population in the process of generating Foxp3− Th2 cells (ex-Treg cells), we employed a lineage tracing approach using a Rosa26 Stop-flox YFP reporter (R26YFP/YFP) crossed with a Foxp3-directed Cre recombinase (Foxp3EGFPCre) on WT and Il4raF709 background. Sensitization of Il4raF709Foxp3EGFPCreR26YFP/YFP mice, but not Foxp3EGFPCreR26YFP/YFP, with OVA-SEB rendered them susceptible to robust anaphylaxis upon oral challenge with OVA (data not shown). IL-4 expression by Treg cells (EGFP+YFP+), ex-Treg cells (EGFP−YFP+) and CD4+ Tconv cells (EGFP−YFP−) was examined by flow cytometry. Sensitized Il4raF709 mice manifested a modest increase in the number of ex-Treg cells as compared to similarly treated WT mice (Figures 4C and 4D). However, those ex-Treg cells did not secrete IL-4, indicating that Th2-like Treg cells did not contribute to the generation of Tconv Th2 cells. These results also indicate that Th2-like Treg cells are unlikely to emerge from activated Tconv Th2 cells with transient Foxp3 expression, as such a process would also lead to the accumulation of IL-4+EGFP−YFP+ cells (Figure 4E).
Figure 4. Treg cells of food allergic Il4raF709 mice undergo pathogenic Th2 cell-like reprogramming.
(A) IL-4 expression by MLN CD4+Foxp3+ Treg cells from OVA-SEB-sensitized WT and Il4raF709 mice. (B) Frequencies (upper panel) and absolute numbers (bottom panel) of IL-4 expressing CD4+Foxp3+ Treg cells isolated from the MLN of PBS and OVA-SEB-sensitized WT and Il4raF709 mice. (C) Flow cytometric analysis of EGFP and YFP expression in CD4+ T cells isolated from the MLN of OVA-SEB-sensitized Foxp3EGFPCreR26YFP/YFP and Il4raF709Foxp3EGFPCreR26YFP/YFP mice. (D) Percentages (top panel) and numbers of Treg (EGFP+YFP+) and ex-Treg cells (EGFP−YFP+) in the MLN of the mouse groups described in panel (C). (E) Numbers of IL-4 secreting Tconv (EGFP−YFP−), ex-Treg (EGFP−YFP+) and Treg (EGFP+YFP+) CD4+ T cells isolated from the MLN of OVA-SEB sensitized Foxp3EGFPCreR26YFP/YFP and Il4raF709Foxp3EGFPCreR26YFP/YFP mice. (F–H) Flow cytometric analysis (F), frequencies (G) and numbers (H) of GATA-3+ or IRF-4+ Treg cells isolated from the MLN of PBS and OVA-SEB-sensitized WT and Il4raF709 mice. Results are representative of 2–3 independent experiments. N=3–10 mice/group; *p<0.05, **p<0.01 and ***p<0.001 by 1- and 2-way ANOVA with post-test analysis and Student’s unpaired two tailed t test.
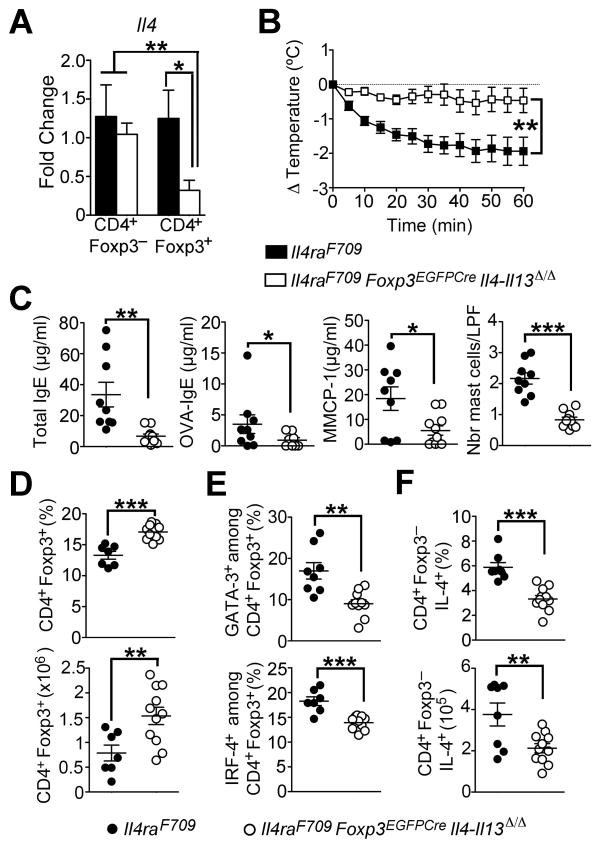
Further characterization of the Th2-like Treg cells in Il4raF709 mice showed that expression of the pro-Th2 cell transcription factor GATA-3 was dramatically increased in a fraction of ex vivo isolated Il4raF709 Treg cells, both at baseline and especially following OVA sensitization, as compared to WT Treg cells (Figures 4F and 4G). The pro-Th2 cell factor IRF4 was also more highly expressed in Il4raF709 Treg cells (Figures 4F and 4G). To determine the contribution of Th2 cell cytokine production by reprogrammed Treg cells to the food allergic response, we employed Il4raF709 mice with targeted deletion of both Il4 and Il13 in Treg cells, achieved using a floxed Il4/Il13 gene cassette and a Foxp3-directed Cre recombinase (Il4raF709Foxp3EGFPCreIl4/Il13Δ/Δ mice) (Figure 5A). Results revealed that OVA-SEB-sensitized Il4raF709Foxp3EGFPCreIl4/Il13Δ/Δ mice were protected against anaphylaxis following OVA challenge as compared to Il4raF709Foxp3EGFPCre control mice (Figures 5B and 5C). Furthermore, Foxp3-directed Il4 and Il13 deletion corrected the deficit in CD4+Foxp3+ Treg cells in sensitized Il4raF709 mice and reversed their Th2-reprogramming as assessed by GATA-3 and IRF-4 expression (Figures 5D and 5E). It also reduced IL-4 production by Tconv Th2 cells (Figure 5F). Collectively, these results indicated that Il4raF709 Treg cells became Th2 cell reprogrammed following oral allergic sensitization and contributed to disease pathogenesis through Th2 cell cytokine expression.
Figure 5. Th2 cell cytokine production by Th2-reprogrammed Treg cells is critical to the food allergic response.
(A) Real time PCR analysis of Il4 mRNA transcripts in Tconv (CD4+Foxp3−) and Treg cells (CD4+Foxp3+) sorted from spleens of Il4raF709 and Il4raF709 Foxp3EGFPCreIl4-Il13Δ/Δ. (B) Core body temperature changes following OVA challenge of OVA-SEB sensitized Il4raF709 and Il4raF709Foxp3EGFPCreIl4-Il13Δ/Δ mice. (C) Serum total IgE, OVA-specific IgE and MMCP-1 concentrations post anaphylaxis of mice from panel (B). (D) Percentages and numbers of CD4+Foxp3+ Treg cells in the MLN of OVA-SEB-sensitized Il4raF709 and Il4raF709 Foxp3EGFPCre Il4-Il13Δ/Δ mice. (E) Frequencies of GATA-3+ or IRF-4+ Treg cells isolated from the MLN of OVA-SEB-sensitized Il4raF709 and Il4raF709 Foxp3EGFPCre Il4-Il13−/− mice. (F) Percentages (top panel) and numbers (bottom panel) of CD4+ Tconv cells producing IL-4 in the MLN of OVA-SEB-sensitized Il4raF709 and Il4raF709 Foxp3EGFPCre Il4-Il13Δ/Δ mice. Results are representative of 2 independent experiments. N=3–18 mice/group; *p<0.05, **p<0.01 and ***p<0.001 by 1- and 2-way ANOVA with post-test analysis and Student’s unpaired two tailed t test.
IL-4 production by activated mast cells is critical in directing the Th2 cell response in food allergy (Figures S5A and S5B) (Burton et al., 2014). Consistent with this observation, a key mechanism driving Th2 cell reprogramming of Il4raF709 Treg cells involved IgE-dependent mast cell expansion and activation. OVA-SEB-sensitized Il4raF709Fcer1a−/− double mutant mice, which lack the alpha chain of the high affinity receptor for IgE (FcεRIα), were completely protected from anaphylaxis following oral OVA challenge, consistent with IgE and mast cell dependence of the anaphylactic response (Figure S5C). Significantly, FcεRIα deficiency profoundly impaired total and OVA-specific IgE antibody responses and suppressed mast cell expansion and activation (Figures S5D and S5E). Concurrent FcεRIα deficiency completely corrected the deficit in CD4+ Foxp3+ Treg cells and reversed Treg cell Th2-reprogramming in OVA-SEB-sensitized Il4raF709 mice, indicating an essential role for dysregulated IgE-FcεRIα signaling in amplifying the Th2 cell response and inducing Th2 cell reprogramming of Treg cells in these mice (Figures S5F and S5G).
Allergen-specific Treg cells of human subjects with food allergy exhibit Th2 cell reprogramming
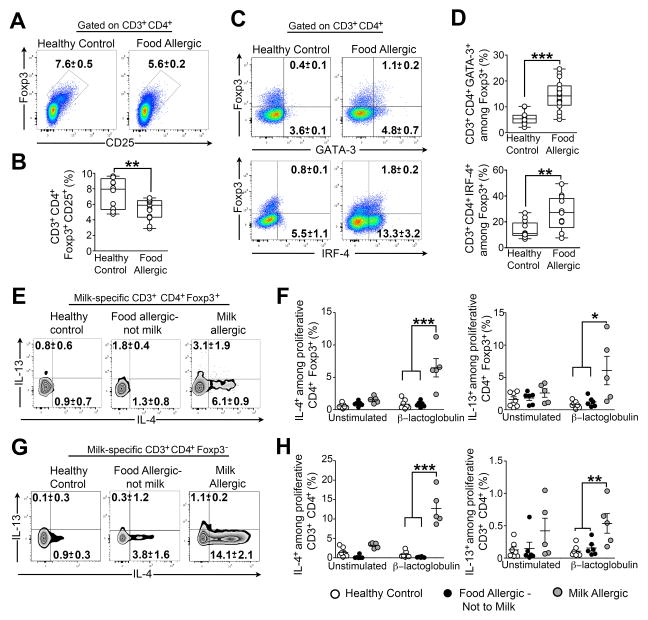
We examined children with food allergy for evidence of Th2 cell reprogramming of their Treg cells (Table S1). Peripheral blood Treg cells of food allergic children were decreased in numbers and had increased expression of GATA-3 and IRF-4 as compared to those of control subjects (Figures 6A–6D). We also examined Th2 cell cytokine expression in milk-specific Treg cells of food allergic and control subjects, identified by their proliferation to milk allergens in in vitro cultures of freshly isolated peripheral blood mononuclear cells (PBMC) (Figure S6A). Only CD4+Foxp3+ and CD4+Foxp3− cells of milk allergic children, but not those of healthy controls or of subjects allergic to foods other than milk, proliferated in response to milk allergen stimulation (Figures S6B–S6E). Furthermore, milk allergic subjects exhibited increased frequencies of milk-specific Treg cells that expressed IL-4 upon allergen stimulation (Figures 6E and 6F). The same pattern was also observed in the CD4+Foxp3− T cell compartment (Figures 6G and 6H). Hence, Treg cells of human food allergic subjects underwent Th2 cell reprogramming similar to those of Il4raF709 food allergic mice.
Figure 6. Allergen-specific Treg cells of food allergic human subjects acquire a Th2-like phenotype.
(A,B) Flow cytometric analysis (A) and frequencies (B) of circulating CD4+CD25+Foxp3+ Treg cells in the peripheral blood of food allergic children and age-matched healthy controls. Cells were gated on live CD3+CD4+ then analyzed for Foxp3 and CD25 expression. (C, D) Flow cytometric analysis (C) and frequencies (D) of GATA-3+ and IRF-4+ Treg cells in food allergic and control subjects. (E, F) Flow cytometric analysis (E) and frequencies (F) of IL-4+ and IL-13+ allergen-specific Treg cells in milk-stimulated PBMC cultures from healthy control, food allergic and milk allergic subjects. (G, H) Flow cytrometric analysis (G) and frequencies (H) of IL-4 and IL-13 expressing CD4+Foxp3− Tconv cells in PBMC cultures of the respective groups shown in (E, F). Numbers in the flow plots represents means ± SEM of positive cells. N=5–17; *p<0.05, **p<0.01 and ***p<0.001 by Student’s unpaired two tailed t test and 1-way ANOVA with post-test analysis.
Rescue of Treg cell deficiency with Il4raF709 CD4+ T cells confers susceptibility to food allergy
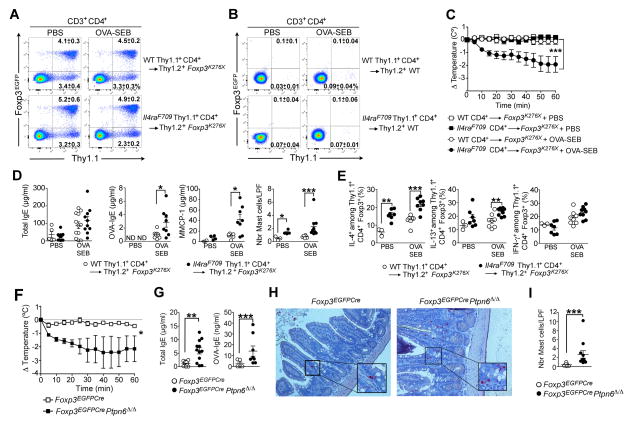
To demonstrate that Il4raF709 Treg cells confer susceptibility to food allergy, we reconstituted Thy1.2+Foxp3K276x newborn pups, lacking functional Treg cells due to a nonsense mutation in Foxp3, with CD4+ T cells from either Thy1.1+WT or Thy1.1+Il4raF709 Foxp3EGFP mice (as a source of nTreg cells and Tconv cell precursors of iTreg cells) (Haribhai et al., 2011). Rescued Foxp3K276X mice grew into adulthood and harbored similar frequencies of grafted donor WT and Il4raF709 Thy1.1+Treg cells, while their CD4+ Tconv cells remain overwhelmingly (≥97%) of recipient host origin (Thy1.2+) (Figure 7A). In contrast, donor Thy1.1+CD4+ T cells injected into Thy1.2+ WT newborn recipients failed to engraft (Figure 7B). At 7 weeks, the rescued Foxp3K276X mice were sham or OVA-SEB-sensitized and then challenged with OVA. Only the OVA-SEB-sensitized Foxp3K276X mice rescued with Thy1.1+Il4raF709 Foxp3EGFP cells underwent anaphylaxis (Figure 7C). Mice rescued with Il4raF709 CD4+ T cells exhibited increased OVA-specific IgE responses and dysregulated mast cell expansion and activation (Figure 7D). Following OVA sensitization, Il4raF709 Treg cells exhibited increased IL-4 and to a lesser extent IL-13, but not IFN-γ, expression as compared to WT Treg cells (Figure 7E). Thus, disease susceptibility was imparted by the rescue of Foxp3K276X mice with donor Il4raF709 Treg cell lineage.
Figure 7. Lineage specific upregulation of IL-4R signaling in Treg cells confers susceptibility to food allergy.
(A). Flow cytometric analysis of MLN congenic Treg cells in Foxp3K276X mice rescued at birth with either WT or Il4raF709 Thy1.1+CD4+ T cells from Foxp3EGFP mice then sham or OVA-SEB-sensitized and challenged with OVA. (B). Flow cytometric analysis of MLN cells of WT Foxp3 competent mice injected at birth with either WT or Il4raF709 Thy1.1+CD4+ T cells isolated from Foxp3EGFP mice then sham or OVA-SEB-sensitized and challenged with OVA. (C) Core body temperature changes in PBS and OVA-SEB-sensitized rescued Foxp3K276X mice following OVA challenge. (D) Total and OVA-specific serum IgE concentrations, intestinal mast cell count and MMCP-1 release post-anaphylaxis in PBS and OVA-SEB-sensitized rescued Foxp3K276X mice. (E) Frequencies of IL-4, IL-13, IFN-γ and IL-9-expressing CD4+Foxp3+ Treg cells in the MLN of PBS and OVA-SEB-sensitized Foxp3K276X rescued mice. (F) Core body temperature changes in Foxp3EGFPCre and Foxp3EGFPCrePtpn6Δ/Δ mice sensitized and challenged with OVA. (G–I) Serum total and OVA-specific IgE concentrations (G), chloroacetate esterase staining of jejunal mast cells (revealed in red) (H) and mast cells counts (I) of OVA-SEB-sensitized Foxp3EGFPCre and Foxp3EGFPCrePtpn6Δ/Δ mice. Results representative of two independent experiments. N=3–10 mice per group. *p<0.05; **p<0.01; ***p<0.001 by 1-and 2-way ANOVA with post-test analysis and Student’s unpaired two tailed t test.
Treg cell-specific deletion of Ptpn6 confers a Th2 cell-like phenotype and promotes food allergy
The Il4raF709 mutation disrupts the interaction of the phosphotyrosine phosphatase Shp1 with IL4-Rα (Tachdjian et al., 2010). Consistent with the critical function of Shp1 in regulating Th2 cell responses, lineage-specific deletion of Ptpn6 (encoding Shp1) in CD4+ T cells enhanced IL-4R signaling and induced Th2 cell skewing (Johnson et al., 2013). We generated transgenic mice in which Ptpn6 was specifically deleted in Treg cells using a floxed Ptpn6 allele and Foxp3EGFPCre, both on C57BL/6 background (Figure S7A). Ptpn6 transcripts were profoundly reduced (>95%) in Treg, but not Tconv, cells of Foxp3EGFPCrePtpn6Δ/Δ mice (Figure S7B). The mice had otherwise normal growth, with no evidence of immune dysregulation (Figures S7C–S7G). Shp1-deficient Treg cells, but not Shp1-sufficient Tconv cells, exhibited enhanced amounts of phosphorylated STAT6 (pSTAT6) phosphorylation in response to IL-4 (Figure S7F). They also exhibited increased serum IgE concentrations at baseline, indicative of Th2 cell skewing (Figure S7H). Foxp3EGFPCre Ptpn6Δ/Δ mice orally sensitized with OVA-SEB anaphylaxed upon oral OVA challenge (Figure 7F). They exhibited elevated total and OVA-specific serum IgE concentrations and increased mast cell expansion in the SI as compared to controls (Figures 7G–7I). Furthermore, Treg cells of OVA-SEB-sensitized Foxp3EGFPCrePtpn6Δ/Δ mice exhibited higher expression of IL-4, GATA-3 and IRF-4 as compared to those of control mice (Figures S7I–S7M). These results indicated that Shp1 deficiency in Treg cells, with its attendant enhanced IL-4R signaling, was sufficient to render mice susceptible to food allergy.
Discussion
Our results show that failure of Treg cell-mediated oral tolerance, in the context of a Th2-polarized gut environment, is fundamental to the pathogenesis of food allergy. By employing a murine model that replicates key aspects of human food allergy, including susceptibility to oral sensitization and response to oral challenge with IgE-mediated anaphylaxis, we demonstrated that formation of allergen-specific Treg cells was decreased due to enhanced IL-4R-STAT6 signaling. We also demonstrated that allergen-specific Treg cells undergo Th2 cell reprogramming driven by dysregulated IgE: FcεRIα signaling and mast cell expansion. This reprogramming, which was also observed in human food allergic subjects, was shown to be sufficient to impart disease susceptibility. These findings outline a cascade of cellular and molecular events that underlie the failure of Treg cells to maintain oral tolerance in food allergy.
Impaired formation of food allergen-specific Il4raF709 iTreg, evident in both in vitro and in vivo models, reflected the influence of excessive IL-4R signaling and was corrected by Stat6 deletion. Il4raF709 allergen-specific iTreg cells proliferated less and were more prone to apoptosis as compared to their WT counterparts. Their deficiency was compounded by ineffective disease suppressing function that was completely corrected by blockade of the STAT6 pathway, indicative of Treg cell subversion by excessive IL-4R-STAT6 signaling. Consistent with this finding, Treg cells of allergen-sensitized Il4raF709 mice exhibited intense Th2 cell-like reprogramming, evidenced by heightened expression of GATA-3, IRF-4 and IL-4 in mutant as compared to WT Treg cells. Previous studies have demonstrated that Treg cells appropriate partial or “aborted” forms of the transcriptional programs of target T helper (Th) cells by expressing their master transcription factors, such as T-bet for Th1 cells and IRF-4 for Th2 cells, and coopting their function (Koch et al., 2012; Zheng et al., 2009). GATA-3 in particular plays a cardinal role in Treg cell homeostasis: it enables their accumulation at sites of inflammation and prevents their polarization into Th17 cells (Rudra et al., 2012; Wang et al., 2011; Wohlfert et al., 2011). Whereas under physiological conditions such partial Th cell programming remains restrained, such restraint is lost under the influence of heightened STAT6 signaling, leading to pathogenic reprogramming of Treg cells into Th2-like cells. The production by the reprogrammed Treg cells of IL-4 directly contributes to disease, evidenced by its amelioration upon Treg cell lineage-specific deletion of Il4/Il13. Aberrant reprogramming was also evident in allergen-specific Treg cells of food allergic children, indicating that it is a pathogenic event common to experimental and human food allergy.
The requisite role of enhanced IL-4R signaling in Treg cells in disease pathogenesis was demonstrated in two experimental models. First, engraftment of an Il4raF709 Treg cell lineage in Foxp3-deficient mice transferred disease susceptibility. In the second approach, Treg cell-specific Shp1 deficiency, which recapitulates the effects of the Il4raF709 ITIM mutation, was sufficient to induce the reprogramming of Treg cells into Th2-like cells and impart susceptibility to food allergy. These findings indicated that Treg cells are a key locus of action of enhanced IL-4R signaling in mediating susceptibility to food allergy.
The mechanism driving the acquisition by Treg cells of a Th2 cell-like phenotype involved a dysregulated IgE-FcεRIα-mast cell axis. Mast cell expansion induced by allergen sensitization of Il4raF709 mice was abrogated upon FcεRIα deficiency, and both the conventional Th2 cell response and the reprogramming of Treg cell into Th2-like cells were inhibited. Moreover, in vitro suppression of mast cell activation by Il4raF709 Treg cells was impaired in the presence of IL-4, a defect that was corrected by STAT6 deletion. These findings outline a scenario in which failure of Il4raF709 Treg cells to suppress mast activation and expansion results in mast cell-driven conventional Th2 cell skewing and Treg cell reprogramming into Th2-like cells. Consistent with this model is our recent demonstration that suppression of mast cell activation and IL-4 production restores tolerance and promotes Treg cell induction (Burton et al., 2014). Accordingly, blockade of IL-4-IL-4Rα pathway or depletion of IgE and/or mast cells may augment allergen-specific iTreg cell generation in food allergic subjects and prevent their pathogenic reprogramming, leading to lasting oral tolerance.
Experimental Procedures
Animals
BALB/cByJ (WT) and all the following strains, except where indicated, were obtained from or rederived at the JAX lab. C.129X1-Il4ratm3.1Tch (Il4raF709), C.Cg-Foxp3tm2Tch/J (Foxp3EGFP), C.Cg-Foxp3tm1Tch (Foxp3K276X) and the BALB/c congenics Thy1.1Foxp3EGFP and DO11.10+Foxp3EGFP have been previously described (Burton et al., 2014; Haribhai et al., 2007; Mathias et al., 2011; Noval Rivas et al., 2013; Tachdjian et al., 2010). NOD/ShiLt-Tg(Foxp3-EGFP-cre)1cJbs/J (Foxp3EGFPCre) and B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J (R26YFP/YFP) were backcrossed 12 generations on BALB/cBYJ (Zhou et al., 2008; Srinivas et al. 2001). Foxp3EGFPCre mice were similarly backcrossed on C57BL/6J (B6.Foxp3EGFPCre). C.129S2-Fcer1atm1Knt (FcerIa−/−) and C.129P2(Cg)-Il4-Il13tm1.1Lky (Il4-Il13fl/fl) mice, both BALB/c congenics, were crossed with Il4raF709 and Il4raF709Foxp3EGFPCre mice as indicated (Dombrowicz et al., 1993; Voehringer et al., 2009). C.129S2-Stat6tm1Gru (Stat6−/−) mice were crossed with DO11.10+Foxp3EGFP and DO11.10+Il4raF709Foxp3EGFP mice (Kaplan et al., 1996). B6.129P2-Ptpn6tm1Rsky (Ptnp6fl/fl) mice were crossed with B6.Foxp3EGFPCre mice (Pao et al., 2007). C.129S6(B6)-Rag2tm1Fwa (Rag2−/−) (Shinkai et al., 1992) mice were obtained from Taconic and crossed with DO11.10, Foxp3EGFP or Il4raF709Foxp3EGFP (JAX) to generate the respective transgenic mice. Mice were maintained under specific pathogen-free conditions and used according to the guidelines of the institutional Animal Research Committee at the Boston Children’s Hospital.
Sensitization and challenge protocol
Mice were treated intragastrically with either sterile PBS or OVA (Sigma-Aldrich) (250μg) together with 10μg SEB (Toxin Technology) in PBS (Treg) once weekly for 8 weeks. In some experiments, mice were gavaged with SEB (10 μg) alone. On week 9, mice were challenged intragastrically with 150 mg of OVA. Anaphylaxis was assessed by measuring changes in total body core temperature with transponders placed subcutaneously 2 days before challenge (IPTT-300; Bio Medic Data Systems) and a DAS-6001 console (Bio Medic Data Systems).
TGF-β-mediated in vitro iTreg cell induction
Sorted naïve CD4+CD62L+Foxp3EGFP− T cells (1×106/ml) were cultured with plate-bound anti-CD28 (5μg/ml, Biolegend), anti-CD3 (5μg/ml, Biolegend), recombinant TGF-β Sigma-Aldrich) and in the presence of a gradient of recombinant IL-4 (Perpotech). After 3 days, the induced iTreg cells were analyzed by flow cytometry and/or re-sorted based upon EGFP fluorescence.
In vivo iTreg cells conversion
For in vivo iTreg cells conversion experiments, naïve CD4+ DO11.10+Foxp3EGFP− cells were isolated from WT or DO11.10+Il4raF709Rag2−/−Foxp3EGFP mice by MACS selection (purity >95%). The cells were labeled with Violet CellTrace Dye (Invitrogen) and adoptively transferred (3×106) retro-orbitally into Il4raF709 mice that had been previously OAV-SEB-sensitized during 8 weeks. The mice were then further sensitized with OVA-SEB for 4 more weeks. After 150mg OVA challenge, the MLN were collected and analyzed by flow cytometry for the fluorescence of Violet CellTrace Dye, DO11.10 and EGFP.
Treg cells adoptive transfer
CD4+DO11.10+ Foxp3EGFP+ iTreg were derived in vitro as detailed above. For treatment of established disease, Il4raF709 recipients were sensitized with OVA-SEB during 8 weeks. At week 9, the sensitized mice were given retro-orbitally 5×106 WT or Il4raF709 CD4+ D011.10+Foxp3EGFP+ iTreg cells, sensitized with OVA-SEB for 4 more weeks and challenged at week 5 (150mg OVA). For food allergy prevention, CD4+ DO11.10+Foxp3EGFP+ Treg cells were cell-sorted from WT DO11.10+Foxp3EGFP and DO11.10+Il4raF709Foxp3EGFP STAT6-sufficient or deficient mice. Il4raF709 mice were given retro-orbitally 0.5×106 of WT or Il4raF709 CD4+ D011.10+Foxp3EGFP+ Treg or in vitro-derived iTreg cells on day 0 of the sensitization protocol. The mice were then sensitized with OVA-SEB for 8 weeks then challenged with OVA.
Human study population
Three groups of subjects, aged from 6 months to 13 years were recruited under a protocol approved by the Institutional Review Board at the Boston Children’s Hospital: 1) healthy subjects without a history of food allergy (n=12), 2) subjects who have other food allergies (with no clinical history of milk allergy; n=8) and 3) subjects who have milk allergy (n=8), as determined by the World Allergy Organization diagnostic criteria (2010). Subject demographics and allergen reactivity are detailed in Table S1, and inclusion and exclusion criteria are detailed in the Supplemental Information section.
Foxp3K276X mice rescue
Splenic and lymph nodes CD4+ T cells were isolated from WT Th1.1+Foxp3EGFP and Thy1.1+Il4raF709Foxp3EGFP by MACS (Miltenyi Biotec). For the rescue of Thy1.2+Foxp3K276X mice, 200μl of purified 5×106 Thy1.1+CD4+ T cells were injected into the peritoneal cavity of pups within the first 30 hours after birth. Rescued Foxp3K276X/K276X females and Foxp3K276X males were maintained and mated to generate litters in which all progeny were Foxp3-deficient.
Statistical Analysis
Anaphylaxis temperature curves were analyzed by using 2-way ANOVA. Student’s unpaired two tailed t test were used for 2 groups comparisons. For more than 2 groups, 1-way ANOVA with Bonferroni post-test analysis were used. Results are presented as means (horizontal lines or rectangular bars) and SEM where each point represents one sample. Differences in mean values were considered significant at a p < 0.05.
Supplementary Material
Acknowledgments
We thank Drs. Calvin B Williams and Dipica Haribhai for discussions. This work was supported by NIH NIAID grants R01 AI065617 and R01 AI085090 (T.A.C.), R56 AI100889 (H.C.O.), T32 AI007512 (O.T.B.), by the Boston Children’s Hospital Translational Research Program (TAC, R.R and H.C.O.), by the Rao Chakravorti Family Fund (H.C.O.), and by the Bunning Foundation (H.C.O., T.A.C.).
Footnotes
Supplemental Information. Supplemental Information includes one table, seven figures, and Supplemental Experimental Procedures and can be found with this article online.
Authors Contributions.
M.N.R and T.A.C designed experiments; M.N.R and O.T.B performed experiments and developed experimental models; P.W, L-M C. and P.G provided technical assistance and developed assays; H.C.O provided scientific advice; R.R. recruited food allergic and control subjects and provided patient histories and blood samples; M.N.R. and T.A.C wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amoli MM, Hand S, Hajeer AH, Jones KP, Rolf S, Sting C, Davies BH, Ollier WER. Polymorphism in the STAT6 gene encodes risk for nut allergy. Genes Immun. 2002;3:220–224. doi: 10.1038/sj.gene.6363872. [DOI] [PubMed] [Google Scholar]
- Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berin MC, Mayer L. Can we produce true tolerance in patients with food allergy? J Allergy Clin Immunol. 2013;131:14–22. doi: 10.1016/j.jaci.2012.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- Brown P, Nair B, Mahajan SD, Sykes DE, Rich G, Reynolds JL, Aalinkeel R, Wheeler J, Schwartz SA. Single nucleotide polymorphisms (SNPs) in key cytokines may modulate food allergy phenotypes. Eur Food Res Technol. 2012;235:971–980. doi: 10.1007/s00217-012-1827-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BS, KG, BS, CVN, MD, AH, PhD, RPS, Xunrong Luo P., MD, PhD, PJB Impairing oral tolerance promotes allergy and anaphylaxis: A new murine food allergy model. Journal of Allergy and Clinical Immunology. 2009;123:231–238. e234. doi: 10.1016/j.jaci.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, Chatila TA, Oettgen HC. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol. 2013;6:740–750. doi: 10.1038/mi.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton OT, Rivas MN, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, Roers A, Houshyar H, Crackower MA, Chatila TA, et al. Immunoglobulin E Signal Inhibition during Allergen Ingestion Leads to Reversal of Established Food Allergy and Induction of Regulatory T Cells. Immunity. 2014;41:141–151. doi: 10.1016/j.immuni.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowicz D, Flamand V, Brigman KK, Koller BH, Kinet JP. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell. 1993;75:969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: A new murine food allergy model. J Allergy Clin Immunol. 2009;123:231–238. e4. doi: 10.1016/j.jaci.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grützkau A, Smorodchenko A, Lippert U, Kirchhof L, Artuc M, Henz BM. LAMP-1 and LAMP-2, but not LAMP-3, are reliable markers for activation-induced secretion of human mast cells. Cytometry A. 2004;61:62–68. doi: 10.1002/cyto.a.20068. [DOI] [PubMed] [Google Scholar]
- Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, Li SH, Simpson PM, Chatila TA, Williams CB. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182:3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DJ, Pao LI, Dhanji S, Murakami K, Ohashi PS, Neel BG. Shp1 regulates T cell homeostasis by limiting IL-4 signals. J Exp Med. 2013;210:1419–1431. doi: 10.1084/jem.20122239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Burks AW, Dupont C. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J Allergy Clin Immunol. 2014;133:318–323. doi: 10.1016/j.jaci.2013.12.1040. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med. 2004;199:1679–1688. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard SA, Martos G, Wang W, Nowak-Węgrzyn A, Berin MC. Oral immunotherapy induces local protective mechanisms in the gastrointestinal mucosa. J Allergy Clin Immunol. 2012;129:1579–1587. e1. doi: 10.1016/j.jaci.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, Massing M, Cohn RD, Zeldin DC. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126:798–806. e13. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias CB, Hobson SA, Garcia Lloret M, Lawson G, Poddighe D, Freyschmidt EJ, Xing W, Gurish MF, Chatila TA, Oettgen HC. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J Allergy Clin Immunol. 2011;127:795–805. e1–6. doi: 10.1016/j.jaci.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TP, Vickery BP, Burks AW. Oral and sublingual immunotherapy for food allergy: current progress and future directions. Curr Opin Immunol. 2013;25:781–787. doi: 10.1016/j.coi.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noval Rivas M, Burton OT, Wise P, Zhang YQ, Hobson SA, Garcia Lloret M, Chehoud C, Kuczynski J, Desantis T, Warrington J, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131:201–212. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Rudra D, deRoos P, Chaudhry A, Niec RE, Arvey A, Samstein RM, Leslie C, Shaffer SA, Goodlett DR, Rudensky AY. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol. 2012;13:1010–1019. doi: 10.1038/ni.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt EG, Haribhai D, Williams JB, Aggarwal P, Jia S, Charbonnier LM, Yan K, Lorier R, Turner A, Ziegelbauer J, et al. IL-10 produced by induced regulatory T cells (iTregs) controls colitis and pathogenic ex-iTregs during immunotherapy. J Immunol. 2012;189:5638–5648. doi: 10.4049/jimmunol.1200936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Sicherer SH, Wood RA, Stablein D, Burks AW, Liu AH, Jones SM, Fleischer DM, Leung DYM, Grishin A, Mayer L, et al. Immunologic features of infants with milk or egg allergy enrolled in an observational study (Consortium of Food Allergy Research) of food allergy. J Allergy Clin Immunol. 2010;125:1077–1083. e1078. doi: 10.1016/j.jaci.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicherer SH, Wood RA, Vickery BP, Jones SM, Liu AH, Fleischer DM, Dawson P, Mayer L, Burks AW, Grishin A, et al. The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol. 2014;133:492–499. doi: 10.1016/j.jaci.2013.12.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, Campbell DJ. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivinas S, Watanabe T, Chyun-Sheng L, William CM, Tanabe Y, Jesell T, Constantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachdjian R, Khatib Al S, Schwinglshackl A, Kim HS, Chen A, Blasioli J, Mathias C, Kim HY, Umetsu DT, Oettgen HC, et al. In vivo regulation of the allergic response by the IL-4 receptor alpha chain immunoreceptor tyrosine-based inhibitory motif. J Allergy Clin Immunol. 2010;125:1128–1136.e1128. doi: 10.1016/j.jaci.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson TR, Linane A, Moes N, Anover S, Mateo V, Rieux-Laucat F, Hermine O, Vijay S, Gambineri E, Cerf-Bensussan N, et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology. 2007;132:1705–1717. doi: 10.1053/j.gastro.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Uyttenhove C, Van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Wu D, Liang HE, Locksley RM. Efficient generation of long-distance conditional alleles using recombineering and a dual selection strategy in replicate plates. BMC Biotechnol. 2009;9:69. doi: 10.1186/1472-6750-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723-42-S1. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R, et al. GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J Clin Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. Pediatric Allergy and Immunology. 2010;21:1–125. doi: 10.1111/j.1399-3038.2010.01068.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.