Abstract
Background
Chronic disease can contribute to functional disability, which can degrade quality of life. However, the prevalence of functional disability and its association with outcomes among patients with heart failure (HF) requires further study.
Methods and Results
Southeastern Minnesota residents with HF were enrolled from September 2003 through January 2012 into a cohort study with follow-up through December 2012. Difficulty with 9 ADLs was assessed by questionnaire. Patients were divided into 3 categories of ADL difficulty (no/minimal, moderate, severe). The associations of ADL difficulty with mortality and hospitalization were assessed using Cox and Andersen-Gill models. Among 1128 patients (mean age 74.7 years, 49.2% female), a majority (59.4%) reported difficulty with one or more ADLs at enrollment, with 272 (24.1%) and 146 (12.9%) experiencing moderate and severe difficulty, respectively. After a mean (SD) follow-up of 3.2 (2.4) years, 614 (54.4%) patients had died. Mortality increased with increasing ADL difficulty; the HR (95% CI) for death was 1.49 (1.22–1.82) and 2.26 (1.79–2.86) for those with moderate and severe difficulty, respectively, compared to those with no/minimal difficulty (ptrend <0.001). Patients with moderate and severe difficulty were at increased risk for all-cause and non-cardiovascular hospitalization. In a second assessment, 17.7% of survivors reported more difficulty with ADLs and patients with persistently severe or worsening difficulty were at increased risk for death (HR 2.10, 95% CI 1.71–2.58, p<0.001) and hospitalization (HR 1.51, 95% CI 1.31–1.74, p<0.001).
Conclusions
Functional disability is common in patients with HF, can progress over time, and is associated with adverse prognosis.
Keywords: heart failure, mortality, survival, morbidity, epidemiology
Physical disability and loss of independence can complicate the care of patients with chronic diseases such as heart failure (HF)1, and can degrade their quality of life2. The ability to perform activities of daily living (ADLs) is often used as a surrogate for the more difficult-to-measure construct of functional disability. Despite the ease of estimating functionality through patient-reported outcomes, very little is known about the prevalence and severity of ADL deficits and functional disability is in patients with HF.
Our limited understanding of functional disability among patients with HF is problematic for several reasons. First, disease-specific treatment options depend upon a patient’s functional capabilities. Second, while functional disability has been associated with increased mortality in general elderly populations3, 4, its association with outcomes including mortality and hospitalization in patients with HF is unclear.
To address these gaps in knowledge, we investigated the prevalence and progression of difficulty with ADLs and their associations with mortality and hospitalization in a community-based cohort of patients with HF.
Methods
Study Design
This study was a cohort study conducted in southeastern Minnesota. This area is relatively isolated from other urban centers, and a limited number of providers, the largest of which is the Mayo Clinic, deliver the vast majority of medical care to local residents. The Rochester Epidemiology Project5, a medical record linkage system, allows the indexing of medical care for residents of southeastern Minnesota, thus enabling the comprehensive capture of health-related events for the community’s residents.
Patient Population
Potential patients with HF were identified using natural language processing of the electronic medical record text6. Following a clinical visit, documentation is transcribed and appears in the electronic record within 24 hours, making prompt identification of patients with HF possible. The search was restricted to patients at least 20 years old who were residents of Olmsted, Dodge, and Fillmore Counties in Minnesota. This approach yielded 100% sensitivity compared with billing data, which is the desired methodology for case finding6. Medical records were then reviewed by experienced research nurses to determine whether patients had active HF meeting Framingham criteria7. Patients were approached to provide consent to participate in the study from September 2, 2003 through January 31, 2012, which included an echocardiogram and blood draw. Hospitalized patients were contacted in the hospital, and patients seen in the outpatient setting were approached at their next scheduled clinical encounter. All patients provided written authorization to participate in the study, which was approved by the Mayo Clinic Institutional Review Board.
Data Collection
Patient Baseline Characteristics
Patient baseline characteristics were collected from the medical record. A physician’s diagnosis was used to define a history of cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), dementia and malignancy. A history of hypertension was defined as systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg or use of antihypertensive medications. Diabetes mellitus was defined using American Diabetes Association criteria8 or use of diabetes medications. Body mass index (BMI) was calculated using the last outpatient weight prior to study enrollment and earliest adult height. Estimated glomerular filtration rate (eGFR) was calculated based on the creatinine level at study enrollment9. Anemia was defined as hemoglobin <12 mg/dL in women or <13 mg/dL in men10. The Charlson comorbidity index was used to assess the degree of comorbidity11. Resting left ventricular ejection fraction (EF) was collected from transthoracic echocardiograms performed within 6 months prior to 2 months after study enrollment. Preserved EF was defined as ≥50%. Marital status was obtained from a self-administered annual survey. Resuscitation preference was defined as either “Full Code” or “DNR” based on documentation in the electronic medical record12.
Activities of Daily Living
A patient’s ability to perform ADL is requested annually for all patients at the Mayo Clinic by a self-administered survey sent to the patient’s home address. During a hospital admission, patients are asked to complete the survey if they have not completed one within the past 6 months. Patients are asked to identify whether they have difficulty performing the following on their own: feeding themselves, dressing, using the toilet, housekeeping, climbing stairs, bathing, walking, using transportation, and managing medications. The response options are binary (Yes/No). Similar activity-based items with binary response options have been used for functional assessment in other populations13, 14. As this was self-administered, proxy responses were allowed, but their frequency could not be assessed. To be eligible for inclusion in this analysis, participants were required to have responded to the survey near the time of study enrollment (6 months before to 3 months after). Marital status was obtained from the same annual survey.
Ascertainment of All-Cause Mortality and Hospitalizations
Participants were followed through December 31, 2012, for death from any cause and for all-cause hospitalization. Mortality follow-up was available on all patients; hospitalization data was available in 95% of patients and those without hospitalization data were excluded from that portion of the analysis. The date of death was determined using death certificates filed in Olmsted County, obituary notices, and electronic files of death certificates obtained from the State of Minnesota Department of Vital and Health Statistics. Information on hospitalizations in Olmsted County was obtained using local administrative sources. Transfers between hospitals were considered a single hospitalization. The principal discharge diagnosis was categorized based on the primary International Classification of Diseases (ICD-9) code as HF (ICD9 428) other cardiovascular/ renal (ICD9 390–459, 580–89) or non-cardiovascular (all other codes). Patients who were alive at last follow-up were censored at their date of last medical contact.
Statistical Analysis
Negative binomial regression was used to examine the predictors of the number of ADLs for which patients had difficulty. In order to obtain a parsimonious multivariable model, all potential predictors shown in Table 1 (with the exception of Charlson comorbidity index) were entered into the model and backward selection was performed retaining only significant predictors of difficulty with ADLs. Rasch analysis was used to identify the order of difficulty of ADLs15. Rasch analysis is a 1-parameter item response theory model for analyzing categorical data, such as questionnaire responses. The probability of a respondent providing a positive answer to an item is a function of the respondent’s ability and the item’s difficulty. Item difficulties are determined not only by the frequency with which an item gets a positive response but also by the ability levels of subjects who have a positive response. For example, when applied to educational tests, easier test items should be answered correctly by most subjects, including those with limited proficiency in the subject matter, whereas the probability of a correct response to more difficult questions would be much higher in those test-takers with high proficiency. In this way, difficult items are those items that are generally given positive responses by those with high-ability levels and negative responses by those with low-ability levels. Similarly, when applied to ADL difficulties, Rasch analysis identifies the level of difficulty of each ADL based upon the response patterns of patients with varying levels of functionality. Once ADL difficulties were established with Rasch analysis, subjects were assigned a numeric grade based on the least difficult ADL that a subject could not perform. Other groups, such as those who developed the physical function item bank for the Patient Reported Medical Information System, have used item response theory-based approaches to model physical function as a unidimensional construct – an approach similar to ours – without the requirement for subdomains such as applied cognition and mobility16. Patients were then divided into one of 3 categories (minimal, moderate, severe difficulty) based upon their difficulty with ADLs. Kaplan Meier curves and Cox proportional hazard regression models were used to examine the association between ADL difficulty and mortality. We checked the proportional hazard assumption using the scaled Schoenfeld residuals and it was found to be valid. To examine the association between ADL difficulty and hospitalization, we used Andersen-Gill models, which account for the repeated nature of hospitalizations17, 18. Complete case analysis was used in all modeling. A p value <0.05 was used as the level of significance for all analyses. Analyses were performed using SAS Version 9.3(Cary, NC) and WinSteps software (Rasch analysis).
Table 1.
Patient Baseline Characteristics
| Patient Characteristic | Missing (No.) |
Overall (n=1128) |
|---|---|---|
| Age (years) | 0 | 74.7 (13.2) |
| Male, n(%) | 0 | 573 (50.8) |
| Married, n(%) | 3 | 577 (51.3) |
| Preserved EF, n(%) | 23 | 571 (51.7) |
| Hypertension, n(%) | 0 | 986 (87.4) |
| Diabetes mellitus, n(%) | 3 | 411 (36.5) |
| Peripheral vascular disease, n(%) | 0 | 299 (26.5) |
| Cerebrovascular disease, n(%) | 0 | 337 (29.9) |
| Dementia, n(%) | 0 | 57 (5.1) |
| COPD, n(%) | 0 | 318 (28.2) |
| Morbid obesity (BMI ≥35kg/m2) | 0 | 209 (18.5) |
| eGFR<60 mL/min, n(%) | 0 | 717 (63.6) |
| Anemia, n(%) | 5 | 534 (47.6) |
| Charlson comorbidity index, median (IQR) | 2 | 4 (2,6) |
| No. ADLs reporting difficulty, median (IQR) | 0 | 1 (0,3) |
ADL= activities of daily living, BMI= body mass index, COPD= chronic obstructive pulmonary disease, EF= ejection fraction, eGFR= estimated glomerular filtration rate, IQR= interquartile range
Results
A total of 2331 patients were invited to participate in the study, and 1476 patients were enrolled (63.3% consent rate). Of those, 1128 (76.4%) provided information on their ability to perform ADLs around the time of enrollment (median 17 days prior), and are included in analysis. The majority of questionnaires assessing ADL difficulty were collected in the outpatient setting (69%), while the others were collected while the patient was hospitalized. The baseline characteristics of the study population are shown in Table 1. Patients were elderly (mean age 74.7 years), 50.8% were men, 51.7% had preserved EF, and comorbidities such as hypertension, diabetes, and cerebrovascular disease were common. Patients who were not eligible for inclusion in the analysis because they lacked ADL information or did not consent to participate were more often older (78.2 vs. 74.7 years, p<0.001) and female (55.1% vs. 49.2%, p=0.004). At the time of enrollment, a majority of patients (n=670, 59.4%) had difficulty with one or more ADLs.
Predictors of Difficulty with ADLs
The associations between patient clinical characteristics and the number of ADLs for which patients reported difficulty are shown in Table 2. The independent predictors of difficulty with ADLs were older age, male sex, diabetes, cerebrovascular disease, dementia, anemia, morbid obesity, and unmarried status. Dementia was one of the factors most strongly associated with ADL difficulty; adjusting for other factors, patients with dementia have a more than 2-fold increase in the number of ADLs for which they report difficulty compared with a patient without dementia. For example, if the average patient without dementia reported difficulty with 2 ADLs, adjusting for other factors, on average, patients with dementia would report difficulty with 4.3 ADLs.
Table 2.
Predictors of Difficulty with Activities of Daily Living
| Variable |
*Relative Difference in No. ADLs (95% CI) |
P value |
|---|---|---|
| Age (per 10 year increase) | 1.17 (1.11, 1.23) | <0.001 |
| Female sex | 1.59 (1.37, 1.85) | <0.001 |
| Not married | 1.22 (1.05, 1.43) | 0.011 |
| Diabetes mellitus | 1.50 (1.29, 1.75) | <0.001 |
| Cerebrovascular disease | 1.19 (1.02, 1.38) | 0.031 |
| Dementia | 2.17 (1.64, 2.89) | <0.001 |
| Morbid obesity (BMI≥ 35kg/m2) | 1.32 (1.09, 1.60) | 0.005 |
| Anemia | 1.25 (1.09, 1.45) | 0.002 |
Estimates the expected relative increase in number of ADLs for which patients report difficulty per unit change in the predictor. For example, diabetics would be expected to report difficulty with 50% more ADLs than non-diabetics. All variables shown were included in the multivariable model. BMI= body mass index
Hierarchy of Difficulty with ADLs
The difficulty hierarchy of the 9 ADLs was found to be in order from easiest to most difficult: eating, toileting, dressing, taking medications, bathing, transportation, housekeeping, walking, and climbing stairs (Table 3 and Figure 1). All 9 items adequately fit the Rasch model based on mean squares infit values, which ranged from 0.78–1.26. The internal consistency of the scale was good (Cronbach’s alpha=0.80). The order and level of ADL difficulty were similar in both men and women. For all ADLs more difficult than eating, the majority of patients who could perform each ADL could perform all easier ADLs (Table 3). The ADL that performed the least well in this regard was walking, as 26.5% of persons who could walk without assistance could not complete all easier ADLs.
Table 3.
Hierarchy of Difficulty with Activities of Daily Living
| Item | No. (%) Patients Reporting Difficulty |
*Difficulty Based on Rasch Model |
†Mean Squares Infit |
% Patients Reporting no Difficulty who Can Complete all Easier Items |
|---|---|---|---|---|
| Eating | 40 (3.5) | −4.40 | 1.26 | -- |
| Toileting | 67 (5.9) | −2.20 | 0.81 | 98.6 |
| Dressing | 127 (11.3) | −0.77 | 0.86 | 98.1 |
| Taking Medications | 162 (14.4) | −0.01 | 1.14 | 92.5 |
| Bathing | 171 (15.2) | 0.09 | 0.78 | 89.1 |
| Transportation | 175 (15.5) | 0.20 | 1.00 | 84.7 |
| Housekeeping | 289 (25.6) | 1.48 | 0.94 | 84.6 |
| Walking | 327 (29.0) | 1.83 | 1.14 | 73.5 |
| Climbing Stairs | 518 (45.9) | 3.78 | 1.04 | 75.1 |
Estimated difficulty of each item assessed by the Rasch model. Easier items have lower (more negative) values. Items close in value have similar difficulty.
Mean squares infit values indicate how well the data fit the Rasch model. A value of 1.0 indicates a perfect fit, with values of 0.5–1.5 considered acceptable.
Figure 1. Proportion of Patients Completing Activities of Daily Living without Difficulty.
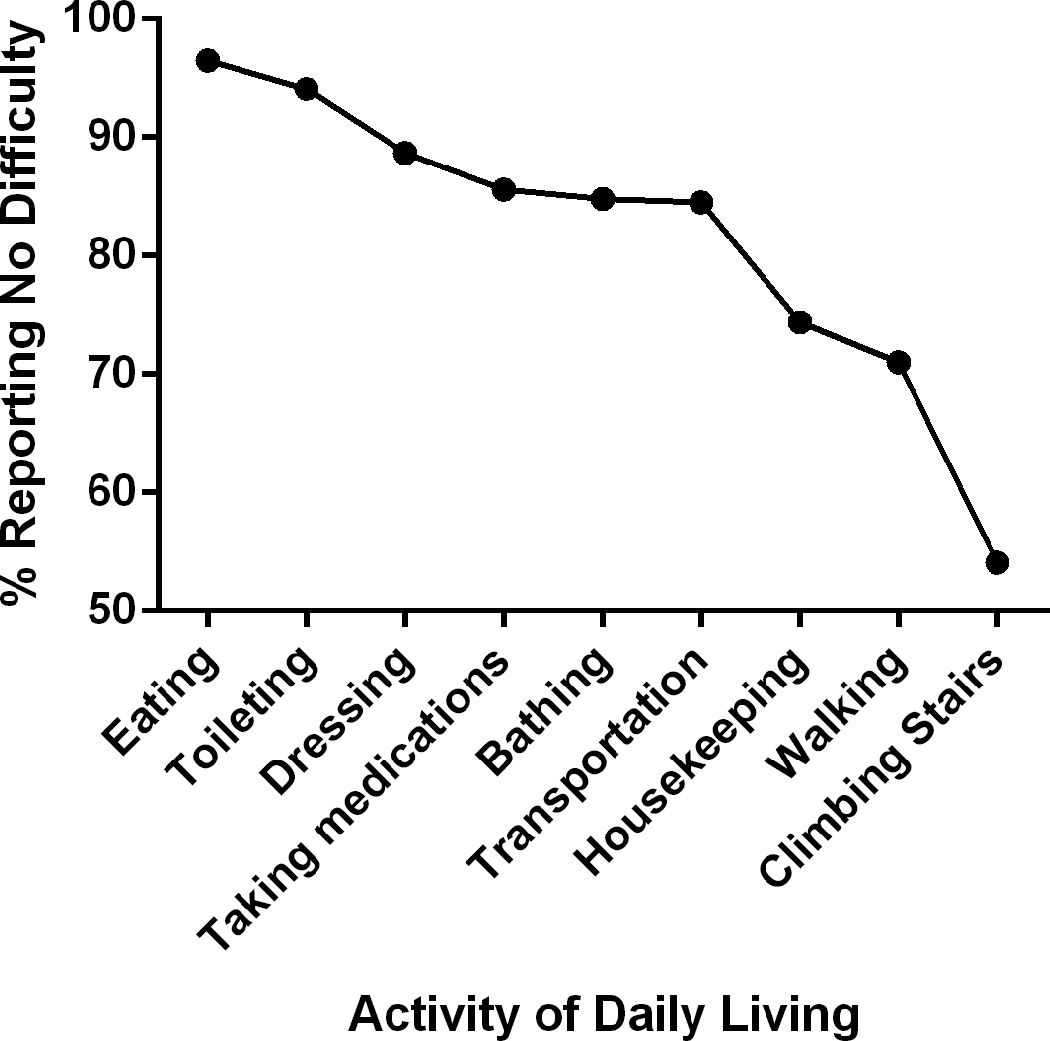
The proportion of patients with no reported difficulty performing each activity of daily living is shown.
Difficulty with ADLs, Hospitalizations, and Death
After a mean (SD) follow-up of 3.2 (2.4) years, 614 (54.4%) patients had died and 910 patients had been hospitalized a total of 4024 times (median 2 hospitalizations per person, 25th–75th percentile 1–5, 38.1% cardiovascular). The most common reasons for hospitalizations were heart failure (ICD9 428, N=741 hospitalizations, 18.4% all hospitalizations), arrhythmia (ICD9 427, N=187, 4.7% all hospitalizations), and pneumonia (ICD9 486, N=174, 4.3% all hospitalizations). When patients were categorized by the easiest ADL that they had difficulty completing, we found similar mortality risk in patients who had difficulty with eating, toileting, and dressing (severe ADL difficulty= highest risk), taking medications, bathing, transportation, and housekeeping (moderate ADL difficulty= moderate risk), and walking, climbing stairs or no difficulty with any ADLs (minimal ADL difficulty= lowest risk). The Kaplan Meier curves demonstrating the relationship between difficulty with ADL groups and death are shown in Figure 2. The estimated 2-year mortality for those with minimal, moderate, and severe difficulty with ADLs at study enrollment was 21.4%, 36.3%, and 54.6%, respectively. The median survival for those with minimal, moderate, and severe difficulty was 5.6, 3.0, and 1.5 years. Adjusting for age, sex, Charlson comorbidity index, and in-hospital completion of ADL questionnaires, the HR (95% CI) for mortality was 1.49 (1.22–1.82) for those with moderate difficulty and 2.26 (1.79–2.86) for those with severe difficulty compared to those with minimal difficulty with ADLs (ptrend <0.001, Table 4).
Figure 2. Difficulty with Activities of Daily Living and Death.
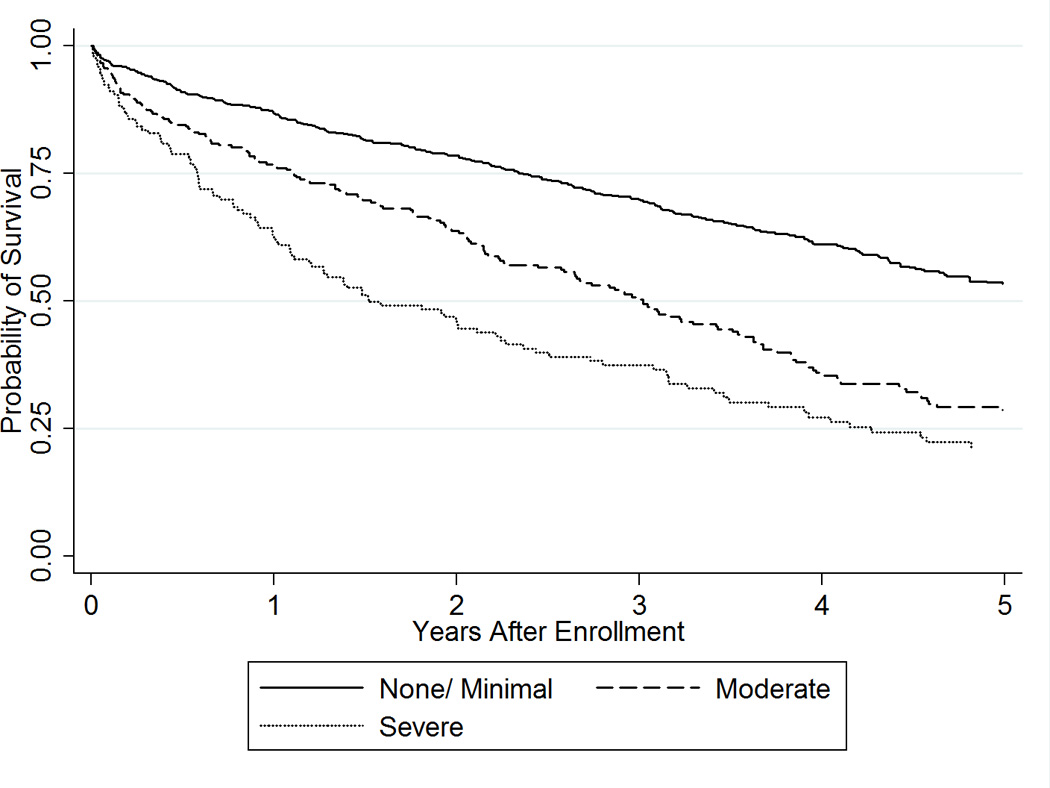
Kaplan Meier curves demonstrating the time to death in patients with heart failure according to their level of difficulty with activities of daily living (none/minimal, moderate, severe) at enrollment are shown.
Table 4.
Activities of Daily Living and Outcomes
| Outcome | Level of Difficulty with ADLs at Enrollment, Hazard Ratio (95% CI) |
P for trend |
||
|---|---|---|---|---|
| No/Minimal | Moderate | Severe | ||
| Mortality | ||||
| Unadjusted | 1 (referent) | 1.98 (1.65–2.37) | 2.81 (2.26–3.48) | <0.001 |
| *Adjusted | 1 (referent) | 1.49 (1.22–1.82) | 2.26 (1.79–2.86) | <0.001 |
| All-Cause Hospitalization | ||||
| Unadjusted | 1 (referent) | 1.53 (1.32–1.78) | 1.45 (1.19–1.75) | <0.001 |
| *Adjusted | 1 (referent) | 1.37 (1.17–1.59) | 1.22 (1.00–1.49) | 0.001 |
| HF Hospitalization | ||||
| Unadjusted | 1 (referent) | 1.43 (1.12–1.82) | 1.45 (1.04–2.01) | 0.002 |
| *Adjusted | 1 (referent) | 1.32 (1.03–1.69) | 1.17 (0.82–1.67) | 0.115 |
| Other cardiovascular/ renal hospitalization | ||||
| Unadjusted | 1 (referent) | 1.09 (0.89–1.35) | 0.88 (0.66–1.17) | 0.065 |
| *Adjusted | 1 (referent) | 1.12 (0.90–1.41) | 0.86 (0.64–1.16) | 0.769 |
| Non-cardiovascular hospitalization | ||||
| Unadjusted | 1 (referent) | 1.51 (1.27–1.78) | 1.45 (1.18–1.78) | <0.001 |
| *Adjusted | 1 (referent) | 1.33 (1.13–1.58) | 1.31 (1.06–1.62) | <0.001 |
Adjusted for age, sex, Charlson comorbidity index, and location of questionnaire completion (hospital vs. outpatient)
The risk of hospitalization was increased in those with moderate and severe ADL difficulty. The rates of hospitalization per 100 person years of follow-up for those with minimal, moderate, and severe difficulty were 100.4, 162.4, and 158.6, respectively. After adjusting for age, sex, and Charlson comorbidity index the HR (95% CI) for hospitalization was 1.37 (1.17–1.59) for those with moderate difficulty and 1.22 (1.00–1.49) for those with severe difficulty, indicating that the patients with moderate difficulty may be at the highest risk of hospitalization (Table 4). However, this difference was partially attributable to the fact that the patients with severe difficulty with ADLs were at highest risk for out-of-hospital death, such that they may die without being hospitalized. When we performed a sensitivity analysis where we assumed that patients were hospitalized on their date of death, the HR for hospitalization for those with moderate and severe difficulty with ADLs were similar (1.57 vs. 1.57). The association between ADL difficulty and HF hospitalization followed a similar pattern to all-cause hospitalization, with a stronger association in those with moderate difficulty. Patients with both moderate and severe ADL difficulty were at increased risk for non-cardiovascular hospitalization. ADL difficulty did not predict hospitalization for other cardiovascular/ renal causes.
Change in Independence with ADLs Over Time
In total, 823 (73.0%) patients completed a second ADL questionnaire during follow-up. The median time between the first and second reports of ADL difficulty was 9 (25th–75th percentile 7–13) months. Over half of patients (55.1%) who did not have a second ADL report died within 18 months of enrollment; they may not have had an opportunity to complete a second assessment. Using Rasch analysis, the order of difficulty of ADLs was similar to the first assessment, with a difficulty hierarchy (easiest to most difficult) of eating, toileting, dressing, bathing, medications, transportation, housekeeping, walking, and climbing stairs. Only bathing and medications were reversed in order of difficulty in the second assessment, underscoring that these two items are likely of similar difficulty. Using the previously established categories of minimal, moderate, and severe difficulty, ADL difficulty was stable in most patients (73.5%, Figure 3), while only a small fraction (8.8%) improved, and 17.7% worsened.
Figure 3. Change in Independence with Activities of Daily Living Over Time.
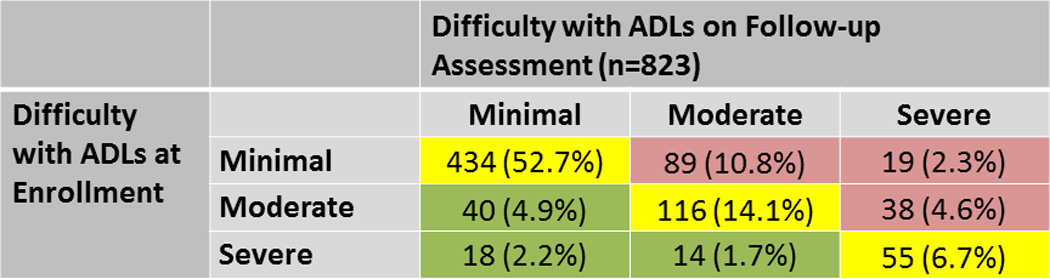
Patients (N, %) are categorized by their level of difficulty with activities of daily living at study enrollment and during the follow-up assessment (performed a median of 9 months later). Patients with stable difficulty over time are shown in yellow, less difficulty in green, and increasing difficulty in red.
For those with persistently severe or worsening difficulty with ADLs over time (went from minimal to moderate/severe or went from moderate to severe, n=201, 24.4% of patients with serial ADL assessments), the risk of death (unadjusted HR 2.44, 95% CI 2.00–2.98, p<0.001) and readmission (HR 1.64, 95% CI 1.41–1.91, p<0.001) were markedly increased. Adjusting for age, sex, Charlson comorbidity index, and time between surveys, patients with persistently severe or worsening difficulty with ADLs were at increased risk of subsequent mortality (HR 2.10, 95% CI 1.71–2.58, p<0.001) and readmission (HR 1.51, 95% CI 1.31–1.74, p<0.001).
Association Between ADL Difficulty and Resuscitation Preferences
Information on resuscitation preferences was available in the 517 patients enrolled after September 2007. At enrollment, 390 (75.4%) were Full Code, 73 (14.1%) elected to be DNR, and 54 (10.4%) had unknown resuscitation preference. Patients reporting moderate or severe difficulty with ADLs more often elected DNR status at enrollment (22.0% vs. 12.5%, p=0.009). Among those who were Full Code at enrollment, patients reporting moderate or severe difficulty with ADLs were more likely to change to DNR during follow-up (40.8% vs. 24.7%, p<0.001).
Discussion
In this large community cohort of patients with HF, the majority of patients had difficulty with one or more ADLs. A clear hierarchy in the order of ADL difficulty existed, which allowed patients to be categorized as having minimal, moderate and severe difficulty with ADLs. Within this framework, there was an increase in mortality and hospitalization risk with increasing difficulty with ADLs. While most patients reported similar functionality a year later, persistently severe or worsening difficulty with ADLs was associated with increased mortality and hospitalization risk.
The ability to perform ADLs is a commonly used method of assessing a person’s functionality19. ADLs include daily self-care activities that a person would routinely encounter in their residence or when interacting with their external environment. ADLs are sometimes divided into basic, which include self-care tasks such as eating, dressing, and bathing, and instrumental, which allow a person to live independently, such as housekeeping, managing medications, and using transportation. Our assessment included a combination of both basic and instrumental ADLs. By contrast, another commonly used form of ADL assessment, the Katz ADL index13, includes only 6 basic ADLs (eating, continence, transferring, toileting, dressing, bathing). While the ADLs we assessed differed slightly from the Katz index, there was consistency in the order of difficulty among the common ADLs compared with previous reports, with eating being the easiest of the 6 Katz ADLs and bathing requiring the highest level of function to complete20, 21. These data, which define a meaningful hierarchy of ADL difficulty, also suggest an expected pattern of functional decline or recovery that may be of clinical utility. Accordingly, there was consistency to the order of difficulty with ADLs in a second assessment, with eating, toileting and dressing representing the easiest items, and walking/climbing stairs being the most difficult items to complete. This hierarchy of difficulty will enable clinicians to easily assess a patient’s current level of function, and to provide insight into the types of activities that would represent signs of decline or improvement in function in individuals.
We found that moderate or severe difficulty with ADLs was very common in community patients with HF. We suspect that the decreased functionality observed is not entirely attributable to HF, as most patients had other comorbidities, such as diabetes22, peripheral vascular disease23, and cerebrovascular disease24, all of which can impact a person’s mobility and ability to perform self-care activities. In the contemporary era, most patients with HF are elderly and have multi-morbidity, and care needs to be comprehensive, and include consideration of their functional status and comorbidity burden.
Some patients experienced a progression in functional disability over time. Anticipating the needs of patients is an important component in providing patient-centered care. Functional independence is highly valued by patients and increasing difficulty with ADLs has been associated with worse health-related quality of life25, which can in turn affect disease management and impact outcomes. Further work is needed to address whether there are interventions that could be performed to prevent progression. Care by disease-specific subspecialists26 and physical therapy interventions27 have been demonstrated to slow or halt functional decline in other disease states, but their role in patients with HF remains to be explored.
In addition to its potential impact on quality of life and care needs, difficulty with ADLs was associated with an increased risk of hospitalization and death. HF is a clinical syndrome with an overall poor prognosis, with a 5 year survival of only 50% in community studies28. However, the prognosis can be highly variable based on the clinical characteristics of the patient, and prognostication can be difficult. While risk scores have been developed, they are often complex and can be challenging to implement in a busy clinical practice. Herein, we observed that patients who reported severe difficulty with ADLs had a dismal median survival of only 1.5 years, thus representing a very high risk subgroup of patients. Ascertainment of information on ability to perform ADLs may thus serve several clinically-relevant functions. In addition to supplying information that may help to anticipate care needs of the patient, it may also provide a quick idea of a patient’s global prognosis, which can help in guiding discussions and determining the appropriateness of therapeutic options.
While we saw a clear graded increase in the risk of death with increasing ADL difficulty, the relationship between ADL difficulty and hospitalization was more complex. Taking into account the potential impact of out-of-hospital death on risk, patients with both moderate and severe difficulty had a 57% increased risk for hospitalization compared with those with no or minimal difficulty. These findings suggest that while patients with functional limitations are at higher risk for hospitalization than those who are functionally independent, the level of difficulty does not necessarily determine risk. There are several factors that may contribute to this observation. First, one of the strongest predictors of ADL difficulty was dementia. It may be that many patients with severe ADL difficulty are known to have a poor prognosis and have made choices to avoid hospitalization or be cared for in settings that limit their likelihood of hospitalization. Additionally, the ADLs associated with poor outcomes in this study, such as self-feeding, are those characterized by integrated multi-step tasks that must be accurately sequenced and executed through higher level cognitive processes. Walking and stair climbing, the ADLs least associated with poor outcomes, in contrast, require intact neuromuscular function but impose limited cognitive demands. It is possible that the construct most strongly associated with death and hospitalization among patients with HF is a cognitive one; related to, but imperfectly reflected by ADL performance and a diagnosis of dementia. Enhanced predictive capability and more targeted therapeutic interventions may be achieved through efforts to more accurately characterize functional domains that are causally linked to poor outcomes in HF.
A similar combination of basic and instrumental ADLs has been used to assess older populations in a variety of settings29–32. In the Cardiovascular Health Study (participants age 65 and older)30, the Medicare Current Beneficiary Survey (age 65 and older)29, 32, and a large Swedish cohort (age 70–76 years)31, the prevalence of difficulty with one or more ADLs ranged from 17–26%, much lower than the 59% observed in our study. However, the prevalence of ADL difficulty was similar to that reported in patients age 70 and older discharged from a general medical service after an acute hospitalization33, where 59% reported difficulty with one or more ADLs. In each of these studies a graded increase in mortality with increasing difficulty with ADLs was observed, similar to our findings. These results suggest that while community patients with HF may have a higher burden of ADL difficulty than the general elderly population, the associated increase in mortality risk with ADL difficulty is likely generalizable to patients in a variety of settings.
Limitations and Strengths
There are limitations to acknowledge to aid in interpretation of these data. First, as in any prospective study, participation rates must be taken into account in interpreting study results. Difficulty with ADLs may be underestimated as participants, on average, were younger than non-participants. As the questionnaire used to assess ADLs is self-administered, it is unclear how often it was completed by a proxy informant, rather than the patient. This may be of particular relevance for the subset of patients with dementia, where self-report of ADL difficulty may be less accurate. Hospitalizations occurring outside of the county were not captured. However, comparison with Medicare data would suggest that only 5% of all hospitalizations for elderly patients living in Olmsted County occur outside of the county. Conversely, this study has several notable strengths. We were able to capture serial ADL information on a large community cohort of patients with HF who are well characterized and have extensive longitudinal follow-up. This allowed us to characterize functionality and its association with outcomes, which to the best of our knowledge, has not yet been reported. This information is of critical value in planning care for the growing population of elderly patients with HF, and had been previously lacking from the literature.
Clinical Applicability
These data have important clinical implications. The ability to complete ADLs can be easily assessed during an office visit and may be helpful in real-time prognostication to inform patient-provider discussions about goals of care and expected functional trajectory. Furthermore, if a patient is identified as having difficulty with one or more ADLs or has a progression in dysfunction over time, this may represent an important trigger to pursue a more thorough assessment and physical therapy evaluation.
Conclusions
In summary, we found that difficulty with ADLs was common in patients with HF, progressed over time in many individuals, and was a powerful marker of adverse prognosis. These findings provide new insight into the burden of functional disability in patients with HF. Routine assessment of patient-reported difficulty with ADLs may aid in prognostication and tailoring of therapeutic options.
Acknowledgments
Sources of Funding
This work was supported by grants from the National Institutes of Health (R01 HL72435 [VL Roger] and K23 HL116643 [SM Dunlay])
Footnotes
Disclosures
None.
References
- 1.Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: The women's health and aging study. J Clin Epidemiol. 1999;52:27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 2.Singh JA, Borowsky SJ, Nugent S, Murdoch M, Zhao Y, Nelson DB, Petzel R, Nichol KL. Health-related quality of life, functional impairment, and healthcare utilization by veterans: Veterans' quality of life study. J Am Geriatr Soc. 2005;53:108–113. doi: 10.1111/j.1532-5415.2005.53020.x. [DOI] [PubMed] [Google Scholar]
- 3.Millan-Calenti JC, Tubio J, Pita-Fernandez S, Gonzalez-Abraldes I, Lorenzo T, Fernandez-Arruty T, Maseda A. Prevalence of functional disability in activities of daily living (adl), instrumental activities of daily living (iadl) and associated factors, as predictors of morbidity and mortality. Arch Gerontol Geriatr. 2010;50:306–310. doi: 10.1016/j.archger.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Ramos LR, Simoes EJ, Albert MS. Dependence in activities of daily living and cognitive impairment strongly predicted mortality in older urban residents in brazil: A 2-year follow-up. J Am Geriatr Soc. 2001;49:1168–1175. doi: 10.1046/j.1532-5415.2001.49233.x. [DOI] [PubMed] [Google Scholar]
- 5.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the rochester epidemiology project: Half a century of medical records linkage in a us population. Mayo Clin Proceed. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pakhomov SV, Buntrock J, Chute CG. Prospective recruitment of patients with congestive heart failure using an ad-hoc binary classifier. J Biomed Inform. 2005;38:145–153. doi: 10.1016/j.jbi.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 7.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 8.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 10.WHO. Nutritional anemias: Report of a who scientific group. WHO Technical Support Series. 1968;405:1. [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Dunlay SM, Swetz KM, Redfield MM, Mueller PS, Roger VL. Resuscitation preferences in community patients with heart failure. Circ Cardiovasc Qual Outcomes. 2014;7:353–359. doi: 10.1161/CIRCOUTCOMES.113.000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of adl: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 14.Reijneveld SA, Spijker J, Dijkshoorn H. Katz' adl index assessed functional performance of turkish, moroccan, and dutch elderly. J Clin Epidemiol. 2007;60:382–388. doi: 10.1016/j.jclinepi.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 15.Wright B, Mok M. Rasch models overview. J Appl Meas. 2000;1:83–106. [PubMed] [Google Scholar]
- 16.Fries JF, Witter J, Rose M, Cella D, Khanna D, Morgan-DeWitt E. Item response theory, computerized adaptive testing, and promis: Assessment of physical function. J Rheumatol. 2014;41:153–158. doi: 10.3899/jrheum.130813. [DOI] [PubMed] [Google Scholar]
- 17.Andersen PK, Gill RD. Cox's regression model for counting processes: A large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 18.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz S. Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 20.Gerrard P. The hierarchy of the activities of daily living in the katz index in residents of skilled nursing facilities. J Geriatr Phys Ther. 2013;36:87–91. doi: 10.1519/JPT.0b013e318268da23. [DOI] [PubMed] [Google Scholar]
- 21.Stineman MG, Ross RN, Granger CV, Maislin G. Predicting the achievement of 6 grades of physical independence from data routinely collected at admission to rehabilitation. Arch Phys Med Rehabil. 2003;84:1647–1656. doi: 10.1053/s0003-9993(03)00317-4. [DOI] [PubMed] [Google Scholar]
- 22.Gregg EW, Beckles GL, Williamson DF, Leveille SG, Langlois JA, Engelgau MM, Narayan KM. Diabetes and physical disability among older u.S. Adults. Diabetes Care. 2000;23:1272–1277. doi: 10.2337/diacare.23.9.1272. [DOI] [PubMed] [Google Scholar]
- 23.Garg PK, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, Tan J, McDermott MM. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation. 2009;119:251–260. doi: 10.1161/CIRCULATIONAHA.108.791491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007;68:1583–1587. doi: 10.1212/01.wnl.0000260967.77422.97. [DOI] [PubMed] [Google Scholar]
- 25.JP B, WW T, MM Z, GL K, W H-J, SE B. Multiple chronic medical conditions and health-related quality of life in older adults, 2004–2006. Prev Chronic Dis. 2013;10:120282. doi: 10.5888/pcd10.120282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward MM, Leigh JP, Fries JF. Progression of functional disability in patients with rheumatoid arthritis. Associations with rheumatology subspecialty care. Arch Intern Med. 1993;153:2229–2237. [PubMed] [Google Scholar]
- 27.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–1074. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 28.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 29.Lubitz J, Cai L, Kramarow E, Lentzner H. Health, life expectancy, and health care spending among the elderly. N Engl J Med. 2003;349:1048–1055. doi: 10.1056/NEJMsa020614. [DOI] [PubMed] [Google Scholar]
- 30.Peralta CA, Katz R, Newman AB, Psaty BM, Odden MC. Systolic and diastolic blood pressure, incident cardiovascular events, and death in elderly persons: The role of functional limitation in the cardiovascular health study. Hypertension. 2014;64:472–480. doi: 10.1161/HYPERTENSIONAHA.114.03831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonn U. Longitudinal studies of dependence in daily life activities among elderly persons. Scand J Rehabil Med Suppl. 1996;34:1–35. [PubMed] [Google Scholar]
- 32.Federal Interagency Forum on Aging Related Statistics. Older americans 2012: Key indicators of well-being. [Accessed October 15, 2014]; http://www.agingstats.gov/agingstatsdotnet/Main_Site/Data/2012_Documents/Docs/EnterChartbook.pdf. [Google Scholar]
- 33.Walter LC, Brand RJ, Counsell SR, Palmer RM, Landefeld CS, Fortinsky RH, Covinsky KE. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]


