Abstract
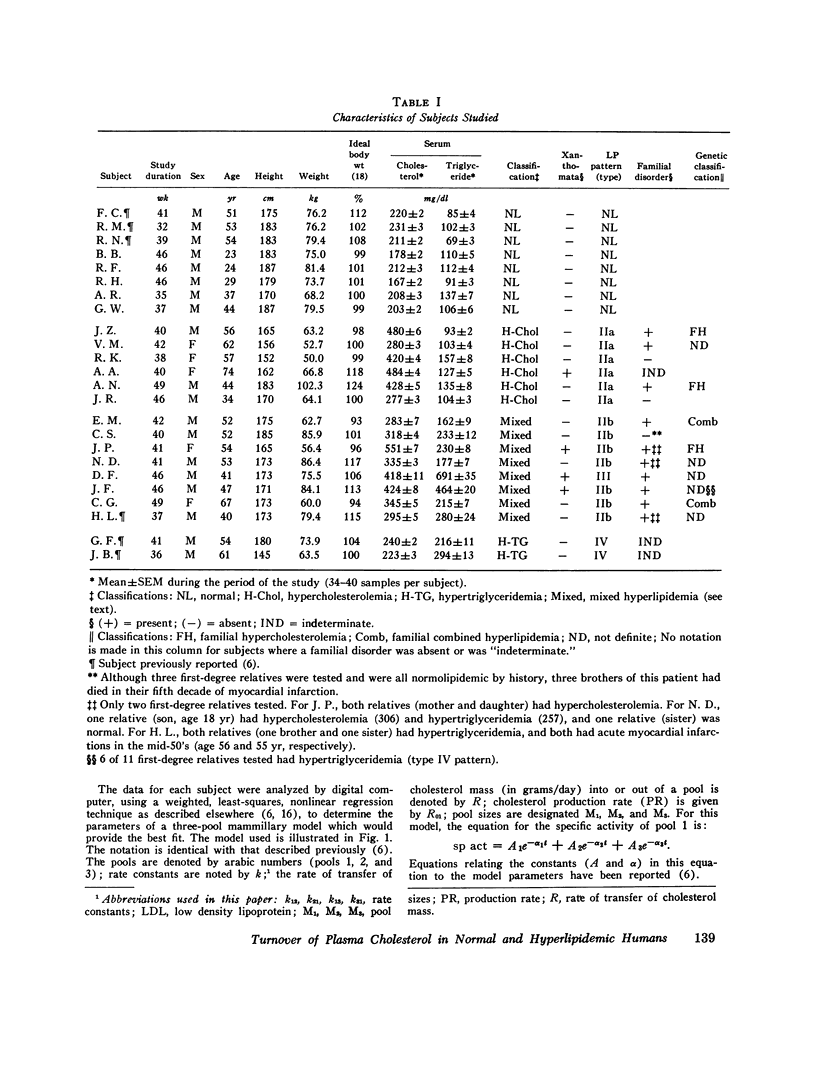
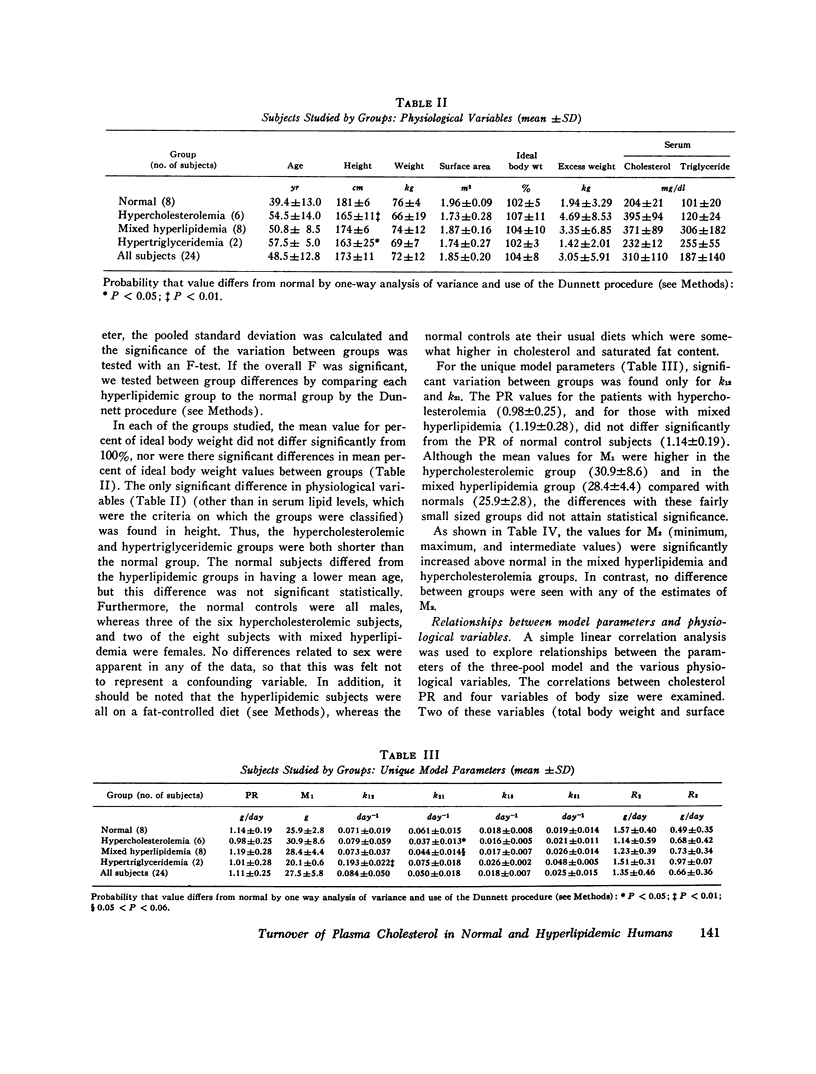
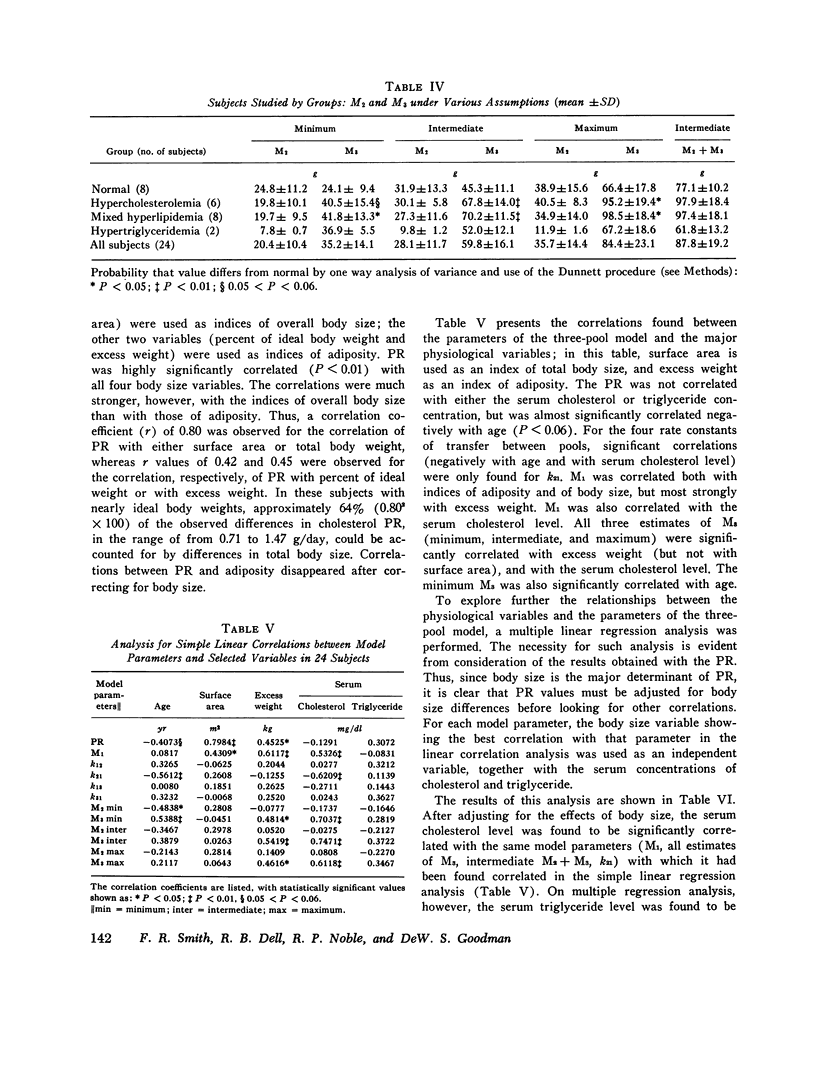
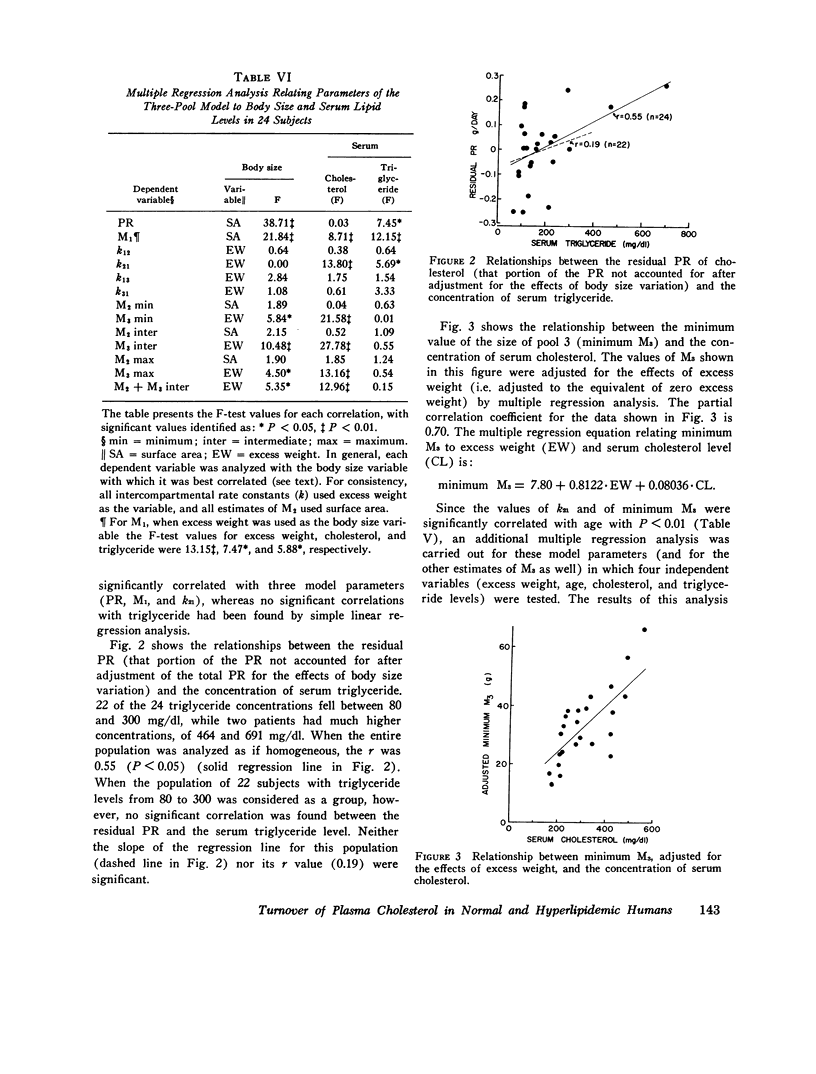
Long-term studies (32-49 wk) of the turnover of plasma cholesterol were conducted in 24 subjects. Eight subjects were normilipidemic, six had hypercholesterolemia, eight had hypercholesterolemia and hypertriglyceridemia, and two had hypertriglyceridemia alone. 10 of the hyperlipidemic patients had a definite familial disorder. In all subjects (except one for whom complete data were not available), the same three-pool model previously described gave the best fit for the data. The parameters of the three-pool model observed in the normal subjects were compared with the model parameters found in the patients with the different kinds of hyperlipidemia. In addition, single and multiple regression analyses were conducted to explore the relationships between the model parameters and various physiological variables, including age, body size, and serum lipid concentrations. Using this approach, significant differences between groups, or correlations with serum lipid levels were seen for several parameters of the three-pool model: the production rate (PR); the size of the rapidly exchanging pool 1 (M1); all estimates of the size of the most slowly equilibrating pool 3 (M3); and the rate constant k21. The PR in normal subjects (1.14 +/- 0.19 g/day, mean +/- SD) was not significantly different from that found in patients with hypercholesterolemia, with or without hypertriglyceridemia. The major determinant of cholesterol PR was overall body size, expressed either as total body weight or as surface area. The correlations between PR and indices of adiposity (percent ideal weight and excess weight), although statistically significant, were much weaker in this nonobese population. After adjustment for body size variation, cholesterol PR was not correlated with the serum cholesterol concentration but was probably (P less than 0.05) correlated with the triglyceride concentration. When the two patients with very high triglyceride concentrations were excluded, however, no correlation was observed between adjusted PR and triglyceride level. It is probable that hypertriglyceridemic patients represent a heterogeneous population, in which the majority do not show increased cholesterol PR. M1 was correlated with all body size variables, but most strongly with excess weight. After adjusting for the effects of body size, M1 was also correlated and triglyceride. Major differences were found in the relationships between the physiological variables and the sizes of pools 2 and 3. M2 was correlated neither with any of the indices of body size or adiposity, nor with the serum levels of either cholesterol or triglyceride. In contrast, all estimates of M3 were correlated with indices of adiposity (but not of overall body size) and with the serum cholesterol concentration. Thus, the amount of cholesterol in slowly equilibrating tissue sites appears to particularly increase with elevations of the serum cholesterol level. The results also confirm previous data that adipose tissue cholesterol is an important part of pool 3.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaumont J. L., Carlson L. A., Cooper G. R., Fejfar Z., Fredrickson D. S., Strasser T. Classification of hyperlipidaemias and hyperlipoproteinaemias. Bull World Health Organ. 1970;43(6):891–915. [PMC free article] [PubMed] [Google Scholar]
- CHOBANIAN A. V., HOLLANDER W. Body cholesterol metabolism in man. I. The equilibration of serum and tissue cholesterol. J Clin Invest. 1962 Sep;41:1732–1737. doi: 10.1172/JCI104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse J. R., Grundy S. M., Ahrens E. H., Jr Cholesterol distribution in the bulk tissues of man: variation with age. J Clin Invest. 1972 May;51(5):1292–1296. doi: 10.1172/JCI106924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsson K., Hellström K., Kallner M. Bile acid kinetics in relation to sex, serum lipids, body weights, and gallbladder disease in patients with various types of hyperlipoproteinemia;. J Clin Invest. 1974 Dec;54(6):1301–1311. doi: 10.1172/JCI107876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIELD H., Jr, SWELL L., SCHOOLS P. E., Jr, TREADWELL C. R. Dynamic aspects of cholesterol metabolism in different areas of the aorta and other tissues in man and their relationship to atherosclerosis. Circulation. 1960 Oct;22:547–558. doi: 10.1161/01.cir.22.4.547. [DOI] [PubMed] [Google Scholar]
- Fredrickson D. S., Levy R. I., Lees R. S. Fat transport in lipoproteins--an integrated approach to mechanisms and disorders. N Engl J Med. 1967 Jan 26;276(4):215–contd. doi: 10.1056/NEJM196701262760406. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Hazzard W. R., Schrott H. G., Bierman E. L., Motulsky A. G. Hyperlipidemia in coronary heart disease. I. Lipid levels in 500 survivors of myocardial infarction. J Clin Invest. 1973 Jul;52(7):1533–1543. doi: 10.1172/JCI107331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Schrott H. G., Hazzard W. R., Bierman E. L., Motulsky A. G. Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest. 1973 Jul;52(7):1544–1568. doi: 10.1172/JCI107332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. S., Noble R. P., Dell R. B. The effects of colestipol resin and of colestipol plus clofibrate on the turnover of plasma cholesterol in man. J Clin Invest. 1973 Oct;52(10):2646–2655. doi: 10.1172/JCI107457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. S., Noble R. P., Dell R. B. Three-pool model of the long-term turnover of plasma cholesterol in man. J Lipid Res. 1973 Mar;14(2):178–188. [PubMed] [Google Scholar]
- Goodman D. S., Noble R. P. Turnover of plasma cholesterol in man. J Clin Invest. 1968 Feb;47(2):231–241. doi: 10.1172/JCI105719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr Measurements of cholesterol turnover, synthesis, and absorption in man, carried out by isotope kinetic and sterol balance methods. J Lipid Res. 1969 Jan;10(1):91–107. [PubMed] [Google Scholar]
- Grundy S. M. Effects of polyunsaturated fats on lipid metabolism in patients with hypertriglyceridemia. J Clin Invest. 1975 Feb;55(2):269–282. doi: 10.1172/JCI107930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan S. N., Connor W. E., Baker W. H., Bhattacharyya A. K. The turnover of cholesterol in human atherosclerotic arteries. J Clin Invest. 1974 Aug;54(2):366–377. doi: 10.1172/JCI107772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke B. A. Difference in bile acid excretion. Primary hypercholesteremia compared to combined hypercholesteremia and hypertriglyceridemia. Circulation. 1969 Jul;40(1):13–20. doi: 10.1161/01.cir.40.1.13. [DOI] [PubMed] [Google Scholar]
- Langer T., Strober W., Levy R. I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen T. A. Cholesterol production in obesity. Circulation. 1971 Nov;44(5):842–850. doi: 10.1161/01.cir.44.5.842. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Hunter J. D. Differences in bile acid excretion in subjects with hypercholesterolaemia, hypertriglyceridaemia and overweight. Aust N Z J Med. 1974 Oct;4(5):491–496. doi: 10.1111/j.1445-5994.1974.tb03223.x. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Schreibman P. H., Ahrens E. H., Jr Cholesterol metabolism in human obesity. J Clin Invest. 1973 Oct;52(10):2389–2397. doi: 10.1172/JCI107428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P. J., Whyte H. M., Goodman D. S. Distribution and turnover of cholesterol in humans. J Clin Invest. 1969 Jun;48(6):982–991. doi: 10.1172/JCI106079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble R. P., Campbell F. M. Improved accuracy in automated fluorometric determination of plasma triglycerides. Clin Chem. 1970 Mar;16(3):166–170. [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Perl W., Samuel P. Input-output analysis for total input rate and total traced mass of body cholesterol in man. Circ Res. 1969 Aug;25(2):191–199. doi: 10.1161/01.res.25.2.191. [DOI] [PubMed] [Google Scholar]
- Salen G., Ahrens E. H., Jr, Grundy S. M. Metabolism of beta-sitosterol in man. J Clin Invest. 1970 May;49(5):952–967. doi: 10.1172/JCI106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel P., Lieberman S. Improved estimation of body masses and turnover of cholesterol by computerized input--output analysis. J Lipid Res. 1973 Mar;14(2):189–196. [PubMed] [Google Scholar]
- Samuel P., Perl W. Long-term decay of serum cholesterol radioactivity: body cholesterol metabolism in normals and in patients with hyperlipoproteinemia and atherosclerosis. J Clin Invest. 1970 Feb;49(2):346–357. doi: 10.1172/JCI106243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibman P. H., Dell R. B. Human adipocyte cholesterol. Concentration, localization, synthesis, and turnover. J Clin Invest. 1975 May;55(5):986–993. doi: 10.1172/JCI108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi H. S., Kudchodkar B. J. Catabolism of cholesterol in hypercholesterolemia and its relationship to plasma triglycerides. Clin Chim Acta. 1973 Jun 28;46(2):161–171. doi: 10.1016/0009-8981(73)90024-7. [DOI] [PubMed] [Google Scholar]
- Sodhi H. S., Kudchodkar B. J. Synthesis of cholesterol in hypercholesterolemia and its relationship to plasma trigylcerides. Metabolism. 1973 Jul;22(7):895–912. doi: 10.1016/0026-0495(73)90062-0. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. The measurement of the exchangeable pools of cholesterol in the baboon. J Clin Invest. 1970 Apr;49(4):655–665. doi: 10.1172/JCI106277. [DOI] [PMC free article] [PubMed] [Google Scholar]



