Abstract
Background
Depression and stress have each been found to be associated with poor prognosis in coronary heart disease (CHD) patients. A recently offered ‘Psychosocial Perfect Storm’ conceptual model hypothesizes amplified risk will occur in those with concurrent stress and depressive symptoms. We tested this hypothesis in a large sample of U.S. adults with CHD.
Methods and Results
Participants included 4487 adults with CHD from the REasons for Geographic and Racial Differences in Stroke (REGARDS) study, a prospective cohort study of 30,239 Black and White adults. We conducted Cox proportional hazards regression with the composite outcome of myocardial infarction (MI) or death and adjustment for demographic, clinical, and behavioral factors. Overall, 6.1% reported concurrent high stress and high depressive symptoms at baseline. Over a median 5.95-years of follow-up, 1,337 events occurred. In the first 2.5-years of follow-up, participants with concurrent high stress and high depressive symptoms had increased risk for MI or death (adjusted hazard ratio [HR]=1.48, [95% CI: 1.08–2.02]) relative to those with low stress and low depressive symptoms. Those with low stress and high depressive symptoms (HR=0.92, [95% CI: 0.66–1.28]) or high stress and low depressive symptoms (HR=0.86, [95% CI: 0.57–1.29]) were not at increased risk. The association on MI or death was not significant after the initial 2.5-years of follow-up (HR=0.89, [95% CI: 0.65–1.22]).
Conclusions
Our results provide initial support for a ‘Psychosocial Perfect Storm’ conceptual model; the confluence of depressive symptoms and stress on medical prognosis in adults with CHD may be particularly destructive in the shorter-term.
Keywords: stress, depression, coronary heart disease, death, prognosis, U.S.A.
Stress and depression are each associated with increased risk for incident and recurrent coronary heart disease (CHD),1–19 but the two likely have a complex inter-relationship,20 and only a limited number of prior studies have investigated their effect on risk for myocardial infarction (MI) and mortality when both are present. In one of the largest cross-national studies on this topic, adults who reported permanent home/work stress and depression had a two-fold higher odds for a history of MI when compared to their never stressed and not depressed counterparts.21 Relatedly, extant research on the conjoint effect of anxiety and depression on adverse cardiac outcomes has produced conflicting results. The presence of both depression and anxiety were associated with increased risk for recurrent MI, cardiovascular death, or all-cause mortality in some studies,22–25 while others have not found evidence of higher cardiovascular risk in the presence of both psychosocial factors.26, 27 A recent conceptual model of the occurrence of an MI used a Perfect Storm metaphor to note that MIs are not caused by a single or a few factors, but rather result from the confluence of many situations and underlying risk factors.28 We have recently presented a Psychosocial Perfect Storm model of MI and mortality, and have suggested that it may take an underlying chronic psychosocial vulnerability, such as depression, in the presence of a more transient situation or trigger, such as psychological stress, for clinical events to occur.29
We tested our Psychosocial Perfect Storm29 model for understanding CHD prognosis by examining whether the confluence of high stress and high depressive symptoms amplifies shorter-term risk (< 2.5-years) for MI or death in individuals with CHD. We hypothesized that among individuals with CHD, those with concurrent high stress and high depressive symptoms would exhibit an increased risk of MI or death while their counterparts with high stress only or depressive symptoms only would not have an increased risk of MI or death.
Methods
Data Source and Sample
The REasons for Geographic and Racial Differences in Stroke (REGARDS) study is examining the reasons for higher stroke mortality among blacks and residents of the Southeastern United States (US). CHD outcomes are being obtained with support from an ancillary study (REGARDS-MI).30 Details of the REGARDS study have been described in detail previously.31 In brief, 30,239 Black and White adults ≥ 45 years of age from throughout the continental US were enrolled between January 2003 and October 2007. Blacks and residents of the stroke buckle (coastal North Carolina, South Carolina and Georgia) and stroke belt (the remainder of North Carolina, South Carolina and Georgia and Alabama, Mississippi, Louisiana, Tennessee and Arkansas) were over-sampled. The current analysis was limited to REGARDS study participants with a history of CHD at baseline defined as a self-reported history of MI or coronary revascularization procedure (percutaneous coronary interventions or coronary artery bypass grafting) or evidence of MI on the study electrocardiogram.
Of the 5,314 participants with a history of CHD, we excluded 28 participants who were missing data on depressive symptoms or perceived stress at baseline, 81 participants who were missing follow-up data for outcomes, and 718 participants who were missing covariate information, leaving 4,487 participants for analysis. The Institutional Review Boards at the participating centers approved the REGARDS study protocol, and all participants provided written informed consent.
Data Collection
Baseline data were collected through a computer-assisted telephone interview, an in-home examination, and a self-administered questionnaire. Information was collected on demographics, education, income, alcohol consumption, marital status, general self-rated health, cigarette smoking, physical activity and medication adherence during the telephone interview. The in-home examination was conducted by trained health professionals and included a physical examination, collection of biological samples and a pill bottle review. For the pill bottle review, participants were asked to provide all prescription and non-prescription medications they had taken in the past two weeks prior to the in home-examination and medication names were recorded and coded into drug classes. During the in-home examination, an electrocardiogram was performed.
Depressive symptoms
The 4-item Center for Epidemiologic Studies Depression (CES-D) scale, collected as part of the computer-assisted telephone interview, was used to assess depressive symptoms. The four scale items assess how often over the prior week the participant felt depressed, felt lonely, had crying spells and felt sad; as such these 4-items assess cognitive and not somatic symptoms of depression. Response options for each item were less than 1 day (no points), 1–2 days (1 point), 3–4 days (2 points) and 5–7 days (3 points). After summing the points from the four items, high depressive symptoms were defined as having a score ≥ 4. This definition has been found in previous studies to have 79.2% sensitivity and 81.2% specificity when compared to depressive symptoms on the 20-item CES-D.32, 33 The internal consistency of the 4-item CES-D in this sample was high (α = 0.82).
Perceived stress
Perceived stress was ascertained using the 4-item version of the Perceived Stress Scale (PSS) as part of the computer-assisted telephone interview.34 The PSS assesses the degree to which participants felt they were unable to control important things in their life, confidence in their ability to handle personal problems, how often they felt that things were going their way and how often difficulties were overwhelming over the past month. Each of the four items is scored using a 4-point scale resulting in an overall score that can range from 0 to 16 with higher scores indicating the presence of more stress. There is no accepted cut-point for defining high stress on the PSS. In primary analyses, high stress was defined as a PSS ≥ 8 in order to achieve a prevalence similar to high depressive symptoms. In secondary analyses, high stress was defined as a PSS ≥ 7 to approximate the highest 20% of scores. The internal consistency of the 4-item PSS in this sample was acceptable (α = 0.68).
Covariates
We included demographics, region of residence, CHD risk factors, health behaviors, and medication use, ascertained at baseline as covariates. Self-reported race, age, sex, region of residence, education (less than high school education vs. at least a high school diploma), income (<$20,000 vs. ≥$20,000), marital status (married vs. not married), and general self-rated health (excellent vs. very good, good, fair or poor) were included as covariates. We adjusted for region of residence because of the oversampling of REGARDS study participants in the stroke belt and stroke regions of the US. Heavy alcohol consumption was defined as > 14 alcoholic beverages per week for men (> 7 for women). Current smoking was defined as responding affirmatively to the following questions “Have you smoked at least 100 cigarettes in your lifetime?” and “Do you smoke cigarettes now, even occasionally?” Physical inactivity was defined as answering “none” to the question “How many times per week do you engage in intense physical activity, enough to work up a sweat?” Level of medication adherence was assessed using the 4-item Morisky Medication Adherence Scale (MMAS).35 Participants who reported poor medication taking behaviors on ≥ 2 of the items on the MMAS were categorized as having low adherence. Height and weight, measured during the study visit, were used to calculate body mass index (BMI). Blood pressure was measured two times and hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, and/or self-reported use of antihypertensive medication. Diabetes was defined as a serum glucose ≥ 126 mg/dL for participants who had fasted prior to their in-home study visit, serum glucose ≥ 200 mg/dL for participants who had not fasted, or self-report of a prior diagnosis of diabetes with current use of insulin or oral hypoglycemic medications. History of stroke was based on self-report.
Outcomes
The outcomes for the current study included definite/probable MI or death from any cause. Outcome data through December 31, 2011 were used in this analysis. Following the in-home examination, participants were contacted twice yearly by telephone to identify potential events. When a cardiac-related hospitalization was reported, medical records were retrieved and reviewed by a team of trained physicians using a standardized protocol.36, 37 Follow-up time was recorded as the number of days from the baseline in-home visit to the first occurrence of a participant’s confirmed date of death, occurrence of a definite or probable MI, their last REGARDS study telephone contact, or December 31, 2011. Expert-adjudicated definite MI was defined by review of medical records for the presence of ischemic signs or symptoms38, 39 and for positive diagnostic enzymes on electrocardiogram. Probable MIs were defined as those with elevated but not diagnostic enzymes with a positive but not diagnostic electrocardiogram; or with a positive electrocardiogram if enzymes were missing. Deaths were defined by report of next of kin or through online sources (e.g., Social Security Death index) and then confirmed by death certificates.30 Cardiovascular death was examined in a secondary analysis. Using adjudicated outcomes, cardiovascular death was defined as fatal MI, fatal stroke, fatal heart failure, and other fatal cardiovascular-related deaths.
Statistical Analyses
REGARDS participants were categorized into four mutually exclusive groups: low stress and low depressive symptoms, high stress and low depressive symptoms, low stress and high depressive symptoms, and having both high stress and high depressive symptoms.
Characteristics of REGARDS study participants with a history of CHD were calculated for participants in each of these four groups. We examined the association of high depressive symptoms and high stress, separately, and then jointly on MI or death. Analyses for the composite outcome of MI or death were conducted first, then, identical analyses were conducted for MI and for death, separately. Incidence rates were calculated per 1,000 person-years of follow-up. Hazard ratios (HR) were calculated with three progressive levels of adjustment: Model 1 adjusted for race, sex, region of residence (stroke buckle, stroke belt, non-belt), BMI, income, education, marital status, general self-rated health, hypertension, diabetes, history of myocardial infarction and history of stroke; Model 2 additionally adjusted for use of statins, beta blockers, aspirin, antidepressants, clopidogrel and renin angiotensin system inhibitors; Model 3 additionally adjusted for health risk behaviors including heavy alcohol consumption, cigarette smoking, physical inactivity, and low medication adherence. The multiplicative interaction between high stress and high depressive symptoms on outcomes was determined in regression models including main effects for high stress and depressive symptoms and an interaction term (high stress * high depressive symptoms). The proportionality assumption of the Cox regression model was assessed by modeling changes in the HR associated with high stress and high depressive symptoms over follow-up time. The proportionality assumption was violated, indicating that the HRs were not constant over time. Therefore, separate Cox models were fitted for different follow-up time intervals. Consequently, HRs are presented separately for the first 2.5 years of follow-up and follow-up beyond 2.5 years. This cut-point was chosen as ~50% of MIs or deaths occurred during the first 2.5 years of follow-up. In sensitivity analyses, follow-up time was dichotomized at 2 years, and separately, at 3 years.
To assess whether our results were robust to the cut-point chosen for defining high stress, we conducted a sensitivity analysis using PSS ≥7 to define high stress. We also used multiple imputation to explore the consistency of our results after accounting for participants with missing covariate information (n=718). For this analysis, 10 data sets were imputed with chained equations. Multiple imputation was based on observed values from all of the covariates included in the fully-adjusted Cox regression models and the outcome.40, 41 Additionally, to better understand the association of high stress and depressive symptoms with cardiovascular disease, we conducted a supplemental analysis with cardiovascular death as the outcome. Analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC). STATA 13.1 (StataCorp LP, College Station, TX) was used for multiple imputation. The significance level was set at P <0.05 for two-sided analyses.
Results
Participant Characteristics by Stress and Depressive Symptoms Group
Overall, 11.7% (n = 527) had high stress and 13.8% (n = 621) participants had high depressive symptoms. The prevalence of high stress only was 5.6% (n = 253) and the prevalence of high depressive symptoms only was 7.7% (n = 347), while the prevalence of concurrent high stress and high depressive symptoms at baseline was 6.1% (n = 274). The correlation between the scores on the stress scale (PSS) and the depressive symptoms scale (CES-D) was moderately high r = 0.52. Baseline characteristics by stress and depressive symptoms group are presented in Table 1. Mean age, BMI, and most demographic measures varied across stress and depressive symptoms groups. Prevalence of medical comorbidities (stroke, hypertension) and health risk behaviors (smoking, physical inactivity, and medication adherence) also varied by stress and depressive symptoms groups, with the highest prevalence of these medical conditions and health risk behaviors observed among those in the high stress and high depressive symptoms subgroup.
Table 1.
Baseline characteristics of participants with coronary heart disease by stress and depressive symptoms groups, REGARDS (N = 4,487).
| Low stress and low depressive symptoms (n =3,613) |
High stress and low depressive symptoms (n =253) |
Low stress and high depressive symptoms (n =347) |
High stress and high depressive symptoms (n =274) |
p-value | ||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, Mean (SD), years | 69.1 (8.7) | 67.3 (9.6) | 67.2 (9.1) | 63.6 (9.6) | < 0.001 | |
| Black | 30.9% | 50.6% | 46.4% | 51.8% | < 0.001 | |
| Male | 65.0% | 40.3% | 49.0% | 35.0% | < 0.001 | |
| Region of residence | 0.22 | |||||
| Non-belt | 45.0% | 39.9% | 42.4% | 38.3% | ||
| Stroke belt | 33.7% | 37.9% | 36.9% | 36.5% | ||
| Stroke buckle | 21.3% | 22.1% | 20.8% | 25.2% | ||
| Income <$20,000 | 19.4% | 41.2% | 42.4% | 51.9% | < 0.001 | |
| High school graduate | 85.9% | 75.1% | 73.8% | 67.9% | < 0.001 | |
| Body mass index, Mean (SD) | 29.2 (5.8) | 30.6 (6.8) | 30.7 (6.4) | 30.8 (6.9) | < 0.001 | |
| Married | 65.8% | 55.3% | 39.8% | 43.1% | < 0.001 | |
| Less than excellent general health | 90.3% | 98.0% | 96.5% | 98.2% | < 0.001 | |
| Comorbidities | ||||||
| Hypertension | 71.7% | 76.7% | 80.4% | 80.7% | < 0.001 | |
| Diabetes mellitus | 31.2% | 47.0% | 43.5% | 41.2% | < 0.001 | |
| Myocardial infarction | 70.8% | 75.5% | 70.6% | 77.0% | 0.07 | |
| Stroke | 10.8% | 19.8% | 15.0% | 20.4% | < 0.001 | |
| Medication Use | ||||||
| Statins | 61.0% | 55.3% | 53.6% | 49.6% | < 0.001 | |
| Beta blockers | 47.8% | 43.5% | 50.4% | 52.9% | 0.13 | |
| Aspirin | 71.4% | 67.2% | 70.9% | 64.2% | 0.05 | |
| Antidepressants | 12.8% | 19.0% | 24.5% | 38.7% | < 0.001 | |
| Clopidogrel | 15.2% | 16.2% | 19.0% | 19.0% | 0.13 | |
| Renin angiotensin system inhibitors | 53.9% | 57.3% | 57.1% | 54.0% | 0.52 | |
| Health Risk Behaviors | ||||||
| No physical activity | 35.2% | 48.6% | 51.3% | 53.7% | < 0.001 | |
| Heavy alcohol consumption | 3.2% | 0.8% | 2.3% | 3.3% | < 0.001 | |
| Current cigarette smoker | 13.4% | 16.6% | 21.6% | 34.7% | < 0.001 | |
| Low medication adherence | 6.3% | 11.1% | 10.4% | 19.3% | < 0.001 | |
| Stress and depressive symptoms | ||||||
| CESD score, Median (25th – 75th percentile) | 0 (0–1) | 1 (0–2) | 5 (4–7) | 6 (5–9) | < 0.001 | |
| PSS score, Median (25th – 75th percentile) | 2 (0–4) | 8 (8–9) | 5 (3–6) | 9 (8–10) | < 0.001 | |
CESD- Centers for epidemiological studies of depression. PSS – Perceived stress scale.
High depressive symptoms were defined by Center for Epidemiological Studies Depression Scale score ≥ 4. High stress was defined by Cohen’s Perceived Stress Scale score ≥ 8.
REGARDS (Reason for Geographic and Racial Differences in Stroke). Stroke buckle includes coastal North Carolina, South Carolina and Georgia. Stroke belt includes the remainder of North Carolina, South Carolina and Georgia and Alabama, Mississippi, Louisiana, Tennessee and Arkansas.
Association of Stress and Depressive Symptoms with MI or Death
Over a median 5.95-years of follow-up, 1,337 events (1,094 deaths and 614 MI events) occurred (Supplemental Table 1). Participants with high stress had a marginally higher risk for MI or death in the first 2.5 years of follow-up than participants with low stress (HR=1.22, [95% Confidence Interval [CI]: 0.94–1.57]) (Table 2). Similarly, participants with high depressive symptoms relative to those with low depressive symptoms had a higher risk for MI or death (HR=1.30, 95% CI: 1.02–1.64). After the initial 2.5-years of follow-up, no statistically significant association was present for high stress, or for high depressive symptoms, with MI or death.
Table 2.
Association of high stress and depressive symptoms evaluated separately on myocardial infarction or death, REGARDS (N = 4,487).
| Hazard ratio (95% confidence interval) | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| First 2.5 years of follow-up | |||
| High stress versus low stress | 1.22 (0.94 – 1.57) | 1.20 (0.93 – 1.55) | 1.20 (0.93 – 1.55) |
| High depressive symptoms versus low depressive symptoms | 1.30 (1.02 – 1.64) | 1.22 (0.96 – 1.55) | 1.16 (0.92 – 1.47) |
| > 2.5 years of follow-up | |||
| High stress versus low stress | 1.03 (0.83 – 1.28) | 1.01 (0.81 – 1.25) | 1.01 (0.81 – 1.25) |
| High depressive symptoms versus low depressive symptoms | 0.98 (0.80 – 1.21) | 0.92 (0.75 – 1.13) | 0.88 (0.72 – 1.09) |
Model 1 adjusted for race, age, sex, region of residence, body mass index, income, education, marital status, general self-rated health, hypertension, diabetes, history of myocardial infarction and stroke.
Model 2 adjusted for all covariates in Model 1 and use of statins, beta blockers, aspirin, antidepressants, clopidogrel and renin angiotensin system inhibitors.
Model 3 adjusted for all in Model 1 and Model 2 and physical activity, alcohol consumption, cigarette smoking, and medication adherence.
REGARDS (Reason for Geographic and Racial Differences in Stroke).
Joint presence of high stress and high depressive symptoms
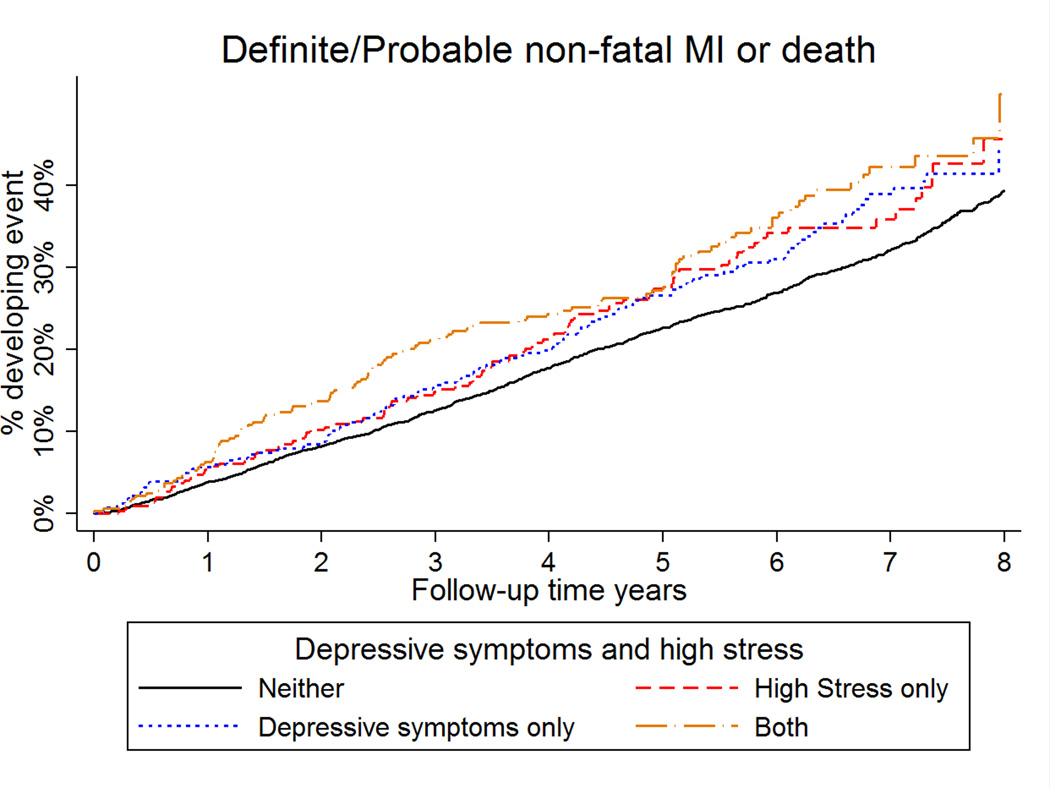
The incidence rate for MI or death was 42.8, 42.2, 51.4 and 77.5 per 1,000 person-years in the low stress and low depressive symptoms group, high stress and low depressive symptoms group, low stress and high depressive symptoms group, and concurrent high stress and high depressive symptoms group, respectively (Supplemental Table 2). After adjustment for age, race, sex, region of residence, BMI, income, education, marital status, general self-rated health, hypertension, diabetes, history of myocardial infarction and stroke and during the first 2.5 years of follow-up, participants with concurrent high stress and high depressive symptoms relative to participants with low stress and low depressive symptoms had an increased risk of MI or death (Table 3) (HR = 1.64, 95% CI: 1.20–2.24). In contrast, those with low stress and high depressive symptoms (HR = 1.03, [95% CI: 0.74–1.42]), and those with high stress and low depressive symptoms (HR = 0.83, [95% CI: 0.55–1.25]) were not at increased risk for MI or death (Figure 1). These associations remained present after further multivariable adjustment. In the full multivariable adjusted model, the p-value for the high stress * high depressive symptoms interaction term during the first 2.5 years of follow-up was 0.04. After the initial 2.5-year follow-up period, concurrent high stress with high depressive symptoms were not significantly associated with MI or death. The interaction between high stress * high depressive symptoms was not statistically significant after 2.5 years of follow-up (p=0.66). Results were similar when follow-up time was dichotomized at 2 years (Supplemental Table 2) and 3 years (Supplemental Table 3).
Table 3.
Association of concurrent stress and depressive symptoms with myocardial infarction or death, REGARDS (N = 4,487).
| Hazard ratio (95% confidence interval) | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| First 2.5 years of follow-up | |||
| Low stress and low depressive symptoms | 1 (ref) | 1 (ref) | 1 (ref) |
| High stress and low depressive symptoms | 0.83 (0.55 – 1.25) | 0.84 (0.56 – 1.27) | 0.86 (0.57 – 1.29) |
| Low stress and high depressive symptoms | 1.03 (0.74 – 1.42) | 0.97 (0.70 – 1.35) | 0.92 (0.66 – 1.28) |
| High stress and high depressive symptoms | 1.64 (1.20 – 2.24) | 1.54 (1.13 – 2.11) | 1.48 (1.08 – 2.02) |
| p-value for interaction | 0.03 | 0.03 | 0.04 |
| > 2.5 years of follow-up | |||
| Low stress and low depressive symptoms | 1 (ref) | 1 (ref) | 1 (ref) |
| High stress and low depressive symptoms | 1.09 (0.82 – 1.44) | 1.09 (0.82 – 1.44) | 1.10 (0.83 – 1.46) |
| Low stress and high depressive symptoms | 1.00 (0.78 – 1.29) | 0.94 (0.73 – 1.21) | 0.90 (0.70 – 1.16) |
| High stress and high depressive symptoms | 0.98 (0.71 – 1.33) | 0.91 (0.67 – 1.25) | 0.89 (0.65 – 1.22) |
| p-value for interaction | 0.65 | 0.65 | 0.66 |
Model 1 adjusted for race, age, sex, region of residence, body mass index, income, education, marital status, general self-rated health, hypertension, diabetes, history of myocardial infarction and stroke.
Model 2 adjusted for all covariates in Model 1 and use of statins, beta blockers, aspirin, antidepressants, clopidogrel and renin angiotensin system inhibitors.
Model 3 adjusted for all in Model 1 and Model 2 and physical activity, alcohol consumption, cigarette smoking, and medication adherence.
REGARDS (Reason for Geographic and Racial Differences in Stroke).
Figure 1.
Cumulative incidence of myocardial infarction or death by stress and depressive symptoms group
Individual Outcomes
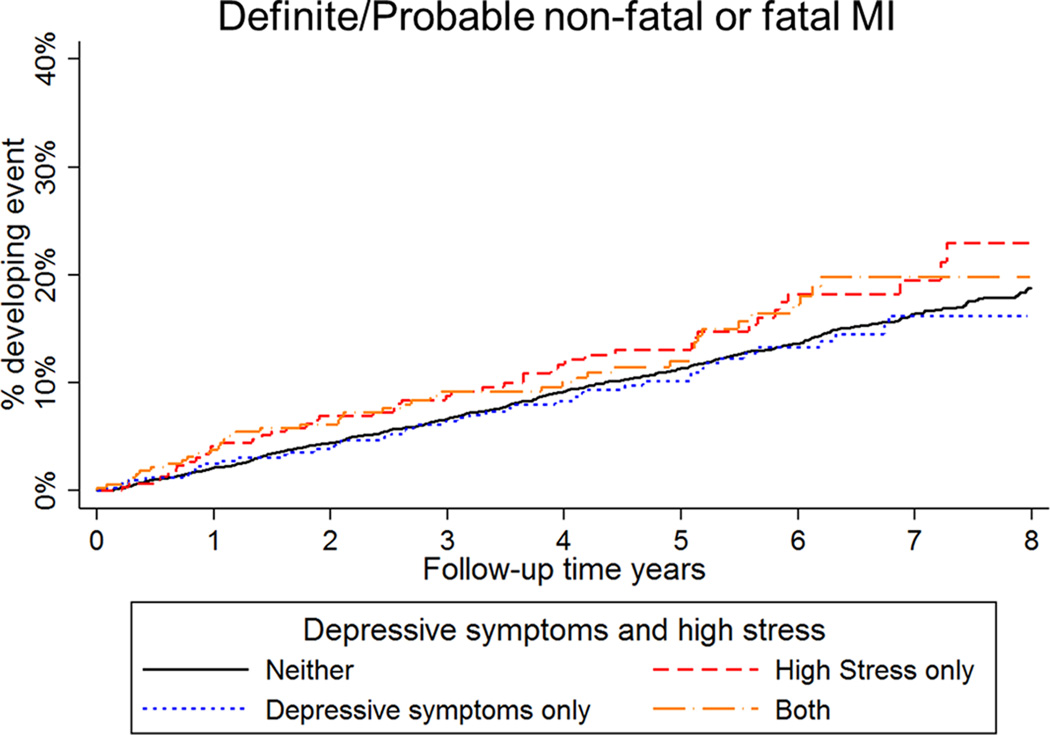
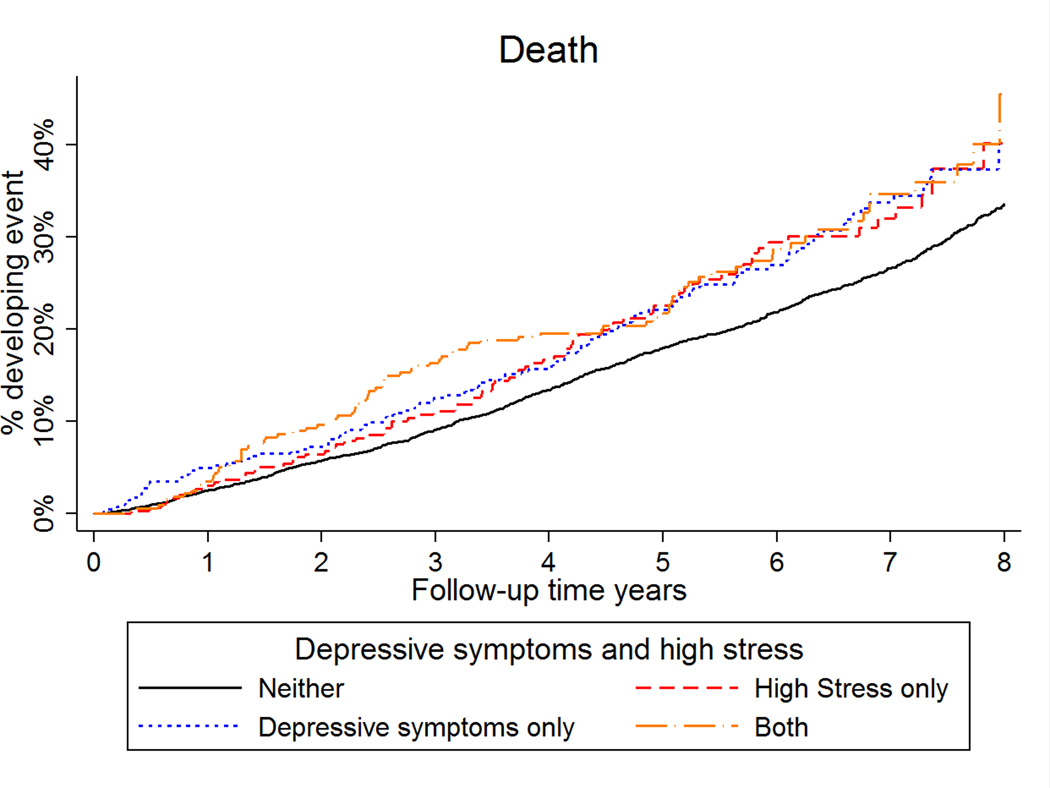
High stress and high depressive symptoms, separately and jointly, were not associated with risk of MI during the first 2.5 years or beyond 2.5 years of follow-up (Supplemental Tables 4 and 5; Figure 2). When examined individually, high depressive symptoms were associated with an increased risk of death in the first 2.5 years of follow-up in models with minimal adjustment for confounders (Supplemental Table 6; Figure 3). This association was attenuated after full multivariable adjustment. Only those with both high stress and high depressive symptoms had an increased risk for death in the first 2.5 years of follow-up, although the interaction term was not statistically significant (Supplemental Table 7). Consistent with our main results, high stress and high depressive symptoms, separately and jointly, were not associated with risk of death after the first 2.5 years of follow-up.
Figure 2.
Cumulative incidence of myocardial infarction by stress and depressive symptoms group
Figure 3.
Cumulative incidence of death by stress and depressive symptoms group.
Sensitivity Analyses
The HRs for MI/death associated with high stress and high depressive symptoms did not change appreciably when PSS ≥ 7 score was used to define high stress (Supplemental Table 8). Similarly, point estimates with the multiple imputation analysis did not change appreciably (Supplemental Table 9). During the first 2.5 years of follow-up and compared to participants with low stress and low depressive symptoms, the multivariable adjusted hazard ratio for cardiovascular death was 1.14 (95% CI 0.61 – 2.13), 0.89 (95% CI 0.51 – 1.56) and 1.38 (95% CI 0.80 – 2.36) for participants with high stress and low depressive symptoms, low stress and high depressive symptoms, and high stress and high depressive symptoms (Supplemental Table 10; p-value for interaction = 0.53).
Discussion
Consistent with the Psychosocial Perfect Storm29 model, and prior research in this area,21 our results demonstrate that the joint presence of high stress and high depressive symptoms compared to low stress and low depressive symptoms is associated with a 48% increased short-term risk for MI or death in a large sample of US adults with CHD. The heightened risk associated with concurrent high stress and high depressive symptoms was robust and consistent across models that adjusted for demographics, medical history, medication use, and health risk behaviors. We also show that the risk of MI or death associated with concurrent high stress and high depressive symptoms during the 2.5-year high vulnerability period is markedly higher than that of stress or depressive symptoms alone. Further, when we disaggregated the composite outcome, those with concurrent high stress and high depressive symptoms relative to their counterparts were at increased risk for death, but not MI; thus the combined effect of high stress and high depressive symptoms may be most pronounced for risk of death. Although not statistically significant, the point estimates suggest an elevated risk for cardiovascular death.
Prior studies have examined whether exposure to stress or the presence of depression, independent of each other, were associated with incident CHD or death, with some finding support for an independent main effect of stress and depression,19 and others finding a main effect for one and not the other.27 In INTERHEART, a case-control study conducted in 52 countries, depressed people who reported permanent stress at home or at work had higher odds of having ischemic heart disease when compared to their never stressed and not depressed counterparts.21 While prior studies have focused on the health risk associated with the confluence of both depression and anxiety,25 with up to a three-fold increased risk of cardiovascular death in some studies, our results provide initial empirical evidence of the applicability of the Psychosocial Perfect Storm29 model specifically for understanding the association of stress and depression to medical prognosis (specifically death, and potentially cardiovascular death) in people with established CHD.
The results from the current study imply that prior research using measures of stress or depressive symptoms likely overestimated the effect of each individual risk factor on medical prognosis and all-cause mortality when the other was not considered. Thus, within the field of behavioral cardiology, research should more consistently consider the combined, rather than independent, effect of psychosocial factors (such as depression and stress) on CHD prognosis. More importantly, our results highlight that the adverse health consequences, specifically death, associated with the joint presence of high stress and high depressive symptoms are particularly pronounced during an early high vulnerability period even after adjustment for known confounders, and may not exert an influence beyond that critical period. Indeed, a prior analysis of the REGARDS dataset found that the effect of high depressive symptoms on risk of MI or death was not sustained after adjustment for established behavioral confounders when this vulnerability period (< 2.5 years) was not considered.18
These findings serve as initial validation of dimensions of the Psychosocial Perfect Storm conceptual model, where the co-occurrence of multiple psychosocial risk factors each in tandem contribute to elevated risk for poor medical prognosis in adults with CHD. Our results indicate that the deleterious health consequences associated with concurrent high stress and high depressive symptoms in the past-month may be specific to death, and may be time dependent. However, we do not have any information on the persistence and course of the concurrent depressive symptoms - stress phenotype for REGARDS participants, and as such cannot account for the remitting and relapsing nature of depressive symptoms and stress over time. We also do not have information in REGARDS about specific pathophysiological pathways (i.e., thrombogenicity, coronary perfusion, arrhythmia) implicated in the Perfect Storm model.29 Thus, we are unable to determine whether the interaction of stress exposure (chronic vs. acute), depression, and specific pathophysiological pathways (i.e., thrombogenicity, coronary perfusion, arrhythmia) is particularly cardiotoxic. It is possible that the presence of all three factors-- psychosocial, behavioral, and pathophysiological-- in an early vulnerability period, confers greatest risk for cardiovascular events (recurrent MI, cardiovascular death), and that increased all-cause mortality risk is observed only when the confluence of two psychosocial factors are considered. Future research using the Perfect Storm framework should clarify whether specific combinations of psychosocial exposures (acute versus chronic stress, depression), behaviors, and pathophysiologic factors, generate differential prognostic outcomes and risk estimates over time.
Limitations
Our study is not without limitations. First, the presence of stress and depressive symptoms was measured at a single time point. Future research should examine the conditions under which high depressive symptoms contribute to excess risk of MI and death (e.g., in the context of stress exposure, the persistence of stress exposure). Just as regional wall motion abnormalities are identified by echocardiography only under exercise or pharmacologic stress, it may be that psychosocial ‘stress’ in the depressed CHD patient reveals the mechanisms responsible for depression-related prognostic risk.29 Second, the REGARDS study is observational and we are unable to make causal inferences. We were also unable to conduct additional subgroup analyses because of the available sample size. Our supplementary analyses with the individual components of the composite outcome suggest that those with concurrent stress and depressive symptoms are at greatest risk for all-cause mortality, and potentially cardiovascular mortality, in the first 2.5 years; larger samples are required to explore these individual outcomes more definitively as we were underpowered. Future research should also replicate these findings in independent samples adequately powered to examine effect modification by levels of stress and depressive symptoms; to facilitate replication by independent researchers, we have included the initial REGARDS manuscript proposal as a supplemental file. While those with concurrent high stress and high depressive symptoms had the highest risk for adverse outcomes, this represented only 6% of the study sample, a small population subgroup. Thus, further work is needed to determine the net public health benefit of focusing on this potentially low probability occurrence. Third, health behaviors were assessed using self-report which is subject to reporting biases. Future research should use objective measures of health behaviors as opposed to self-report to determine if the Perfect Storm is partially explained by behavior. We also used a self-report measure of general health as a proxy for overall medical comorbidity. Although self-rated health is shown to be associated with mortality beyond provider assessments of health,42 future research in this area could benefit from use of a standardized instrument such as the Charlson comorbidity index to account for overall medical comorbidity and disease severity. Fourth, the 4-item version of the CES-D that was used in REGARDS assesses only cognitive symptoms of depression, and thus does not assess somatic symptoms of depression. Therefore, we are unable to determine whether specific clusters of depression symptoms in combination with high stress would yield similar estimates of prognostic risk.
Conclusion
The current results provide initial empirical evidence to support a Psychosocial Perfect Storm conceptual model for understanding how psychosocial variables might be associated with medical prognosis in people with established CHD.29 While the prevalence of concurrent high stress and high depressive symptoms was only 6.0% among REGARDS participants with established CHD, a low probability occurrence, the impact of the confluence of psychosocial risk factors on the overall burden of disease, and mortality risk, was high. In particular, we found that concurrent high stress and high depressive symptoms was associated with an increased risk of MI or death, specifically death, in the first 2.5 years of follow-up, and that risk for outcomes was not elevated for participants with either of these conditions alone during this critical period. These results provide a challenge to traditional research paradigms that have focused on identifying if depression confers excess prognostic risk in post-MI patients, and instead places a newfound focus on identifying under what conditions poor prognosis occurs for CHD patients with high depressive symptoms, or conversely, that for stressed CHD patients, it requires the presence of depressive symptoms to indicate high mortality risk. The Psychosocial Perfect Storm model may inform research into the development, testing, and implementation of novel primary and secondary prevention strategies that focus on the treatment of stress and depression during this high vulnerability period. Thus, behavioral interventions that teach individuals with CHD how to adaptively manage stress and depressive symptoms during the high vulnerability period might be particularly important for lowering risk of death, and potentially cardiovascular death, in the shorter-term. By identifying the specific conditions under which depressed and stressed patients exhibit poor medical prognosis, we might also be better able to identify the modifiable mechanisms (biological, psychological, social, and environmental) by which depression and stress increase risk of MI and mortality and create targeted interventions to mitigate the excess risk.
Supplementary Material
Acknowledgments
Carmela Alcántara, Ph.D. led all aspects of manuscript development, and made substantial contributions to the conception and design, interpretation of data, and writing of the manuscript. Paul Muntner, Ph.D. made substantial contributions to the conception, design, acquisition, analysis, interpretation of the data, and made critical revisions to the manuscript for important intellectual content. Donald Edmondson, Ph.D., Nicole Redmond, M.D., Ph.D., M.P.H. and Monika M. Safford, M.D. were involved with data interpretation, and critical revisions of the manuscript. Lisandro D. Colantonio, M.D., M.Sc. made substantial contributions to the analysis and interpretation of the data, and was involved with critical revision of the manuscript. Karina W. Davidson, Ph.D. served as senior author and made substantial contributions to the conception, design, interpretation of data, and writing of the manuscript. This manuscript is not under consideration elsewhere, and none of its contents have been previously published. All authors have read and approved the manuscript.
Funding Sources
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS (REasons for Geographic and Racial Differences in Stroke) study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. This work was also supported by the National Heart Lung, and Blood Institute at the National Institutes of Health (3R01HL115941-01S1 to C.A., HL47540 to D.E., HL088117 to K.W.D., 3R01HL080477-07S1 to N.R.).
Dr. Safford and Dr. Muntner receive salary support from Amgen, Inc., for research studies; she has served as consultant for DiaDexus.
Footnotes
Disclosures
All other authors have reported that they have no conflicts of interest to disclose.
References
- 1.Davidson KW, Burg MM, Kronish IM, Shimbo D, Dettenborn L, Mehran R, Vorchheimer D, Clemow L, Schwartz JE, Lesperance F, Rieckmann N. Association of anhedonia with recurrent major adverse cardiac events and mortality 1 year after acute coronary syndrome. Arch Gen Psychiatry. 2010;67:480–488. doi: 10.1001/archgenpsychiatry.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121:S20–S27. doi: 10.1016/j.amjmed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Frasure-Smith N, Lesperance F. Recent evidence linking coronary heart disease and depression. Can J Psychiatry. 2006;51:730–737. doi: 10.1177/070674370605101202. [DOI] [PubMed] [Google Scholar]
- 4.Rugulies R. Depression as a predictor for coronary heart disease. a review and meta-analysis. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 5.Mostofsky E, Maclure M, Sherwood JB, Tofler GH, Muller JE, Mittleman MA. Risk of acute myocardial infarction after the death of a significant person in one's life: the determinants of myocardial infarction onset study. Circulation. 2012;125:491–496. doi: 10.1161/CIRCULATIONAHA.111.061770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kivimaki M, Nyberg ST, Batty GD, Fransson EI, Heikkila K, Alfredsson L, Bjorner JB, Borritz M, Burr H, Casini A, Clays E, De Bacquer D, Dragano N, Ferrie JE, Geuskens GA, Goldberg M, Hamer M, Hooftman WE, Houtman IL, Joensuu M, Jokela M, Kittel F, Knutsson A, Koskenvuo M, Koskinen A, Kouvonen A, Kumari M, Madsen IE, Marmot MG, Nielsen ML, Nordin M, Oksanen T, Pentti J, Rugulies R, Salo P, Siegrist J, Singh-Manoux A, Suominen SB, Vaananen A, Vahtera J, Virtanen M, Westerholm PJ, Westerlund H, Zins M, Steptoe A, Theorell T. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet. 2012;380:1491–1497. doi: 10.1016/S0140-6736(12)60994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PloS One. 2012;7:e38915. doi: 10.1371/journal.pone.0038915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edmondson D, Newman JD, Whang W, Davidson KW. Emotional triggers in myocardial infarction: do they matter? Eur Heart J. 2013;34:300–306. doi: 10.1093/eurheartj/ehs398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson S, Shaffer JA, Falzon L, Krupka D, Davidson KW, Edmondson D. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol. 2012;110:1711–1716. doi: 10.1016/j.amjcard.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frasure-Smith N. In-hospital symptoms of psychological stress as predictors of long-term outcome after acute myocardial infarction in men. Am J Cardiol. 1991;67:121–127. doi: 10.1016/0002-9149(91)90432-k. [DOI] [PubMed] [Google Scholar]
- 11.Iso H, Date C, Yamamoto A, Toyoshima H, Tanabe N, Kikuchi S, Kondo T, Watanabe Y, Wada Y, Ishibashi T, Suzuki H, Koizumi A, Inaba Y, Tamakoshi A, Ohno Y. Perceived mental stress and mortality from cardiovascular disease among japanese men and women: the japan collaborative cohort study for evaluation of cancer risk sponsored by monbusho (jacc study) Circulation. 2002;106:1229–1236. doi: 10.1161/01.cir.0000028145.58654.41. [DOI] [PubMed] [Google Scholar]
- 12.Arnold SV, Smolderen KG, Buchanan DM, Li Y, Spertus JA. Perceived stress in myocardial infarction: Long-term mortality and health status outcomes. J Am Coll Cardiol. 2012;60:1756–1763. doi: 10.1016/j.jacc.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aboa-Eboule C, Brisson C, Maunsell E, Masse B, Bourbonnais R, Vezina M, Milot A, Theroux P, Dagenais GR. Job strain and risk of acute recurrent coronary heart disease events. JAMA. 2007;298:1652–1660. doi: 10.1001/jama.298.14.1652. [DOI] [PubMed] [Google Scholar]
- 14.Orth-Gomer K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA. Marital stress worsens prognosis in women with coronary heart disease: the stockholm female coronary risk study. JAMA. 2000;284:3008–3014. doi: 10.1001/jama.284.23.3008. [DOI] [PubMed] [Google Scholar]
- 15.Eaker ED, Sullivan LM, Kelly-Hayes M, D'Agostino RB, Sr, Benjamin EJ. Does job strain increase the risk for coronary heart disease or death in men and women? the framingham offspring study. Am J Epidem. 2004;159:950–958. doi: 10.1093/aje/kwh127. [DOI] [PubMed] [Google Scholar]
- 16.Macleod J, Smith GD, Heslop P, Metcalfe C, Carroll D, Hart C. Are the effects of psychosocial exposures attributable to confounding? Evidence from a prospective observational study on psychological stress and mortality. J Epidemiol Community Health. 2001;55:878–884. doi: 10.1136/jech.55.12.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore L, Meyer F, Perusse M, Cantin B, Dagenais GR, Bairati I, Savard J. Psychological stress and incidence of ischaemic heart disease. Int J Epidemiol. 1999;28:652–658. doi: 10.1093/ije/28.4.652. [DOI] [PubMed] [Google Scholar]
- 18.Ye S, Muntner P, Shimbo D, Judd SE, Richman J, Davidson KW, Safford MM. Behavioral mechanisms, elevated depressive symptoms, and the risk for myocardial infarction or death in individuals with coronary heart disease: the regards (reason for geographic and racial differences in stroke) study. J Am Coll Cardiol. 2013;61:622–630. doi: 10.1016/j.jacc.2012.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mausbach BT, Patterson TL, Rabinowitz YG, Grant I, Schulz R. Depression and distress predict time to cardiovascular disease in dementia caregivers. Health Psychol. 2007;26:539–544. doi: 10.1037/0278-6133.26.5.539. [DOI] [PubMed] [Google Scholar]
- 20.Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 21.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the interheart study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 22.Phillips AC, Batty GD, Gale CR, Deary IJ, Osborn D, MacIntyre K, Carroll D. Generalized anxiety disorder, major depressive disorder, and their comorbidity as predictors of all-cause and cardiovascular mortality: the vietnam experience study. Psychosom Med. 2009;71:395–403. doi: 10.1097/PSY.0b013e31819e6706. [DOI] [PubMed] [Google Scholar]
- 23.Chamberlain AM, Vickers KS, Colligan RC, Weston SA, Rummans TA, Roger VL. Associations of preexisting depression and anxiety with hospitalization in patients with cardiovascular disease. Mayo Clin Proc. 2011;86:1056–1062. doi: 10.4065/mcp.2011.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doering LV, Moser DK, Riegel B, McKinley S, Davidson P, Baker H, Meischke H, Dracup K. Persistent comorbid symptoms of depression and anxiety predict mortality in heart disease. Int J Cardiol. 2010;145:188–192. doi: 10.1016/j.ijcard.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watkins LL, Koch GG, Sherwood A, Blumenthal JA, Davidson JR, O'Connor C, Sketch MH. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. 2013;2:e000068. doi: 10.1161/JAHA.112.000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frasure-Smith N, Lesperance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry. 2008;65:62–71. doi: 10.1001/archgenpsychiatry.2007.4. [DOI] [PubMed] [Google Scholar]
- 27.Frasure-Smith N, Lesperance F. Depression and other psychological risks following myocardial infarction. Arch Gen Psychiatry. 2003;60:627–636. doi: 10.1001/archpsyc.60.6.627. [DOI] [PubMed] [Google Scholar]
- 28.Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute coronary events. Circulation. 2012;125:1147–1156. doi: 10.1161/CIRCULATIONAHA.111.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burg MM, Edmondson D, Shimbo D, Shaffer JA, Kronish IM, Whang W, Alcantara C, Schwartz JE, Muntner P, Davidson KW. The ‘perfect storm’ and acute coronary syndrome onset: do psychosocial factors play a role? Prog Cardiovasc Dis. 2013;55:601–601. doi: 10.1016/j.pcad.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G REGARDS Investigators. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 32.Radloff LS. The ces-d scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 33.Melchior LA, Huba G, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas. 1993;53:1117–1125. [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 35.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med. Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 37.Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 38.Prineas RJ, Crow RS, Blackburn HW. The minnesota code manual of electrocardiographic findings: standards and procedures for measurement and classification. Boston, MA: John Wright; 1982. [Google Scholar]
- 39.Prineas RJ, Crow RS, Zhang Z-M. The minnesota code manual of electrocardiographic findings: Including measurement and comparison with the novacode; standards and procedures for ecg measurement in epidemiologic and clinical trials. London: Springer; 2010. [Google Scholar]
- 40.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 42.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.