Abstract
Objectives
To examine the association between parental human papillomavirus (HPV) awareness and HPV vaccine initiation/completion based on 13–17 year old US adolescent children and to explore whether these associations were mediated by provider recommendation.
Methods
We used publicly available National Immunization Survey-Teen 2011 data (11,236 adolescent girls and 12,328 boys).
Results
Weighted logistic regression analysis showed that parental HPV awareness and provider recommendation predicted HPV vaccine initiation and completion separately among both girls and boys, after adjusting for demographic and healthcare utilization variables. When provider recommendation and parental HPV awareness were entered in the model simultaneously, only provider recommendation independently associated with HPV vaccine initiation and completion, demonstrating a mediation effect of provider recommendation.
Conclusions
Future studies are needed to better understand why physicians may not provide a recommendation for the HPV vaccine as well as to identify strategies to improve the provider’s ability to effectively communicate their recommendation.
Keywords: Human papillomavirus (HPV), parental HPV vaccine awareness, provider recommendation, HPV Vaccine initiation, HPV vaccine completion, mediation effects
Introduction
Human papillomavirus (HPV) is responsible for almost all cervical cancers and 90% of genital warts, as well as a substantial number of other anogenital and head and neck cancers.1–4 The HPV vaccine can protect against 70% of cervical cancer cases and 90% of genital warts.2,5 However, nationally representative data from 2011 showed that HPV vaccine uptake (≥1 dose) among 13–17 year old girls (53%) and boys (8%) was much lower than other vaccines administered to this age group (Tdap 78%; meningococcal 71%).6
Parental HPV vaccine awareness, parental acceptability and provider recommendation are key factors for higher HPV vaccine uptake among adolescents.7–11 Yet, HPV vaccine uptake in adolescent girls has not substantially increased over time while parental awareness has increased.6,12,13 There is a need to understand how parental HPV vaccine awareness affects vaccine initiation and completion and whether vaccine uptake is mediated by other factors. The objective of this study was to examine the association between parental HPV vaccine awareness and vaccine initiation/completion among 13–17 year old US adolescents, and to explore whether these associations were mediated by provider recommendation.
METHODS
The National Immunization Survey–Teen (NIS-Teen), conducted by the Centers for Disease Control and Prevention, is a continuous cross-sectional survey of provider-verified vaccination information on 13–17 year-old US adolescents. NIS-Teen methodology has been reported elsewhere.14 Household interviews for 39,839 teens were conducted in 2011; of which 23,564 had provider-verified HPV vaccination information (excluding U.S. Virgins Islands).
We considered provider-verified HPV vaccine initiation (received at least one dose of HPV vaccine) or completion (received ≥ 3 doses of HPV vaccine) as dependent variable, parental HPV vaccine awareness as an independent variable, and provider recommendation as a mediator variable. Parental HPV vaccine awareness was assessed using the question “have you ever heard of the cervical cancer vaccine, HPV shot, or Gardasil?” The response options were “yes”, “no”, “don’t know” and “refused”. Those who responded “yes” were considered as having HPV vaccine awareness. Information on whether the participant had provider recommendation for the HPV vaccine was obtained using the following question with the similar response options. “Had or has doctor or other health care professional ever recommended that teen receive HPV shots?” Similarly information on total number of HPV vaccine doses received was obtained. Socio-demographic characteristics included information about the teen, mother, and household. Respondents provided data on their age as well as their relationship to the adolescent (mother, father, other) and the adolescent’s age, race/ethnicity, eligibility for the Vaccine For Children (VFC) program, influenza vaccination before age 13 years, health care coverage and region of residence.
Statistical analysis
STATA 12 svy commands (STATA Corporation, College Station, TX) were used for data analysis by incorporating probability sampling weights in conjunction with strata and primary sampling units generated by NIS-Teen survey design. We used logistic regression models to examine the mediation effect of mediator variable on the association between independent variable and dependent variable based on standard criteria:15,16 (1) statistical significant relationship between independent variable and dependent variable (2) statistical significant relationship between independent variable and mediator variable, (3) existence of significant effect of mediator variable on the dependent variable in the presence of independent variable, and (4) the magnitude of the effect of independent variable on the dependent variable diminishes when mediator variable is controlled. All multivariable models were adjusted for socio-demographic covariates.
RESULTS
Provider-verified HPV vaccination data were available for 11,236 adolescent girls and 12,328 boys. Overall, 53.0% (95% confidence interval (CI), 51.4–54) and 34.8% (95% CI, 33.2–36.4) of girls (weighted values) reported initiating and completing the 3-dose series in 2011, respectively (Table 1). The respective figures for boys were 8.3% (95% CI, 7.4–9.3) and 1.3% (95% CI, 1.1–1.7). Ninety percent of parents with a girl and 81.5% of parents with a boy were aware of the HPV vaccine, while 58.8% and 14.2%, respectively, reported that their provider had recommended this vaccine for their child. Distribution of socio-demographic variables was comparable between respondents with a girl and a boy (P>.05 for all comparisons).
Table 1.
Socio-demographic characteristics of adolescent children by gender, National Immunization Survey-Teen Study, 2011
| Characteristics | Adolescent girls (n=11,236) % | Adolescent boys (n=12,328) % |
|---|---|---|
| Age, year | ||
| 13 | 19.9 | 19.1 |
| 14 | 19.9 | 20.0 |
| 15 | 20.8 | 20.5 |
| 16 | 21.1 | 21.2 |
| 17 | 18.3 | 19.3 |
| Respondent’s age, year | ||
| ≤34 | 11.5 | 10.3 |
| 35–44 | 44.2 | 45.5 |
| ≥45 | 44.3 | 44.2 |
| Race/ethnicity | ||
| Non-Hispanic white | 56.2 | 58.3 |
| Non-Hispanic black | 14.8 | 14.0 |
| Hispanic | 21.1 | 19.0 |
| Non-Hispanic other* | 7.8 | 8.7 |
| Marital status of mother | ||
| Married | 65.6 | 66.7 |
| Never married/divorced, widowed/separated/deceased | 34.4 | 33.3 |
| Education of the respondent | ||
| <HS | 14.0 | 13.6 |
| HS graduate | 24.8 | 25.8 |
| Some college hours | 27.3 | 26.4 |
| College graduate | 33.9 | 34.2 |
| Family income (% of the federal poverty line) | ||
| <100% | 23.7 | 22.8 |
| 100% to <200% | 21.2 | 21.6 |
| ≥200% | 55.1 | 55.6 |
| Eligible for VFC program | 39.4 | 39.4 |
| Have healthcare coverage | 92.9 | 92.4 |
| Relationship of respondent to the teen | ||
| Mother | 75.9 | 73.2 |
| Father, grandparent, other | 24.1 | 26.8 |
| Provider-confirmed seasonal influenza vaccination | 14.9 | 14.1 |
| Moved from different state | ||
| No | 76.5 | 75.3 |
| Yes | 23.5 | 24.7 |
| HPV initiation, (95% CI) | 53.0 (51.4–54.7) | 8.3 (7.4–9.3) |
| HPV completion, (95% CI) | 34.8 (33.2–36.4) | 1.3 (1.1–1.7) |
| Had HPV vaccine awareness, (95% CI) | 90.0 (88.7–91.1) | 81.5 (80.1–82.9) |
| Had provider recommendation, (95% CI) | 58.8 (57.1–60.5) | 14.2 (13.1–15.4) |
| Region | ||
| Northeast | 17.2 | 17.1 |
| Midwest | 21.9 | 21.9 |
| South | 36.9 | 36.9 |
| West | 24.1 | 24.1 |
HS=High school; VFC=Vaccine for Children; CI=confidence intervals; All values are weighted
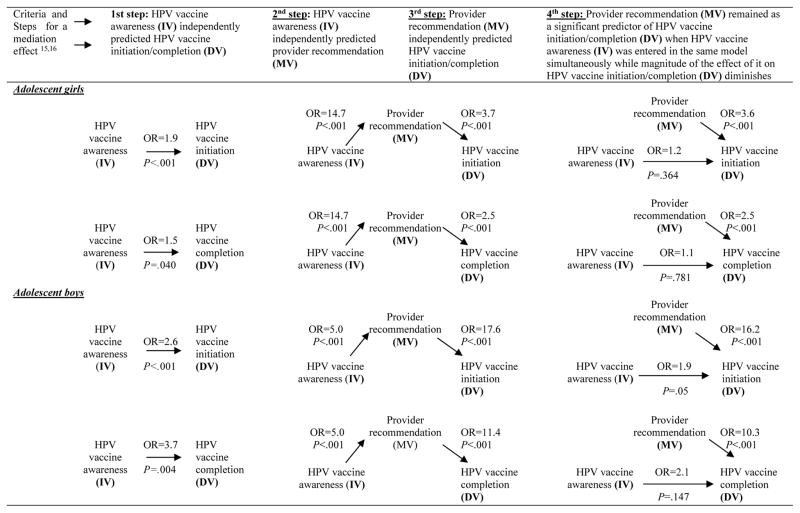
We summarized mediation analyses in Figure 1. Adjusted logistic regression analyses showed that parental HPV vaccine awareness and provider recommendation separately predicted HPV vaccine initiation and completion among girls and boys. However, when they were entered in the logistic models simultaneously, only provider recommendation independently predicted vaccine initiation and completion, demonstrating a mediation effect of provider recommendation. Moreover, changes in odds ratio from statistically significant to statistically non-significant indicated the presence of full mediation.
Figure 1.
Provider recommendation as a mediator of the association between HPV vaccine awareness and HPV vaccine initiation and completion among 13–17 year old adolescent children, the National Immunization Survey-Teen Study, 2011
OR=Odds Ratio; OR listed in this figures are adjusted for socio-demographic variables listed in Table 1 and based on weighted analysis; IV=Independent variable; DV=Dependent variable; MV= Mediator variable
DISCUSSION
Published reports show that both parental HPV vaccine awareness and provider recommendation are strong predictors of HPV vaccine uptake.7–11 However, the mechanism through which they increase vaccine uptake is not understood. This study observed that provider recommendation mediated the association between parental HPV vaccine awareness and HPV vaccine initiation and completion.
While HPV vaccine awareness has reached 90% and 82% among parents with an adolescent girl and boy in 2011, respectively, the rates of HPV vaccine uptake have remained stagnant. This suggests that other factors may play an important role in translating parental HPV vaccine awareness into vaccine uptake. On the other hand, only 59% and 14% of parents reported that they had ever received a provider recommendation to vaccinate their adolescent girls and boys, respectively. However, very low provider recommendation for adolescent boys might be due to the fact that the Advisory Committee on Immunization Practices recommendation for the use of quadrivalent HPV vaccine in males was made in October 2011. This situation reveals that there is a huge room available to improve provider recommendation for this vaccine. As we observed that provider recommendation is actually a mediator of the association between HPV vaccine awareness and HPV vaccine uptake, irrespective of gender, there are reasons to believe that improvement in provider recommendation would improve HPV vaccine initiation and completion.
It is also important to examine barriers to provider recommendation for this vaccine in future studies as almost half of the respondent with a girl and 7 out of 8 respondents with a boy mentioned that they did not get provider recommendation for this vaccine. Many physicians believe that parental refusal is an important barrier as they ignore physician recommendation. Future studies are also needed to better understand why physicians may not provide a recommendation for the HPV vaccine as well as to identify strategies to improve the provider’s ability to effectively communicate their recommendation.
Conclusion
The results of this study, based on nationally representative data from the US, demonstrate the importance of incorporating provider recommendation into all programs promoting HPV vaccine uptake in adolescent children. In addition, studies to identify barriers to effective communication between providers and parents of eligible children also are needed. Increasing provider’s recommendations for HPV vaccine may help increase HPV vaccine initiation and completion rates among adolescent children in the US.
Acknowledgments
Dr. McGrath is supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program-BIRCWH) from the Office of Research on Women’s Health (ORWH), the Office of the Director (OD), the National Institute of Allergy and Infectious Diseases (NIAID), and NICHD at the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
Footnotes
Conflict of interest: None
References
- 1.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, de Sanjose SS. Human papillomavirus and cervical cancer – burden and assessment of causality. J Natl Cancer Inst Monogr. 2003;31:3–13. doi: 10.1093/oxfordjournals.jncimonographs.a003479. [DOI] [PubMed] [Google Scholar]
- 3.Greer CE, Wheeler CM, Ladner MB, et al. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol. 1995;33:2058–63. doi: 10.1128/jcm.33.8.2058-2063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center for Disease Control and Prevention. [Accessed January 25, 2013];Genital HPV: The Facts. 2010 Available at http://www.cdc.gov/std/hpv/the-facts/HPV_English_2011_508.pdf.
- 5.Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: Analysis of placebo arm of 2 randomized phase III trials of quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199:805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13–17 years-United States, 2011. MMWR. 2012;61:671–677. [PubMed] [Google Scholar]
- 7.Zimet GD, Perkins SM, Sturm LA, et al. Predictors of STI vaccine acceptability among parents and their adolescent children. J Adolesc Health. 2005;37:179–86. doi: 10.1016/j.jadohealth.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Dorell C, Yankey D, Kennedy A, Stokley S. Factors That Influence Parental Vaccination Decisions for Adolescents, 13 to 17 Years Old: National Immunization Survey-Teen, 2010. Clin Pediatr (Phila) 2013;52:162–70. doi: 10.1177/0009922812468208. [DOI] [PubMed] [Google Scholar]
- 9.Reiter PL, Brewer NT, Gottlieb SL, McRee AL, Smith JS. Parents’ health beliefs and HPV vaccination of their adolescent daughters. Soc Sci Med. 2009;69:475–80. doi: 10.1016/j.socscimed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. Am J Public Health. 2013;103:164–9. doi: 10.2105/AJPH.2011.300600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caskey R, Lindau ST, Alexander GC. Knowledge and early adoption of the HPV vaccine among girls and young women: Results of a national survey. J Adolesc Health. 2009;45:453–62. doi: 10.1016/j.jadohealth.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Rahman M, McGrath CJ, Berenson AB. Geographic variation in human papillomavirus vaccination uptake among 13–17 year old adolescent girls in the United States. Vaccine. 2014;32:1394–98. doi: 10.1016/j.vaccine.2014.02.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC). . National and state vaccination coverage among adolescents aged 13–17 years--United States, 2012. MMWR. 2013;62:685–93. [PMC free article] [PubMed] [Google Scholar]
- 14.Jain N, Singleton JA, Montgomery M, Skalland B. Determining accurate vaccination coverage rates for adolescents: the National Immunization Survey-Teen 2006. Public Health Rep. 2009;124:642–51. doi: 10.1177/003335490912400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 16.MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Evaluation Review. 1993;17:144–158. [Google Scholar]