Abstract
Normalization of fluorodeoxyglucose positron emission tomography (FDG PET) imaging prior to high-dose therapy and autologous stem cell transplantation (ASCT) improves outcomes in relapsed and refractory (RR) Hodgkin lymphoma (HL), but many patients refractory to platinum-based salvage regimens are unable to achieve this goal. We therefore investigated whether brentuximab vedotin (BV) could normalize FDG PET in platinum-refractory HL prior to ASCT. Fifteen consecutive patients with RR HL and FDG PET positive disease after platinum-based salvage therapy were treated with a median of 4 cycles of BV. Normalization of FDG PET (Deauville ≤2) occurred in 8/15 (53%) patients but was only observed in patients that had achieved partial remission or stable disease after platinum-based salvage therapy. All patients eventually proceeded to ASCT, regardless of FDG PET status. Our data suggest that BV can normalize FDG PET in a subset of patients with platinum-refractory HL prior to ASCT.
Keywords: brentuximab vedotin, Hodgkin lymphoma, autologous transplant
Introduction
Classical Hodgkin lymphoma (HL) relapses after or is refractory to (RR) upfront chemotherapy in approximately 20–30% of patients [1]. For medically fit patients with RR HL, a standard first-salvage approach includes a platinum-based chemotherapy regimen such as ifosfamide, carboplatin, and etoposide (ICE). Consolidation ASCT is then typically pursued for those with chemosensitive disease. Functional imaging with fluorodeoxyglucose positron emission tomography (FDG PET) after platinum-based salvage therapy and prior to ASCT predicts the long-term success of ASCT; patients with FDG PET negative disease at this juncture have superior outcomes [2,3]. Patients that achieve FDG PET normalization after a second non-cross-resistant salvage regimen have comparably favorable post-ASCT outcomes [4]. Second-line salvage therapy is therefore commonly administered before ASCT to convert FDG PET positive disease; however, the optimal treatment regimen in this setting remains to be defined [5–7].
Brentuximab vedotin (BV) is an antibody drug conjugate that targets the cytotoxin monomethyl auristatin E to cells expressing CD30. It is approved as monotherapy for patients with HL recurring after ASCT or, for patients ineligible for ASCT, after two prior multi-agent salvage chemotherapy regimens [8]. Limited data exist regarding the use of BV as salvage therapy prior to ASCT.
We hypothesized that BV could normalize functional imaging in transplant-naïve HL patients with persistently positive FDG PET status following platinum-based salvage therapy. To this end, we performed a retrospective analysis to evaluate the efficacy of BV in this setting.
Methods
Consecutive patients over the period of 2009 to 2013 with RR HL treated at the University of Washington (UW) Medical Center, Fred Hutchinson Cancer Research Center (FHCRC), and Seattle Cancer Care Alliance that received BV for FDG PET positive disease following platinum-based salvage therapy and prior to ASCT were identified. All patients had histologically confirmed CD30 positive tumors, received BV following the platinum-based salvage regimen, and underwent follow-up functional imaging. The primary endpoint of this retrospective analysis was the conversion to FDG PET negative status. Secondary endpoints included disease response to subsequent therapy in patients that relapsed after or were refractory to BV, toxicity, ability to proceed to ASCT, and survival and progression-free status.
Deauville criteria were used to evaluate FDG PET status [9]. Scores of 1 (correlating to no residual uptake) and 2 (correlating to residual uptake less than or equal to the mediastinal blood pool), were regarded as FDG PET negative. Scores of 3 (correlating to residual uptake greater than the mediastinal blood pool but not greater than the liver) and greater were regarded as positive. Additional information regarding therapeutic response was assessed according to the Revised Response Criteria for Malignant Lymphoma on the basis of clinical and imaging assessments [10]. Toxicities were assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events. The UW/FHCRC Institutional Review Board approved this work.
Results
Fifteen patients with RR HL and FDG PET positive disease following platinum-based salvage therapy met our inclusion criteria. As shown in Table 1, the median age at diagnosis was 31 years (range, 16–64), and the number of patients with stage III and IV disease at diagnosis was 8 (63%). Ten (67%) patients had primary refractory disease and five (33%) patients had relapsed disease after a median of 8 months (range, 2–29 months). Thirteen patients (87%) received doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as first-line therapy. The median number of regimens received prior to BV was 2 (range, 2–4) and the best response to any prior chemotherapy was complete remission (CR) in two (12%), partial response (PR) in six (38%), stable disease (SD) in five (33%), and progressive disease (PD) in two (12%).
Table 1.
Patient demographics and clinical characteristics according to response to BV as salvage therapy for RR HL after a platinum-based salvage regimen
| All patients (n = 15) |
After BV | ||
|---|---|---|---|
| FDG PET negative (n = 8) |
FDG PET positive (n = 7) |
||
| Median age, years (range) | 31 (16–64) | 28 (17–64) | 34 (16–40) |
| Female gender, n (%) | 5 (33) | 2 (25) | 3 (43) |
| Stage III/IV at diagnosis, n (%) | 8 (53) | 5 (63) | 3 (43) |
| ABVD induction, n (%) | 13 (87) | 6 (75) | 7 (100) |
| Primary refractory disease, n (%) | 10 (67) | 6 (75) | 4 (57) |
| Median number of regimens prior to BV, n (range) | 2 (2–4) | 2 (2–4) | 2 (2–4) |
| Prior radiation, n (%) | 2 (13) | 1 (13) | 1 (14) |
| Platinum-based therapy, n (%) | |||
| ICE/RICE | 10 (67) | 5 (63) | 5 (71) |
| TEC | 3 (20) | 2 (25) | 1 (14) |
| Other | 1 (13) | 1 (13) | 1 (14) |
| Response to platinum-based salvage, n (%) | |||
| PR | 4 (27) | 2 (25) | 2 (29) |
| SD | 9 (60) | 6 (75) | 3 (43) |
| PD | 2 (13) | 0 (0) | 2 (29) |
| Mean bulk on CT prior to BV (largest lesion), cm (range) | 2.3 (1.0–5.5) | 2.0 (1–3.3) | 3.8 (2.1–5.5) |
| Median baseline pre-BV Deauville score, n (range) | 5 (4–5) | 5 (4–5) | 5 (4–5) |
| Median number of BV cycles, n (range) | 4 (2–7) | 5 (2–5) | 3 (2–7) |
| Median post-BV Deauville score, n (range) | 1 (1–5) | 1 (1) | 5 (5) |
| Post-BV salvage chemotherapy, n (%) | 3 (20) | 0 (0) | 3 (43) |
| GVD | 2 (13) | 0 (0) | 2 (29) |
| IGEV plus Bendamustine | 1 (7) | 0 (0) | 1 (14) |
| Proceeded to ASCT, n (%) | 15 (100) | 8 (100) | 7 (100) |
| Median time from last BV to ASCT, days (range) | 53 (38–137) | 44.5 (28–55) | 88 (47–131) |
| FDG PET positive at ASCT, n (%) | 7 (47) | 0 (0) | 7 (100) |
| Alive at time of analysis, n (%) | 14 (93) | 8 (100) | 6 (86) |
| Progression-free at time of analysis, n (%) | 12 (80) | 7 (88) | 5 (71) |
| Median follow-up, months (range) | |||
| Date of last contact since last BV | 17.3 (2.4–48.7) | 20.0 (4.2–26.8) | 10.7 (2.4–48.7) |
| Date of last contact since ASCT | 15.8 (0.3–44.9) | 18.5 (2.9–25.0) | 7.7 (0.3–44.9) |
Qualifying platinum-based salvage therapies included ICE or rituximab plus ICE (n = 10); bendamustine, etoposide, carboplatin (TEC, n = 3); gemcitabine, carboplatin, dexamethasone (GCD, n = 1); and cisplatin, high-dose cytarabine, dexamethasone (DHAP, n = 1). All patients underwent stem cell collection following chemomobilization with the platinum-based regimen. The response to platinum-based therapy was PR, SD, and PD in four (27%), nine (60%), and two (13%) patients, respectively. All patients had FDG PET positive disease at the time that BV was initiated with a mean bulk of 2.3 cm (range, 1–5.5 cm) on CT. The median baseline Deauville score prior to BV was 5 (range, 4–5).
A median of 4 (range, 2–7) cycles of BV was administered at a standard dose of 1.8 mg/kg intravenously every 3 weeks over 30 min on an outpatient basis. One patient developed a documented grade 2 or higher adverse event (peripheral neuropathy) that did not require discontinuation of BV.
Treatment with BV converted eight (53%) patients to FDG PET negative status. Stratified by prior response to platinum-based salvage therapy, BV achieved FDG PET negative status in two of four (50%), six of nine (67%), and zero of two patients with, respectively, PR, SD, and PD. Of the patients with primary refractory disease, six (60%) converted to FDG PET negative status.
All eight patients that converted to FDG PET negativity proceeded directly to consolidative ASCT. The median time between last dose of BV and ASCT in this group was 44.5 days (range, 28–55). Of the seven patients that had persistently FDG PET avid disease after BV, three went on to receive additional salvage therapy [gemcitabine, vinorelbine, and doxorubicin (GVD) in two patients; ifosfamide, gemcitabine, and vinorelbine (IGEV) followed by bendamustine in one patient] and four proceeded directly to ASCT. In each of these three patients that received additional, post-BV salvage therapy, persistent FDG PET positive disease was present at the time of ASCT. The median time between last dose of BV and ASCT in the seven patients with FDG PET positive disease after BV was 88 days (range, 47–137). At the time of our analysis, 100% of patients had proceeded to ASCT. Fourteen (94%) patients were alive; one patient died from a lethal cardiac arrhythmia as an apparent complication of high-dose therapy on day 18 after ASCT. Median engraftment of neutrophils post-ASCT occurred at on day 12 (range, 11–22) for the 13 patients for whom data was available.
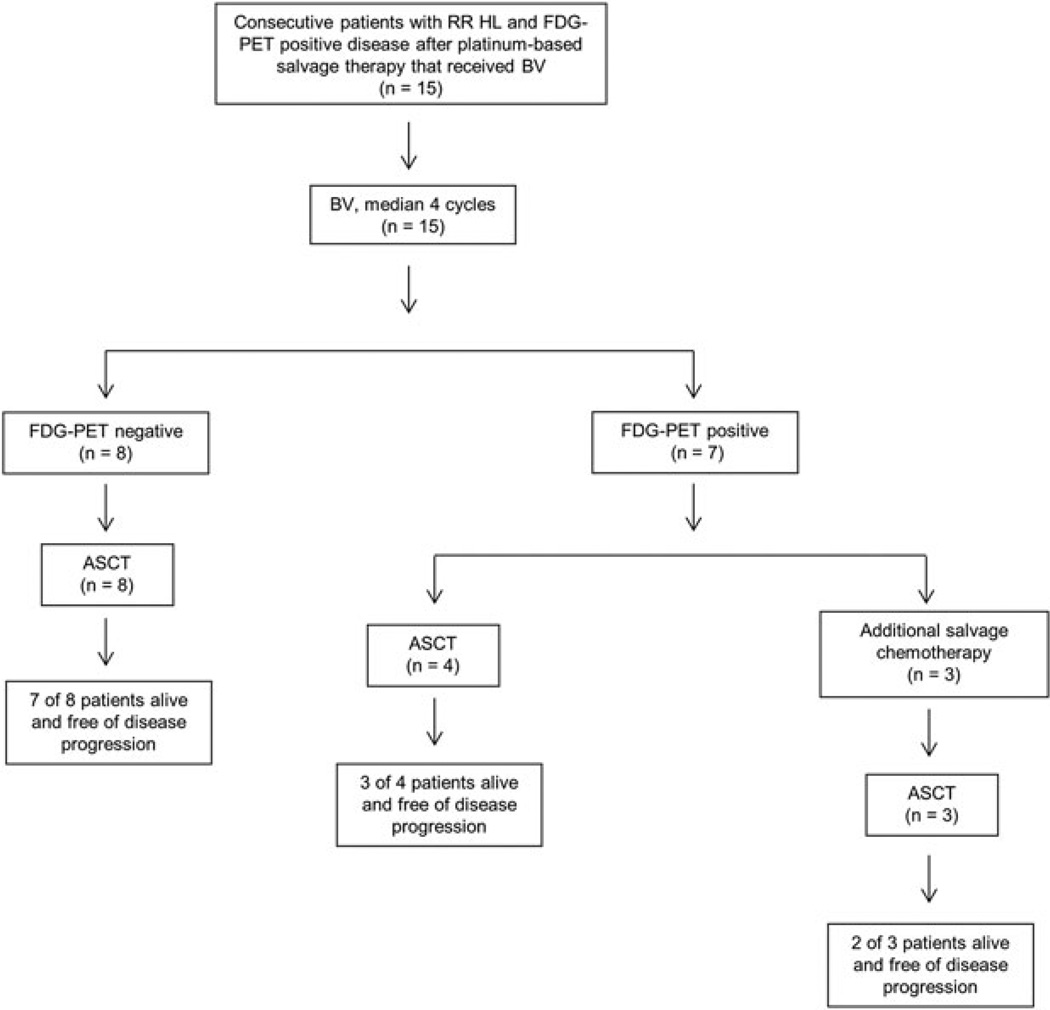
At the most recent restaging imaging with FDG PET/CT, 12 patients (80%) were documented to be progression-free, including 7 (88%) patients who achieved FDG PET negativity after BV and 5 (71%) patients who did not. The median follow-up since the last dose of BV and ASCT was 17.3 months and 15.8 months, respectively. The outcomes of these patients are summarized in Figure 1.
Figure 1.
Diagram of treatments and responses following BV given for RR HL after a platinum-based salvage regimen
Discussion
Our data suggest that BV can normalize FDG PET in a substantial fraction of patients with RR HL after platinum-based salvage therapy and prior to ASCT. BV may be relatively more effective as a second-line salvage therapy for those patients that achieve at least SD following platinum-based salvage therapy, as the two patients with PD to platinum-based salvage did not respond to BV.
Of the seven patients that did not convert to FDG PET negativity following BV, the three that went on to receive additional salvage chemotherapy continued to have persistent FDG PET positive disease. It is possible, therefore, that FDG PET normalization to BV monotherapy in the post-platinum salvage setting reflects relatively more favorable, chemo-sensitive disease. Indeed, a 52% FDG PET normalization rate, comparable to the 53% described here, has been reported with post-platinum, second-line salvage multi-agent chemotherapy [4]. Similar to prior studies, we found that BV was well tolerated without the characteristic toxicities of multi-agent salvage regimens and that no treatment-limiting toxicities were observed. Moreover, there has been no observed negative impact on the ability of patients to safely undergo ASCT after receiving BV.
Our findings are limited by their retrospective nature and the small number of patients studied. Nevertheless, this represents the largest series reported to date describing the use of BV in the setting of multiply-relapsed, platinum-refractory disease. Chen et al. described a consecutive case series of 11 patients with RR HL that received BV as first salvage [11], that is, after an ABVD-based regimen only. Of the eight patients evaluable for a response by FDG PET/CT, an OR was achieved in seven and a CR in four. At the time of the analysis, 6 of the 11 patients had proceeded to ASCT and thereafter achieved CR. Other groups are currently evaluating BV in various salvage settings of RR HL, including with an FDG PET adapted strategy [12] and in combination regimens, including, at our Center, an ongoing study of BV plus ICE in the first-salvage setting. These investigations are aimed at exploring the incorporation of BV in RR HL pre-ASCT with the goal of improving the rate of attaining FDG PET negative CR and reducing cumulative exposure to multi-agent chemotherapies.
Conclusion
While the optimal use of BV prior to ASCT is not known, the results from our analysis suggest that BV may represent a strategy to achieve FDG PET negativity in patients with platinum-refractory HL prior to consolidative ASCT. Longer follow up of this dataset and prospective studies are needed to confirm these findings and determine if FDG PET negative CR achieved with BV predicts the same post-ASCT outcomes as responses to conventional salvage regimens.
Acknowledgement
The authors thank all the patients who participated in the study. This work was supported by research funding from NCI P01CA44991, K12CA076930, Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium Cancer Center Support Grant P30 CA015704, and gifts from Frank and Betty Vandermeer. A.K.G. is a Scholar in Clinical Research of the Leukemia and Lymphoma Society. We would also like to thank Marlisa Isom, Seema Murthy, Malcolm Mack, and Heather Rasmussen.
Conflict of interest
L.H. has served as a consultant for Seattle Genetics, Allos, and Genzyme; as a speaker for Educational Concepts; and received research funding from Seattle Genetics, Millenium, Merck, Otsuka, and Sanofi. A.S. has served as a consultant and received honoraria and research funding from Seattle Genetics. J.M.P. has equity ownership of Seattle Genetics. O.W.P. has served as a consultant for Roche/Genentech. A.K.G. has served as a speaker and consultant for Seattle Genetics and Millennium Pharmaceuticals, and received research support from Seattle Genetics.
References
- 1.Copeland A, Younes A. Current treatment strategies in Hodgkin lymphomas. Curr Opin Oncol. 2012;24(5):466–474. doi: 10.1097/CCO.0b013e32835689a3. [DOI] [PubMed] [Google Scholar]
- 2.Moskowitz AJ, Yahalom J, Kewalramani T, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116(23):4934–4937. doi: 10.1182/blood-2010-05-282756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smeltzer JP, Cashen AF, Zhang Q, et al. Prognostic significance of FDG-PET in relapsed or refractory classical Hodgkin lymphoma treated with standard salvage chemotherapy and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(11):1646–1652. doi: 10.1016/j.bbmt.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119(7):1665–1670. doi: 10.1182/blood-2011-10-388058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baetz T, Belch A, Couban S, et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin’s disease: a phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 2003;14(12):1762–1767. doi: 10.1093/annonc/mdg496. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez de Larrea C, Martinez C, Gaya A, et al. Salvage chemotherapy with alternating MINE-ESHAP regimen in relapsed or refractory Hodgkin’s lymphoma followed by autologous stem-cell transplantation. Ann Oncol. 2010;21(6):1211–1216. doi: 10.1093/annonc/mdp487. [DOI] [PubMed] [Google Scholar]
- 7.Gopal AK, Press OW, Shustov AR, et al. Efficacy and safety of gemcitabine, carboplatin, dexamethasone, and rituximab in patients with relapsed/refractory lymphoma: a prospective multi-center phase II study by the Puget Sound Oncology Consortium. Leuk Lymphoma. 2010;51(8):1523–1529. doi: 10.3109/10428194.2010.491137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biggi A, Gallamini A, Chauvie S, et al. International validation study for interim PET in ABVD-treated, advanced-stage hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. J Nucl Med. 2013;54(5):683–690. doi: 10.2967/jnumed.112.110890. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 11.Chen RW, Palmer J, Siddiqi T, et al. Brentuximab Vedotin As First Line Salvage Therapy in Relapsed/Refractory HL. ASH Annu Meeting Abstr. 2012;120(21):3699. [Google Scholar]
- 12.Moskowitz A, Schoder H, Gerecitano JF, et al. FDG-PET Adapted Sequential Therapy With Brentuximab Vedotin and Augmented ICE Followed By Autologous Stem Cell Transplant For Relapsed and Refractory Hodgkin Lymphoma. Blood. 2013;122(21):2099. [PubMed] [Google Scholar]