Abstract
Chronic pain is a major characteristic feature of sickle cell disease (SCD). The refractory nature of pain and the development of chronic pain syndromes in many patients with SCD suggest that central neural mechanisms contribute to pain in this disease. We used HbSS-BERK sickle mice, which show chronic features of pain similar to those observed in SCD, and determined whether sensitization of nociceptive neurons in the spinal cord contributes to pain and hyperalgesia in SCD. Electrophysiological recordings of action potential activity were obtained from single, identified dorsal horn neurons of the spinal cord in anesthetized mice. Compared to control HbAA-BERK mice, nociceptive dorsal horn neurons in sickle mice exhibited enhanced excitability as evidenced by enlarged receptive fields, increased rate of spontaneous activity, lower mechanical thresholds, enhanced responses to mechanical stimuli, and prolonged after-discharges following mechanical stimulation. These changes were accompanied by increased phosphorylation of mitogen activated protein kinases (MAPKs) in the spinal cord that are known to contribute to neuronal hyperexcitability, including c-Jun N-terminal kinase (JNK), p44/p42 extracellular signaling-regulated kinase (ERK), and p38. These findings demonstrate that central sensitization contributes to pain in SCD.
1. Introduction
Chronic pain is a major defining characteristic of sickle cell disease (SCD) which can start in infancy and continue throughout life [2]. SCD is one of the world's most common inherited diseases, and is due to a point mutation in hemoglobin that leads to the polymerization of hemoglobin S which gives red blood cells (RBCs) their sickle shape. Sickle red blood cells occlude blood vessels, leading to impaired oxygen supply and ischemia/reperfusion-induced vascular dysfunction, inflammation, oxidative stress and pain [15,25]. As a result, patients often suffer from unpredictable, devastating acute pain episodes due to vaso-occlusive crises (VOC) that are believed to be a consequence from sickling of RBCs and vascular occlusion, leading to hospitalization and poor analgesic outcomes [2]. While VOC-induced pain is episodic, unpredictable and varies in frequency, a vast number of patients with SCD experience persistent chronic pain [9]. Opioids, particularly morphine, have been the primary treatment for managing pain but pose a major challenge because of the many undesirable side-effects, including tolerance, dependence, sedation, respiratory depression, nausea, constipation, and pruritus and may not always be effective [2,9].
Newer analgesic strategies are required to effectively treat the life-long pain experienced by patients with SCD, but the mechanisms underlying pain in SCD are not understood, thus limiting the development of more effective therapies. However, transgenic mice expressing exclusively human sickle hemoglobin and showing the major features found in human SCD [37], offer a unique opportunity to investigate pathophysiological mechanisms that contribute to pain in this disease. Transgenic homozygous HbSS-BERK sickle mice exhibit major hematologic features and organ damage found in human SCD [37]. We previously showed that these mice exhibit cutaneous and deep tissue hyperalgesia [5,27] similar to that observed in patients with SCD [4], and that hyperalgesia was increased following hypoxia/reoxygenation [5]. Thus, homozygous HbSS-BERK sickle mice show chronic and acute pain during VOC, observed as tonic hyperalgesia and following hypoxia/reoxygenation, respectively [5,16,27,47].
The cause of persistent pain that is difficult to treat remains an enigma in SCD. Central sensitization contributes to persistent pain in inflammatory and neuropathic conditions [28,48] and may contribute to pain in SCD. We showed that hemizygous hBERK1 mice expressing a relatively small % of sickle human Hb as compared to homozygous HbSS-BERK mice, had elevated levels of TLR4, COX-2, IL-6, phosphorylated signal transducer and activator of transcription 3 (STAT3), and phosphorylated mitogen-activated protein kinases (MAPKs) p38, and p44/p42 extracellular signal-regulated kinase (ERK), in the spinal cord, each of which can contribute to sensitization of nociceptive dorsal horn neurons [21,27,46]. The hBERK1 mice also show severe inflammation and hyperalgesia [15,27]. It is therefore likely that in HbSS-BERK sickle mice that show characteristic features of sickle pain, hyperalgesia may be due to central sensitization. In the present study, we determined whether enhanced excitability of dorsal horn neurons contributes to pain in SCD. Electrophysiological approaches were used to directly record response properties of identified nociceptive dorsal horn neurons in transgenic homozygous HbSS-BERK sickle mice.
2. Materials and Methods
2.1. Mice
Adult (4-12 months old weighing 25-50g) male, HbSS-BERK (n=14) sickle mice that express exclusively (>99%) human sickle hemoglobin and age/gender-matched controls (HbAABERK control mice; n=14) expressing normal human hemoglobin A (HbA) were used. Mice were bred in our AALAC approved mouse colony. Mice were genotyped by qPCR for the transcripts of mouse alpha and beta chains and human alpha and beta-S and human HbA transgenes, using tail snips (Transnetyx). HbSS-BERK mice showed the presence of human alpha and beta-s transcripts and absence of mouse alpha and beta transcripts. Mice were phenotyped by isoelectric focusing for the presence or absence of HbS exclusively or human HbA in HbSS-BERK and HbAA-BERK mice, respectively as described by us [47]. These mice show severe features of human SCD including anemia, reticulocytosis, hemolysis, organ damage, reduced life-span and pain [27,37]. All procedures were approved and performed in compliance with the Institutional Animal Care and Use Committee of the University of Minnesota.
2.2. Behavioral measure of mechanical hyperalgesia
To assess for mechanical hyperalgesia, mice were placed on an elevated mesh platform, covered with a glass container, and allowed to acclimate for at least 15 min. A von Frey (Semmes-Weinstein) monofilament (Stoelting Co, Wood Dale, IL) with a calibrated bending force of 12.8 mN was applied for 1-2 s to the mid-plantar surface of each hind paw at random locations for 10 trials on each paw (excluding the toes). Only HbSS-BERK sickle mice that exhibited hyperalgesia, defined as vigorous paw withdrawals in at least 50% of the trials, and HbAA-BERK controls which did not, were used for electrophysiological experiments [5,27].
2.3. Electrophysiological recording from spinal dorsal horn neurons
Mice were anesthetized with 2.5% isoflurane, and given dexamethasone (5 mg/kg, sq) to reduce swelling and continuous saline (0.2 ml/hr sq) to maintain hydration. Respiration rate and blood pressure were monitored continuously. Core body temperature was maintained at ~37°C using a feedback-controlled heating pad (Physitemp Instruments, Inc., Clifton, NJ). Following removal of hair, an incision in the skin was made over the thoracic and lumbar parts of the vertebral column, a laminectomy was performed to expose the lumbar enlargement at L4-L5, and mice were secured in a spinal frame. Anesthesia was then reduced to 0.8-1.2% and a reservoir around the spinal column was made of vinyl polysiloxane dental impression material (3M ESPE Dental Products, St. Paul, MN) and filled with warm mineral oil. The dura mater was removed and extracellular recordings from dorsal horn neurons with receptive fields located on the plantar surface of the hind paw were obtained using glass microelectrodes (~1 mΩ; Kation Scientific, Minneapolis, MN) that were lowered into the spinal cord in 3-μm steps using a hydraulic microdrive (Kopf, Tujunga, CA). Action potential activity was amplified, audiomonitored, and displayed on a storage oscilloscope. Receptive fields (RFs) of dorsal horn neurons were searched for by gently stroking the skin and applying mild pressure with the experimenter's fingers. Once identified, neurons were classified functionally as low threshold (LT), wide dynamic range (WDR) and high threshold (HT) cells using mechanical stimulation of graded intensities (brushing, pressure using a large arterial clip, and pinch with forceps) as described previously [26]. RF areas were mapped using suprathreshold von Frey monofilaments, drawn on a schematic of the hind paw, and measured using Image J (NIH, Bethesda, MD). Only nociceptive WDR and HT neurons with easily discriminated action potentials were studied.
2.4. Experimental design
Following identification and characterization of a nociceptive dorsal horn neuron, the discharge rate (impulses/s) of ongoing, spontaneous activity was determined for a period of 3 minutes. Subsequently, mechanical response thresholds were determined using von Frey monofilaments. Mechanical threshold (mN) was defined as the smallest von Frey monofilament that evoked a clear response or increase above any ongoing activity in at least 50% of the trials (8-10 trials). Next, an increasing series of three von Frey monofilaments (37.3, 73.6, and 135.3 mN) were applied in ascending order. Each stimulus was applied three times, each for 5 s, to random locations in the RF with an inter-stimulus interval of at least 30 s. Heat (34-50°C) and cold (28-0°C) stimuli were applied in 2° C increments every 60 s using a Peltier-type thermode starting from a base temperature of 30°C. Neuronal activity, discriminated action potentials, time of mechanical stimulation, and digitized traces of heat and cold stimuli were recorded using specialized data acquisition software (Spike II; Cambridge Electronic Design, Cambridge, UK) and stored on a computer for off-line analyses.
2.5. Histological verification of recording sites
At the end of each experiment, current (20 μA for 20 s) was passed through the recording electrode to produce a small lesion. Mice received an overdose of Euthasol (Virbac, Fort Worth, TX) and perfused with phosphate buffered saline followed by 10% formalin. Serial transverse 50-μm sections of the spinal cord were stained with neutral red. Recording sites were identified by small microlesions.
2.6. Western Immunoblotting for MAPKs
Whole spinal cord lysates containing 30 μg of protein were resolved on a 3-15% gradient SDS-PAGE gel and transferred to a polyvinylidene difluoride membrane (Immobilon, Millipore, Bedford, MA) as described previously [27]. For immunoblotting, we used antibodies to phospho-JNK (Thr183/Tyr185 (1:250), total JNK, phospho-p38 (Thr180/Tyr182 (1:250), total p38 MAPK (1:500), and phospho-p44/42 ERK (Thr202/Tyr204 (1:500), total p44/42 ERK, (all from Cell Signaling Technology, Beverly, MA). Immunoreactive proteins were visualized using a species-specific antibody linked to alkaline phosphate followed by development of chemiluminescent signals using the ECF Western blotting system (Amersham Life Sciences, Buckinghamshire, UK). Chemiluminescent signals were acquired using a Storm 860 PhosphoImager (Molecular Dynamics, Sunnyvale, CA) and densitometric analysis was performed using Image J software (NIH, Bethesda, MD).
2.7. Data analyses
A difference in the frequency of paw withdrawal (mechanical hyperalgesia) between sickle and control mice was determined using an unpaired t-test. Receptive field areas of WDR and HT cells in control and sickle mice were also compared using unpaired t-tests. A Chi-square test was used to compare the proportions of nociceptive neurons that exhibited ongoing spontaneous activity between control and sickle mice. Differences in the rates of spontaneous activity (impulses/s) of cells in control and sickle mice were determined using the Mann-Whitney test. Mechanical response thresholds of WDR and HT were compared between control and sickle mice using t-tests. The number of impulses evoked by mechanical (von Frey) stimuli was determined by subtracting ongoing activity during 10 s prior to the stimulus from the response that occurred during the stimulus (5 s) and for 5 s after. For each cell, responses evoked by each stimulus were defined as the mean response of three trials. The discharge rate (impulses/s) evoked by each of the monofilaments for HT and WDR neurons was compared between groups by using a two-way analysis of variance (ANOVA) with repeated measures. The rates of afterdischarges evoked by the von Frey monofilaments were determined for a period of 30 s following the response obtained during the stimulus. Afterdischarge was defined as ongoing activity (impulses/s) beginning 2 s after removal of the von Frey stimulus that was greater than 50% of the ongoing activity for 10 s prior to application of the stimulus. Rates and durations of afterdischarges of WDR and HT cells were compared between control and sickle mice using one-way ANOVA with repeated measures. All post-hoc comparisons were made using Bonferroni t-tests. Unpaired t-tests were used to determine differences in densitometric profiles of JNK, p38 and p44/p42 ERK MAPKs in control and sickle mice. For all statistical analyses, a probability value of <0.05 was considered significant. Mean (±SEM) values are shown for all data unless otherwise stated.
3. Results
3.1. Mechanical hyperalgesia in sickle mice
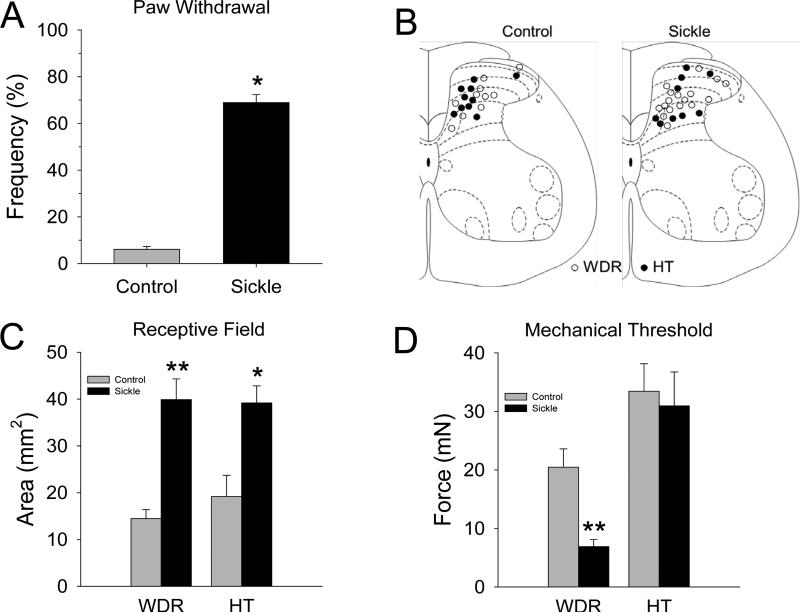
Consistent with our earlier findings [5,27], HbSS-BERK sickle mice exhibited robust mechanical hyperalgesia as defined by an increase in paw withdrawal frequency evoked by a von Frey monofilament with a bending force of 12.8 mN (Figure 1A).
Figure 1.
A) Sickle mice exhibited mechanical hyperalgesia. None of the control mice (n=14) exhibited a withdrawal response frequency greater than 15% (range: 0 to 15%) to this force, whereas sickle mice (n=14) had response frequencies that ranged between 50-100%. The mean (±SEM) frequency of paw withdrawal differed between the groups (t-test, p<0.001). B) Schematic representation of the location of recording sites in the spinal dorsal horn. The location of recording sites for WDR and HT neurons were similar for control (left) and sickle (right) mice and were located throughout the dorsal horn. The mean (±SEM) depths of recording sites from the surface of the spinal cord did not differ between control and sickle mice (307.1 ±32.6 μm and 399.1 ±33.3 μm, respectively). C) The mechano-sensitive receptive field areas of dorsal horn neurons differed between control and sickle mice. Receptive field areas were larger for both WDR and HT neurons in sickle mice (t-tests; *P < 0.05; **P < 0.01). D) Mechanical thresholds of WDR neurons were lower in mice with SCD as compared to controls (t-tests; **P < 0.01).
3.2. Nociceptive dorsal horn neurons in sickle mice exhibit increases in spontaneous activity, receptive field areas, and sensitivity to mechanical stimulation
A total of 50 nociceptive dorsal horn neurons (30 WDR and 20 HT) were studied. Of these, 13 WDR and 11 HT cells were studied in HbAA control mice and 17 WDR and 9 HT cells were obtained from HbSS sickle mice. Figure 1B shows the location of the recording sites for 46 neurons that were recovered histologically. The distribution of recording sites for WDR and HT cells throughout the dorsal horn was similar for control and sickle mice.
Spontaneous activity
Since very few HT cells exhibited ongoing activity, spontaneous ongoing discharge rates of WDR and HT cells were combined. The proportion of nociceptive dorsal horn neurons with ongoing spontaneous activity was similar for HbAA control and HbSS sickle mice (χ2 = 0.062, p < 0.80). Thirty eight percent (5/13) of WDR cells and 9% (1/11) of HT cells in control mice had ongoing activity; 35% (6/17) of WDR cells and 33% (3/9) of HT cells in sickle mice exhibited ongoing spontaneous activity. However, discharge rates of spontaneous activity were significantly higher in sickle mice. The median (25th and 75th percentile) discharge rates of spontaneous activity was 0.08 (0.008 and 0.64) impulses/s in control mice and 5.88 (1.1 and 20.3) impulses/s in sickle mice (Mann-Whitney U Statistic = 3.0, p < 0.03). Increased ongoing spontaneous activity may be related to ongoing, spontaneous pain in patients with SCD.
Receptive field areas
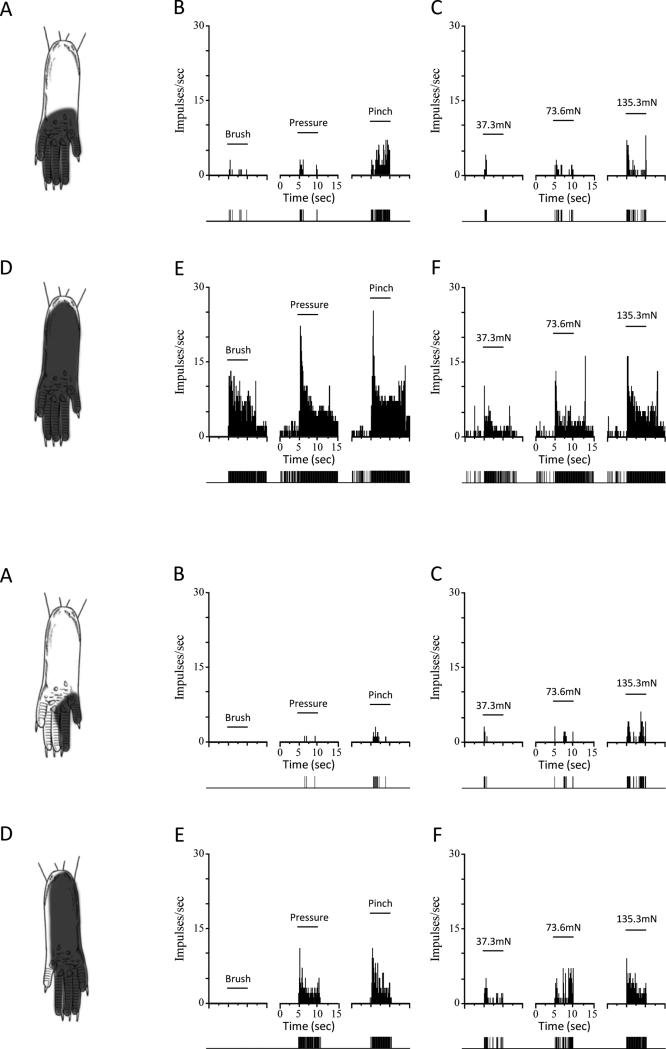
Since enlarged RFs occur with central sensitization [8,29,41,42], we measured the mechano-sensitive RF area for all neurons. All RFs were located on the plantar surface of the hind paw. Both WDR and HT cells in HbSS sickle mice had significantly larger RF areas as compared to those in HbAA control mice (Figure 1C). RF areas for WDR neurons ranged from 4.4 - 25.9 mm2 in control mice (n=13) and from 10.4 - 59.4 mm2 in sickle mice (n=17). The ranges of RF areas for HT cells were 5.4 - 58.4 mm2 in control mice (n=11) and 20.2 – 58.7 mm2 in sickle mice (n=9). The area of the RF often included most or all of the plantar surface of the hind paw in sickle mice (Figure 2D), which was rare in control mice (Figure 2A). Enlarged mechano-sensitive RF areas are likely to contribute to the mechanical hyperalgesia since a greater number of neurons are excited when an area of skin is stimulated.
Figure 2.
Receptive field (RF) areas and responses of nociceptive neurons evoked by mechanical stimuli are greater in sickle mice. Upper panels: Location of the RF, and peristimulus time histograms showing discharge rates evoked by stimuli used for functional characterization (brush, pressure pinch applied to the RF) and responses evoked by von Frey monofilaments of controlled force for a single WDR neuron from a control (top panels A, B, and C) and from a sickle (lower panels D, E, and F) mouse. Solid horizontal lines represent the time of application of the stimuli (5 s). Discriminated output pulses from a window discriminator are provided below the histograms. Lower panels: Same format as above but RF areas and evoked responses are shown for single HT neurons from a control (A-C) and sickle (D-F) mouse. Bin width for all peristimulus time histograms is 100 ms.
3.3. Responses evoked by mechanical stimulation
Both WDR and HT neurons in HbSS sickle mice exhibited sensitization to mechanical stimuli as evidenced by lower mechanical response thresholds and/or enhanced responses evoked by mechanical stimulation of the RF as compared to control mice. As shown in Figure 1D, the mean mechanical response threshold was lower for WDR neurons in sickle (n = 16) as compared to those in control (n=12) mice whereas thresholds for HT cells did not differ between control (n = 11) and sickle (n = 9) mice.
Responses of HT and WDR cells evoked by the mechanical stimuli (brush, pressure, pinch) used to initially classify nociceptive cells functionally were typically higher in sickle mice (see Figure 2), but these responses were not quantified. To obtain quantified responses of WDR and HT cells to controlled suprathreshold mechanical stimuli, the number of impulses evoked by von Frey monofilaments with calibrated bending forces of 37.3, 73.6, and 135.3 mN were determined. Evoked responses of WDR cells in sickle mice were greater than those in control mice (two-way ANOVA with repeated measures; F1,61 = 22.4. p < 0.001). Mean (±SEM) discharge rates for WDR cells evoked by mechanical stimuli in control and sickle mice are shown in Figure 3A. Discharge rates evoked by the 73.6 and 135.3 mN forces were approximately 3-4-fold greater in sickle mice as compared to control mice.
Figure 3.
Mean (±SE) discharge rates (impulses/s) for all WDR (A) and HT (B) neurons evoked by application of 37.3 mN, 73.6 mN and 135.3 mN von Frey filaments in control and sickle mice. Responses evoked by the 73.6 mN and 135.3 mN stimuli were greater for WDR neurons in sickle mice whereas responses to each of the forces was greater in sickle mice. *P < 0.05; **P < 0.01).
Similarly, HT cells in sickle mice also exhibited greater responses to mechanical stimuli as compared to those in control mice (F1,51 = 25.7, p < 0.001). As shown in Figure 3B, mean discharge rates evoked by each of the forces were greater for HT cells in sickle mice than in control mice. Discharge rates of HT cells evoked by the 37.3, 73.6 and 135.3 mN forces were increased approximately 7-fold, 4-fold and 2.5-fold, respectively, in sickle mice. These data demonstrate that nociceptive neurons in the dorsal horn of sickle mice are sensitized to mechanical stimulation and this may contribute to the mechanical hyperalgesia observed in these mice and in patients with SCD.
3.4. Prolonged afterdischarges to mechanical stimuli in sickle mice
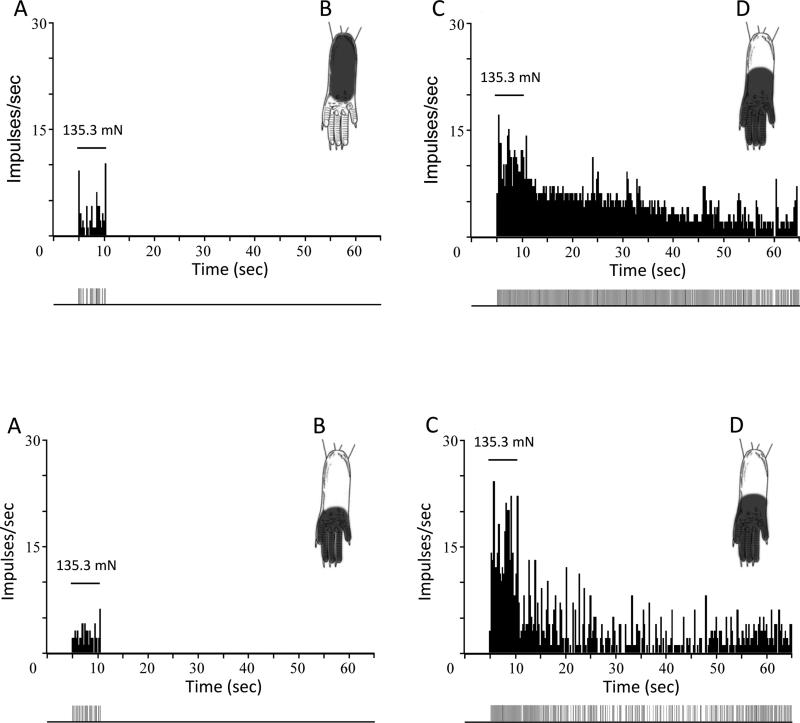
None of the cells (11 WDR and 11 HT) in HbAA control mice exhibited afterdischarge to any of the von Frey monofilaments (37.3, 73.6, and 135.3 mN). Responses evoked by these stimuli ceased within 1-2 s upon removal of the monofilament from the skin. However, in addition to the robust increases in mechanically-evoked responses of nociceptive neurons in HbSS sickle mice, a significant proportion of dorsal horn neurons (11 of 20) neurons also exhibited clear and prolonged afterdischarges following mechanical stimulation with von Frey monofilaments (χ2 = 7.8, p < 0.005), although the discharge rates and durations of afterdischarge varied between trials and between cells. Nine of 12 WDR and 2 of 8 HT cells exhibited afterdischarges to one or more of the von Frey stimuli. Since the von Frey stimuli evoked afterdischarges in only 2 HT cells, data were pooled for WDR and HT cells. One-way ANOVAs with repeated measures revealed that evoked discharge rates and durations of afterdischarge evoked by the three von Frey stimuli did not differ among the stimuli. Mean (±SEM) discharge rates evoked by 37.3, 73.6, and 135.3 mN forces during the 30-s period after removal of the stimulus were 10.5 ±4.8, 14.6 ±10.1, and 9.8 ±2.5 impulses/s, respectively (F2,19 = 0.22, p< 0.81). Similarly, the mean duration of afterdischarge evoked by the various von Frey stimuli did not differ (49.9 ±18.6, 43.7± 16.8, and 76.4 ±37.8 s for forces of 37.3, 73.6, and 135.3 mN, respectively (F2,19 = 0.30, p < 0.74). Because there were no differences in discharge rates or duration of afterdischarge as a function of von Frey force, all rates and durations of afterdischarge were combined for the three von Frey stimuli. The mean (±SEM) rate of afterdischarge evoked by the von Frey stimuli was 11.1 ±2.8 impulses/s (range = 1.4 – 55.1 impulses/s), and the mean (±SEM) duration of afterdischarge was 60.5 ±18.2 s (range = 12.4 – 412.2 s). Examples of afterdischarge for individual dorsal horn neurons in control and sickle mice are shown in Figure 4. These data suggest that mechanical stimulation evokes pain that outlasts the duration of the stimulus, although this has not been investigated in patients with SCD.
Figure 4.
Mechanical stimulation evokes prolonged afterdischarges in nociceptive neurons in sickle mice. Upper panels show responses evoked by the 135.3 mN force in a WDR neuron from a control mouse (A) and from a sickle mouse (B). Similarly, responses of single HT neurons from a control and from a sickle mouse are shown in C and D. Horizontal lines represent the time of stimulus application. The location of the RF is shown for each neuron. Discriminated output pulses from a window discriminator are provided below the histograms. Responses to mechanical stimulation were greater and prolonged in sickle mice. Bin width of the peristimulus time histograms is 250 ms.
3.5. Spinal nociceptive signaling pathways are activated in sickle mice
We examined whether activation of MAPKs in the spinal cord that contribute to central sensitization were increased in HbSS sickle mice. Constitutively, phosphorylation of JNK, p38, and ERK was significantly increased in spinal cords from sickle mice as compared to control mice (Figure 5).
Figure 5. Increased nociceptive signaling in the spinal cord of sickle mice.
Protein bands (and molecular weight) of phosphorylated and total proteins determined using Western immunoblotting and their densitometric profiles are shown for JNK (A), p38 (B), and p44/p42 ERK (C). Density of phosphoprotein bands relative to their specific total protein are shown. Data are expressed as mean ± SEM from 5 mice per group of 5 separate experiments. Statistical significance is denoted by *P < 0.05 and **P < 0.005. Mean age of mice ± SEM in months were 4.04 ± 0.29 for HbAA-BERK and 4.38 ± 0.32 for HbSS-BERK.
4. Discussion
Chronic, severe pain is a common feature of SCD that is superimposed on episodic pain due to VOC which often leads to hospitalization. Genetic as well as environmental factors contribute to the frequency and severity of acute pain produced by VOC [24]. As for environmental factors, a number of studies found an association between cold temperatures and painful episodes [1,20,40]. Moreover, skin cooling due to wind was also associated with acute painful episodes [35], underscoring the exquisite sensitivity of VOC crises to cold. Although pain from VOC and can be severe, require hospitalization and treatment with high doses of opioids, and impact morbidity and mortality [39], persistent pain also occurs in the absence of VOC, with many patients experiencing pain beginning early in life [2].
The underlying basis of persistent and episodic pain in SCD remains unknown, making therapeutic approaches for palliative care a major challenge. The goal of the present study was to determine whether pain in SCD was associated with sensitization of nociceptive neurons in the spinal cord. We used the transgenic HbSS-BERK mouse as a model of SCD because of its close resemblance to the hematologic and pain phenotypes observed in patients with SCD [4,27,34,37]. Our electrophysiological studies show that nociceptive neurons in the dorsal horn constitutively exhibit increased spontaneous activity, enlarged mechanosensitive RF areas, decreased mechanical response thresholds and increased responses to mechanical stimuli applied to the plantar skin. In addition, prolonged afterdischarges following mechanical stimulation were observed in approximately 50% of the neurons. These changes in RF area and response properties of nociceptive dorsal horn neurons suggest that central sensitization contributes, at least in part, to the chronic pain in SCD. Increased spontaneous activity may be related to ongoing pain whereas changes in responses to mechanical stimuli may be related to mechanical hyperalgesia. Afterdischarges evoked by mechanical stimulation could signal pain that persists following removal of a mechanical stimulus. Although the duration of pain evoked by an acute mechanical stimulus has not been studied, it is possible that these prolonged responses of dorsal horn neurons lead to prolonged and refractory pain in SCD.
The mechanisms that underlie sensitization of nociceptive dorsal horn neurons in HbSSBERK mice are unknown. We found that mediators of central sensitization, including the phosphorylation of MAPK family of kinases, were increased in the spinal cord of HbSS-BERK mice as compared to HbAA-BERK mice. MAPKs are activated in primary sensory neurons as well as in the dorsal horn following nociceptive afferent activity and play a major role in neuronal sensitization [7,11,21,22,23,32,36]. For example, activation of ERK can lead to hyperexcitability of dorsal horn neurons [18] by decreasing A-type potassium currents via Kv4.2 potassium channels [17,19], and by contributing to the phosphorylation of the NR1 subunit of N-methyl-D-aspartate (NMDA) receptors [43], which are essential for the induction of central sensitization [49]. ERK also plays a role in transcriptional regulation, particularly via phosphorylation of cAMP-response element binding protein (CREB), which may maintain long-term neuronal plasticity by inducing gene transcription and the formation of new synapses [31]. As for p38, its activation in the spinal cord increases proinflammatory mediators such as COX-2 [45] and iNOS [44] which contribute to sensitization of dorsal horn neurons. In addition to COX-2 and iNOS, we also observed increases in TLR4 and IL-6 in the spinal cord of HbSS mice which also contribute to pain and central sensitization through glial-neuronal interactions [see reviews: 13,30].
The mechanisms that drive and maintain the central sensitization observed in the present study are unknown. However, it is likely that nociceptor sensitization and increased afferent input to the dorsal horn contribute to pain and central sensitization in HbSS mice Early studies showed that visceral [6] and cutaneous [38] afferent fibers are excited during ischemia and hypoxia, including C-fibers [33]. Whether nociceptive afferent fibers in HbSS mice exhibit ongoing activity is unclear. However, electrophysiological studies showed that cutaneous nociceptors in HbSS-BERK mice exhibit greater responses to mechanical stimuli as compared to controls, and this was mediated, at least in part, by TRPV1 receptors [16]. Interestingly, low threshold mechanoreceptors also exhibited greater evoked responses in HbSS-BERK mice as compared to controls [12], and this increased afferent input may play a role in the tactile allodynia and enhanced responses of dorsal horn neurons to mechanical stimuli observed in these mice. In addition, it was recently shown that cold-sensitive C-fiber nociceptors in HbSSBERK mice had lower response thresholds for cold (responded to warmer temperatures), although the proportion of C-fibers that are sensitive to cold stimuli and the magnitude of response to suprathreshold cold stimuli did not differ between sickle and control mice [50]. Thus, lowered response thresholds of nociceptors for cold by contribute to a decrease in the cold pain threshold. Although the mechanisms underlying nociceptor sensitization in SCD are unknown, this may result from persistent activation of mast cells and neurogenic inflammation [47].
It is also possible that a decrease in local spinal inhibitory transmission contributes to central sensitization in HbSS sickle mice. Decreasing inhibitory transmission in the spinal cord under normal conditions leads to hyperalgesia and enhanced responsiveness of nociceptive dorsal horn neurons, and under certain conditions of persistent pain there is a decrease in spinal inhibitory neurotransmission [see reviews: 3,10,14,51]. Additional studies are needed to determine whether changes in inhibitory modulation in the spinal cord contributes to central sensitization observed in HbSS sickle mice.
In conclusion, the use of transgenic mouse models of SCD is beginning to unravel the neural mechanisms of chronic pain associated with this disease. It appears that sensitization of both nociceptors and dorsal horn neurons are involved. Results of the present study provide new and important information on the sickle pathobiology that central sensitization contributes to persistent pain in SCD and perhaps to the poor response to pain therapies. Understanding the cellular mechanisms by which nociceptive neurons become sensitized in SCD may identify novel approaches for alleviating pain in this disease.
Acknowledgements
We thank Barbara Benson, Derek Vang and Stefan Kren for breeding and typing of mice. We also thank Janneta Tabakov for assistance with spinal cord histology. This study was supported by NIH grants R01 HL103773, U01 HL117664-01 and R01 DA011471. Dr. G. Cataldo was supported by a grant from the National Institute of Dental and Craniofacial Research (T90 DE0227232).
Footnotes
Authorship Contributions
G.C. performed all the electrophysiological experiments, analyzed data, and wrote the manuscript; S.R. performed the Western immunoblotting; K.G. co-supervised the study and was responsible for the study concept and experimental design, interpretation of data, and editing the manuscript; D.A.S. supervised and designed the study, collected and interpreted electrophysiology data, and edited the manuscript.
Conflict of Interest
Each of the authors declares no competing financial interests.
References
- 1.Amjad H, Bannerman RM, Judisch JM. Letter: sickling pain and season. Br Med J. 1974;2:54. doi: 10.1136/bmj.2.5909.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120:3647–56. doi: 10.1182/blood-2012-04-383430. [DOI] [PubMed] [Google Scholar]
- 3.Bardoni R, Takazawa T, Tong CK, Choudhury P, Scherrer G, Macdermott AB. Pre- and postsynaptic inhibitory control in the spinal cord dorsal horn. Ann N Y Acad Sci. 2013;1279:90–6. doi: 10.1111/nyas.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol. 2013;88:37–43. doi: 10.1002/ajh.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cain DM, Vang D, Simone DA, Hebbel RP, Gupta K. Mouse models for studying pain in sickle disease: effects of strain, age, and acuteness. Br J Haematol. 2012;156:535–44. doi: 10.1111/j.1365-2141.2011.08977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- 7.Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33:1970–8. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook AJ, Woolf CJ, Wall PD, McMahon SB. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325:151–3. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- 9.Darbari DS, Ballas SK, Clauw DJ. Thinking beyond sickling to better understand pain in sickle cell disease. Eur J Haematol. 2014;93:89–95. doi: 10.1111/ejh.12340. [DOI] [PubMed] [Google Scholar]
- 10.Dickenson AH, Chapman V, Green GM. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen Pharmacol. 1997;28:633–8. doi: 10.1016/s0306-3623(96)00359-x. [DOI] [PubMed] [Google Scholar]
- 11.Edelmayer RM, Brederson JD, Jarvis MF, Bitner RS. Biochemical and pharmacological assessment of MAP-kinase signaling along pain pathways in experimental rodent models: a potential tool for the discovery of novel antinociceptive therapeutics. Biochem Pharmacol. 2014;87:390–8. doi: 10.1016/j.bcp.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Garrison SR, Kramer AA, Gerges NZ, Hillery CA, Stucky CL. Sickle cell mice exhibit mechanical allodynia and enhanced responsiveness in light touch cutaneous mechanoreceptors. Mol Pain. 2012;8:62. doi: 10.1186/1744-8069-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–31. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo D, Hu J. Spinal presynaptic inhibition in pain control. Neurosci. 2014;283:95–06. doi: 10.1016/j.neuroscience.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11:129–51. [PubMed] [Google Scholar]
- 16.Hillery CA, Kerstein PC, Vilceanu D, et al. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118:3376–83. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RW., 4th The kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Hu HJ, Gereau RW., 4th ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. II. Modulation of neuronal excitability. J Neurophysiol. 2003;90:1680–8. doi: 10.1152/jn.00341.2003. [DOI] [PubMed] [Google Scholar]
- 19.Hu HJ, Glauner KS, Gereau RW., 4th ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J Neurophysiol. 2003;90:1671–9. doi: 10.1152/jn.00340.2003. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim AS. Relationship between meteorological changes and occurrence of painful sickle cell crises in Kuwait. Tran Royal Soc Tropical Med and Hygiene. 1980;74:159–61. doi: 10.1016/0035-9203(80)90236-9. [DOI] [PubMed] [Google Scholar]
- 21.Ji RR, Gereau RW, 4th, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–48. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 24.Jones S, Duncan ER, Thomas N, Walters J, Dick MC, Height SE, Stephens AD, Thein SL, Rees DC. Windy weather and low humidity are associated with an increased number of hospital admissions for acute pain and sickle cell disease in an urban environment with a maritime temperate climate. Br J Haematol. 2005;131:530–3. doi: 10.1111/j.1365-2141.2005.05799.x. [DOI] [PubMed] [Google Scholar]
- 25.Kassim AA, DeBaun MR. Sickle cell disease, vasculopathy, and therapeutics. Annu Rev Med. 2013;64:451–66. doi: 10.1146/annurev-med-120611-143127. [DOI] [PubMed] [Google Scholar]
- 26.Khasabov SG, Hamamoto DT, Harding-Rose C, Simone DA. Tumor-evoked hyperalgesia and sensitization of nociceptive dorsal horn neurons in a murine model of cancer pain. Brain Res. 2007;1180:7–19. doi: 10.1016/j.brainres.2007.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohli DR, Li Y, Khasabov SG, et al. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116:456–65. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 30.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–44. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–23. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 32.Ma W, Quirion R. The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opin Ther Targets. 2005;9:699–713. doi: 10.1517/14728222.9.4.699. [DOI] [PubMed] [Google Scholar]
- 33.MacIver MB, Tanelian DL. Activation of C fibers by metabolic perturbations associated with tourniquet ischemia. Anesthesiol. 1992;76:617–23. doi: 10.1097/00000542-199204000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood. 2006;107:1651–8. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nolan VG, Zhang Y, Lash T, Sebastiani P, Steinberg MH. Association between wind speed and the occurrence of sickle cell acute painful episodes: results of a case-crossover study. Br J Haematol. 2008;143:433–8. doi: 10.1111/j.1365-2141.2008.07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74:2643–53. doi: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Pászty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–8. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 38.Pethô G, Pórszász R, Peitl B, Szolcsányi J. Spike generation from dorsal roots and cutaneous afferents by hypoxia or hypercapnia in the rat in vivo. Exp Physiol. 1999;84:1–15. doi: 10.1111/j.1469-445x.1999.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 39.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 40.Redwood AM, Williams EM, Desal P, Serjeant GR. Climate and painful crisis of sickle-cell disease in Jamaica. Br Med J. 1976;1:66–68. doi: 10.1136/bmj.1.6001.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ririe DG, Bremner LR, Fitzgerald M. Comparison of the immediate effects of surgical incision on dorsal horn neuronal receptive field size and responses during postnatal development. Anesthesiol. 2008;109:698–706. doi: 10.1097/ALN.0b013e3181870a32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simone DA, Baumann TK, Collins JG, LaMotte RH. Sensitization of cat dorsal horn neurons to innocuous mechanical stimulation after intradermal injection of capsaicin. Brain Res. 1989;486:185–9. doi: 10.1016/0006-8993(89)91293-6. [DOI] [PubMed] [Google Scholar]
- 43.Slack SE, Pezet S, McMahon SB, Thompson SW, Malcangio M. Brain-derived neurotrophic factor induces NMDA receptor subunit one phosphorylation via ERK and PKC in the rat spinal cord. Eur J Neurosci. 2004;20:1769–78. doi: 10.1111/j.1460-9568.2004.03656.x. [DOI] [PubMed] [Google Scholar]
- 44.Sung CS, Wen ZH, Chang WK, Chan KH, Ho ST, Tsai SK, Chang YC, Wong CS. Inhibition of p38 mitogen-activated protein kinase attenuates interleukin-1beta-induced thermal hyperalgesia and inducible nitric oxide synthase expression in the spinal cord. J Neurochem. 2005;94:742–52. doi: 10.1111/j.1471-4159.2005.03226.x. [DOI] [PubMed] [Google Scholar]
- 45.Svensson CI, Marsala M, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–44. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 46.Tsuda M, Kohro Y, Yano T, et al. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain. 2011;134:1127–39. doi: 10.1093/brain/awr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122:1853–62. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 50.Zappia KJ, Garrison SR, Hillery CA, Stucky CL. Cold hypersensitivity increases with age in mice with sickle cell disease. Pain. 2014;155:2476–85. doi: 10.1016/j.pain.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeilhofer HU, Wildner H, Yévenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev. 2012;92:193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]