Abstract
It is not known why mentally ill persons smoke excessively. Inasmuch as endogenous opioid and dopaminergic systems are involved in smoking reinforcement, it is important to study mu opioid receptor (OPRM1) A118G (rs1799971), dopamine D2 receptor (DRD2) Taq1A (rs1800497) genotypes, and sex differences among patients with schizophrenia or bipolar disorder. Smokers and nonsmokers with schizophrenia (177) and bipolar disorder (113) were recruited and genotyped. They were classified into three groups: current smoker, former smoker, and never smoker by tobacco smoking status self-report. The number of cigarettes smoked per day was used as the major tobacco smoking parameter. In patients with schizophrenia, tobacco smoking prevalence was greater in males than in females as expected, but women had greater daily cigarette consumption (p<0.01). Subjects with schizophrenia who had the OPRM1 *G genotype smoked more cigarettes per day than the AA allele carriers with schizophrenia (p<0.05). DRD2 Taq1A genotype differences had no effect on the number of cigarettes smoked per day. However, female smokers with schizophrenia who were GG homozygous with the DRD2 receptor smoked more than the *A male smokers with schizophrenia (p<0.05). In bipolar patients, there were no OPRM1 and DRD2 Taq1A genotype differences in smoking status. There also were no sex differences for smoking behavior among the bipolar patients. The results of this study indicate that single nucleotide polymorphism (SNP) of the less functional mu opioid receptor increases tobacco smoking in patients with schizophrenia. Alteration of DRD2 receptor function also increased smoking behavior in females with schizophrenia.
Keywords: Schizophrenia, Bipolar, Tobacco Smoking, Genetics, Mu opioid receptors
Introduction
It is well documented that persons with mental illness smoke more than normal controls [1–6]. This includes patients with schizophrenia [7–15], depression [16–19] and bipolar disorder [20–22]. Furthermore, tobacco smoking is the primary cause of preventable diseases and death in the United States [23]. Although many individuals with a mental illness desire to stop using tobacco products [24], they are unable to easily do so, resulting in an increase of serious physical disease risk. For those with a mental illness, up to 25 years of life are lost when compared to the general population with diseases attributable to smoking [25].
Our research group has examined pharmacogenetic variability within the mu opioid receptor and dopamine D2 receptor gene as possible risk factors for cigarette smoking. Both receptors are involved in the reinforcing effects of smoking directly and indirectly [26]. Previous work has shown that the OPRM1 A118G variant results in an amino acid change (Asn40Asp) on the N-glycosylation site of the receptor protein. This amino acid change produces decreased mRNA and receptor protein expression [27]. It has been suggested the G allele is a “loss-of-function” variant. Ray et al. [28] reported that A118G variant carriers had a reduction in the binding potential of free mu opioid receptors in several brain areas involving the reward system. The DRD2 Taq1A (rs1800497) variant is a variant of the ankyrin repeat and kinase domain containing 1 (ANKK1) gene. The minor A1 (A) allele is associated with low expression of DRD2 protein in vitro [29] which regulates dopamine (DA) release as an autoreceptor [30, 31]. This allele in particular has been examined for its role in both of schizophrenia and bipolar disorder [32, 33] and being identified as a marker in antipsychotic drug response [34].
The present study was designed to confirm two hypotheses: 1) Subjects with schizophrenia or bipolar disorder that carry the less active OPRM1 118G allele smoke more compared to subjects with the AA genotype. 2) Subjects with the DRD2 Taq1A A1 (A) allele will exhibit greater tobacco smoking behaviors due to lower DRD2 protein expression resulting in more DA release. These hypotheses were examined with appropriate statistical analyses to compare their smoking differences. In addition, joint OPRM1 and DRD2 Taq1A genotype effects were determined on smoking behavior. These two genes are often discussed together, but the joint gene effects have not been studied well to date. The results imply that greater smoking prevalence in patients with schizophrenia is associated with genetic components. The present study contributes to further understanding of tobacco smoking among persons with mental illness.
Methods
Subjects
The subjects in this study met the following inclusion criteria: 1) DSM-IV diagnosis of schizophrenia, schizophreniform disorder, schizoaffective disorder, or bipolar disorder I or II, 2) ≥18 years old, and 3) treated with antipsychotic or mood-stabilizing medication as clinically indicated for at least six months. Subjects were excluded if they were unable to provide informed consent (assessed using a short questionnaire asking key questions about the study). Study subjects were recruited from ambulatory care mental health clinics and were included in a previous pharmacogenomic study related to the occurrence of atypical antipsychotic associated metabolic complications [35]. Subjects meeting inclusion and exclusion criteria underwent informed consent including a brief assessment of the risks and benefits associated with study participation. Afterwards, a clinical interview, which included the Structured Clinical Interview for DSM Diagnoses (SCID) for schizophrenia patients [36], and the Diagnostic Interview for Genetic Studies (DIGS) for bipolar disorder subjects were completed by a trained research associate and verified through chart review. Two different diagnostic assessments were utilized since subjects with schizophrenia vs. bipolar disorder were initially recruited for separate, but similar, pharmacogenomic studies. The study protocols were approved by the University of Michigan Medical School Institutional Review Board (IRBMED).
Smoking status data collection
Smoking status was assessed by self-report at the time of the study visit. Life time smoking status was classified as 1) current smoker, 2) former smoker, and 3) never smoker based on patient self-report. Former smokers were also identified by self-report and subjects who had quit smoking more than 12 months previously were classified as former smokers. Upon classification more information was obtained from subjects regarding former and current smoking habits as they were asked questions about smoking (number of cigarettes smoked per day, age at when smoking started, and quit date if applicable) to calculate a smoking pack -year history. Whenever possible these data were confirmed through documentation within their medical records.
Genotyping
DNA was extracted from a whole blood sample using a Puregene kit (Qiagen, Valencia California). After the samples were processed, they underwent spectrophotometry to establish purity and yield and were then frozen at −80°C. Polymerase chain reaction (PCR) and sequencing primers for the OPRM1 A118G (rs1799971) and DAD2 Taq1A (rs1800497) were designed using Pyrosequencing SNP Primer Design Version 1.01 software (http://www.pyrosequencing.com). The PCR were performed using One Taq 2X Master Mix with Standard Buffer (Biolabs Inc) with the forward primer (5′-TGA TGC CTT GGC GTA CTC A -3′) and biotinylated reverse primer (5′-5Biosg/GCC GTG ATC ATG GAG GGA CT -3′) for OPRM1 A118G variant. The PCRs were performed using Platinum PCR Super-Mix with the biotinylated forward primer (5′-/5Bio/CAA GGG CAA CAC AGC CAT C -3′) and reverse primer (5′-CAA GGG CAA CAC AGC CAT C-3′) for DRD2 Taq1A variant. PCR products were visualized by electrophoresis on 1.8% agarose gels stained with ethidium bromide before Pyrosequencing. Genotyping was done with Pyrosequencing™ Technology.
Statistical analyses
The OPRM1 genotypes were grouped based on previous studies [26, 37–40] as OPRM1 AA and AG/GG (*G allele carriers). The DRD2 Taq1A genotypes were also classified according to previous studies with respect to the presence of A1 (A) allele as AA, AG and GG; also the genotypes were grouped AA+AG (*A) and GG in the most of statistic comparisons as described previously [41, 42].
The major tobacco smoking parameter used for this study was number of cigarettes smoked per day, as well as current smoking status at the time of assessment. Current and former smokers were included to examine the number of cigarettes smoked per day for all statistical analyses unless otherwise specified because no significant differences were detected in smoking habits in separated groups. The numbers of cigarettes per day were analyzed with a two-tailed student t test and one-way ANOVA for the genotypes and sex differences. A linear regression model was used to estimate the impact of the each factor (e.g. OPRM1 and DRD2 Taq1A genotype, diagnosis, sex, age and race) on tobacco smoking behavior. The genotypes, race and sex frequency differences were examined by χ2 and Hardy Weinberg analyses. All groups in the present study included all sex, medication groups, and ethnicity unless otherwise specified. The nonsmoker data were excluded for all statistical analyses to compare the number of cigarettes unless otherwise mentioned. These analyses were performed with the IBM SPSS (Statistic Package for Social Sciences) statistics version 20 for Windows. A p<.05 was considered significant.
Results
Study Population Characteristics
A total of 290 smoking and nonsmoking patients with schizophrenia (n = 177) or bipolar disorder (n = 113) were recruited. Table 1 summarizes the demographic differences between groups. It is important to note that he number of males and females were significantly different in opposite directions in the diagnostic groups (p< .05 for both groups). Overall their mean age was 45.0 ± 11.7 (range = 19–71) years. Caucasians were the racial majority (n=194, 66.9 %) followed by African Americans (n=75, 25.9 %). Males compromised the majority with a total of 52.8% (n=153). Within the whole group, 135 (46.6%) were current smokers, 65 (22.4%) were previous smokers and 90 (31.0%) were never smokers. Current smokers smoked an average of 17.3 ± 10.8 cigarettes per day which resulted in a mean pack year history of 23.0 ± 21.9. Former smokers smoked an average of 19.0 ± 14.1 cigarettes per day which resulted in a mean pack year history of 14.1 ± 21.8. Although there were no sex differences for the overall demographic variables, surprisingly female subjects with schizophrenia smoked more cigarettes per day (cpd) compared to the male subjects (22.4 ± 13.3 cpd vs. 16.8 ± 10.5 cpd; t(135)=2.621, p=.010; Fig. 1). No sex differences were found among those with bipolar disorder related to number of cigarettes smoked per day.
Table 1.
Demographics for all subjects.
| Classifications | All | SCZ | BP |
|---|---|---|---|
| Age ± SD | 45.0 ± 11.7 | 46.2 ± 11.3 | 43.1 ± 12.0 |
| Sex | |||
| * Male (%) | 153 (52.8) | 114 (64.4) | 39 (34.5) |
| * Female (%) | 137(47.2) | 63 (35.6) | 74 (65.5) |
| Races | |||
| * Caucasian (%) | 194(66.9) | 102 (57.6) | 92 (81.4) |
| * African American (%) | 75(25.9) | 62 (35.0) | 13 (11.5) |
| Other (%) | 21(7.2) | 13 (7.3) | 8 (7.0) |
| Smoking History | |||
| * Current smoker (%) | 135(46.6) | 95 (53.7) | 40 (35.4) |
| Former smoker (%) | 65(22.4) | 44 (24.9) | 21 (18.6) |
| * Never smoker (%) | 90(31.0) | 38 (21.5) | 52 (46.0) |
| Overall pack year history ±SD† | 19.9 ±22.2 | 21.0 ±22.7 | 17.3 ±21.1 |
| Number of cigarettes/ day ±SD† | 17.8 ±12.0 | 18.4 ±11.6 | 16.4 ±13.0 |
The distributions of subjects were statistically different between the classifications in the diagnostic groups (p<.05). Detailed statistic analysis methods and p values were available upon request.
Never smokers were excluded to calculate the number of cigarettes smoked per day.
SCZ: patients with schizophrenia, BP: patients with bipolar disorder.
Figure 1.
Female smokers with schizophrenia had significantly greater daily tobacco cigarette consumption than male smokers with schizophrenia (t(135)=2.621, p=.010). There were no sex differences in smokers with bipolar disorder. SCZ: patients with schizophrenia, BP: patients with bipolar disorder. *Statistical significance in this figure is p<0.05.
OPRM1 A118G genotype
A total of 290 subjects were genotyped for the OPRM1 A118G variant. Three subjects with schizophrenia and three subjects with bipolar disorder were not genotyped due to assay failure. There were 220 subjects (77.5 %) with the AA genotype, 58 subjects (20.4 %) with the GA genotype and 6 subjects (2.1 %) with the GG genotype. The genotypes were within the Hardy Weinberg distribution in the whole and both diagnostic groups. In the entire group, the G allele was more common in the Caucasian subjects (χ2=16.7, p = 0.0002, Table 2). Similar racial differences were found in the whole group and the subjects with schizophrenia (χ2=13.8, p = 0.001, Table 2) but not in the bipolar disorder group. The genotype distributions were not associated with the subject’s sex.
Table 2.
Demographics with OPRM1 A118G genotypes.
| Classifications | All | SCZ | BP | |||
|---|---|---|---|---|---|---|
| OPRM1 | AA | * G | AA | * G | AA | * G |
| N (%) | 220 (77.5) | 64(22.5) | 138(79.3) | 36(20.7) | 82(74.5) | 28(25.5) |
| Sex | ||||||
| Male | 120(42.3) | 30(10.6) | 92(52.9) | 20(11.5) | 28(25.5) | 10(9.1) |
| Female | 100(35.2) | 34(12.0) | 46(26.4) | 16(9.2) | 54(49.1) | 18(16.4) |
| Race | ||||||
| Caucasian | *137(48.2) | *53(18.7) | *72(41.4) | *29(16.7) | 65(59.1) | 24(21.8) |
| African American | *69(24.3) | *4(1.4) | *57(32.8) | *3(1.7) | 12(10.9) | 1(0.9) |
| Other | *14(4.9) | *7(2.5) | *9(5.2) | *4(2.3) | 5(4.5) | 3(2.7) |
| Smoking History | ||||||
| Current smoker | 111(39.1) | 22(7.7) | *81(46.6) | *13(7.5) | 30(27.3) | 9(8.2) |
| Former smoker | 46(16.2) | 17(6.0) | *34(19.5) | *9(5.2) | 12(10.9) | 8(7.3) |
| Never smoker | 63(22.2) | 25(8.8) | *23(13.2) | *14(8.0) | 40(36.4) | 11(10.0) |
| Number of cigarettes smoked / day ± SD† | 17.28 ± 12.1 | 19.91 ± 11.7 | **17.54 ± 11.4 | **22.89 ± 12.1 | 16.58 ± 14.1 | 16.06 ± 10.1 |
Never smokers were excluded to calculate the number of cigarettes smoked per day.
The distributions of subjects were statistically different between the genotypes within the diagnosis group (p<.05). Detailed statistic analyses methods and p value is available upon request.
The number of cigarettes smoked per day by the patients with schizophrenia was statistically different between the OPRM1 genotype (see the results section, Fig. 1).
SCZ: patients with schizophrenia, BP: patients with bipolar disorder.
DRD2 Taq1A genotype
A total of 290 subjects were genotyped for the DRD2 Taq1A variant. There were 26 subjects (9.0 %) with the AA genotype, 87 subjects (30.0 %) with the AG genotype and 177 subjects (61.0 %) with the GG genotype. In the whole group, the G allele was more common in Caucasian subjects (χ2=10.8, p=0.03, Table 3). Also there was a trend for racial differences among the subject with schizophrenia (χ2=14.1, p=0.07, Table 3), but not bipolar disorder. As Parsons et al. previously described [43], a lower prevarence of AA genotype carriers was confirmed in the subjects with schizophrenia (χ2=6.6, p=0.01, Table 3). The Taq1A genotypes were within the Hardy Weinberg distribution for the subjects with bipolar disorder.
Table 3.
Demographics with DRD2 Taq1A genotypes.
| Classifications | All | SCZ | BP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DRD2 Taq1A | AA | AG | GG | AA | AG | GG | AA | AG | GG |
| N (%) | *26(9.0) | *87(30.0) | *177(61.0) | *15(8.5) | *50(28.2) | *112(63.3) | 11(9.7) | 37(32.7) | 65(57.5) |
| Sex | |||||||||
| Male | 9(3.1) | 51(17.6) | 93(32.1) | 7(4.0) | 36(20.3) | 71(40.1) | 2(1.8) | 15(13.3) | 22(19.5) |
| Female | 17(5.9) | 36(12.4) | 84(29.0) | 8(4.5) | 14(7.9) | 41(23.2) | 9(8.0) | 22(19.5) | 43(38.1) |
| Race | |||||||||
| Caucasian | *11(3.8) | *55(19.0) | *128(44.1) | *4(2.3) | *24(13.6) | *74(41.8) | 7(6.2) | 31(27.4) | 54(47.8) |
| African American | *11(3.8) | *24(8.3) | *40(13.8) | *8(4.5) | *20(11.3) | *34(19.2) | 3(2.7) | 4(3.5) | 6(5.3) |
| Other | *4(1.4) | *8(2.8) | *9(3.1) | *3(1.7) | *6(3.4) | *4(2.3) | 1(0.9) | 2(1.8) | 5(4.4) |
| Smoking History | |||||||||
| Current smoker | 15(5.2) | 42(14.5) | 78(26.9) | 9(5.1) | 29(16.4) | 57(32.2) | 6(5.3) | 13(11.5) | 21(18.6) |
| Former smoker | 5(1.7) | 16(5.5) | 44(15.2) | 4(2.3) | 8(4.5) | 32(18.1) | 1(0.9) | 8(7.1) | 12(10.6) |
| Never smoker | 6(2.1) | 29(10.0) | 55(19.0) | 2(1.1) | 13(7.3) | 23(13.0) | 4(3.5) | 16(14.2) | 32(28.3) |
| Number of cigarettes smoked/ day ±SD† | 15.25 ± 7.5 | 16.87 ± 10.2 | 18.68 ± 13.2 | 15.20 ± 7.6 | 16.65 ± 10.6 | 19.55 ± 12.3 | 15.36 ± 7.96 | 17.25 ± 9.65 | 16.34 ± 15.4 |
Never smokers were excluded to calculate the number of cigarettes smoked per day.
The distributions of subjects were statistically different between the genotypes within the diagnosis group (p<.05). Detailed statistic analyses methods and p value is available upon request.
SCZ: patients with schizophrenia, BP: patients with bipolar disorder.
Correlations between genotypes and tobacco smoking
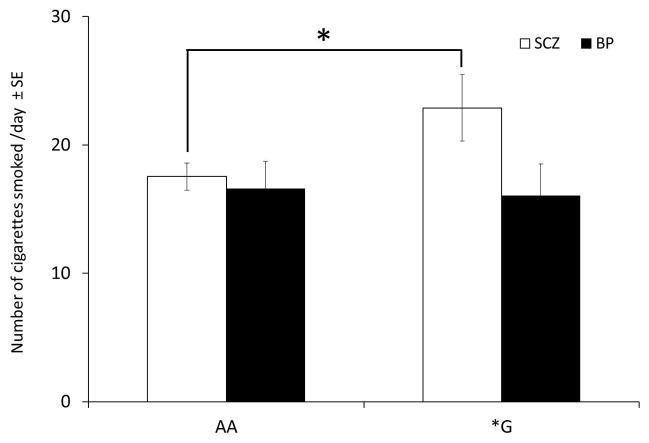
When examining the group as a whole, no differences in number of cigarettes smoked per day were found with either genotype. However when separated by diagnosis, the schizophrenia subjects, including current and former smokers, who had the OPRM1 *G allele, smoked more cigarettes per day compared to the AA genotype group (22.9 ± 12.1 cpd compared to AA who smoked 17.5 ± 11.4 cpd (t(135)=2.000, p=.047; Fig. 2). The subjects diagnosed with the bipolar disorder carrying the *G allele vs the AA genotype smoked a similar number of cigarettes per day (16.1 ± 10.1 and 16.6 ± 14.1, respectively).
Figure 2.
OPRM1 *G genotype smokers with schizophrenia consumed more cigarettes per day than AA genotype smokers with schizophrenia (t(135)=2.000, p=.047). There were no genotype differences in smokers with bipolar disorder. SCZ: patients with schizophrenia, BP: patients with bipolar disorder. *Statistical significance in this figure is p<0.05.
Regarding the DRD2 Taq1A genotype, the mean ± SD number of cigarettes smoked per day by the patients with schizophrenia with AA, AG and GG genotypes was 15.19 ± 7.6, 16.65 ± 10.6 and 19.55 ± 12.3, respectively, which was not statistically different. Following the approach by Munafo et al. [41], the genotypes were grouped as AA+AG (*A allele carriers) and GG alleles. The data indicate that females with schizophrenia and the GG genotype smoked more cigarettes than male smokers with schizophrenia and the *A genotype (F(3, 135)=3.114, p=.028); Fig. 3). The GG female schizophrenia patients, including current and former smokers, smoked 24.1 ± 14.4 cpd compared to the male A allele carriers who smoked 15.8 ± 9.5 cpd. With the bipolar patients, there was no difference in the number of cigarettes smoked per day based upon the DRD2 Taq1A genotype.
Figure 3.
DRD2 Taq1A GG female smokekrs with schizophrenia consumed more cigarettes than *A male with schizophrenia (F=(3, 135)=3.114, p=.028). There were no differences found in smokers with bipolar disorder. SCZ: patients with schizophrenia, BP: patients with bipolar disorder. *Statistical significance in this figure is p<0.05.
The relationship between current smoking status (current, previous, never) and genotype were examined. Subjects with schizophrenia and the OPRM1 G allele seemed more likely to be never or former smokers, compared to AA subjects with schizophrenia: 13% of those with the G allele were current smokers compared to 21% of former smokers and 38% of never smokers (χ2=9.32, p=0.009; Table 2). No DRD2 Taq1A genotype effect on smoking status was found among those with schizophrenia and bipolar disorder. OPRM1 and DRD2 Taq1A joint genotype effect on tobacco smoking was found. The joint genotypes were classified into 4 groups; 1) OPRM1 AA + DRD2 Taq1A *A, 2) OPRM1 AA + DRD2 Taq1A GG, 3) OPRM1 *G + DRD2 Taq1A *A and 4) OPRM1 *G + DRD2 Taq1A GG. The numbers of cigarettes smoked per day were compared using one-way ANOVA. The results showed that the OPRM1 *G + DRD2 Taq1A GG group had the highest consumption (26.27±12.74 cpd) and the OPRM1 AA + DRD2 Taq1A *A group had the lowest consumption (15.98±10.02 cpd) in smokers with schizophrenia but not those with bipolar disorder (F(3, 135)=2.671, p=.050).
Racial differences for tobacco smoking and genotype distributions
Caucasian was majority ethnic group (n=194, 66.9 %) followed by African Americans (n=75, 25.9 %), and others including Asian and Hispanic (n=21, 7.2 %). Within each ethnic group, no genetic and sexual effects on tobacco smoking were found. However the amount of daily tobacco cigarette consumption by current and former smokers was significantly different between ethnic groups. Caucasians smoked more than the African American current and former smokers in the whole group (F(2, 193)=6.646, p=.002) as well as the subjects with schizophrenia (F(2, 134)=6.000, p=.003). The number of cigarettes smoked per day was 20.1 ± 13.4 and 13.6 ± 7.3 for the Caucasians and African Americans respectively in the whole group. A difference in daily tobacco consumption was not found among the bipolar patients. The genotype distributions of the two genes in each ethnicity groups were consistent with the previous studies [44, 45].
Linear Regression Analyses
Variables such as diagnosis, sex, OPRM1 genotype, DRD2 Taq1A genotype and race were used as independent factors to determine the number of cigarettes smoked per day. The threshold for variable entry was p< .05 and that of retention was p < .10 in the model. The most fitted model was described by predictors such as sex, age, race, OPRM1 and DRD2 Taq1A genotypes for the subjects with schizophrenia (adjusted R2=.170, p=.000). For the entire group, the daily tobacco consumption was well predicted by diagnosis, sex, age, race, OPRM1 and DRD2 Taq1A genotypes. When the whole group included all smoking status, adjusted R2=0.86, p=.000. When only current and former smokers were included, adjusted R2=.155, p=.000. Surprisingly the demographic and genetic factors did not have significant effects for the number of cigarettes per day in the bipolar group. Medication effects were not considered as a factor of tobacco smoking because there was no significant difference between the daily tobacco consumption and the smoking status among each medication groups. However, the groups studied may not be large enough to detect any effects.
Discussion
This study has two limitations: 1) lack of medication control groups and 2) self-reporting smoking data collection instead of biological smoking markers. Nevertheless OPRM1 and DRD2 Taq1A genotypes, sex and race were significant predictors for tobacco smoking in the present study. Female smokers with schizophrenia consumed more cigarettes than the males per day despite a smaller number of current and former smokers. Smokers with schizophrenia and the OPRM1 G allele smoke more cigarettes than the AA genotype patients. Furthermore females with schizophrenia and DRD2 Taq1A GG genotype consumed more cigarettes per day than males with schizophrenia and the *A genotype. Surprisingly in the bipolar patients there were no differences between genotype and sex. The tobacco reward system in patients with bipolar disorder appears to differ. Although many social and environmental factors have been associated with why people start smoking, the results indicate that genetic differences related to the opioid and dopamine neurotransmitter systems are implicated in tobacco smoking among subjects with schizophrenia.
Sex differences and tobacco smoking status
Sex differences in smoking behavior among the general population have been reported by many researchers [46–48]. Males have been reported to smoke more than females [49]. Female smokers have more difficulties to quit smoking [50] and seem to be less sensitive to nicotine cues than males and are more influenced with non-nicotine factors [51]. Surprisingly, in the present study the females with schizophrenia smoked more than the male patients. Interestingly Benowitz and Hatsukami [50] reported that within the general population females smoked more than males when they were under stress. It is possible that the diagnosis of schizophrenia is related to higher stress levels in females. Unfortunately, we did not obtain standardized rating scales, such as the Brief Psychiatry Rating Scale (BPRS) to estimate current symptom severity which may contribute to potential stress. Also in the present study, the number of males and females were significantly different in the two diagnostic groups. Especially in the group of subjects with bipolar disorder, females were majority. Many studies have been reported the influence of menstrual cycle phases on female’s smoking behavior [52–56]. Unfortunately there were no menstrual phase controls in this study. Thus methodological bias may contribute to the negative results in the bipolar disorder group.
Ethnic differences for the genotypes and smoking behavior
It is well known that there are significant ethnic genetic distributions. For the OPRM1 genotype, there are almost 50 % of *G genotype in the Japanese while in the African American population, the genotype is less than 5 % [44]. The DRD2 Taq1A genotype is also differently distributed among various ethnicities [45]. In the present study, the OPRM1 and DRD2 Taq1A genotype distributions in the ethnic population were consistent with previous studies. The number of daily cigarettes, pack year and smoking status were compared with genotypes within the ethnic groups, but significant differences were not found. However the number of cigarettes smoked per day of the African American group was significantly less than that of the Caucasian group. This difference was found in only the current smoker group, and the group combined current and former smokers in whole group and group with schizophrenia. After regrouping by race, the sample data is too small to examine the smoking behavior in the each ethnic group.
OPRM1 A118G genotype differences in tobacco smoking
Although the function of the OPRM1 A118G variant is still very controversial, it is well accepted that the mu (μ) opioid receptor on the GABAergic interneuron is associated with the dopaminergic reward system [57, 58]. Alcohol and nicotine release endogenous opioids [58–61] which activate mu opioid receptors on GABAergic interneurons [62]. Opioid receptor activation inhibits GABAergic interneurons (inhibition of inhibition) to increase DA release in the ventral tegmental area (VTA) [63]. In vitro, this variant demonstrates increased binding affinity with β-endorphin [64, 59] and greater inhibition of cAMP accumulation in HEK293 and AV-12 cells [65]. On the other hand, Ramchandani et al. [66] reported humans and humanized mice with the GG genotype had greater DA response to alcohol than the AA genotype. Furthermore mentally normal smokers with OPRM1 *G have more striatal DA release in response to average nicotine tobacco cigarette smoking compared with those homozygous for the A allele [67]. Based on the latter very limited data, the *G variant is associated with greater DA release. Hence the presence of this allele may result in greater daily cigarettes consumption. Certainly other possible explanations for the greater cigarettes consumption among *G patients may also exist. According to the study of Ray et al., female smokers with the OPRM1*G were less likely to distinguish the difference between denicotinized and average nicotine cigarettes [39]. These authors concluded that the G allele is associated with a fewer number of mu opioid receptors, resulting in being less sensitive to nicotine which may contribute to the greater cigarettes consumption because the *G allele subjects need more nicotine to satisfy their brain needs.
In addition, the OPRM1 118G carriers with schizophrenia did not seem to start smoking and they were likely to have stopped smoking when compared to the AA genotype subjects with schizophrenia. Although at first these results may seem contradictory, these data are consistent with previous animal work showing that mu opioid receptor knockout mice are less likely to exhibit nicotine preference [68]. Recently Verhage et al. published a meta-analysis about smoking initiation, nicotine dependence and smoking cessation. They concluded that subjects with the OPRM1 AA genotype had higher risk for nicotine dependence [69]. Furthermore Lerman et al. [37] and Ray et al. [39] have previously reported that the G allele carriers were more likely to quit smoking successfully using nicotine replacement therapy. Thus, the present data indicate additional positive evidence showing that the OPRM1 genotype affects smoking behavior and nicotine dependence.
Surprisingly, contrary to our hypothesis, there were no OPRM1 genotype smoking differences in the bipolar patients due to possible changes in dopamine neurotransmission which may be dependent on the subject’s current mood state [70]. All of the bipolar patients included in this study were currently euthymic. The relationship between bipolar disorder moods, cigarette smoking and the dopaminergic reward system deserves further study.
DRD2 Taq1A genotype difference in tobacco smoking
DRD2 Taq1A is one of the genes associated with alcohol, drug abuse and nicotine dependence [71–73]. Many studies have examined the relationship between the TaqA1 allele and tobacco smoking [41, 42, 74–77], but its relationship still is controversial. Our hypothesis was that the Taq1A AA genotype would be associated with greater smoking reinforcement due to the less inhibition of DA release. However the present data indicated a trend that the GG genotype is associated with greater daily tobacco consumption in patients with schizophrenia. In addition, female smokers with schizophrenia with GG genotype smoked more cigarettes than male smokers with the A allele (Fig. 3). The mean ± SD number of cigarettes smoked by male *A allele carriers was 15.8 ± 9.5 cpd and the GG female patients smoked 24.1 ± 14.4 cpd. These results agree with previous studies that female *A carriers were less likely to quit smoking with nicotine replacement therapy [41] and that GG females have higher risk for smoking [78]. Although Pearlson et al. [79] demonstrated that the DRD2 Bmax value is elevated in those with bipolar disorder and schizophrenia compared to controls, the mechanism of increased smoking reinforcement with GG allele is obscure. In contrast, Comings [75], Comings and MacMurray [74] and Lee et al. [42] found that males with the AG allele had a higher smoking prevalence among Korean patients with schizophrenia. Also Pohjalainen et al. [29] reported significantly decreased availability of DRD2 in the AG genotype. However in the present study, the AG genotype patients smoked a similar number of cigarettes per day among those with schizophrenia and bipolar disorder. Also no significant genotype and sex differences were found among those with bipolar disorder.
OPRM1 and DRD2 genes are often discussed together for nicotine dependence and tobacco cessation [69, 80–83]. However most studies have not evaluated combined gene effects. In the present study, the OPRM1 and DRD2 Taq1A genotypes additively affected smoking habits. Integrated gene effects have been found in methadone maintenance therapy [84], and alcohol use and parental rule setting [85] to date. However Hardman et al. reported a lack of association between these genes in obesity [86]. The present study is preliminary, however it contributes positive evidence of combined gene effects for tobacco smoking.
Acknowledgments
The present study was supported in part by the Department of Pharmacology Psychopharmacology Research Fund C361024, Education and Research Development Fund 276157 for EFD and NIMH (R01 MH082784), NIH-NCCR, GCRC Program (UL1RR024986), the Chemistry Core of the Michigan Diabetes Research and Training Center (NIH5P60 DK 20572), (all Bethesda, Maryland) and the Washtenaw Community Health Organization (WCHO, Ann Arbor, Michigan), The Brain and Behavior Research Foundation (formerly NARSAD, Great Neck, New York), and the Prechter Longitudinal Study of Bipolar Disorder (Ann Arbor, Michigan) for VLE.
References
- 1.Dome P, et al. Smoking, nicotine and neuropsychiatric disorders. Neuroscience and Biobehavioral Reviews. 2010;34(3):295–342. doi: 10.1016/j.neubiorev.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285. doi: 10.1186/1471-2458-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalman D, Morissette SB, George TP. Co-Morbidity of Smoking in Patients with Psychiatric and Substance Use Disorders. The American Journal on Addictions. 2005;14(2):106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonard S, et al. Smoking and mental illness. Pharmacology Biochemistry and Behavior. 2001;70(4):561–570. doi: 10.1016/s0091-3057(01)00677-3. [DOI] [PubMed] [Google Scholar]
- 5.Lasser KBJWSHDUMDBDH. Smoking and mental illness: A population-based prevalence study. JAMA: The Journal of the American Medical Association. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 6.Hughes JR, et al. Prevalence of smoking among psychiatric outpatients. American Journal of Psychiatry. 1986;143(8):993–7. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- 7.Brunzell DH, McIntosh JM. Alpha7 Nicotinic Acetylcholine Receptors Modulate Motivation to Self-Administer Nicotine: Implications for Smoking and Schizophrenia. Neuropsychopharmacology. 2012;37(5):1134–1143. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee SA, et al. Effects of the nicotinic receptor antagonist mecamylamine on ad-lib smoking behavior, topography, and nicotine levels in smokers with and without schizophrenia: a preliminary study. Schizophrenia Research. 2009;115(2–3):317–24. doi: 10.1016/j.schres.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams JM, et al. Higher Nicotine and Carbon Monoxide Levels in Menthol Cigarette Smokers With and Without Schizophrenia. Nicotine and Tobacco Research. 2007;9(8):873–881. doi: 10.1080/14622200701484995. [DOI] [PubMed] [Google Scholar]
- 10.Williams JM, et al. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophrenia Research. 2005;79(2–3):323–335. doi: 10.1016/j.schres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Tidey JW, et al. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug and Alcohol Dependence. 2005;80(2):259–65. doi: 10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 12.de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia Research. 2005;76 (2–3):135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 13.de Leon J, et al. Schizophrenia and tobacco smoking: a replication study in another US psychiatric hospital. Schizophrenia Research. 2002;56(1–2):55–65. doi: 10.1016/s0920-9964(01)00192-x. [DOI] [PubMed] [Google Scholar]
- 14.ÜÇOk ALP, et al. Cigarette smoking among patients with schizophrenia and bipolar disorders. Psychiatry and Clinical Neurosciences. 2004;58(4):434–437. doi: 10.1111/j.1440-1819.2004.01279.x. [DOI] [PubMed] [Google Scholar]
- 15.Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biological Psychiatry. 1997;42 (1):1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- 16.Diaz FJ, et al. Tobacco smoking behaviors in bipolar disorder: a comparison of the general population, schizophrenia, and major depression. Bipolar Disorders. 2009;11(2):154–165. doi: 10.1111/j.1399-5618.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 17.Breslau N, et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry. 1998;55(7):626–32. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 18.Glassman AH. Cigarette smoking: implications for psychiatric illness. American Journal of Psychiatry. 1993;150(4):546–53. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- 19.Glassman AhHJECLS, et al. Smoking, smoking cessation, and major depression. JAMA: The Journal of the American Medical Association. 1990;264(12):1546–1549. [PubMed] [Google Scholar]
- 20.Gonzalez-Pinto A, et al. Tobacco smoking and bipolar disorder. Journal of Clinical Psychiatry. 1998;59(5):225–8. doi: 10.4088/jcp.v59n0503. [DOI] [PubMed] [Google Scholar]
- 21.Ostacher MJ, et al. Cigarette smoking is associated with suicidality in bipolar disorder. Bipolar Disorders. 2009;11(7):766–771. doi: 10.1111/j.1399-5618.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein BI, et al. Substance use disorders among adolescents with bipolar spectrum disorders. Bipolar Disorders. 2008;10(4):469–478. doi: 10.1111/j.1399-5618.2008.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC. CDC - Chronic Disease - Tobacco - At A Glance 2011. 2012 Nov 16; [cited 2013 11th July]; Available from: http://www.cdc.gov/chronicdisease/resources/publications/aag/osh.htm.
- 24.Hall SM, Prochaska JJ. Treatment of smokers with co-occurring disorders: emphasis on integration in mental health and addiction treatment settings. Annu Rev Clin Psychol. 2009;5:409–31. doi: 10.1146/annurev.clinpsy.032408.153614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prochaska JJ. Smoking and mental illness--breaking the link. New England Journal of Medicine. 2011;365(3):196–8. doi: 10.1056/NEJMp1105248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins KA, et al. Dopamine and opioid gene variants are associated with increased smoking reward and reinforcement owing to negative mood. Behavioural Pharmacology. 2008;19(5–6):641–9. doi: 10.1097/FBP.0b013e32830c367c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, et al. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. Journal of Biological Chemistry. 2005;280(38):32618–24. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 28.Ray R, et al. Human Mu Opioid Receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proceedings of the National Academy of Sciences. 2011;108(22):9268–9273. doi: 10.1073/pnas.1018699108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pohjalainen T, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Molecular Psychiatry. 1998;3(3):256–60. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 30.Tang L, Todd RD, O’Malley KL. Dopamine D2 and D3 receptors inhibit dopamine release. Journal of Pharmacology and Experimental Therapeutics. 1994;270(2):475–9. [PubMed] [Google Scholar]
- 31.Pothos EN, et al. D2-Like dopamine autoreceptor activation reduces quantal size in PC12 cells. Journal of Neuroscience. 1998;18(15):5575–85. doi: 10.1523/JNEUROSCI.18-15-05575.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsutsumi A, et al. The genetic validation of heterogeneity in schizophrenia. Behavioral and Brain Functions. 2011;7:43. doi: 10.1186/1744-9081-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li T, et al. Association analysis between dopamine receptor genes and bipolar affective disorder. Psychiatry Research. 1999;86(3):193–201. doi: 10.1016/s0165-1781(99)00034-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhang JP, Lencz T, Malhotra AK. D2 receptor genetic variation and clinical response to antipsychotic drug treatment: a meta-analysis. American Journal of Psychiatry. 2010;167(7):763–72. doi: 10.1176/appi.ajp.2009.09040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellingrod VL, et al. Risk factors associated with metabolic syndrome in bipolar and schizophrenia subjects treated with antipsychotics: the role of folate pharmacogenetics. Journal of Clinical Psychopharmacology. 2012;32(2):261–5. doi: 10.1097/JCP.0b013e3182485888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spitzer RL, et al. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of General Psychiatry. 1992;49(8):624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 37.Lerman C, et al. The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics Journal. 2004;4(3):184–192. doi: 10.1038/sj.tpj.6500238. [DOI] [PubMed] [Google Scholar]
- 38.Munafo MR, et al. Association of the mu-opioid receptor gene with smoking cessation. Pharmacogenomics Journal. 2007;7(5):353–361. doi: 10.1038/sj.tpj.6500432. [DOI] [PubMed] [Google Scholar]
- 39.Ray R, et al. Association of OPRM1 A118G variant with the relative reinforcing value of nicotine. Psychopharmacology. 2006;188(3):355–363. doi: 10.1007/s00213-006-0504-2. [DOI] [PubMed] [Google Scholar]
- 40.Huang P, et al. A common single nucleotide polymorphism A118G of the mu opioid receptor alters its N-glycosylation and protein stability. Biochemical Journal. 2012;441(1):379–86. doi: 10.1042/BJ20111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munafo MR, et al. Lack of association of DRD2 rs1800497 (Taq1A) polymorphism with smoking cessation in a nicotine replacement therapy randomized trial. Nicotine and Tobacco Research. 2009;11(4):404–7. doi: 10.1093/ntr/ntp007. [DOI] [PubMed] [Google Scholar]
- 42.Lee H-S, et al. Gender-specific molecular heterosis of dopamine D2 receptor gene (DRD2) for smoking in schizophrenia. American Journal of Medical Genetics. 2002;114(6):593–597. doi: 10.1002/ajmg.10641. [DOI] [PubMed] [Google Scholar]
- 43.Parsons MJ, et al. A dopamine D2 receptor gene-related polymorphism is associated with schizophrenia in a Spanish population isolate. Psychiatric Genetics. 2007;17(3):159–63. doi: 10.1097/YPG.0b013e328017f8a4. [DOI] [PubMed] [Google Scholar]
- 44.Kreek MJ, et al. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacological Reviews. 2005;57(1):1–26. doi: 10.1124/pr.57.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Geer LY, et al. The NCBI BioSystems database. Nucleic Acids Res. 2010;38(Database issue):D492–6. doi: 10.1093/nar/gkp858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dluzen DE, Anderson LI. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neuroscience Letters. 1997;230(2):140–142. doi: 10.1016/s0304-3940(97)00487-4. [DOI] [PubMed] [Google Scholar]
- 47.Zeman MV, Hiraki L, Sellers EM. Gender differences in tobacco smoking: higher relative exposure to smoke than nicotine in women. Journal of Women’s Health and Gender-Based Medicine. 2002;11(2):147–53. doi: 10.1089/152460902753645281. [DOI] [PubMed] [Google Scholar]
- 48.Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine and Tobacco Research. 2008;10(7):1245–50. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- 49.Lopez AD, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tobacco Control. 1994;3(3):242–7. [Google Scholar]
- 50.Benowitz NL, Hatsukami D. Gender differences in the pharmacology of nicotine addiction. Addiction Biology. 1998;3(4):383–404. doi: 10.1080/13556219871930. [DOI] [PubMed] [Google Scholar]
- 51.Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine and Tobacco Research. 1999;1(4):301–15. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- 52.Allen SS, et al. Smoking- and menstrual-related symptomatology during short-term smoking abstinence by menstrual phase and depressive symptoms. Addictive Behaviors. 2014;39 (5):901–906. doi: 10.1016/j.addbeh.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carpenter MJ, et al. Menstrual Cycle Phase Effects on Nicotine Withdrawal and Cigarette Craving: A Review. Nicotine & Tobacco Research. 2006;8(5):627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- 54.Allen SS, et al. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine & Tobacco Research. 2000;2(3):231–241. doi: 10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- 55.Perkins KA, et al. Tobacco withdrawal in women and menstrual cycle phase. Journal of Consulting and Clinical Psychology. 2000;68(1):176. doi: 10.1037/0022-006X.68.1.176. [DOI] [PubMed] [Google Scholar]
- 56.Pomerleau CS, et al. The effects of menstrual phase and nicotine abstinence on nicotine intake and on biochemical and subjective measures in women smokers: a A preliminary report. Psychoneuroendocrinology. 1992;17(6):627–638. doi: 10.1016/0306-4530(92)90021-x. [DOI] [PubMed] [Google Scholar]
- 57.Wise RA, Rompre PP. Brain dopamine and reward. Annual Review of Psychology. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 58.Walters CL, et al. μ-Opioid Receptor and CREB Activation Are Required for Nicotine Reward. Neuron. 2005;46(6):933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Davenport KE, Houdi AA, Van Loon GR. Nicotine protects against μ-opioid receptor antagonism by β-funaltrexamine: Evidence for nicotine-induced release of endogenous opioids in brain. Neuroscience Letters. 1990;113(1):40–46. doi: 10.1016/0304-3940(90)90491-q. [DOI] [PubMed] [Google Scholar]
- 60.Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129(2):99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 61.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends in Pharmacological Sciences. 1992;13(0):177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 62.Mansour A, et al. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends in Neurosciences. 1995;18(1):22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 63.Nestler EJ. Is there a common molecular pathway for addiction? Nature Neuroscience. 2005;8(11):1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 64.Bond C, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: Possible implications for opiate addiction. Proceedings of the National Academy of Sciences. 1998;95(16):9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kroslak T, et al. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. Journal of Neurochemistry. 2007;103(1):77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- 66.Ramchandani VA, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Molecular Psychiatry. 2011;16(8):809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Domino EF, et al. Tobacco smoking produces greater striatal dopamine release in G-allele carriers with mu opioid receptor A118G polymorphism. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;38(2):236–240. doi: 10.1016/j.pnpbp.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. Journal of Neuroscience. 2002;22(24):10935–40. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verhagen M, Kleinjan M, Engels RC. A systematic review of the A118G (Asn40Asp) variant of OPRM1 in relation to smoking initiation, nicotine dependence and smoking cessation. Pharmacogenomics. 2012;13(8):917–33. doi: 10.2217/pgs.12.76. [DOI] [PubMed] [Google Scholar]
- 70.Abler B, et al. Abnormal Reward System Activation in Mania. Neuropsychopharmacology. 2007;33(9):2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Ruyck K, et al. Genetic variation in three candidate genes and nicotine dependence, withdrawal and smoking cessation in hospitalized patients. Pharmacogenomics. 2010;11(8):1053–63. doi: 10.2217/pgs.10.75. [DOI] [PubMed] [Google Scholar]
- 72.Freire MTMV, et al. Polymorphisms in the DBH and DRD2 gene regions and smoking behavior. European Archives of Psychiatry and Clinical Neuroscience. 2006;256(2):93–97. doi: 10.1007/s00406-005-0610-x. [DOI] [PubMed] [Google Scholar]
- 73.Mroziewicz M, Tyndale RF. Pharmacogenetics: a tool for identifying genetic factors in drug dependence and response to treatment. Addict Sci Clin Pract. 2010;5(2):17–29. [PMC free article] [PubMed] [Google Scholar]
- 74.Comings DE, MacMurray JP. Molecular heterosis: a review. Molecular Genetics and Metabolism. 2000;71(1–2):19–31. doi: 10.1006/mgme.2000.3015. [DOI] [PubMed] [Google Scholar]
- 75.Comings DE. Molecular heterosis as the explanation for the controversy about the effect of the DRD2 gene on dopamine D2 receptor density. Molecular Psychiatry. 1999;4(3):213–5. doi: 10.1038/sj.mp.4000500. [DOI] [PubMed] [Google Scholar]
- 76.Comings DE, et al. The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics. 1996;6(1):73–9. doi: 10.1097/00008571-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 77.Verde Z, et al. ‘Smoking genes’: a genetic association study. PLoS ONE. 2011;6(10):e26668. doi: 10.1371/journal.pone.0026668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamajima N, et al. Association between smoking habits and dopamine receptor D2 taqI A A2 allele in Japanese males: a confirmatory study. Journal of Epidemiology. 2002;12(4):297–304. doi: 10.2188/jea.12.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pearlson GD, et al. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Archives of General Psychiatry. 1995;52(6):471–7. doi: 10.1001/archpsyc.1995.03950180057008. [DOI] [PubMed] [Google Scholar]
- 80.Chatkin JM. The influence of genetics on nicotine dependence and the role of pharmacogenetics in treating the smoking habit. J Bras Pneumol. 2006;32(6):573–9. doi: 10.1590/s1806-37132006000600016. [DOI] [PubMed] [Google Scholar]
- 81.Lerman CE, Schnoll RA, Munafo MR. Genetics and smoking cessation improving outcomes in smokers at risk. Am J Prev Med. 2007;33(6 Suppl):S398–405. doi: 10.1016/j.amepre.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Munafo MR, et al. Pharmacogenetics and nicotine addiction treatment. Pharmacogenomics. 2005;6(3):211–23. doi: 10.1517/14622416.6.3.211. [DOI] [PubMed] [Google Scholar]
- 83.NIH. NIH State-of-the-Science Conference on Tobacco Use: Prevention, Cessation, and Control. NIH State-of-the-Science Conference; Bethesda, Maryland: NIH; 2006. [PubMed] [Google Scholar]
- 84.Hung C-C, et al. Impact of genetic polymorphisms in ABCB1, CYP2B6, OPRM1, ANKK1 and DRD2 genes on methadone therapy in Han Chinese patients. Pharmacogenomics. 2011;12 (11):1525–1533. doi: 10.2217/pgs.11.96. [DOI] [PubMed] [Google Scholar]
- 85.Pieters S, et al. The Moderating Effect of Alcohol-Specific Parental Rule-Setting on the Relation between the Dopamine D2 Receptor Gene (DRD2), the Mu-Opioid Receptor Gene (OPRM1) and Alcohol Use in Young Adolescents. Alcohol and Alcoholism. 2012;47(6):663–670. doi: 10.1093/alcalc/ags075. [DOI] [PubMed] [Google Scholar]
- 86.Hardman CA, et al. Lack of association between DRD2 and OPRM1 genotypes and adiposity. Int J Obes. 2014;38(5):730–736. doi: 10.1038/ijo.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]