Abstract
Lymphatic malformations (LM) are characterized by abnormal formation of lymphatic vessels and tissue overgrowth. The lymphatic vessels present in LM lesions may become blocked and enlarged as lymphatic fluid collects, forming a mass or cyst. Lesions are typically diagnosed during childhood, and are often disfiguring and life threatening. Available treatments consist of sclerotherapy, surgical removal and therapies to diminish complications.
We isolated lymphatic endothelial cells (LM-LEC) from a surgically removed microcystic LM lesion. LM-LEC and normal human dermal-LEC (HD-LEC) expressed endothelial (CD31, VE-Cadherin) as well as lymphatic endothelial (Podoplanin, PROX1, LYVE1)-specific markers. Targeted gene sequencing analysis in patient-derived LM-LEC revealed the presence of two mutations in class I phosphoinositide 3-kinases (PI3K) genes. One is an inherited, premature stop codon in the PI3K regulatory subunit PIK3R3. The second is a somatic missense mutation in the PI3K catalytic subunit PIK3CA; this mutation has been found in association with overgrowth syndromes and cancer growth.
LM-LEC exhibited angiogenic properties: both cellular proliferation and sprouting in collagen were significantly increased compared to HD-LEC. AKT-Thr308 was constitutively hyper-phosphorylated in LM-LEC. Treatment of LM-LEC with PI3-Kinase inhibitors Wortmannin and LY294 decreased cellular proliferation and prevented the phosphorylation of AKT-Thr308 in both HD-LEC and LM-LEC. Treatment with the mTOR inhibitor rapamycin also diminished cellular proliferation, sprouting and AKT phosphorylation, but only in LM-LEC. Our results implicate disrupted PI3K-AKT signaling in LEC isolated from a human lymphatic malformation lesion.
Keywords: vascular anomaly, lymphatic vessels, PI3K, rapamycin, AKT
INTRODUCTION
The lymphatic system plays an essential role in fluid homeostasis, fat absorption and immune surveillance. During development lymphatic vessels originate from a subset of Prox1+ endothelial cells located on the dorsal side of the cardinal vein, around mouse embryonic day E9.5 (1–3). The Prox1+ endothelial cells form primary lymph sacs, and from these structures lymphatic vessels subsequently sprout in a process known as lymphangiogenesis.
Lymphatic malformations (LMs), also called lymphangioma or cystic hygroma, are composed of malformed, low-flow lymphatic channels (4–7). LMs are regarded as a developmental defect because of their early onset; they are evident at birth or become evident in early childhood (8). LMs tend to expand during adolescence and the lesions can affect vital organs, destroy bones, contribute to infections and cause disfigurement. The most common treatments are sclerotherapy for macrocystic (deep) LMs and surgical resection for microcystic (superficial) LMs. Lesions often recur after treatment (9–11).
LMs occur sporadically suggesting somatic mutations may be involved, but to date no causative mutation has been reported (12). Class I phosphoinositide 3-kinases (PI3Ks) are critical regulators of cell proliferation that act upon stimulation of upstream receptors by a growth factor or hormone. Class I PI3Ks are heterodimeric molecules composed of a catalytic subunit (p110α, β, γ and δ) combined with a regulatory subunit (p85α, p55α, p50α, p85β and p55γ) (13). Upon stimulation PI3Ks convert phosphatidylinositol-4,5-biphosphate (PIP2) to phosphatidylinositol-3,4,5-triphosphate (PIP3) (14) leading to activation of the PH-domain containing serine-threonine kinase known as AKT. AKT phosphorylation is induced by PIP3-dependent kinase 1 (PDK1) and is responsible for a variety of cellular activities such as cell proliferation, survival, and cell cycle entry (15). PIK3CA, encoding the PI3K catalytic subunit p110α, is one of the most frequently mutated genes in human cancer (16, 17). Dominant activating mutations of PIK3CA have been identified in glioblastoma, breast, lung, and colon cancer (16, 18). The most frequent PIK3CA mutations reported are H1047R, E542K and E545K, and all of them stimulate kinase activity and exert oncogenic activity (19). A somatic activating PIK3CA mutation, H1047L, was also identified in congenital lipomatous overgrowth, vascular malformations, epidermal nevis, spinal/skeletal anomalies/scoliosis (CLOVES) syndrome, a rare congenital disorder characterized by tissue overgrowth in extremities, vascular malformations and skin abnormalities (20). PIK3CA mutations were also detected in infiltrating lipomatosis (21) and in megalencephaly-capillary malformation (MCAP) syndrome (22).
Mutations in the PI3K regulatory subunit genes are also found in tumor samples. PIK3R1 (p85α) mutations were detected in glioblastoma, colorectal, breast and pancreatic tumor samples. Mutations in PIK3R2 (p85β) and PIK3R3 (p55γ) are rare (23). PIK3R1 and PIK3R2 have also been implicated in lymphatic development in mice and dysregulated overgrowth in humans, respectively (22, 24). PIK3R3 function is not well understood, although it is thought to contribute to the growth of highly aggressive glioblastomas by mediating IGF2 receptor signaling to PI3K (25).
Here we show the angiogenic phenotype of lymphatic endothelial cells isolated from a patient-derived microcystic lymphatic malformation lesion (LM-LEC). We identified 2 mutations in these LM-LECs - a somatic mutation in the PI3K catalytic subunit PIK3CA and a germline mutation in the regulatory subunit PIK3R3. LM-LECs exhibited increased cell proliferation and AKT activation compared to human dermal lymphatic endothelial cells (HD-LEC). The PI3K inhibitors LY294 and Wortmannin inhibited cell proliferation and AKT activation in both HD- and LM-LEC, and prevented sprouting from LM-LEC derived spheroids. Of note, the mTOR inhibitor rapamycin decreased LM-LEC proliferation, sprouting, and activation of AKT, while no effect was noted on HD-LEC.
RESULTS
Isolation and characterization of lymphatic malformation endothelial cells (LM-LEC)
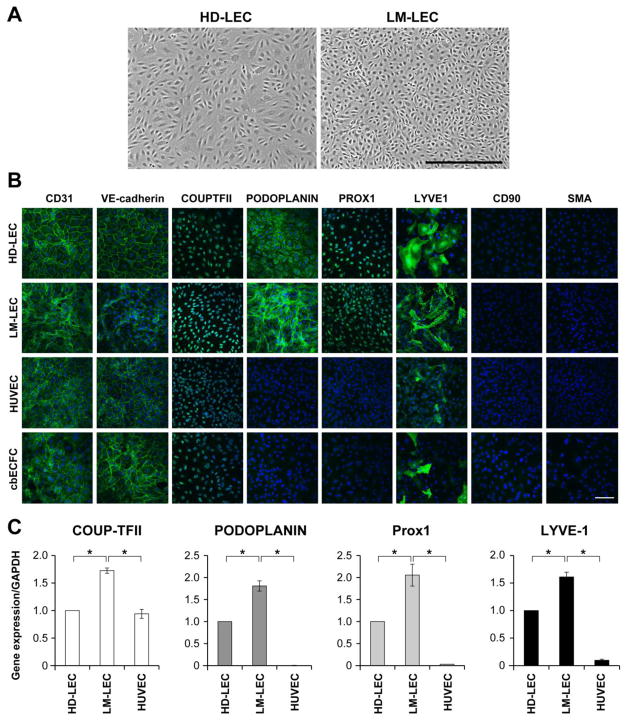
LM-LECs were isolated from surgically resected microcystic LM tissue by sequential anti-CD31 immuno-magnetic beads and anti-Podoplanin antibody selection. CD31+/Podoplanin+ LM-LECs displayed cobblestone morphology typical of endothelial cells although the size of the LM-LECs appeared smaller than the control human dermal lymphatic endothelial cells (HD-LEC) (Fig.1A). Blood and lymphatic endothelial markers were assessed in LM-LECs, in comparison to HD-LECs, human umbilical vein endothelial cells (HUVEC) and cord blood derived endothelial colony forming cells (cbECFC) (Fig.1B). LM-LEC monolayers stained for blood and lymphatic endothelial markers CD31, VE-Cadherin and COUPTFII, and for lymphatic endothelial markers Podoplanin, PROX1 and LYVE1, and were negative for the fibroblast and smooth muscle markers CD90 and α-smooth muscle actin (α-SMA), respectively. Expression of COUP-TFII, Podoplanin, PROX1 and LYVE1 mRNA was confirmed by real-time qPCR in both LM-LEC and HD-LEC, with HUVECs shown for comparison (Fig.1C).
Figure 1. Characterization of LM-LEC.
A. Phase image of HD-LEC and LM-LEC, in vitro. Scale bar 500μm. B. Immunofluorescence staining of HD-LEC, LM-LEC, HUVEC and cbECFC for CD31, VE-Cadherin, COUPTFII, Podoplanin, Prox1, LYVE1, CD90 and αSMA. Scale bar 100μm. C. mRNA expression levels, normalized to GAPDH, of COUPTFII, Podoplanin, Prox1, and LYVE1 in HD-LEC, LM-LEC and HUVEC, analyzed by real-time qPCR. Data expressed as mean ± SDM, *p<0.01.
Germline and somatic PIK3 mutations in LM-LEC
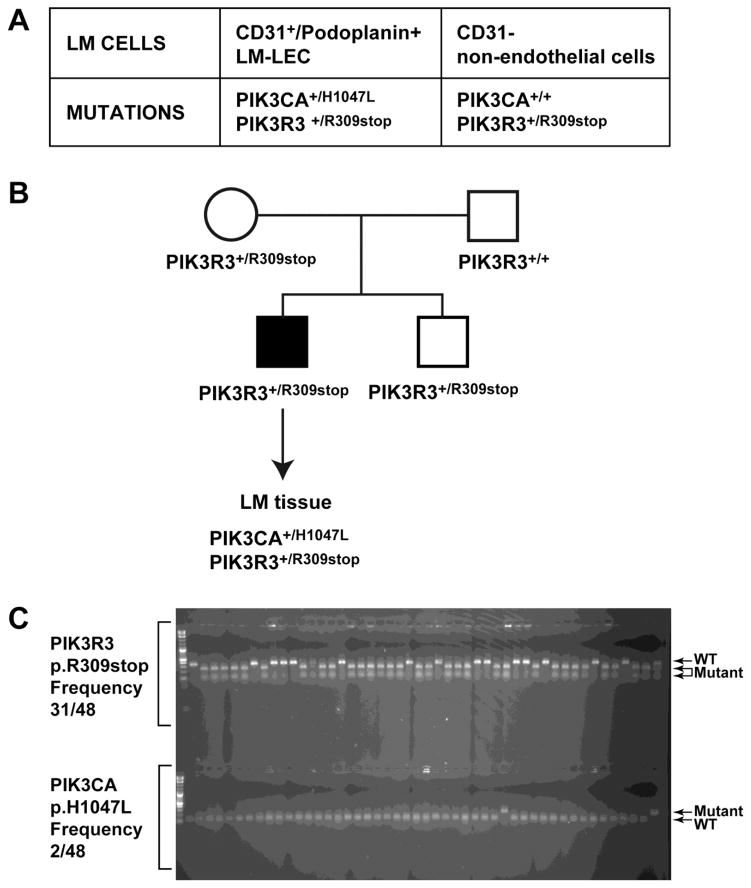
Targeted sequencing of a set of ten genes in the PI3K pathway (AKT1, AKT2, AKT3, PIK3CA, PIK3CB, PIK3CG, PIK3R1, PIK3R2, PIK3R3, PTEN) was performed in the LM-LECs (CD31+/Podoplanin+ LM cells) and returned 169,290 unique reads. Of these, 72,205 reads (49%) aligned to the genes included in the capture. The sample had >100X coverage across 67% of the bases captured. In LM-LECs two mutations were identified in two different genes of the PI3K pathway: c.2140A>T (p.His1047Leu, H1047L) mutation in the PIK3CA gene and c.925C>T (p.Arg309STOP, R309STOP) mutation in the PIK3R3 gene. The mutation in PIK3CA was seen in 9 out of 19 reads (47% variant) and the mutation in PIK3R3 was seen in 126 out of 248 reads (51% variant). LM-LECs and CD31- cells isolated from the same LM patient were then tested for these two mutations by Sanger sequencing. Both the PIK3CA and the PIK3R3 mutations were seen in the LM-LEC. In contrast, in the LM non-endothelial CD31- cells only the PIK3R3 mutation was seen, confirming that the PIK3CA mutation was somatic whereas the PIK3R3 mutation was inherited (Fig.2A). In both cell types, the PIK3R3 mutation appeared to be heterozygous. PIK3CA mutation in LM-LEC appeared to be heterozygous as well.
Figure 2. PIK3 mutations in LM-LECs and in LM patients’ tissue.
A. Table with mutations identified in LM-LEC (CD31+/podoplanin+) and non-endothelial cells (CD31-). B. Pedigree of family of patient with LM and schematic of mutational analysis for mutations in PI3K gene in LM tissue. C. DNA subcloning from patient’s LM tissue, and colony digestion with BspCNI for PIK3R3 mutation, (the mutation creates a restriction enzyme cutting site, frequency 31/48, see 2 lower bands on the gel), and digestion with BsaBI for the PIK3CA mutation (the p.H1047L base change removes a restriction site, frequency 2/48, see upper band in the gel).
DNA samples were obtained from the mother, father, and sibling of the patient. Sanger sequencing for both mutations showed that only the affected family member had the PIK3CA mutation but both the mother and the sibling had the heterozygous change in PIK3R3 (Fig.2B), suggesting that the PIK3CA mutation was somatic whereas the PIK3R3 mutation was inherited.
To confirm that both mutations were present in the patient tissue and were not a result of an advantageous mutation that arose during cell culture, DNA was extracted from LM tissue that had been frozen immediately after surgical removal. Sanger sequencing confirmed the presence of both PIK3CA and PIK3R3 mutations. Furthermore, DNA subcloning and subsequent colony digestion with specific restriction enzymes showed the PIK3R3 mutation with an allelic frequency of 31/48 (65%) (the mutation creates a site for the restriction enzyme BspCNI) and the PIK3CA mutation with an allelic frequency 2/48 (4%) (the mutation removes a site for BsaBI) (Fig.2C). The lower frequency of PIK3CA mutation in the DNA from the frozen tissue is not surprising as no sorting was performed and the relative abundance of endothelial cells is much lower compared to non-endothelial cell types that do not contain the mutation.
Pro-angiogenic properties of LM-LEC
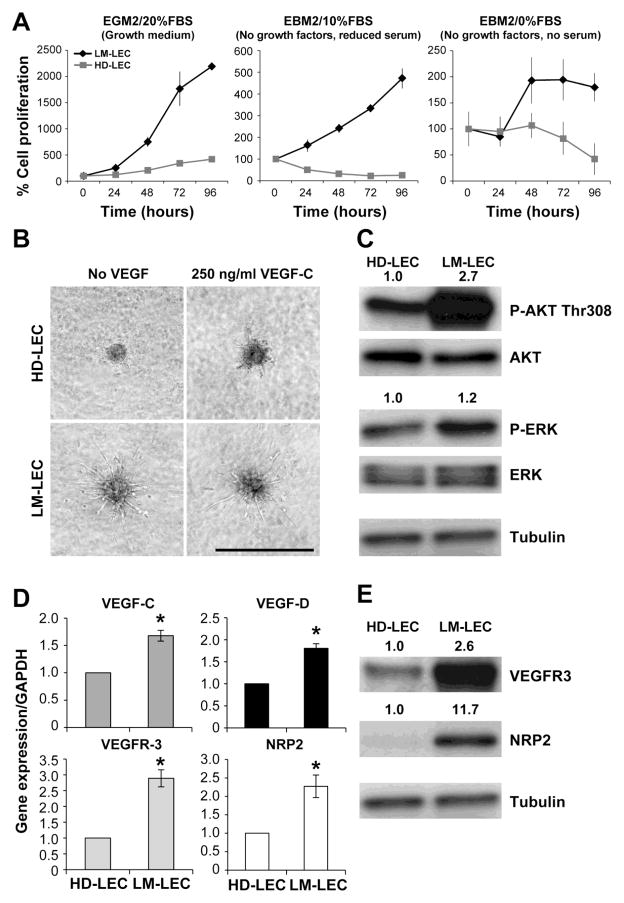
Next we analyzed the angiogenic properties of LM-LEC vs. HD-LEC. LM-LECs proliferated faster than HD-LEC when cultured either in growth (EGM2/20%FBS), starvation (EBM2/no growth factors/10%FBS), and serum-free (EBM2/no growth factors/no FBS) media (Fig.3A). HD-LECs sprouted only in the presence of 250ng/ml of VEGF-C, when re-suspended in 3-dimentional collagen gels as spheroids (Fig.3B). In contrast, LM-LEC extended tubular structures in the presence or absence of the lymphangiogenic factor VEGF-C.
Figure 3. Angiogenic properties of LM-LEC.
A. Cell proliferation evaluated at 24, 48, 72 and 96 hours for HD-LEC and LM-LEC in growth medium (EGM2/20%FBS), starvation medium (EBM2/no growth factors/10%FBS), and serum-free medium (EBM2/no growth factors/no FBS). Cell count at 24 hours after seeding was set to 100% to normalize for differences in initial adherence to the well. Data expressed as mean ± SDM. B. Sprouting assay with HD-LEC and LM-LEC spheroids in collagen gel, after 16 hours in the absence or presence of VEGF-C 250ng/ml. Scale bar 500μm. C. Immunoblot of HD-LEC and LM-LEC for phosphoAKT (P-AKT) Thr308, P-ERK, and relative total AKT and total ERK. Values are normalized ratios P-AKT/AKT and P-ERK/ERK band intensities. Tubulin serves as loading control. D. mRNA expression levels, normalized to GAPDH, for VEGF-C and VEGF-D, in HD-LEC and LM-LEC, measured by real-time qPCR. Data expressed as mean ± SDM, *p<0.01. E. Immunoblot of HD-LEC and LM-LEC for VEGFR-3, NRP2 and the endothelial marker VE-Cadherin. Values are ratios VEGFR-3/Tubulin NRP2/Tubulin and VE-Cadherin/Tubulin band intensities. Tubulin is loading control.
We next analyzed the activation status of AKT, a critical downstream target of PI3K and mediator of angiogenic signals. LM-LEC showed strong upregulation (2.7 fold) of phospho-AKT-Thr308 (P-AKT) compared to HD-LEC (Fig.3C), while levels of the MAP kinase phospho-ERK (P-ERK) were similar. Furthermore, real-time qPCR analysis of the lymphangiogenesis factors VEGF-C and VEGF-D in LM-LEC revealed a 1.5 and 2 fold upregulation of gene expression compared to HD-LEC (Fig.3D). VEGFR-3 and Neuropilin-2 (NRP2) mRNA levels in LM-LEC were higher than HD-LEC, and VEGFR-3 and NRP2 protein expression in LM-LEC were 2.6 and 11.7 times higher than HD-LEC, respectively (Fig.3E). Thus, these results demonstrate that LM-LECs exhibited increased AKT activation and increased expression of lymphangiogenesis factors and receptors, which could explain the enhanced pro-angiogenic activities compared to HD-LEC.
PI3K inhibitors and rapamycin prevent the pro-angiogenic phenotype of LM-LEC
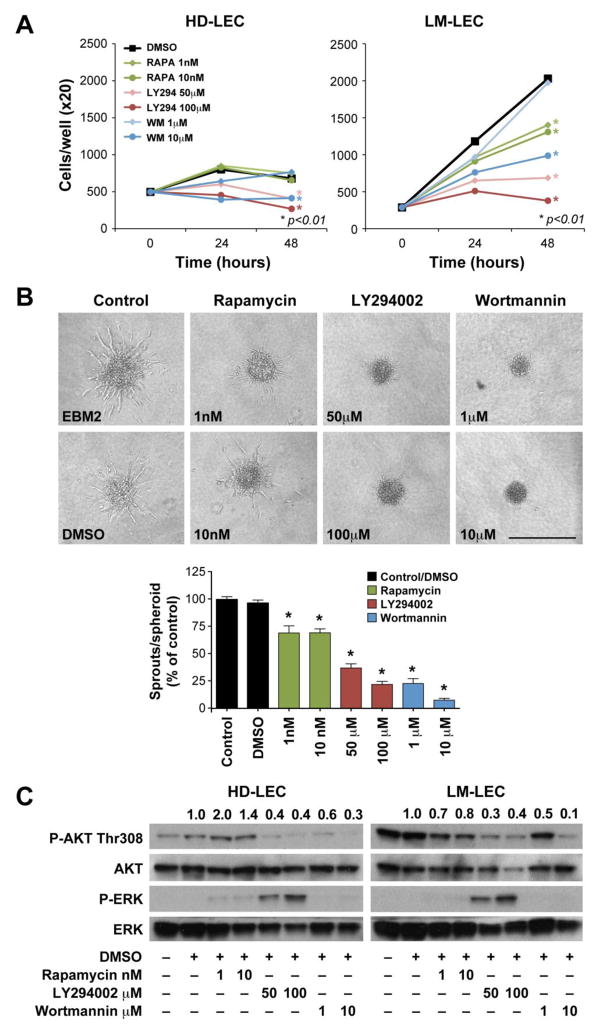
To determine whether inhibition of PI3K pathway would inhibit the pro-angiogenic activities of LM-LEC, we assessed the effects of the PI3K inhibitors LY294 and Wortmannin on LM-LEC proliferation, spheroid sprouting and AKT phosphorylation (Fig.4). We also assessed the effect of the mTOR inhibitor rapamycin since it has been reported that rapamycin suppresses lymphangiogensis and lymphatic metastasis in mice and zebrafish (26–29). LM-LEC proliferation was significantly (p<0.05) decreased in response to 48h treatment with rapamycin, LY294, and Wortmannin 10μM (Fig.4A). In contrast, HD-LEC proliferation was affected by LY294 and Wortmannin 10μM treatment, but not by rapamycin. In a second angiogenesis assay, LM-LEC formed sprouts from spheroids in collagen gels. Each drug caused a significant (p<0.05) reduction of LM-LEC spheroid sprout number. At the highest concentration tested, rapamycin reduced the number of sprouts by 30.5%, LY294 by 78.1%, and Wortmannin by 94.5% (Fig.4B).
Figure 4. Effect of PI3K inhibitors and rapamycin on the pro-angiogenic properties of LM-LEC.
A. Cell proliferation evaluated at 24 and 48 hours for HD-LEC and LM-LEC treated with rapamycin (1, 10nM), LY294 (50, 100μM), and Wortmannin (1, 10 μM). Cells were grown in EBM2/10%FBS. DMSO treatment is the control. Data expressed as mean. B. HD-LEC and LM-LEC spheroids in collagen gels, after 16 hours treatment with EBM2, or EBM2 containing DMSO, rapamycin (1, 10nM), LY294 (50, 100μM), and Wortmannin (1, 10 μM). Graph illustrates quantification of EC sprouts from the spheroids, expressed in % relative to EBM2 alone. Data expressed as mean± SEM. * p≤0.001. Scale bar 500μm. C. Immunoblot of HD-LEC and LM-LEC for phosphoAKT (P-AKT) Thr308, P-ERK, and relative total AKT and ERK. Cells were treated, for 48 hours with rapamycin (1, 10nM), LY294 (50, 100μM), and Wortmannin (1, 10 μM). Values are normalized ratios P-AKT/AKT and P-ERK/ERK band intensities.
Phosphorylation of AKT-Thr308 was significantly lower after LM-LECs treatment for 48 hours with rapamycin, LY294 and Wortmannin (Fig.4C). Conversely, in HD-LEC, the levels of phospho-AKT-Thr308 were affected by the PI3K inhibitors LY294 and Wortmannin, but not by rapamycin. Of interest, in response to LY294, phospho-ERK expression increased in both LM- and HD- LEC; this increased ERK activation was previously shown in HUVECs with RAF1S259A-induced impaired AKT signaling (30, 31).
DISCUSSION
Here we identify two mutations in PI3K pathway genes in LEC from a lymphatic malformation lesion (LM-LEC). Our analyses of the pro-angiogenic properties and the response to specific inhibitors of the patient-derived LM-LEC suggest a role for PIK3 mutations and AKT hyper-activation in lymphatic malformation development. Inhibitors of PI3K and mTOR pathways can diminish AKT phosphorylation and suppress cell proliferation and sprouting in LM-LECs carrying PIK3 mutations.
Lymphatic malformations (LM) are vascular lesions composed of dilated lymphatic channels often disconnected from the normal lymphatic system (32). Lymphatic vessels develop in the embryo from a subset of Prox1+ endothelial cells that, in response to VEGF-C, form lymph sacs that transiently fill with blood until separation from the cardinal vein and formation of lymphovenous valves (2, 3, 33, 34). LMs are a result of a congenital/early defect in the development of the lymphatic system, possibly caused by incomplete maturation of the Prox1+ endothelial cells or migration of a small subpopulation of the Prox1+ cells to the incorrect site. In LMs, dilated channels are filled with lymphatic and blood fluids (35), suggesting there could be an incomplete separation from the blood circulation.
Recently, Turner and colleagues proposed that integrin α5β1 in Prox1+/Pdgfrb+ LEC is required for lymphovenous valve formation, enabling correct lymphatic-blood vessel separation. In fact Itgα5Pdgfrb-cre mice embryos show blood-filled hyperplastic lymphatic vessels, reminiscent of LMs. Integrin α5β1 is required for VEGFR-3 activation (36), therefore disruption of the VEGFR-3 signaling is likely to be responsible for defects in the formation of the lymphatic system. VEGFR-3 cooperates with NRP2 to promote lymphatic vessel development and sprouting (37, 38). In our study we show that LM-LEC overexpress NRP2 and VEGFR-3 and the VEGFR-3 ligands VEGF-C and VEGF-D. These findings suggest that LM-LECs have a pro-lymphangiogenic phenotype; similarly VEGFR-3/NRP2 overexpression has been described in a subset of vascular malformation ECs (39). VEGFR-3 signaling can activate the PI3K/AKT pathway (40) and this signaling cascade has been shown to be critical for lymphatic development in mice (41) and for LEC migration in vitro (42). Whether and to what extent VEGFR-3 and NRP-2 interact with the mutant PIK3R3 and PIK3CA polypeptide products was not addressed in this study.
Germline mutations in VEGFR3 and in genes of the VEGFR-3 signaling pathway are involved in familial lymphatic abnormalities such as primary lymphedema, a defect of lymphatic drainage (for which mutations in VEGF-C, VEGFR3, FOXC2, SOX18, CCBE1, PTPN14, and NEMO have been identified) (43–47). These were not among the 10 PI3K pathway genes that were sequenced in this study, therefore, we cannot rule in or rule out mutations in these genes in the LM-LECs.
LMs are non-familial sporadic lesions, therefore it has been postulated (32) that somatic mutations restricted to the cells in the affected area are the cause for LM. In the LM tissue from one patient, we detected mutations in PIK3R3 and PIK3CA, two genes that are part of the PI3K signaling pathway. The PIK3R3 mutation is a germline mutation as it was also detected in the mother and sibling and it is present in all of the cells of the LM patient. The PIK3CA mutation is a somatic mutation: it was detected at low allelic frequency in the LM tissue, but at ~50% in the LM-LEC, indicating likely heterozygosity. Concurrent with our study, PIK3CA somatic mutations have been identified in a subset of vascular anomalies associated with/comprised of a lymphatic malformation (48).
The PIK3R3 germline mutation detected in the LM patient is a p.R309stop, which would cause premature truncation of the polypeptide and potentially non-sense mediated decay of the mRNA. Therefore, the p.R309stop may be a loss of function mutation. To date there is no report of a PIK3R3 knock-out mouse model, and thus the role of PIK3R3 during development remains elusive. It is possible that, in subjects with only the germline PIK3R3 mutation, genes encoding for other PI3K regulatory subunits (PIK3R1 and PIK3R2) could compensate for the loss of PI3KR3 function and thus, another mutation in the PIK3 pathway is required for LM to develop. Indeed, it has been shown that Pik3r1 is essential for embryonic lymphangiogenesis, and its targeted deletion impairs lymphatic sprouting and maturation in the gut and diaphragm (24).
PIK3CA encodes for the p110α catalytic subunit and is expressed ubiquitously in cells throughout the body. PIK3CA somatic mutations, detected in a wide array of cancers (16, 17), have also been found in association with overgrowth syndromes with a lymphatic or vascular malformation component, such as CLOVES (Congenital Lipomatous asymmetric Overgrowth of the trunk, lymphatic, capillary, venous, and combined-type Vascular malformations, Epidermal nevi, Skeletal and spinal anomalies) (20), MCAP (Megaencephaly-CApillary Malformation syndrome) and FH (Fibroadipose Hyperplasia), respectively (49). Mutations in the some of the PI3K-AKT pathway genes that we sequenced in the LM-LECs, such as PTEN, AKT1, AKT2, and AKT3, have been implicated in other overgrowth syndromes (50–52).
Although LMs are considered a vascular malformation, some investigators regard LMs as a benign neoplasm (53) since LM-LEC have high proliferative potential and can form LM-like lesions when injected into mice (54). The LM-LEC isolated herein, with the PIK3CA p.H1047L and PIK3R3 p.R309stop mutations, exhibit high cellular proliferative and sprouting potential, as well as increased AKT phosphorylation. The PI3K inhibitors Wortmannin and LY294 impaired cellular proliferation and sprouting, and prevented AKT phosphorylation in LM-LECs. These inhibitors also strongly reduced cellular proliferation and AKT activation in normal HD-LEC. Interestingly, strong phospho-ATK inhibition, caused by LY294, increased phospho-ERK levels in both HD-LEC and LM-LEC. Signaling through the ERK pathway was recently shown to be essential for LEC fate specification (55), when phospho-AKT is ablated, ERK signaling is increased, inducing Sox18 and Prox1 expression and subsequent lymphangectasia. This suggests that excessive ERK signaling can also be detrimental for the lymphatic system development.
PI3K inhibitors are currently being tested in clinical trials, however only the p110δ-selective inhibitor (GS-1101/Idelalisib) has been approved by the FDA for treatment of relapsed chronic lymphocytic leukemia (CLL) (56). The mTOR inhibitor rapamycin, compared to the PI3K inhibitors we tested, had a milder effect on reducing AKT phosphorylation, proliferation and sprouting of LM-LEC, but interestingly, in this study, it had no effect on normal HD-LEC. Rapamycin was shown to prevent lymphangiogenesis in a head and neck squamous carcinoma murine model and during wound healing (27–29). In fact, one of the targets of rapamycin in LEC is VEGFR-3 expression (57). A retrospective evaluation of rapamycin effects in 6 patients with life-threatening vascular anomalies showed it is effective and safe (58). Furthermore, a clinical trial for the rapamycin treatment of complicated vascular anomalies, including microcystic lymphatic malformations, is on-going (NCT00975819).
In summary, we demonstrate that mutations in PIK3 can be associated with LMs, and that pharmacological therapies targeting the increased AKT phosphorylation observed in LEC isolated from LMs lesions may be considered, alone or in combination, for the treatment of LMs. Further studies are needed to determine if our results from 1 LM sample can be generalized to other LM tissues with the PIK3CA mutation we identified or other PIK3CA activating mutations. In addition, the contribution of the PIK3R3 mutation to the LM phenotype needs to be considered for future investigations.
MATERIALS AND METHODS
Cell Isolation and Culture
Specimens of LM were obtained under a human subject protocol approved by the Committee on Clinical Investigation, Boston Children’s Hospital. The clinical diagnosis was confirmed in the Department of Pathology at Boston Children’s Hospital. Informed consent was obtained for the specimens, according to the Declaration of Helsinki. Single cell suspensions were prepared from the LM specimens by digesting with collagenase (Roche). Cells were seeded on fibronectin-coated tissue culture dishes in EGM2/20% fetal bovine serum (FBS) (Lonza). When the cells reached 80% confluency, they were purified with anti-CD31 conjugated magnetic beads (Dynal). When the CD31-positive cells were again subconfluent, they were reselected with anti-podoplanin antibody (Covance) followed by magnetic beads conjugated with anti-mouse IgG. Cells were analyzed for lymphatic endothelial cell markers and named lymphatic malformation-lymphatic endothelial cells (LM-LEC). LM-LEC at passage 6 were analyzed for karyotype and found to be normal 46, X,Y. Normal human dermal lymphatic endothelial cells (HD-LEC) were purchased from Lonza. Human umbilical cord endothelial colony forming cells (ECFC) were isolated as previously described (59, 60). HUVECs were a kind gift from Dr. Tanya Mayadas, Vascular Research Division, Brigham and Women’s Hospital. HD-LEC, ECFCs and HUVECs were cultured in the same conditions as LM-LECs.
qRT-PCR
Total RNA was extracted using the RNeasy kit (Qiagen). cDNA was prepared using Superscript II enzyme (Invitrogen Corp.) and 2 μg total RNA. For real-time qPCR analysis, the DyNAmo Sybr-Green-based system (New England BioLabs) was used. Oligonucleotide primers are listed in Supplementary Table S1. Reactions were run on a LightCycler (Roche Applied Science). Each experiment was done in triplicate and repeated two times.
DNA preparation for target capture
DNA was extracted from the cultured LM-LECs and from frozen tissue using the QAIamp DNA Mini Kit (Qiagen). A genomic library was prepared from the LEC DNA as previously described (20). Briefly, 3μg of DNA was mechanically sheared into 100–200 basepair (bp) fragments. A unique 4 bp barcode was added to the ends of the DNA fragments. Following 16 cycles of PCR, the DNA was hybridized for 65 hours to a custom designed capture array (Agilent Technology 1M SureSelect DNA Capture Array). The array contained the coding regions of 10 genes within the PI3K signaling pathway (AKT1, AKT2, AKT3, PIK3CA, PIK3CB, PIK3CG, PIK3R1, PIK3R2, PIK3R3, PTEN). Post-capture, another 17 cycles of PCR were performed. The samples were then sequenced by 100-bp paired end sequencing on an Illimunia HiSeq2 sequencer (Illumina, Inc.).
DNA Sequence Analysis
Paired-end reads from the Illumina HiSeq2 were de-barcoded with Novobarcode (Novocraft Technologies) and aligned to the UCSC Human reference genome (GRCh37) using the Burrows-Wheeler Aligner (version 0.6.1). Pileup files were generated using SAMtools. Variants found in the 1000 Genomes database, the NHLBI Exome Variant Server, or the Database of Common SNPs (dbSNP, build 132) were filtered out.
Mutation Confirmation
Mutations were confirmed with Sanger sequencing and restriction enzyme digest. Sanger sequencing was performed by PCR amplification of the DNA around the mutation. In addition, both mutations changed the cut sites of unique enzymes. The PIK3CA p.H1047L base change removes a restriction site for BsaBI. The PIK3R3 base change creates a restriction site for BspCNI. DNA fragments were amplified by PCR then inserted into a plasmid vector using the TOPO TA Cloning Kit (Life Technologies). One Shot TOP-10 chemically competent E.coli were transformed and colonies cultured. Enzyme digests were performed with DNA from individual colonies.
Immunocytochemistry
LM-LECs, HD-LECs, HUVECs and cbECFCs were cultured until subconfluent, fixed with cold methanol and stained with anti- CD31 (1:100, Dako), VE-Cadherin (1:100, Santa Cruz), COUPTFII (1:100, R&D Systems), Podoplanin (1:100, Covance), Prox1 (1:100, Angiobio), LYVE1 (1:100, Abcam), CD90 (1:100, BD Biosciences), and αSMA (1:1000, Sigma). Cells were then incubated with FITC-labeled secondary antibody (1:200, Vector Laboratories) and nuclei counterstained with DAPI (Vector Laboratories).
Microscope Image acquisition
Fluorescence images were taken with Leica TCS SP2 Acousto-Optical Beam Splitter confocal system equipped with DMIRE2 inverted microscope (Diode 405 nm, Argon 488 nm, HeNe 594 nm; Leica Microsystems), Leica Confocal Software Version 2.61, Build 1537. Images were taken at room temperature (about 20 C) and files always exported as 8 bit format.
Assays for In Vitro Cellular Proliferation
LEC proliferation was assessed after seeding the cells at 104 cell/cm2 on 48-well plates. Following attachment (24 h), plating efficiency was determined, and cell number was determined after 24, 48, 72, and 96hs, using a Coulter Counter® (Beckman) or by manual cell counting with hemocytometer.
Spheroid-based lymphangiogenesis assay
Early passage LM-LECs and HD-LECs were suspended and aggregated overnight to form cellular spheroids (500 cells/spheroid). LEC spheroids were embedded into collagen gels and either left untreated or treated for 16h with 250 ng/ml VEGF-C. Inhibitors were mixed with the collagen gel before polymerization and images were taken after 16 hours. In vitro angiogenesis was quantified by measuring the number of sprouts grown out of each spheroid using NIH ImageJ software. Ten to fifteen spheroids per experimental group were analyzed.
Immunoblot
Cells were lysed with RIPA buffer (Boston Bioproducts), containing a phosphatase inhibitor cocktail (Roche). Lysates were subjected to SDS-PAGE and transferred to Immobilon-P membrane. Membranes were incubated with antibodies against the following: VEGFR-3 (1:1000, BD Bioscience), NRP2 and VE-Cadherin (both 1:500 Santa Cruz Biotech), phospho-AKT (Thr308), AKT, phospho-ERK, ERK (all in 1:1000, Cell Signaling Technology), Tubulin (1:5000, Sigma-Aldrich). Membranes were incubated with peroxidase-conjugated secondary antibodies (1:5000, Vector Laboratories). Antigen-antibody complexes were visualized using ECL and chemiluminescent sensitive film (Pierce). Band intensity was analyzed with ImageJ software.
Inhibitors
The inhibitors used in this study were rapamycin at 1 and 10nM (LC Laboratories), LY294 at 50 and 100μM and Wortmannin at 1 and 10μM (Sigma Aldrich).
Statistical Analysis
The data were expressed as means ± s.d.m. or means ± s.e.m. and analyzed by ANOVA followed by Student’s t-test where appropriate. Differences were considered significant at p values < 0.05.
Supplementary Material
Acknowledgments
Research reported in this manuscript was supported by a Translational Research Program Pilot Study Grant from Boston Children’s Hospital (J.B.), the Charles Hood Foundation (E.B.), the Manton Center for Orphan Disease Research (E.B.), and the National Heart, Lung, and Blood Institute, part of the National Institutes of Health, under Award Number R01 HL117952 (E.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Dr. Steven Fishman, and Lan Huang for helpful discussions, Drs. Camille L. Stewart and Annie Kulungowski for initial characterization of the LM-LEC, Dr. Tanya Mayadas for providing HUVECs, the Cytogenetics Core of Dana Farber Harvard Cancer Center (P30 CA006516), Jill Wylie-Sears for technical assistance and Kristin Johnson for the preparation of figures.
Non-standard abbreviations
- LM
lymphatic malformation
- LEC
lymphatic endothelial cells
- PI3K
phosphoinositide 3-kinase
Footnotes
The authors have declared that no conflict of interest exists.
Boscolo E.: AKT hyperphosphorylation in Lymphatic Malformation.
AUTHOR CONTRIBUTIONS
E.B., J.B. and M.L.W. designed the research. E.B. and S.C. performed the in vitro experiments, V.L.L. performed targeted sequencing. A.G., M.K. and M.L.W. assisted with data analysis and review of the manuscript. E.B. and J.B. wrote the manuscript.
ETHICAL STANDARDS
The experiments in this manuscript comply with the current laws of the United States of America.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
BIBLIOGRAPHY
- 1.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21(7):1505–13. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98(6):769–78. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21(19):2422–32. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulliken JB, Glowacki J. Classification of pediatric vascular lesions. Plast Reconstr Surg. 1982;70(1):120–1. [PubMed] [Google Scholar]
- 5.Padwa BL, Hayward PG, Ferraro NF, Mulliken JB. Cervicofacial lymphatic malformation: clinical course, surgical intervention, and pathogenesis of skeletal hypertrophy. Plast Reconstr Surg. 1995;95(6):951–60. [PubMed] [Google Scholar]
- 6.Whimster IW. The pathology of lymphangioma circumscriptum. Br J Dermatol. 1976;94(5):473–86. doi: 10.1111/j.1365-2133.1976.tb05134.x. [DOI] [PubMed] [Google Scholar]
- 7.Brouillard P, Vikkula M. Vascular malformations: localized defects in vascular morphogenesis. Clinical genetics. 2003;63(5):340–51. doi: 10.1034/j.1399-0004.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 8.Garzon MC, Huang JT, Enjolras O, Frieden IJ. Vascular malformations - Part I. J Am Acad Dermatol. 2007;56(3):353–70. doi: 10.1016/j.jaad.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 9.Fageeh N, Manoukian J, Tewfik T, Schloss M, Williams HB, Gaskin D. Management of head and neck lymphatic malformations in children. J Otolaryngol. 1997;26(4):253–8. [PubMed] [Google Scholar]
- 10.Hancock BJ, Stvil D, Luks FI, Dilorenzo M, Blanchard H. Complications of Lymphangiomas in Children. J Pediatr Surg. 1992;27(2):220–6. doi: 10.1016/0022-3468(92)90316-y. [DOI] [PubMed] [Google Scholar]
- 11.Jackson IT, Carreno R, Potparic Z, Hussain K. Hemangiomas, Vascular Malformations, and Lymphovenous Malformations - Classification and Methods of Treatment. Plast Reconstr Surg. 1993;91(7):1216–30. doi: 10.1097/00006534-199306000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Uebelhoer M, Boon LM, Vikkula M. Vascular Anomalies: From Genetics toward Models for Therapeutic Trials. Csh Perspect Med. 2012;2(8) doi: 10.1101/cshperspect.a009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annual review of biochemistry. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 14.Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332(6165):644–6. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 15.Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? Journal of cell science. 2001;114(Pt 16):2903–10. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 16.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 17.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Current opinion in oncology. 2006;18(1):77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 18.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318(5853):1108–13. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 19.Bader AG, Kang S, Vogt PK. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci U S A. 2006;103(5):1475–9. doi: 10.1073/pnas.0510857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurek KC, Luks VL, Ayturk UM, Alomari AI, Fishman SJ, Spencer SA, Mulliken JB, Bowen ME, Yamamoto GL, Kozakewich HP, et al. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am J Hum Genet. 2012;90(6):1108–15. doi: 10.1016/j.ajhg.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maclellan RA, Luks VL, Vivero MP, Mulliken JB, Zurakowski D, Padwa BL, Warman ML, Greene AK, Kurek KC. PIK3CA activating mutations in facial infiltrating lipomatosis. Plast Reconstr Surg. 2014;133(1):12e–9e. doi: 10.1097/01.prs.0000436822.26709.7c. [DOI] [PubMed] [Google Scholar]
- 22.Riviere JB, Mirzaa GM, O'Roak BJ, Beddaoui M, Alcantara D, Conway RL, St-Onge J, Schwartzentruber JA, Gripp KW, Nikkel SM, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet. 2012;44(8):934–40. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–9. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouta-Bellum C, Kirov A, Miceli-Libby L, Mancini ML, Petrova TV, Liaw L, Prudovsky I, Thorpe PE, Miura N, Cantley LC, et al. Organ-specific lymphangiectasia, arrested lymphatic sprouting, and maturation defects resulting from gene-targeting of the PI3K regulatory isoforms p85alpha, p55alpha, and p50alpha. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238(10):2670–9. doi: 10.1002/dvdy.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soroceanu L, Kharbanda S, Chen R, Soriano RH, Aldape K, Misra A, Zha J, Forrest WF, Nigro JM, Modrusan Z, et al. Identification of IGF2 signaling through phosphoinositide-3-kinase regulatory subunit 3 as a growth-promoting axis in glioblastoma. Proc Natl Acad Sci U S A. 2007;104(9):3466–71. doi: 10.1073/pnas.0611271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flores MV, Hall CJ, Crosier KE, Crosier PS. Visualization of embryonic lymphangiogenesis advances the use of the zebrafish model for research in cancer and lymphatic pathologies. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239(7):2128–35. doi: 10.1002/dvdy.22328. [DOI] [PubMed] [Google Scholar]
- 27.Huber S, Bruns CJ, Schmid G, Hermann PC, Conrad C, Niess H, Huss R, Graeb C, Jauch KW, Heeschen C, et al. Inhibition of the mammalian target of rapamycin impedes lymphangiogenesis. Kidney international. 2007;71(8):771–7. doi: 10.1038/sj.ki.5002112. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S, Kishimoto T, Kamata S, Otsuka M, Miyazaki M, Ishikura H. Rapamycin, a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. Cancer science. 2007;98(5):726–33. doi: 10.1111/j.1349-7006.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel V, Marsh CA, Dorsam RT, Mikelis CM, Masedunskas A, Amornphimoltham P, Nathan CA, Singh B, Weigert R, Molinolo AA, et al. Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Res. 2011;71(22):7103–12. doi: 10.1158/0008-5472.CAN-10-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Y, Atri D, Eichmann A, Simons M. Endothelial ERK signaling controls lymphatic fate specification. J Clin Invest. 2013;123(3):1202–15. doi: 10.1172/JCI63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren B, Deng Y, Mukhopadhyay A, Lanahan AA, Zhuang ZW, Moodie KL, Mulligan-Kehoe MJ, Byzova TV, Peterson RT, Simons M. ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. J Clin Invest. 2010;120(4):1217–28. doi: 10.1172/JCI39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brouillard P, Boon L, Vikkula M. Genetics of lymphatic anomalies. J Clin Invest. 2014;124(3):898–904. doi: 10.1172/JCI71614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francois M, Short K, Secker GA, Combes A, Schwarz Q, Davidson TL, Smyth I, Hong YK, Harvey NL, Koopman P. Segmental territories along the cardinal veins generate lymph sacs via a ballooning mechanism during embryonic lymphangiogenesis in mice. Dev Biol. 2012;364(2):89–98. doi: 10.1016/j.ydbio.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 34.Hagerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams RH, Alitalo K, Andresen V, Schulte-Merker S, Kiefer F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 2013;32(5):629–44. doi: 10.1038/emboj.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: Diagnosis and management. Seminars in pediatric surgery. 2014;23(4):178–85. doi: 10.1053/j.sempedsurg.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Groopman JE, Wang JF. Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin alpha5beta1. Journal of cellular physiology. 2005;202(1):205–14. doi: 10.1002/jcp.20106. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, Larrivee B, Del Toro R, Suchting S, Medvinsky A, et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol. 2010;188(1):115–30. doi: 10.1083/jcb.200903137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129(20):4797–806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 39.Partanen TA, Vuola P, Jauhiainen S, Lohi J, Salminen P, Pitkaranta A, Hakkinen SK, Honkonen K, Alitalo K, Yla-Herttuala S. Neuropilin-2 and vascular endothelial growth factor receptor-3 are up-regulated in human vascular malformations. Angiogenesis. 2013;16(1):137–46. doi: 10.1007/s10456-012-9305-x. [DOI] [PubMed] [Google Scholar]
- 40.Coso S, Zeng Y, Opeskin K, Williams ED. Vascular endothelial growth factor receptor-3 directly interacts with phosphatidylinositol 3-kinase to regulate lymphangiogenesis. PLoS One. 2012;7(6):e39558. doi: 10.1371/journal.pone.0039558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou F, Chang Z, Zhang L, Hong YK, Shen B, Wang B, Zhang F, Lu G, Tvorogov D, Alitalo K, et al. Akt/Protein kinase B is required for lymphatic network formation, remodeling, and valve development. Am J Pathol. 2010;177(4):2124–33. doi: 10.2353/ajpath.2010.091301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20(17):4762–73. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon K, Schulte D, Brice G, Simpson MA, Roukens MG, van Impel A, Connell F, Kalidas K, Jeffery S, Mortimer PS, et al. Mutation in vascular endothelial growth factor-C, a ligand for vascular endothelial growth factor receptor-3, is associated with autosomal dominant milroy-like primary lymphedema. Circ Res. 2013;112(6):956–60. doi: 10.1161/CIRCRESAHA.113.300350. [DOI] [PubMed] [Google Scholar]
- 44.Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, Vikkula M. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet. 2000;67(2):295–301. doi: 10.1086/303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irrthum A, Devriendt K, Chitayat D, Matthijs G, Glade C, Steijlen PM, Fryns JP, Van Steensel MA, Vikkula M. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Hum Genet. 2003;72(6):1470–8. doi: 10.1086/375614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connell F, Kalidas K, Ostergaard P, Brice G, Homfray T, Roberts L, Bunyan DJ, Mitton S, Mansour S, Mortimer P, et al. Linkage and sequence analysis indicate that CCBE1 is mutated in recessively inherited generalised lymphatic dysplasia. Human genetics. 2010;127(2):231–41. doi: 10.1007/s00439-009-0766-y. [DOI] [PubMed] [Google Scholar]
- 47.Au AC, Hernandez PA, Lieber E, Nadroo AM, Shen YM, Kelley KA, Gelb BD, Diaz GA. Protein tyrosine phosphatase PTPN14 is a regulator of lymphatic function and choanal development in humans. Am J Hum Genet. 2010;87(3):436–44. doi: 10.1016/j.ajhg.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osborn AJ, Dickie P, Neilson DE, Glaser K, Lynch KA, Gupta A, Hsi Dickie B. Activating PIK3CA Alleles and Lymphangiogenic Phenotype of Lymphatic Endothelial Cells Isolated from Lymphatic Malformations. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu505. In press. [DOI] [PubMed] [Google Scholar]
- 49.Lindhurst MJ, Parker VE, Payne F, Sapp JC, Rudge S, Harris J, Witkowski AM, Zhang Q, Groeneveld MP, Scott CE, et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat Genet. 2012;44(8):928–33. doi: 10.1038/ng.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussain K, Challis B, Rocha N, Payne F, Minic M, Thompson A, Daly A, Scott C, Harris J, Smillie BJ, et al. An activating mutation of AKT2 and human hypoglycemia. Science. 2011;334(6055):474. doi: 10.1126/science.1210878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, Turner J, Cannons JL, Bick D, Blakemore L, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med. 2011;365(7):611–9. doi: 10.1056/NEJMoa1104017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poduri A, Evrony GD, Cai X, Elhosary PC, Beroukhim R, Lehtinen MK, Hills LB, Heinzen EL, Hill A, Hill RS, et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron. 2012;74(1):41–8. doi: 10.1016/j.neuron.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang HY, Ho CC, Huang PH, Hsu SM. Co-expression of VEGF-C and its receptors, VEGFR-2 and VEGFR-3, in endothelial cells of lymphangioma. Implication in autocrine or paracrine regulation of lymphangioma. Lab Invest. 2001;81(12):1729–34. doi: 10.1038/labinvest.3780386. [DOI] [PubMed] [Google Scholar]
- 54.Lokmic Z, Mitchell GM, Koh Wee Chong N, Bastiaanse J, Gerrand YW, Zeng Y, Williams ED, Penington AJ. Isolation of human lymphatic malformation endothelial cells, their in vitro characterization and in vivo survival in a mouse xenograft model. Angiogenesis. 2014;17(1):1–15. doi: 10.1007/s10456-013-9371-8. [DOI] [PubMed] [Google Scholar]
- 55.Deng Y, Atri D, Eichmann A, Simons M. Endothelial ERK signaling controls lymphatic fate specification. The Journal of clinical investigation. 2013;123(3):1202–15. doi: 10.1172/JCI63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nature reviews Drug discovery. 2014;13(2):140–56. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo Y, Liu L, Rogers D, Su W, Odaka Y, Zhou H, Chen W, Shen T, Alexander JS, Huang S. Rapamycin inhibits lymphatic endothelial cell tube formation by downregulating vascular endothelial growth factor receptor 3 protein expression. Neoplasia. 2012;14(3):228–37. doi: 10.1593/neo.111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammill AM, Wentzel M, Gupta A, Nelson S, Lucky A, Elluru R, Dasgupta R, Azizkhan RG, Adams DM. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57(6):1018–24. doi: 10.1002/pbc.23124. [DOI] [PubMed] [Google Scholar]
- 59.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106(5):1525–31. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 60.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109(11):4761–8. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.