Abstract
Polycystic ovarian syndrome (PCOS) is the most common female endocrine disorder with a prevalence as high as 8–15% depending on ethnicity and the diagnostic criteria employed. The basic pathophysiology and mode of inheritance remain unclear, but environmental factors such as diet, stress and chemical exposures are thought to be contributory. Developmental exposure to endocrine disrupting compounds (EDCs) have been hypothesized to exacerbate risk, in part because PCOS hallmarks and associated metabolic co-morbidities can be reliably induced in animal models by perinatal androgen exposure. Here we show that lifetime exposure to a soy diet, containing endocrine active phytoestrogens, but not developmental exposure (gestational day 6 – lactational day 40) to the endocrine disrupting monomer Bisphenol A (BPA), can induce key features of PCOS in the rat; results which support the hypothesis that hormonally active diets may contribute to risk when consumed throughout gestation and post-natal life.
Key terms: phytoestrogens, genistein, endocrine disruptors, ovary, development
1. Introduction
Polycystic ovarian syndrome (PCOS) is the most common female endocrinopathy with a prevalence as high as 8–15% depending on ethnicity and the diagnostic criteria employed [1]. PCOS tends to cluster in families but the basic pathophysiology and mode of inheritance are unclear, as are the contributing roles of environmental factors such as diet, stress and chemical exposures. The incidence appears to be increasing [2], prompting the hypothesis that environmental factors substantively contribute to disease risk, but few studies have directly tested this possibility. Developmental exposure to endocrine disrupting compounds (EDCs) has been raised as a concern [3, 4], in part because PCOS hallmarks can be reliably induced in animal models by perinatal androgen exposure [5–7]. Here we show that a soy phytoestrogen rich diet, but not developmental exposure to the synthetic monomer Bisphenol A (BPA), can induce key features of PCOS in the rat; results which support the hypothesis that hormonally active diets may contribute to risk.
The symptoms and severity of PCOS varies greatly among affected women, but the criteria established in 2004 by the Rotteram European Society of Human Reproduction/American Society for Reproductive Medicine (ESHRE/ASRM) requires at least two of three key features for PCOS diagnosis: oligo-anovulation (resulting in irregular menstruation or amenorrhea), polycystic ovaries and clinical hyperandrogenism [8]. These are frequently accompanied by metabolic abnormalities including hyperinsulinemia (50–70% of patients), impaired glucose tolerance, and obesity [2, 9, 10]; features which emphasize that PCOS is not simply a “reproductive” disorder. Historically, prenatal androgen exposure has most frequently been used, in a variety of animal models including non-human primates [5–7], to induce PCOS features, demonstrating that developmental perturbation of gonadal hormone levels contributes to disease risk. Estrogen-induction experiments, however, have typically used older animals. Thus the impact of perinatal estrogen remain of concern but unclear [7]. EDC exposure has been implicated as a potential contributor to the rising prevalence of the syndrome [3, 4, 11–13]. Most EDCs are anthropogenic but many, including soy phytoestrogens, are naturally occurring and thus exposure to these compounds is typically higher than to BPA and other synthetic compounds, particularly in populations which rely on soy as a primary protein source. Using a rat model, the present study sought to determine if perinatal exposure to BPA, a soy phytoestrogen rich diet, or both, at levels considered human-relevant, induces hallmarks of PCOS. The synthetic estrogen ethinyl estradiol (EE; found in birth control pills) was used to model estrogenic effects.
Soy phytoestrogens and BPA are well characterized EDCs with a myriad of steroid hormone disrupting effects including effects on follicular development and fertility [14–18]. Early life exposure to soy phytoestrogens or BPA have previously been shown, in a variety of species, to confer a suite of adverse reproductive effects in females, including ovarian malformations and cycle dysregulation [19], changes in body weight [20], elevated androgen production [21], and suppressed fecundity, collectively suggestive of PCOS [11, 13, 22] (reviewed in [14] and [17]). Elevated serum BPA levels have also been associated with PCOS in human patients [23, 24] raising concern that exposure, particularly during development when the ovary is differentiating, contributes to disease risk. Rats exposed to BPA, at levels considerably higher than considered typical for humans, over the first 10 days of life by subcutaneous injection subsequently developed hallmarks of the disease, including elevated androgens and ovarian cysts [21]. Although these available data suggest a potential association between BPA exposure and PCOS symptoms, evidence from studies employing exposure conditions that are more human relevant (oral exposure, lower doses) remain needed to adequately evaluate the potential relationship between BPA exposure and PCOS risk [3, 4]. The BPA dose selected for the present studies serves as a starting point to fill that information void.
Soy-based foods contain numerous isoflavone phytoestrogens (genistein (GEN), daidzein, etc.) shown to have endocrine disrupting effects on the developing female reproductive system in numerous species including rodents, sheep, and humans [14, 17, 25–28]. Although use of soy phytoestrogens have been suggested as a potential therapeutic for PCOS patients because of their ability to mitigate aspects of the co-morbid metabolic features [29], intake during development may contribute to disease risk via endocrine disrupting actions in the organizing ovary. Additionally, concerns have been raised about the long term reproductive health consequences of soy infant formula because neonatal life is a critical period of neuroendocrine development, and also because isoflavone levels in soy formula are high compared to other soy-rich foods [30, 31]. Consequently, serum isoflavone levels in soy formula-fed infants far exceed what is typically seen in adults, even those consuming a soy-rich diet [14, 32–34]. Although soy formula intake has been associated with adult female reproductive disorders including an increased risk of menstrual irregularities and uterine fibroids [35, 36], available data regarding reproductive health effects is sparse and controversial [14, 37, 38]. The potential relationship between developmental isoflavone exposure and PCOS has not yet been explored and was thus a primary goal of the present study.
Because they are so ubiquitous in the environment, humans are invariably exposed to a mixture of EDCs from a variety of sources. These exposures are age and lifestyle dependent. For example infants reared on soy formula are exposed to soy isoflavones from the formula but also chemicals, such as BPA, leaching from food containers and other sources. Understanding how EDCs interact to modulate the physiology of endocrine regulated events, including reproductive development, is thus of seminal importance. Because BPA and soy isoflavones have both been shown to perturb estrogenic activity within the developing female reproductive system [16, 18, 37, 39], we hypothesized that they might act synergistically to adversely impact female reproductive development and thereby induce hallmarks of the PCOS phenotype. Alternatively, it is plausible that one could mitigate the impact of the other. For example the soy isoflavone GEN has been shown to counteract epigenetic changes conferred by early life BPA exposure in the Agouti mouse [40] and, in a companion study to the present one, we reported that a soy rich diet can prevent the anxiogenic phenotype induced by BPA [41]. Importantly, a soy-rich diet has been shown to modulate BPA-related effects on meiotic processes in the periovulatory oocyte [42] although the mechanisms by which this occurs remain unclear. Understanding how dietary and environmental exposures interact to influence reproductive health and development is of seminal importance, particularly given that soy consumption is growing in popularity among Western populations and BPA exposure is ubiquitous.
2. Materials and Methods
2.1 Animal Care and Handling
All animals used for this study were also used for an earlier, companion study, so all study design details including husbandry, exposure conditions, and tissue collection, have been published previously [41]. Animal care, maintenance and surgery were conducted in accordance with the applicable portions of the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for the Care and use of Laboratory Animals” and were approved by the North Carolina State University (NCSU) Institutional Animal Care and Use Committee (IACUC).
These experiments used four cohorts of Wistar rats bred in house (approximately 1 month apart) in conditions specifically designed to minimize exposure to environmental endocrine disruptors (use of glass water bottles with filtered water, thoroughly washed polysulfone caging and woodchip bedding), and reared on a phytoestrogen-free diet (Teklad 2020; Harlan) over several generations. All dams were housed individually in two humidity-and-temperature controlled rooms (segregated by diet), each with a 14h:10h light, dark cycle (lights on from 0200 to 1400 h) at 23°C and 50% average relative humidity at the Biological Resource Facility at NCSU. Food and water consumption were monitored throughout the duration of the experiment. For each measure, only a subset of animals (randomly selected) from each cohort was used so the numbers of pups per endpoint are not identical.
2.2 Exposure
There were a total of 5 exposure groups: Soy-Free (soy-free diet; n = 6 dams); BPA (soy-free diet plus BPA via drinking water; n = 5) Soy (soy diet; n = 8 dams); BPA + Soy (soy diet plus BPA via drinking water; n = 5 dams); and EE (soy-free diet plus EE via drinking water; n = 4). The dams were randomly assigned to their respective diet at least a week prior to mating and were kept on their diets for the duration of the experiment. The soy-based diet was Purina 5001 and the soy-free diet was Teklad 2020. The Purina 5001 diet was used because phytoestrogen levels are considered human-relevant, and the pharmacokinetics of GEN and other phytoestrogens delivered by this diet have been well characterized in the rat [14, 43–45]. Additionally, the energy density of the two diets is similar (3.1 kcal/g for the Teklad 2020 diet and 3.02 kcal/g for the Purina 5001 diet). We previously reported that serum GEN levels in the dams consuming the soy-based diet averaged 20 ng/ml, which is approximately equivalent to levels seen in vegetarians, but lower than those reported in infants reared on soy formula [41].
Exposure to EE or BPA began on gestational day (GD) 6 and continued until the day of sacrifice or postnatal day (PND) 40 (GD 0 defined as day of sperm plug detection; PND 0 defined as day of birth). Sex ratio and weight was obtained for each litter on PND 12. On PND 21 pups were weaned into same sex and exposure groups (3–5 pups per cage), and ear punched for identification. A subset of these was sacrificed just after puberty on the day of estrus (approximately PND 34) for ovarian analysis. At 3 months of age the remaining animals were separated into same-sex pairs and sacrificed at four months of age on the day of estrus (approximately PND 120).
BPA ((2,2-bis(4-hydroxyphenyl)propane; CAS No. 80-05-7; Lot 11909; USEPA/NIEHS standard provided to HBP); 1mg/L of water) and the positive control ethinyl estradiol (EE; 50μg/L) were administered via drinking water. This dose of BPA was chosen based on prior studies utilizing this method of exposure [46–48] to produce serum levels in the human range. We previously reported that serum BPA levels for the dams and pups were 1.5 ng/ml or lower [41], which is within or below serum BPA levels reported in humans [18, 49]. Dosing via this method delivers approx. 0.18 mg/kg to the dams during gestation, 0.35 mg/kg to the dams during lactation, and 0.44 mg/kg to the pups during adolescence (post-weaning). Given the scope and complexity of the experiment, we elected to use only this single dose of BPA with the rationale that any evidence of PCOS-like symptoms would warrant further, follow-up studies to better characterize the dose response. Animals were sacrificed on the day of estrus by transcardial perfusion with 4% paraformaldehyde. For each endpoint, animal (pup) numbers are indicated in the figures. Because only a subset were selected (randomly) for each endpoint the numbers differ across endpoints.
2.3 Assessment of Vaginal Opening and Estrus Cycle Regularity
Age at pubertal maturation was assessed by checking for the day of vaginal opening (VO) using well established criteria, as previously described [50, 51]. Beginning the week of VO (week 4), estrous cyclicity was monitored in a subset of females by vaginal lavage [52]. Samples were collected 5 days a week, every week, until week 10, and then biweekly until time of sacrifice (week 28). Normal estrus was defined as progression through a typical 4–5 day estrous cycle characterized by having distinct diestrus (D), proestrus (P) and estrus (E) stages [19, 52–54].
2.4 Glucose Challenge
A glucose challenge test was performed just prior to sacrifice (approx. PND 120 ± 10 days; day of estrus). Consistent with our prior work [55], animals were fasted for 12 hours prior to testing in fresh cages to minimize coprophagia. To ensure that the β-form, which is better transported, was predominant, the glucose solution was prepared the night before testing by dissolving 5g D-glucose (Sigma) in 10 mL purified water and incubated at 37°C. All animals were weighed at the time of assessment, tested for baseline blood glucose (time zero) from a tail nick using glucometer (OneTouch Ultra; Lifescan), intraperitoneally injected with 2 g/kg bw glucose solution and returned to the home cage. Subsequent glucose measures (10, 30, 60 and 120 min) were obtained from the tail nick while the animal was in the home cage to minimize handling stress. For each animal, the area under the curve (AUC) was calculated with SYSTAT 13 (Systat Software, Inc) using the trapezoidal rule.
2.5 Ovarian Histology
Ovaries were collected and coded at sacrifice, post-fixed in 4% paraformaldehyde for 24h at 4°C, stored at 4°C in 70% EtOH, and subsequently processed by the Histology Core at the NCSU College of Veterinary Medicine. For each animal, both ovaries were adjacently paraffin embedded, and sliced into 5 μm sections (at 100 μm intervals). Sections were slide mounted (Superfrost Plus, Fisher, Pittsburgh, PA) and two mid-level slides per animal were deparaffinized, stained with H&E, coverslipped, and qualitatively examined for histological abnormalities (primordial follicle clusters, hemorrhagic and multi-oocyte follicles) using a standard approach similar to what we and others have done previously [19, 21, 56, 57]. Corpora lutea (CL), antral follicles and cystic follicles were then quantified on each section, averaged to obtain a value for each animal, then validated by a second individual, each blind to exposure group.
2.6 Serum Total Testosterone
Serum testosterone (samples obtained on the day of sacrifice; approx. PND 120 ± 10 days; day of estrus) was quantified via radioimmunoassay (RIA) using the Coat-A-Count Total Testosterone Kit (Siemens) as previously described [58, 59]. Analytical sensitivity was 2 ng/dl and samples were run in duplicate.
2.7 Statistical Analysis
The primary goal of the statistical approach was to establish the effect of diet (soy or soy-free) and exposure (BPA, or none) as well as any significant interactions. Because the EE group served as the positive control for effect, inclusion would, by definition, bias the statistical analysis (see [60] for a more detailed explanation). Thus it was not included in the overall analysis. Instead, the EE group was compared to the unexposed (no BPA) females reared on the soy-free diet by a two-tailed homoscedastic Student’s t-test to the control group on the same diet (soy-free) to establish which “estrogenic” effects were statistically significant.
For PND 12 body weight an average per litter (females only) was obtained and used as the representative measure for that litter. For all other endpoints, the pup was the statistical unit but litter and cohort were accounted for in the statistical approach. Animal numbers for each endpoint are indicated in the figures. The data were first analyzed by ANOVA for main effects of litter and cohort, but none were found for any endpoint examined. Single outliers were identified and removed using a Grubbs Test (1 animal in the blood glucose test). The data were then analyzed using two-way ANCOVA with exposure and diet as the factors, and dam and litter as covariates (no EE group). Significant effects and interactions were followed up with a protected Fisher’s Least Significant Difference (LSD) test. For the glucose challenge test, and weight gain curves, the area under the curve (AUC) was calculated using the trapezoidal rule and compared across groups using ANCOVA as described. Repeated measures ANOVA was not appropriate because animals were sacrificed at different time points; thus not all of the animals are represented at each time point. Body weight differences between controls on the soy and soy-free diets were compared at each age by a two-tailed homoscedastic Student’s t-test because a significant interaction between diet and BPA was observed in the AUC assessment. Comparisons of additional groups of interest were done by t-test, where indicated. All analyses were two tailed and results were considered significantly different when P = 0.05. Statistics were run in Systat 13 and the graphs generated in SigmaPlot 10 or GraphPad Prism 5.
3. Results
3.1 Litter Data and Preweaning Measures
Litter size and sex ratio was reported in our prior study [41] and did not statistically differ among groups, although sex ratio was skewed towards females in all groups (Table 1). For the present studies this outcome is beneficial because it minimizes exposure to prenatal androgens from male siblings [61]. There was a significant effect of BPA exposure (F(1,126) = 9.6; P ≤ 0.002) and diet (F(1,126) = 8.27; P ≤ 0.005) on PND 12 body weight but no significant interaction (Table 1). BPA exposed animals were significantly heavier than unexposed animals, regardless of diet, and animals on the soy diet were significantly heavier than animals on the soy-free diet, regardless of BPA exposure. EE exposed animals were significantly lighter than controls on the same diet (Soy-Free; P ≤ 0.02).
Table 1.
Preweaning measures. Groups on the soy diet are shaded in gray. Bolded groups are significantly different than controls on the same diet. Litter size and ratio was not impacted by diet or exposure. Compared to the Soy-Free controls, PND 12 weight was elevated by BPA exposure (aP ≤ 0.05) or soy diet (bP ≤ 0.006) but there was no significant interaction between the two factors. EE exposed animals were lighter than conspecifics on the same diet (Soy-Free; cP ≤ 0.02)
| Exposure Group | Litters (n) | Litter Size (Mean ± SEM) | Percent Males (Mean ± SEM) | PND 12 Weight (g) |
|---|---|---|---|---|
| Soy-Free | 6 | 13.33 ± 0.84 | 46.69 ± 6.92 | 23.00 ± 0.52 |
| BPA | 5 | 10.40 ± 1.25 | 41.73 ± 3.78 | 25.03 ± 0.73a |
| Soy | 7 | 11.29 ± 1.04 | 40.53 ± 6.66 | 24.87 ± 0.86b |
| BPA + Soy | 5 | 11.20 ± 0.37 | 46.83 ± 7.01 | 27.39 ± 0.58ab |
| EE | 4 | 11.00 ± 1.73 | 36.31 ± 4.70 | 20.45 ± 1.19c |
3.2 Pubertal Outcomes and Ovarian Histology
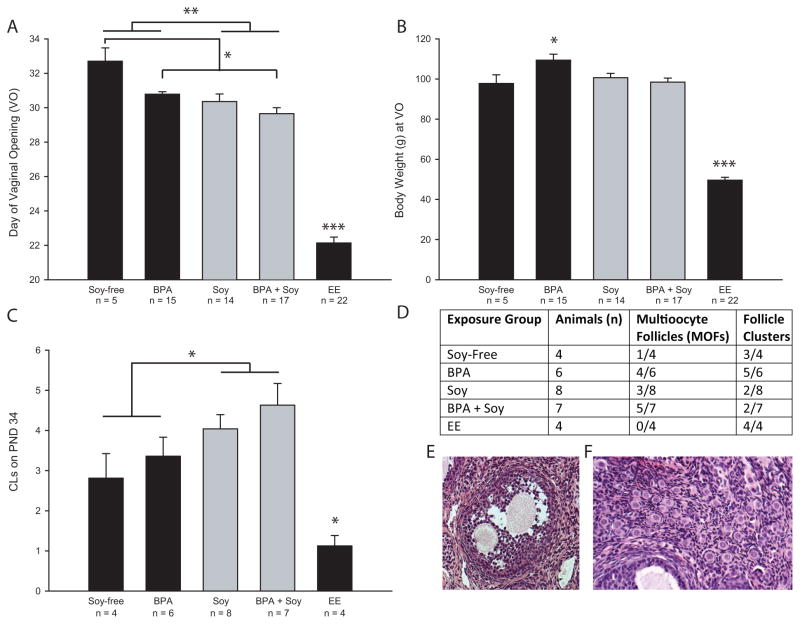
There was a main effect of exposure (F(1,33) = 4.08; P ≤ 0.04) and diet (F(1,33) = 8.51; P ≤ 0.006), but no significant interaction between the two, on age at VO, with soy diet (P ≤ 0.006) and BPA (P ≤ 0.05) both advancing age at VO (Figure 1A). Weight at VO was significantly impacted by diet (F(1,99) = 4.46; P ≤ 0.04). The animals reared on the soy diet weighed less than those reared on the soy-free diet (P ≤ 0.04; Figure 1B) so elevated body weight was not a contributing factor for advanced VO in the soy-fed groups. In contrast, among the females on the soy-free diet, the BPA exposure group was significantly heavier at VO than conspecifics on the same diet (P ≤ 0.03; Figure 1B) suggesting that weight gain could contribute to accelerated puberty in the BPA exposed females. EE exposed animals were significantly lighter than soy-free controls (P ≤ 0.001; Figure 1B) and all other groups.
Figure 1.
Indices of pubertal maturation. (A) Age at vaginal opening (DVO; puberty) was significantly advanced by the soy diet and BPA exposure but there was no significant interaction so exposure to both did not augment the effect. As expected, DVO was significantly earlier in the positive control (EE) group. (B) Body weight on DVO was elevated in the BPA exposed animals reared on the soy-free diet compared to controls on the same diet. EE exposed animals were significantly lighter. (C) Number of CLs on PND 34 were significantly greater in animals reared on soy but there was no interaction with BPA. Age matched EE exposed animal has fewer CLs. (D) Numbers of multioocyte follicles (depicted in E), and primordial cell clusters (depicted in F) observed in each exposure group. Graphs depict group means ±SE; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001
There was a main effect of diet on average number of CLs on PND 34 (F(1,21) = 6.325; P ≤ 0.02; Figure 1C) but no effect of, or interaction with, BPA exposure. Animals reared on soy had significantly more CLs than those reared on the soy-free diet, regardless of BPA exposure. EE exposed animals had fewer CLs compared to controls (P ≤ 0.04). The presence of morphological abnormalities was qualitatively assessed, with multioocyte follicles (MOFs) and primordial follicle clusters observed in some individuals (Figure 1D). While only 1 of 4 control females on the casein diet had MOFs, 4 of 6 exposed to BPA on the same diet and 5 of 7 exposed to BPA on the soy diet had MOFs. Primordial follicle clusters are unusual in animals of this age (approx. PND 34) but observed in 3 of 4 soy-free controls. By contrast, 3 of 6 animals exposed to BPA and reared on the casein diet had more than one. Although, qualitatively, the clusters in the BPA-exposed animals also appeared to be larger, the histological material was insufficient to assess this difference quantitatively.
3.3 Post-Weaning Body Weight
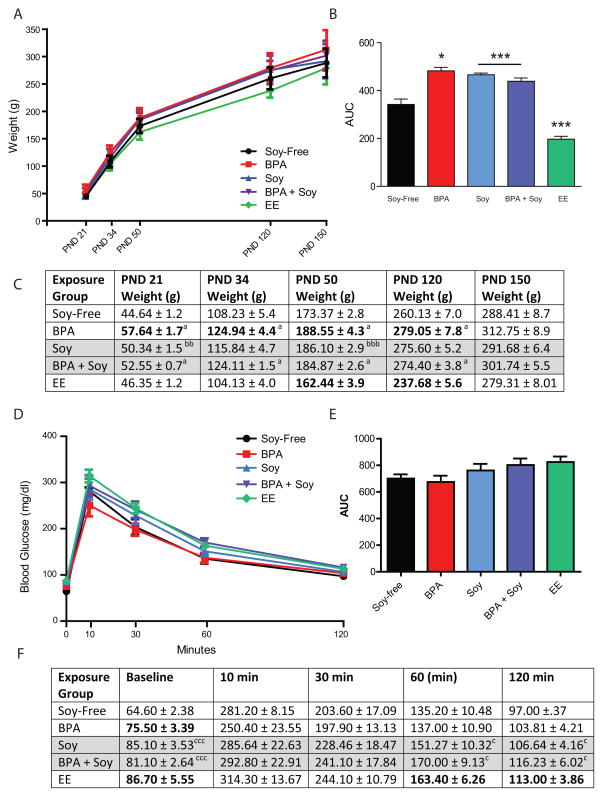
BPA exposure enhanced post-weaning body weight but, unexpectedly, the interaction between the two was not synergistic. At PND 21 (weaning), there was a significant effect of exposure (F(1,104) =17.038; P ≤ 0.001) as well as a significant interaction between diet and exposure (F(1,104) = 8.88; P ≤ 0.004; Figure 2) on body weight. Females exposed to BPA were heavier than unexposed conspecifics if reared on the soy-free diet (P ≤ 0.001) but not the soy diet (P = 0.17). Similarly, a main effect of BPA, and a significant interaction with diet was found for all subsequent ages except PND 150 (Figure 2). Body weights at each age examined are depicted in Figure 2A and summarized in Figure 2C.
Figure 2.
Body weight gain post-weaning. (A) Plotted weight gain between PNDs 21 and 150. (B) Area under the curve (AUC) was significantly greater in the soy fed animals, regardless of BPA exposure. Among the BPA-exposed animals, weight was only significantly elevated in the group reared on the soy-free diet. EE exposed animals were lighter than controls on the same (soy-free) diet. *P ≤ 0.05, ***P ≤ 0.001 (C) Body weights for each group at each age examined. Groups on the soy diet are shaded in gray. Bolded groups are significantly different than controls on the same diet. Main effects of BPA exposure were found for all ages except PND 150: aP ≤ 0.05, aaaP ≤ 0.001. No statistically significant main effect of soy diet was observed at any age in the 2-way ANOVA, but t-tests revealed that controls on soy diet were significantly heavier than controls on soy-free diet on PNDs 21 and 50: bbP ≤ 0.01, bbbP ≤ 0.001. EE animals were lighter than same-diet conspecifics on PNDs 50 and 120. (D) Plotted blood glucose levels across the 120 minute test. (E) No significant differences of diet, BPA or EE were found for glucose AUC but differences at the time points were observed and summarized in (F). Bolded groups are significantly different than controls on the same diet. Main effect of soy diet: cP ≤ 0.05, cccP ≤ 0.001
No main effect of diet was found for any age between PND 21 and PND 150. Follow up t-tests revealed, however, that unexposed females reared on the soy diet were heavier than females reared on the soy-free diet on PNDs 21 (P ≤ 0.01) and 50 (P ≤ 0.001). This result is consistent with what was observed on PND 12. To more comprehensively assess cumulative weight gain over time, weight from PND 21 (weaning) through PND 120 (adulthood) was plotted (Figure 2A) and the AUC was calculated and compared between groups. AUC was significantly greater in animals reared on the soy diet (F(1,77) = 22.32; P ≤ 0.001) compared to those reared on the soy-free diet (Figure 2B). A t-test also found the EE animals to be significantly lighter than unexposed females on the same diet (P ≤ 0.001). The main effect of BPA exposure did not reach statistical significance (P = 0.08), but there was a significant interaction between exposure and diet (F(1,79) = 6.37; P ≤ 0.01). BPA exposed animals on the soy-free diet had a greater AUC than unexposed controls on the soy-free diet (F(1,30) = 4.91; P ≤ 0.03).
Collectively, these data reveal that both soy and BPA promoted weight gain but instead of having an additive effect on body weight (as hypothesized), the soy diet mitigated the BPA-induced weight gain to some degree.
3.4 Adult Glucose Challenge Results
Baseline glucose levels were significantly higher in animals reared on the soy diet (F(1,32 = 13.99; P ≤ 0.001) and there was a significant interaction with exposure (F(1,32) = 4.53; P ≤ 0.04; Figure 2D). Among the BPA exposed animals, females reared on the soy diet had elevated baseline glucose levels compared to those reared on the soy-free diet (F(1,13) = 4.50; P ≤ 0.05). These results suggest that soy, not BPA, was driving the effect on baseline glucose levels. No significant group differences were observed at 10 or 30 minutes (although there was a modest trend for an effect of diet at 30 minutes (F(1,32) = 3.45; P = 0.07), but an effect of diet again emerged at 60 min (F(1,32) = 4.89; P ≤ 0.03) and remained at 120 min (F(1,32) = 4.35; P ≤ 0.04) with animals reared on the soy diet having higher blood glucose levels than those reared on the soy-free diet. No interaction with BPA was identified. Thus, again, the effect on blood glucose was driven by diet, not BPA exposure. Effects at each time point are summarized in Figure 2F.
AUC was calculated for each group and statistically compared to better characterize the impact of diet and BPA exposure on the time course of glucose metabolism. AUC did not significantly differ with exposure or diet, although there was a modest trend for an effect of diet (F(1,32) = 3.28; P = 0.08) with animals on the soy diet having a greater AUC than those on the casein diet (Figure 2E). This analysis thus confirmed what was revealed by comparing serum glucose levels at each individual time point.
In the EE animals, glucose levels were significantly higher than conspecifics on the same diet at baseline (P ≤ 0.02), 60 min (P ≤ 0.03) and 120 min (P ≤ 0.03) but only a trend was observed at 30 min (P ≤ 0.057). Body weight at the time of testing was not statistically compared because testing was conducted across multiple days.
3.5 Estrous Cycle Regularity
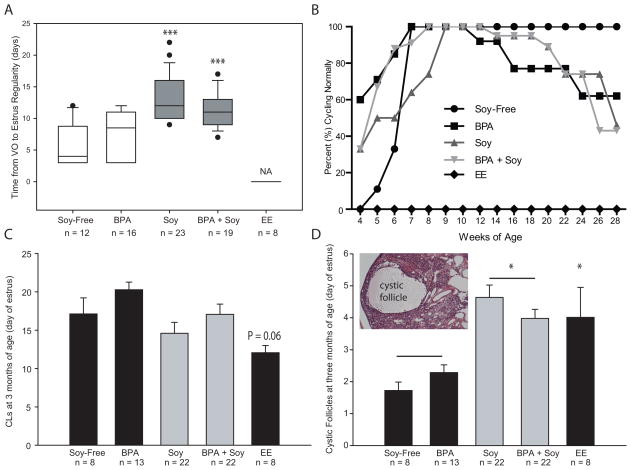
Time from VO to cycle regularity differed by groups (Figure 3A). Soy-reared females (F(1,66) = 47.00; P ≤ 0.001) took significantly longer to establish a regular estrous cycle than animals on the soy-free diet. Additionally, an interaction between diet and BPA exposure was identified (F(1,66) = 4.98; P ≤ 0.03) revealing that BPA animals on the soy diet took longer to establish regular estrus than BPA animals on the soy-free diet. None of the EE animals began cycling and remained in persistent estrus for the duration of the experiment.
Figure 3.
Adult indices of ovarian development and function. (A) Soy-fed animals took longer to establish a regular estrus cycle but BPA had no effect and EE animals were in persistent estrus from the time of VO. (B) Percentage of cycling females in each group at each age examined. All groups except the EE group ultimately established a regular estrus cycle but by 28 weeks of age only 46% of the soy-reared females, 43% of the Soy + BPA females, and 56% of the BPA females had regular 4–5 day estrous cycles compared to 100% of the soy-free controls. (C) Numbers of CLs did not statistically differ between groups. (D) Soy-fed animals had greater numbers of cystic follicles (depicted in the inset) compared to conspecifics on the soy-free diet regardless of BPA exposure. Graphs depict group means ±SE; *P ≤ 0.05, ***P ≤ 0.001
By seven weeks of age, all of the females on the soy-free diet displayed and maintained a regular estrous cycle for the remainder of the experiment (Figure 3B). By 28 weeks of age only 46% of the soy-reared females, and 43% of the Soy + BPA females had regular 4–5 day estrous cycles compared to 100% of the soy-free controls. All of the acyclic females in the soy fed group were in persistent estrus, as were 21% of the acyclic Soy + BPA females. The remainder displayed prolonged (3 days or longer) estrus. Similarly, only 56% of the BPA exposed females on the soy-free diet were regularly cycling. Acyclic females in the BPA group were not in persistent estrous, but rather, a prolonged diestrus (3 days or longer) where some cornified cells remained and a few rounded cells, more characteristic of proestrus were present, resulting in a cellular mix not distinctive of any specific stage.
3.6 Adult Ovarian Histology
CL numbers in the adult ovaries (collected on the day of estrus) were not significantly impacted by diet or BPA exposure but CL numbers in the EE group trended lower (P = 0.06; Figure 3C). By contrast, there was a main effect of diet (F(1,61) = 11.09; P ≤ 0.001), but not exposure, on the number of cystic follicles with animals reared on the soy diet having significantly more cysts (P ≤ 0.001; Figure 3D). Compared to soy-free controls, EE exposed animals also had significantly more cysts (P ≤ 0.03). Histologically, no MOFs or primordial follicle clusters were observed in any of the animals.
3.7 Adult Circulating Androgen Levels
Circulating androgen levels in adulthood (sampled on the day of estrus) were not significantly impacted by exposure or diet nor was there a significant interaction (data not shown).
4. Discussion
Some PCOS-related hallmarks (cystic follicles, irregular estrus) and co-morbidities (elevated body weight and baseline serum glucose) were induced by soy diet, but BPA-related effects at the single dose employed were not consistent with a PCOS phenotype. Soy-attributable differences in glucose metabolism were small, with soy diet modestly increasing time to which levels returned to baseline. Animals were tested while still fairly young, however, so it is possible that this effect could be exacerbated by age. Hyperandrogenemia was not observed in any group but was only assessed at a single time point and is not consistently reported in rat models of PCOS [7]. The etiology of PCOS remains obscure and although BPA and other EDCs have been hypothesized to be potentially contributory [3, 4] the present results support a potential role for hormonally active diets but not BPA.
Available literature regarding the impact of BPA on rat ovarian development is limited but effects consistent with PCOS have previously been reported [19, 62]. These studies used significantly higher doses, shorter exposure windows (neonatal only) and direct injection, rather than oral administration, which can lead to higher internal doses of unconjugated (biologically active) BPA than was attained here (1.5 ng/ml or lower) or reported in humans (levels are controversial but approx. 4 ng/ml or lower) [49, 63, 64]. In the only published rodent study specifically examining a potential link between BPA and PCOS, Fernandez and colleagues reported that Sprague Dawley rat pups subcutaneously injected with 500 μg BPA per day (approx. 25 – 62.5 mg/kg) over the first 10 days of life had features consistent with PCOS including hyperandrogenemia, infertility, undersized ovaries, lower numbers of CLs, and atretic, cystic follicles [21]. Similar, but less pronounced, features were observed at 50 μg BPA (approx. 2.5 – 6.2 mg/kg) but none were noted at 5 μg BPA (approx. 0.25 – 0.62 mg/kg) a dose more consistent than the one used for the present studies (approx. 0.18 mg/kg to the dams during gestation, 0.35 mg/kg to the dams during lactation, and 0.44 mg/kg adolescent oral exposure to the pups). Because, for the present study, BPA was administered orally to the dam, and lactational transfer of BPA appears to be minimal [65], neonatal exposure was likely substantially lower in this study than in the one published by Fernandez and colleagues. Collectively, available rodent data remains limited but do not suggest elevated PCOS risk following developmental BPA exposure within a human-relevant range. Follow-up work spanning a broader dose range would enhance confidence in that reassuring but tenuous conclusion.
In PCOS patients, BPA levels have been positively associated with serum testosterone and androstenedione [12, 66], but this may be a consequence, rather than a cause, of PCOS because hyperandrogenemia has been shown to decrease BPA clearance [21]. Co-morbidities including elevated inflammation, increased body mass index, and insulin resistance have also been associated with higher serum BPA levels (reviewed in [15]). The significance of these relationships is difficult to interpret, however, because they are not prospective and thus cannot account for developmental exposure when BPA is hypothesized to most significantly impact risk. Further work is needed to better elucidate how EDCs that interfere with sex hormone signaling, particularly during ovarian development, may contribute to PCOS risk.
In contrast to BPA where effects are universally presumed to be “adverse,” soy, although well known to be endocrine disrupting, has been evaluated as a potential therapeutic for PCOS [67, 68]. This conflicting attitude about the pros and cons of soy is not atypical and at least partly attributable to the view that because it is “natural” it must be “healthy” [14]. So far, soy has not been found to be effective for treating any aspects of PCOS and the data generated herein suggest that hormonally active diets may, in fact, exacerbate risk. Isoflavones including GEN, daidzein and their respective metabolites, can cross the placenta and are secreted in mammalian milk so the potential for developmental exposure is higher than for BPA, particularly lactational exposure. Although it is appealing to hypothesize that the estrogenic isoflavones found in soy are the causal agents of the effects reported herein, further work will be needed to ascertain specifically how soy produces these features and over what dose and age range. That isoflavones such as GEN can impact female reproduction including ovarian development, ovulation, and fertility in rodents, sheep, and even humans is known (reviewed in [14] and [17]) but their potential relationship to PCOS is not well explored.
Equol, a metabolite of daidzein generated by intestinal microflora, has a higher estrogenic potency than GEN and other soy isoflavones and induces uterine hypertrophy [69, 70]. The single reproductive development study done to date, however, found no evidence that perinatal exposure to either of the two enantiomeric forms (250 mg/kg diet) compromises fertility or alters follicular features in young (PNDs 21 or 50) female rats [70]. One study in male rats has shown that equol can have anti-androgenic properties by binding, and thus deactivating, dihydrotestosterone [71]. Whether or not this happens in humans is unknown. Antiandrogenic action would presumably reduce PCOS risk, but equol has been recognized since the 1940’s to contribute to Clover Disease in legume-grazing sheep [72]; a condition characterized by impaired fertility, endometriosis, dystocia, abortion, and other female reproductive abnormalities. Although rats efficiently metabolize daidzein to equol, this is not the case for humans. At best, only 20–30% of Westerners and 50–60% of Asians are capable of making this bioconversion [73–75].
PCOS prevalence and symptomology have well characterized ethnic differences with the disorder appearing to be more common among Asians; populations in which soy consumption (and equol production) is historically high. In general, patients of Asian descent have a lower body mass index and mild hyperandrogenemia compared to Caucasians. South Asians, however, are at greater risk for type 2 diabetes, insulin resistance and metabolic syndrome [1]. PCOS is known to run in families, but the relative contributions of genetics and environment (including diet) remain poorly understood [76]. The present data suggest it is at least plausible that soy may contribute to risk, and could contribute to multigenerational prevalence; results which emphasize the need for further work exploring potential relationships between PCOS and hormonally active diets.
Further work in rat models should rule out related disorders which produce similar symptoms, such as hyperprolactinemia and thyroid dysfunction, particularly since soy is known to be goiterogenic and has other modes of action including inhibition of tyrosine kinases [14]. Species differences in PCOS-related characteristics should also be considered (reviewed in [7]). Notably, elevated androgen levels are not consistently observed in neonatally androgenized rats but reliably produced in non-human primates. Similarly, although levels of lutenizing hormone (LH) are typically higher in PCOS patients, and can thus be considered diagnostic, they are not consistently elevated in androgenized rodents. The Rotterdam consensus panel considered routine assessment of LH levels unnecessary, and they could not be measured here due to limited serum quantities. Rodent models using estrogen-induction have typically exposed older animals, but the resulting phenotype does not include altered follicular dynamics or the predicted metabolic co-morbidities so early life exposure to estrogen has not been implicated as a risk factor for PCOS. Here we found no clear EE-related PCOS phenotype, thus perinatal estrogen appears to be inadequate to generate a human-relevant model of PCOS in rats.
Finally, consistent with prior studies by us and others, evidence of early puberty was observed in both soy and BPA exposed females [19, 77, 78]. Soy fed females also had elevated CL numbers at puberty, an observation which suggests they were ovulating earlier, even though their estrous cycles were not yet regular, than conspecifics on a soy-free diet. It is unclear if this would have translated to an earlier age at first pregnancy or premature follicular depletion. Obesity can accelerate puberty and thus must be considered as a potentially contributory factor. In general, body weight was elevated by soy diet but the effect was transient and lost by early adulthood. This may indicate accelerated growth and development rather than increased adiposity. By contrast, elevated body weight in the BPA exposed animals was observed at all ages, and the soy diet mitigated this to some degree. Although concordant with prior work in rodents also showing BPA-related weight gain, and the working hypothesis that BPA is an “obesogen” [4, 79], the present data are insufficient to distinguish if BPA is enhancing adiposity or overall growth and development.
4.1 Conclusions
In conclusion, soy diet induces some ovarian and metabolic hallmarks of PCOS in rats, a phenotype not seen with BPA at the single, but environmentally-relevant, dose employed. The effects of soy may be attributable to the endocrine disrupting actions of isoflavones such as GEN and their respective metabolites, most notably equol, but further work is needed to more comprehensively explore this possibility. Effects were not entirely recapitulated by EE exposure suggesting a mode of action other than interference with estrogen signaling.
Research Highlights.
Polycystic ovarian syndrome (PCOS) has a prevalence as high as 8–15% depending on ethnicity.
Developmental exposure to endocrine disruptors is hypothesized to exacerbate risk.
We show that a soy-based diet, but not BPA, induces PCOS-like features in rats.
Exposure was oral throughout gestation and posnatal development, and in a human-relevant range.
We conclude that hormonally active diets throughout gestation and postnatal life may contribute to risk.
Acknowledgments
We thank Jessica Neville for her help with the dosing and animal husbandry as well as the staff of the NCSU Biological Resources Facility. We are also appreciate Wendy Jefferson for her guidance regarding the ovarian histology and Brian Horman for assisting with the ovarian phenotyping. This work was supported by NIEHS R21ES021233 to HPB.
Abbreviations
- AUC
area under the curve
- BPA
bisphenol A
- CL
corpora lutea
- EDC
endocrine disrupting compounds
- EE
ethinyl estradiol
- GD
gestational day
- GEN
genistein
- PCOS
polycystic ovarian syndrome
- PND
postnatal day
- VO
vaginal opening
Footnotes
Author Contributions
HP conceived of the project, contributed to the study design, data acquisition, analysis, data interpretation and drafted the manuscript; NM collected data and performed the experiments with the assistance of HBP and AWS. All authors provided comments and revisions of the manuscript for organization, completeness and intellectual content.
Conflict of interest
H Patisaul serves on the Science Advisory Board of the Glass Packaging Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Consensus on women’s health aspects of polycystic ovary syndrome (PCOS) Hum Reprod. 2012;27:14–24. doi: 10.1093/humrep/der396. [DOI] [PubMed] [Google Scholar]
- 2.Shayya R, Chang RJ. Reproductive endocrinology of adolescent polycystic ovary syndrome. BJOG. 2010;117:150–5. doi: 10.1111/j.1471-0528.2009.02421.x. [DOI] [PubMed] [Google Scholar]
- 3.Rutkowska A, Rachon D. Bisphenol A (BPA) and its potential role in the pathogenesis of the polycystic ovary syndrome (PCOS) Gynecol Endocrinol. 2014;30:260–5. doi: 10.3109/09513590.2013.871517. [DOI] [PubMed] [Google Scholar]
- 4.Barrett ES, Sobolewski M. Polycystic ovary syndrome: do endocrine-disrupting chemicals play a role? Semin Reprod Med. 2014;32:166–76. doi: 10.1055/s-0034-1371088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott DH, Zhou R, Bird IM, Dumesic DA, Conley AJ. Fetal programming of adrenal androgen excess: lessons from a nonhuman primate model of polycystic ovary syndrome. Endocr Dev. 2008;13:145–58. doi: 10.1159/000134831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manneras L, Cajander S, Holmang A, Seleskovic Z, Lystig T, Lonn M, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–91. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 7.Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod. 2012;86:149, 1–12. doi: 10.1095/biolreprod.111.097808. [DOI] [PubMed] [Google Scholar]
- 8.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 9.Lane DE. Polycystic ovary syndrome and its differential diagnosis. Obstet Gynecol Surv. 2006;61:125–35. doi: 10.1097/01.ogx.0000197817.93201.04. [DOI] [PubMed] [Google Scholar]
- 10.Carmina E. Diagnosis of polycystic ovary syndrome: from NIH criteria to ESHRE-ASRM guidelines. Minerva Ginecol. 2004;56:1–6. [PubMed] [Google Scholar]
- 11.Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, et al. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90:911–40. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96:E480–4. doi: 10.1210/jc.2010-1658. [DOI] [PubMed] [Google Scholar]
- 13.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Frontiers in Neuroendocrinology. 2010;31:400–19. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–55. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 16.NTP Multigenerational Reproductive Study of Genistein (CAS No. 446-72-0) in Sprague-Dawley Rats (Feed Study) Natl Toxicol Program Tech Rep Ser. 2008:1–266. [PubMed]
- 17.Jefferson WN, Patisaul HB, Williams CJ. Reproductive consequences of developmental phytoestrogen exposure. Reproduction. 2012;143:247–60. doi: 10.1530/REP-11-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FAO/WHO. Toxicological and Health Aspects of Bisphenol A: Report of Joint FAO/WHO Expert Meeting and Report of Stakeholder Meeting on Bisphenol A. World Health Organization; 2011. [Google Scholar]
- 19.Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal Bisphenol-A Exposure Alters Rat Reproductive Development and Ovarian Morphology Without Impairing Activation of Gonadotropin Releasing Hormone Neurons. Biol Reprod. 2009;81:690–9. doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newbold RR, Padilla-Banks E, Jefferson WN. Environmental estrogens and obesity. Mol Cell Endocrinol. 2009;304:84–9. doi: 10.1016/j.mce.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect. 2010;118:1217–22. doi: 10.1289/ehp.0901257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamanti-Kandarakis E, Christakou C, Marinakis E. Phenotypes and enviromental factors: their influence in PCOS. Current Pharmaceutical Design. 2012;18:270–82. doi: 10.2174/138161212799040457. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J. 2004;51:165–9. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- 24.Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. The Journal of Clinical Endocrinology and Metabolism. 2011;96:E480–4. doi: 10.1210/jc.2010-1658. [DOI] [PubMed] [Google Scholar]
- 25.Jefferson WN, Doerge D, Padilla-Banks E, Woodling KA, Kissling GE, Newbold R. Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environ Health Perspect. 2009;117:1883–9. doi: 10.1289/ehp.0900923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the female reproductive system by the phytoestrogen genistein. Reprod Toxicol. 2007;23:308–16. doi: 10.1016/j.reprotox.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust Vet J. 1946;22:2. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- 28.Adams NR. Detection of the effects of phytoestrogens on sheep and cattle. Journal of Animal Science. 1995;73:1509–15. doi: 10.2527/1995.7351509x. [DOI] [PubMed] [Google Scholar]
- 29.Jungbauer A, Medjakovic S. Phytoestrogens and the metabolic syndrome. J Steroid Biochem Mol Biol. 2014;139:277–89. doi: 10.1016/j.jsbmb.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia J, Greer F. Use of soy protein-based formulas in infant feeding. Pediatrics. 2008;121:1062–8. doi: 10.1542/peds.2008-0564. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, et al. Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. Journal of Exposure Science & Environmental Epidemiology. 2009;19:223–34. doi: 10.1038/jes.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998;68:1453S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- 33.Irvine CH, Shand N, Fitzpatrick MG, Alexander SL. Daily intake and urinary excretion of genistein and daidzein by infants fed soy- or dairy-based infant formulas. American Journal of Clinical Nutrition. 1998;68:1462S–5S. doi: 10.1093/ajcn/68.6.1462S. [DOI] [PubMed] [Google Scholar]
- 34.Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–7. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 35.Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, et al. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA. 2001;286:807–14. doi: 10.1001/jama.286.7.807. [DOI] [PubMed] [Google Scholar]
- 36.D’Aloisio AA, Baird DD, DeRoo LA, Sandler DP. Association of intrauterine and early-life exposures with diagnosis of uterine leiomyomata by 35 years of age in the Sister Study. Environ Health Perspect. 2010;118:375–81. doi: 10.1289/ehp.0901423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of soy formula. Birth Defects Res B Dev Reprod Toxicol. 2006;77:280–397. doi: 10.1002/bdrb.20086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuohy PG. Soy infant formula and phytoestrogens. J Paediatr Child Health. 2003;39:401–5. doi: 10.1046/j.1440-1754.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 39.Chapin RE, Adams J, Boekelheide K, Gray LE, Jr, Hayward SW, Lees PS, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- 40.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–61. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patisaul HB, Sullivan AW, Radford ME, Walker DM, Adewale HB, Winnik B, et al. Anxiogenic effects of developmental bisphenol a exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One. 2012;7:e43890. doi: 10.1371/journal.pone.0043890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muhlhauser A, Susiarjo M, Rubio C, Griswold J, Gorence G, Hassold T, et al. Bisphenol A effects on the growing mouse oocyte are influenced by diet. Biol Reprod. 2009;80:1066–71. doi: 10.1095/biolreprod.108.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–47. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- 44.Cooper S, Latendresse JR, Doerge DR, Twaddle NC, Fu X, Delclos KB. Dietary modulation of p-nonylphenol-induced polycystic kidneys in male Sprague-Dawley rats. Toxicol Sci. 2006;91:631–42. doi: 10.1093/toxsci/kfj171. [DOI] [PubMed] [Google Scholar]
- 45.Thigpen JE, Setchell KD, Ahlmark KB, Locklear J, Spahr T, Caviness GF, et al. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab Anim Sci. 1999;49:530–6. [PubMed] [Google Scholar]
- 46.Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb. 2007;14:245–52. doi: 10.5551/jat.e486. [DOI] [PubMed] [Google Scholar]
- 47.Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 2006;1068:49–55. doi: 10.1016/j.brainres.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 48.Kabuto H, Amakawa M, Shishibori T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 2004;74:2931–40. doi: 10.1016/j.lfs.2003.07.060. [DOI] [PubMed] [Google Scholar]
- 49.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–77. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal Bisphenol-A Exposure Alters Rat Reproductive Development and Ovarian Morphology Without Impairing Activation of Gonadotropin-Releasing Hormone Neurons. Biol Reprod. 2009;81:690–9. doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology. 2008;29:988–97. doi: 10.1016/j.neuro.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 53.Losa-Ward SM, Todd KL, McCaffrey KA, Tsutsui K, Patisaul HB. Disrupted Organization of RFamide Pathways in the Hypothalamus Is Associated with Advanced Puberty in Female Rats Neonatally Exposed to Bisphenol A. Biol Reprod. 2012;87:28. doi: 10.1095/biolreprod.112.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Losa SM, Todd KL, Sullivan AW, Cao J, Mickens JA, Patisaul HB. Neonatal exposure to genistein adversely impacts the ontogeny of hypothalamic kisspeptin signaling pathways and ovarian development in the peripubertal female rat. Reprod Toxicol. 2010;31:280–9. doi: 10.1016/j.reprotox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, et al. Accumulation and endocrine disrupting effects of the flame retardant mixture firemaster((R)) 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27:124–36. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod. 2006;74:161–8. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- 57.Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol Reprod. 2002;67:1285–96. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan AW, Hamilton P, Patisaul HB. Neonatal agonism of ERbeta impairs male reproductive behavior and attractiveness. Horm Behav. 2011;60:185–94. doi: 10.1016/j.yhbeh.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patisaul HB, Burke KT, Hinkle RE, Adewale HB, Shea D. Systemic administration of diarylpropionitrile (DPN) or phytoestrogens does not affect anxiety-related behaviors in gonadally intact male rats. Horm Behav. 2009;55:319–28. doi: 10.1016/j.yhbeh.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haseman JK, Bailer AJ, Kodell RL, Morris R, Portier K. Statistical issues in the analysis of low-dose endocrine disruptor data. Toxicol Sci. 2001;61:201–10. doi: 10.1093/toxsci/61.2.201. [DOI] [PubMed] [Google Scholar]
- 61.Ryan BC, Vandenbergh JG. Intrauterine position effects. Neurosci Biobehav Rev. 2002;26:665–78. doi: 10.1016/s0149-7634(02)00038-6. [DOI] [PubMed] [Google Scholar]
- 62.Kato H, Ota T, Furuhashi T, Ohta Y, Iguchi T. Changes in reproductive organs of female rats treated with bisphenol A during the neonatal period. Reprod Toxicol. 2003;17:283–8. doi: 10.1016/s0890-6238(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 63.Vandenberg LN, Gerona RR, Kannan K, Taylor JA, van Breemen RB, Dickenson CA, et al. A round robin approach to the analysis of bisphenol a (BPA) in human blood samples. Environ Health. 2014;13:25. doi: 10.1186/1476-069X-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doerge DR, Twaddle NC, Vanlandingham M, Brown RP, Fisher JW. Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague-Dawley rats. Toxicol Appl Pharmacol. 2011;255:261–70. doi: 10.1016/j.taap.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 65.Doerge DR, Vanlandingham M, Twaddle NC, Delclos KB. Lactational Transfer of Bisphenol a in Sprague-Dawley Rats. Toxicol Lett. 2010 doi: 10.1016/j.toxlet.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 66.Takeuchi T, Tsutsumi O. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem Biophys Res Commun. 2002;291:76–8. doi: 10.1006/bbrc.2002.6407. [DOI] [PubMed] [Google Scholar]
- 67.Romualdi D, Costantini B, Campagna G, Lanzone A, Guido M. Is there a role for soy isoflavones in the therapeutic approach to polycystic ovary syndrome? Results from a pilot study. Fertil Steril. 2008;90:1826–33. doi: 10.1016/j.fertnstert.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 68.Khani B, Mehrabian F, Khalesi E, Eshraghi A. Effect of soy phytoestrogen on metabolic and hormonal disturbance of women with polycystic ovary syndrome. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences. 2011;16:297–302. [PMC free article] [PubMed] [Google Scholar]
- 69.Setchell KD, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr. 2010;140:1363S–8S. doi: 10.3945/jn.109.119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown NM, Lindley SL, Witte DP, Setchell KD. Impact of perinatal exposure to equol enantiomers on reproductive development in rodents. Reprod Toxicol. 2011;32:33–42. doi: 10.1016/j.reprotox.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lund TD, Munson DJ, Haldy ME, Setchell KD, Lephart ED, Handa RJ. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol Reprod. 2004;70:1188–95. doi: 10.1095/biolreprod.103.023713. [DOI] [PubMed] [Google Scholar]
- 72.Shutt DA, Weston RH, Hogan JP. Quantitative Aspects of Phyto-Oestrogen Metabolism in Sheep Fed on Subterranean Clover (Trifolium-Subterraneum Cultivar Clare) or Red Clover (Trifolium-Pratense) Aust J Agr Res. 1970;21:713. [Google Scholar]
- 73.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 74.Setchell KD, Brown NM, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, et al. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr. 2003;133:1027–35. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 75.Lampe J, Karr S, Hutchins A, Slavin J. Urinary equol excretion with a soy challenge: influence of habitual diet. PSEBM. 1998;217:335–9. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- 76.Palep-Singh M, Picton HM, Vrotsou K, Maruthini D, Balen AH. South Asian women with polycystic ovary syndrome exhibit greater sensitivity to gonadotropin stimulation with reduced fertilization and ongoing pregnancy rates than their Caucasian counterparts. European journal of obstetrics, gynecology, and reproductive biology. 2007;134:202–7. doi: 10.1016/j.ejogrb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 77.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–4. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 78.Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Front Behav Neurosci. 2009;3:10. doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janesick A, Blumberg B. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Res C Embryo Today. 2011;93:34–50. doi: 10.1002/bdrc.20197. [DOI] [PMC free article] [PubMed] [Google Scholar]