Abstract
Infant faces elicit caregiving in adults. In previous research on brain responses to images of infant faces, the faces were unknown to participants. This study investigated EEG in primiparous mothers of 3- to 6-month-old infants viewing their own infant's face compared to an unfamiliar but appearance-matched infant's face. Spectral power was calculated and compared, and power at three EEG bands (delta, theta, and gamma) was found to differ between faces. Brain responses among primiparous mothers distinguish images of their own versus unfamiliar infants.
Keywords: mother-infant interaction, infant face
Human infants are born needing adult supervision and nurturing to survive, and infants possess structural and functional characteristics that help to ensure adult proximity and care, and ultimately, child survival and healthy development [1,2]. Prominent infant features include a facial morphology and a suite of communicative signals (cry, laugh, etc.) that activate sensitive and attuned caregiving behaviors in adults [3,4]. Several studies (e.g., [5,6]) have revealed that in human adults the activation of a specific need for care and affection of infants is modulated by deep cortical/subcortical structures and may be assessed by monitoring autonomic nervous system activity.
Caria et al. [7] reported functional magnetic resonance imaging (fMRI) data that supported specific adult responses. They found that human infant faces uniquely activated several brain systems, including the lateral premotor cortex, supplementary motor area, cingulate cortex, anterior insula, and thalamus; activation of these brain circuits suggests adults' preparation for responding to and communicating with infants as well as attachment and caregiving. Rapid recognition of one's own child facilitates prompt interaction and intervention, and from an evolutionary perspective, such interactions may be essential for child survival. The temporal aspects of own child recognition as well as the general effects of infant faces assume a central role in the investigation of adaptive maternal brain responses, but most previous studies used fMRI, which possesses high spatial resolution but low temporal resolution. It is therefore desirable to investigate maternal responsiveness to own versus unfamiliar infant faces using neuroimaging methods with higher temporal resolution, such as electroencephalography (EEG).
In the present study, we examined the electrophysiological correlates of face processing of own and unfamiliar infants in primipara mothers. The natural circumstance of the great investment of new mothers in their young infants, accompanied by close and consistent motherinfant interaction in the first months, provide an opportunity to evaluate the effects of stimulus familiarity and recollection of a unique and evolutionarily freighted circumstance on components of the EEG thought to be involved in face processing, recognition, and recollection. In a previous study, Bornstein, Arterberry, and Mash [2] reported that mothers of 3- to 6-month-old infants showed equivalent early-wave (N/P1 and N170) responses to own and unfamiliar infant faces, but differentiating late-wave (N/P600) activity to own versus unfamiliar infant faces. However, guided by the foregoing literature, we expected very early global difference in brain activity of mothers between two sets of stimuli. For this reason, we analyzed power spectra in those data to investigate effects of infant faces on fast- and slow-wave brain potentials in different time windows (time-frequency analysis). Thus, mothers of young infants viewed photographs of their own infant and an unfamiliar infant matched in age, skin tone, head shape, and eye and hair color. In this analysis of EEG data, the power of main frequency bands in different time windows activated by the two classes of contrasting stimuli (own versus unfamiliar infant) at different relevant scalp locations (frontal, occipital, parietal, temporal) was assessed to explore different stages in mothers’ processing of perceptual, attentive, and sustained/evaluative processes.
A number of studies point to brain oscillations as a mechanism for brain network integration that can exist across functional domains [8]. In this perspective, different frequency rhythms are associated with each domain. Evidence shows that delta oscillations depend on activity of motivational systems and are associated with salience detection and wakefulness in sustained attention [9]. Theta oscillations are involved in memory and emotional regulation [8-10]. The gamma band is associated with a wide variety of cognitive processes, but limited agreement has emerged so far in assigning a unitary basic function to these oscillations [11]. Nonetheless, it seems that gamma band activity relates to the comparison of memory contents with stimulus-related information and the utilization of signals derived from this comparison [11]. Gamma band oscillations also appear during cross-modal sensory processing involving relatively more conscious perception [12-14].
On the basis of this line of thinking, we expect that, because of the evolutionary need to recognize, preserve, and protect own offspring, own versus other infant faces would elicit electrophysiological brain activity that differs in terms of timing and frequency. We hypothesized that own infant face would elicit early activity relative to unfamiliar infant faces. Nonhuman and human data [11, 15] indicate that slow-wave delta and theta brain activity originating in deep cortical/subcortical structures is related to arousal, whereas fast-wave gamma brain activity, originating in the neocortical mantle, is associated with higher cognitive and regulatory functions.
Twenty-one primiparas of 3-month-old (n = 10) and 6-month-old infants (n = 11), M age = 32.06 years (SD = 4.66), participated. Participants were middle- to upper-middle socioeconomic status [16]. An additional 12 mothers were tested, but their data were not included due to experimenter error or equipment failure (5) or failure to meet the trial criterion for inclusion (6). Preliminary analyses revealed no differences in brain activity as a function of infant gender (n = 11 girls), so all subsequent analyses collapsed by infant gender.
At the start of the laboratory visit, digital photographs of infants’ faces were taken following informed consent. Infants were placed in an upright infant seat that was draped with a gray cloth. A second cloth was wrapped around the infant's neck and torso to eliminate the view of clothing. Multiple photographs were taken to select one in which each infant's facial expression was neutral. Each mother's infant's face (own) was paired with another infant face (unfamiliar) from our laboratory archive of images captured under identical conditions with respect to lighting, background, framing, and camera angle. Based on experimenter consensus, each unfamiliar infant was selected to closely match each mother's own infant's skin tone, head shape, age, and eye and hair color. Face images (12.55° by 15.94°) were presented to mothers on a computer screen against a black background.
Participants sat approximately 65 cm in front of the display and were instructed to minimize head and eye movements while fixating the screen. Mothers viewed 35 trials of the image of their own infant and 35 trials of the image of the unfamiliar infant, for a total of 70 trials presented in a uniquely randomized order for each mother. On each trial, a 100-msec baseline period with a fixation point preceded stimulus presentation. The stimulus appeared for 500 ms and was followed by a variable 1800- to 2200-ms inter-trial interval during which the computer screen was blue.
EEG was recorded with the EGI (Electrical Geodesics Incorporated, Eugene, OR) 128-channel EEG recording system (Net Station 4.1.1, sampling frequency 250 Hz). EEG data of the participants were used for preprocessing and analysis. For each mother, 3 trials of ‘own’ and ‘unfamiliar’ infant faces were used. For each trial, 100 ms of pre-stimulus data were used as baseline. EEG data were first detrended to avoid data drift. Then data were filtered using a bandpass filter of 0.16 Hz-100 Hz. After this, a notch filter around 50 Hz was applied.
EEG data were transformed in the time-frequency domain using Short Time Fourier Transformation (STFT) with a Hanning window of 25 samples (100 ms). Although we computed the entire possible 100 ms interval, starting from time 0 and stepping every 50 ms, responses appeared prominent over 4 time windows (0-100 ms, 700-800 ms, 750-850 ms and 850-950 ms). Furthermore, we analyzed 5 frequencies bands (delta, theta, alpha, beta, and gamma), and responses appeared prominent over 3 frequencies bands: delta (0-4 Hz), theta (4-8 Hz), and gamma (30-60 Hz).
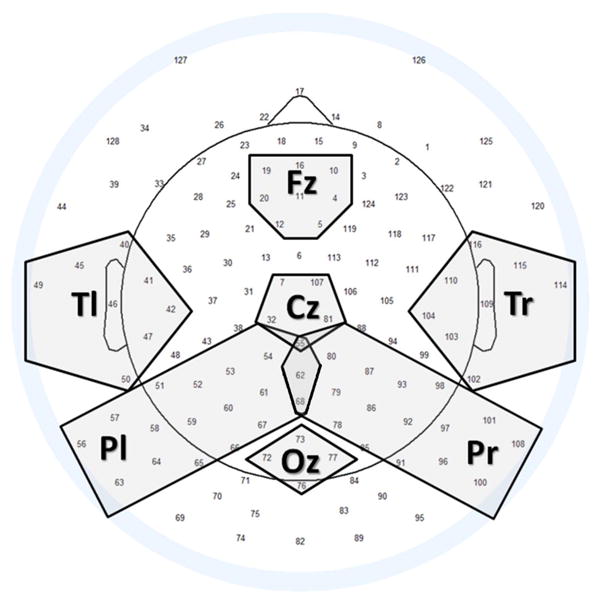
For each mother, averages of power difference in all ‘own’ trials and ‘unfamiliar’ trials were calculated separately for clusters of electrodes in central and lateral areas (Table 1 and Figure 1). Spectral power was averaged over time within widows, over frequencies within bands, and over sensors within clusters. For each cluster, we calculated paired t-tests between ‘own’ and ‘unfamiliar’ face stimuli to determine any statistical significance of differences in response to the two. For t-tests, the alpha value was set to 0.01.
Table 1. Sensor clusters used for ERP measurement Site EGI GSN sensors.
| FZ = 4, 5, 10, 16, 11, 12, 19, 20 |
| CZ = 7, 32, 55, 81, 107 |
| OZ = 72, 73, 76, 77 |
| TL = 40, 41, 42, 45, 46, 47, 49, 50 |
| PL = 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68 |
| TR = 102, 103, 104, 109, 110, 114, 115, 116 |
| PR = 55, 62, 68, 78, 79, 80, 85, 86, 87, 91, 92, 93, 96, 97, 98, 100, 101, 108 |
Fig. 1.

Maps of relative power spectra recorded in delta, theta and gamma band. Panels A, B, and C report relative power recorded during own and unfamiliar infant faces for delta, theta, and gamma frequency band, respectively. Significant differences between power levels measured during own and unfamiliar stimuli display: t*H (where t is the t test value and H is set to 0 or 1 in case of non significant or significant difference, respectively). Red indicates that activation for own infant faces is higher than for unfamiliar infant faces. Blue indicates that activation for unfamiliar infant faces is higher than for own infant faces. Color bar range of significant differences is equally set among all panels [from -4 to 4].
Results
At 0-100 ms, spectral power in the gamma band was greater in response to own (M = .06; SD = 0.12) than unfamiliar (M = -.10; SD = 0.22) infant faces at the midline occipital (Oz) cluster, t(21) = 3.14, p < .01, r2 = .33. No differences were found in this time window for the delta and theta bands.
At 700-800 ms, delta power was greater in response to unfamiliar (M = 2.29; SD = 4.95) than own faces (M = -1.65; SD =5.31) at the right temporal (Tr) cluster, t(21) = -3.44, p < .01, r2 = .38. Theta power at the same site was also greater in response to unfamiliar (M = 1.45; SD = 4.04) than own (M = -1.31; SD = 3.92) faces, t(21) = 3.19, p < .01, r2 = .34. No differences were found in this time window for the gamma band.
At 750-850 ms, delta power was greater in response to unfamiliar (M = 2.13; SD = 4.07) than own faces (M = -1.85; SD = 3.94) at the right temporal (Tr) cluster, t(21) = -3.60, p < .01, r2 = .40. Theta power at the same site was also greater in response to unfamiliar (M = 1.36; SD = 3.18) than own (M = -1.48; SD = 3.02) infant faces, t(21) = 3.42, p < .01, r2 = .37. No differences were found in this time window for the gamma band.
At 850-950 ms, delta power was greater in response to unfamiliar (M = 4.11; SD = 4.55) than familiar (M = 1.94; SD = 4.20) faces at the left temporal (Tl) cluster, t(21) = -3.31, p < .01, r2 = .37. No differences were found in this time window for the theta and gamma bands.
Discussion
Infants elicit caregiving responses in adults. The aims of present study were to investigate very early brain responses of primiparous mothers to own versus unfamiliar infant faces and to evaluate possible EEG power differences between mothers’ brain responses when looking at their own infant's versus an unfamiliar infant's face. EEG data were transformed in the time-frequency domain and analyzed at different scalp sites. Doing so, we observed rhythmic activity in specific ranges of frequency, pointing to specific biological significance associated with the responses.
As hypothesized, own versus other infant faces elicited differentiated brain activity in these frequency domains. Own infant face responses were characterized by lower delta and theta power at temporal sites. Many studies report that the inferior temporal gyrus responds selectively to faces and is involved in face recognition [17-24]. After 850 ms, a difference was found in Tl.
Pfurtscheller and Lopes da Silva [25] reviewed the literature on event-related oscillations and concluded that desynchronization of EEG (i.e., a decrease in power) can result from an increase in the excitability of cortical cells. Therefore, event-related desynchronization (ERD) can be interpreted as cortical activation associated with processing a task event, in the present context with recognizing a familiar face. They reasoned that larger ERDs result from recruitment of larger neural networks in processing. In gamma band frequencies, mothers’ responses were greater to own versus unfamiliar infant faces at Oz (0-100 ms). Oz overlays primary visual cortex and neighbors the associative visual cortex (V3, V4, V5). Consistent with Seeck [26], this result suggests that novel faces may be differentiated from known faces very early. Although there is reason for caution and further research (e.g., [27]), the difference observed here in the pattern of activation measured at Oz appears to reflect familiarity of the stimulus face. Before concluding, it is useful to consider that interpreting the comparison of electrophysiological responses between familiar and unfamiliar faces (as conducted in the present study) includes the possibility that the findings might depend on stimulus familiarity as much as responses uniquely associated with parental behavior. As discussed in [28] the electrophysiological modulation pattern of the N170 may be attributed to reliance on configural information (analysis of spatial relations among facial features) in the processing of a mother's own child's face.
Mothers’ brain responses differed between their own and unfamiliar infants’ faces. When mothers were shown their own infant's face, we detected an immediate, fast brain response; in contrast to when mothers looked at an unfamiliar infant face, the cortical activation patterns observed in the scalp topology were similar, but differed in magnitude in the opposite direction.
Highlights.
Brain responses among mothers distinguish images of their own versus unfamiliar infants
When mothers were shown their own infant's face, we detected an immediate, fast brain response
Unfamiliar infant faces activate similar cortical activation patterns, but opposite in magnitude and direction
Acknowledgments
All participants in this study are gratefully acknowledged. This research was supported by the ST fellowship from Japan Society for the Promotion of Science (JSPS PE09064), Grant-in-aid for Scientific Research from Japan Society for the Promotion of Science (Projects # 24730563 and #2402747), the FPR Program (RIKEN Brain Science Institute), and the Intramural Research Program of the NIH, NICHD (USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bornstein MH. Mother-infant attunement: A multilevel approach via body, brain, and behavior. In: Legerstee M, Haley DW, Bornstein MH, editors. The infant mind: Origins of the social brain. New York: Guilford; 2013. pp. 266–298. [Google Scholar]
- 2.Bornstein MH, Arterberry ME, Lamb ME. Development in infancy: A contemporary introduction. New York: Psychology Press; 2014. [Google Scholar]
- 3.Esposito G, Yoshida S, Ohnishi R, Tsuneoka Y, Rostagno MDC, Yokota S, et al. Infant Calming Responses during Maternal Carrying in Humans and Mice. Curr Bio. 2013;23:739–745. doi: 10.1016/j.cub.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 4.Doi H, Shinohara K. Event-related potentials elicited in mothers by their own and unfamiliar infants's faces with crying and smiling expression. Neuropsychologia. 2012;50:1297–1307. doi: 10.1016/j.neuropsychologia.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Esposito G, Nakazawa J, Ogawa S, Stival R, Kawashima A, Putnick DL, et al. Baby You Light-up My Face: Culture-General Physiological Responses to Infants and Culture-Specific Cognitive Judgements of Adults. PloS One. 2014 doi: 10.1371/journal.pone.0106705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito G, Nakazawa J, Ogawa S, Stival R, Putnick DL, Bornstein MH. Using Infrared Thermography to Assess Emotional Responses to Infants. Early Child Dev Care. 2014 doi: 10.1080/03004430.2014.932153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caria A, de Falco S, Venuti P, Lee S, Esposito G, Rigo P, et al. Species-specific response to human infant faces in the premotor cortex. NeuroImage. 2012;60:884–893. doi: 10.1016/j.neuroimage.2011.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knyazev GG, Slobodskoj-Plusnin JY, Bocharov AV. Event-related delta and theta synchronization during explicit and implicit emotion processing. Neuroscience. 2009;164:1588–1600. doi: 10.1016/j.neuroscience.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 9.Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci Biobehav R. 2007;31:377–395. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroen Clin Neuro. 1994;91:428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends in cognitive sciences. 2004;8(8):347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Kirmizi-Alsan E, Bayraktaroglu Z, Gurvit H, Keskin YH, Emre M, Demiralp T. Comparative analysis of event-related potentials during Go/NoGo and CPT: decomposition of electrophysiological markers of response inhibition and sustained attention. Brain Res. 2006;1104:114–128. doi: 10.1016/j.brainres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Kanayama N, Sato A, Ohira H. Crossmodal effect with rubber hand illusion and gamma-band activity. Psychophysiology. 2007;44:392–402. doi: 10.1111/j.1469-8986.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- 14.Meador KJ, Ray PG, Echauz JR, Loring DW, Vachtsevanos GJ. Gamma coherence and conscious perception. Neurology. 2002;59:847–854. doi: 10.1212/wnl.59.6.847. [DOI] [PubMed] [Google Scholar]
- 15.Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollingshead AB. Unpublished manuscript. Yale University: 1975. The four factor index of social status. [Google Scholar]
- 17.Schweinberger SR, Pickering EC, Jentzsch I, Burton AM, Kaufmann JM. Event-related brain potential evidence for a response of inferior temporal cortex to familiar face repetitions. Cognitive Brain Res. 2002;14:398–409. doi: 10.1016/s0926-6410(02)00142-8. [DOI] [PubMed] [Google Scholar]
- 18.Dobel C, Junghofer M, Gruber T. The Role of Gamma-Band Activity in the Representation of Faces: Reduced Activity in the Fusiform Face Area in Congenial Prosopagnosia. PloS One. 2011;6:5. doi: 10.1371/journal.pone.0019550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukatsu R, Fujii T, Tsukiura T, Yamadori A, Otsuki T. Proper name anomia after left temporal lobectomy: a patient study. Neurology. 1999;52:1096–1099. doi: 10.1212/wnl.52.5.1096. [DOI] [PubMed] [Google Scholar]
- 20.Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61:81–86. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- 21.Grabowski TJ, Damasio H, Tranel D, Boles Ponto LL, Hichwa RD, Damasio AR. A Role for Left Temporal Pole in the Retrieval of Words for Unique Identities. Human Brain Mapping. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haxby JV, Hoffman EA, Grobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Kawashima R, Sato N, Nakamura A, Sugiura M, Kato T, Hatano K, Ito K, Fukuda H, Schormann T, Zilles K. Functional delineation of the human occipito-temporal areas related to face and scene processing: A PET study. Brain. 2000;123:1903–1912. doi: 10.1093/brain/123.9.1903. [DOI] [PubMed] [Google Scholar]
- 24.Ross LA, Olson IR. Social Cognition and the Anterior Temporal Lobes. Neuroimage. 2010;49:3452. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 26.Seeck M, Michel CM, Mainwaring N, Cosgrove R, Blume H, Ives J, et al. Evidence for rapid face recognition from human scalp and intracranial electrodes. Neuroreport. 1997;8:2749–54. doi: 10.1097/00001756-199708180-00021. [DOI] [PubMed] [Google Scholar]
- 27.Caharel S, Ramon M, Rossion B. Face familiarity decisions take 200 msec in the human brain: Electrophysiological evidence from a go/no-go speeded task. J Cognitive Neurosci. 2014;26:81–95. doi: 10.1162/jocn_a_00451. [DOI] [PubMed] [Google Scholar]
- 28.Doi H, Shinohara K. Electrophysiological responses in mothers to their own and unfamiliar child's gaze information. Brain Cognition. 2012;80:266–276. doi: 10.1016/j.bandc.2012.07.009. [DOI] [PubMed] [Google Scholar]


