Abstract
Why do some individuals succumb to stress and develop debilitating psychiatric disorders, whereas others adapt well in the face of adversity? There is a gap in understanding the neural bases of individual differences in the responses to environmental factors on brain development and functions. Here, using a novel approach for screening an inbred population of laboratory animals, we identified two sub-populations of mice: susceptible mice that show mood-related abnormalities compared to resilient mice, which cope better with stress. This approach combined with molecular and behavioral analyses, led us to recognize, in hippocampus, presynaptic mGlu2 receptors, which inhibit glutamate release, as a stress sensitive marker of individual differences to stress-induced mood disorders. Indeed genetic mGlu2 deletion in mice results in a more severe susceptibility to stress, mimicking the susceptible mouse sub-population. Furthermore, we describe an underlying mechanism by which glucocorticoids, acting via mineralocorticoid receptors (MR), decrease resilience to stress via down regulation of mGlu2 receptors. We also provide a mechanistic link between MR and an epigenetic control of the glutamatergic synapse that underlies susceptibility to stressful experiences. The approach and the epigenetic allostasis concept introduced here serve as a model for identifying individual differences based upon biomarkers and underlying mechanisms and also provide molecular features that may be useful in translation to human behavior and psychopathology.
Introduction
It is well known that identical twins differ as they mature in behavior, physiology and susceptibility to disease. This is reflected in patterns of DNA methylation that diverge as a result of non-shared experiences as the lifecourse unfolds (1). Recently, Freund et al. showed that genetically identical mice, living in an enriched environment, displayed differences in exploratory activity that diverged over time, resulting in increasing individual differences that correlated positively with individual differences in adult hippocampal neurogenesis (2). Furthermore, individual differences in anxiety-like behaviors among genetically similar rats, living in the same environments and not previously exposed to experimental manipulations, have been shown to predict lifespan (3) and prefrontal cortical dendritic length (4). Stressful experiences superimposed on top of such individual differences can lead (5, 6) to susceptible individuals developing debilitating stress-related mental and physical health disorders (7, 8, 9); whereas more resilient individuals are able to recover from the same stressors or do not respond to it in the first place (10), displaying cognitive flexibility (11).
However, there is a gap in understanding how the hippocampus and prefrontal cortex (PFC), which are involved in the pathophysiology of mood-related behaviors, integrate the molecular processes of stress responsivity, conferring a different individual susceptibility to psychopathologies (12). Moreover, individual differences in stress sensitivity can have direct effects on the response to pharmacological agents. Stressful events can also precipitate psychopathological episodes in susceptible individuals and aggravate an individual's predisposition for suicidal ideation (13, 14). Thus, understanding the molecular bases of individual stress responsiveness paves the way to a better understanding of individual differences in brain function that subserve successful vs unsuccessful coping. In the case of susceptible individuals, this may provide a mechanistic basis for developing rapidly acting pharmaceutical agents that, together with psychotherapy, will improve mood and reduce the probability of suicide as well as improve the quality of life.
Glutamate, the principal neurotransmitter of the mammalian brain, plays a major role not only in normal brain function but also in the pathophysiology of stress-related mental and neurological disorders (15). Indeed, glutamate homeostasis is mainly regulated by presynaptic mGlu2 receptors, which exert an inhibitory tone on glutamate release into the synaptic space. Recently, we showed that mGlu2 receptors are involved in stress-related disorders and in the mechanisms of action of antidepressant drugs (16). Patients with major depressive disorder (MDD) show a positive correlation between increased glutamate serum levels (17) and the severity of depressive symptoms (18). It is also known that stress-induced dysregulation of glutamate release leads to shrinking of dendrites in CA3 neurons, loss of spines in the CA1 region and suppression of dentate-gyrus neurogenesis, reinforcing the importance of regulating hippocampal glutamatergic activity (19).
Here we addressed the intriguing question as to whether differences in mGlu2 receptors may be involved in the individuality of the brain response to stress. Previously, we discovered that the depressive-like behavior of Flinders Sensitive Line (FSL) rats, a genetic animal model of depression (20), is associated with a selective epigenetically-induced down-regulation of mGlu2 receptors, and that mGlu2 knock-out mice fail to respond to the recently recognized rapidly-acting antidepressant candidate, acetyl-L-carnitine (LAC) (16, 21, 22). Based upon this, we hypothesized that wild type mice might differ in their individual susceptibility to stressors and those mice which are more susceptible might have lower mGlu2 levels. First, we used a chronic unpredictable stressor (CUS), in which we previously showed efficacy of LAC in preventing depressive-like behavior (16), to assess individual differences in mGlu2 receptor expression and in mood-related behavioral traits. We found a subset of wild-type mice showing more depressive-like behavior after CUS with lower mGlu2 expression compared with other mice that were much less affected by CUS and which had higher mGlu2 expression. We then devised a simple, acute screening method for rapidly identifying susceptible vs resilient animals using minimal stress. Finally, we asked whether adrenal steroids, which participate in positive as well as negative actions of stress on the brain (23, 24, 15) would play a key role in the epigenetic regulation of mGlu2 receptors via mineralocorticoid (MR) and/or glucocorticoid (GR) receptors.
Materials and methods
Animals
Male C57black mice (6 wk old, 20–25 g) and mGlu2 receptor knockout mice were housed five per cage under controlled conditions (12-h light/dark cycle, 22°C, food and water ad libitum); they were and individually gently handled daily for 1week before the beginning of the stress procedures. Once a week, body weights were recorded and the condition of their coat and eye conjunctiva was evaluated according to the coat-state rating scale (detailed in SI and SI table 1) by two independent observers before the start of each behavioral test session.
A novel approach to subdivide animals in HS and LS clusters
We developed a novel and acute approach to subdivide animals in high-susceptible (HS) and low-susceptible (LS) clusters. The following statistical approach was used for the assignment of mice to HS and LS clusters. Mice subjected to CUS were divided into high- and low-susceptible subpopulations based on their immobility time at the FST and their sucrose intake. CUS-treated mice, which fell into the standard deviation from the mean of the control group, were considered low-susceptible (LS) animals as they showed a behavior comparable to unstressed mice. Animals that fell outside the standard deviation of the mean of the control group were designated as high-susceptible mice (HS).
Similarly, in the evaluation of mice after the light-dark test, mice falling inside and outside the standard deviation from the mean of the control group were respectively designated as low- and high-susceptible mice. This was done for mice in the acute restraint stress experiment and also for naïve mice after the light-dark test without prior acute stress.
Treatments
The selective MR-antagonist, spironolactone (20 mg kg–1 i.p., Sigma Aldrich, St. Louis, MO, US), and the selective GR-antagonist mifepristone (20 mg kg–1 i.p., formerly known as RU486, Sigma Aldrich, US) were injected i.p. 3 hours prior to the acute restraint stress as schematized in Fig.6A. The doses were selected on the basis of previously published reports (25).
Stress procedures
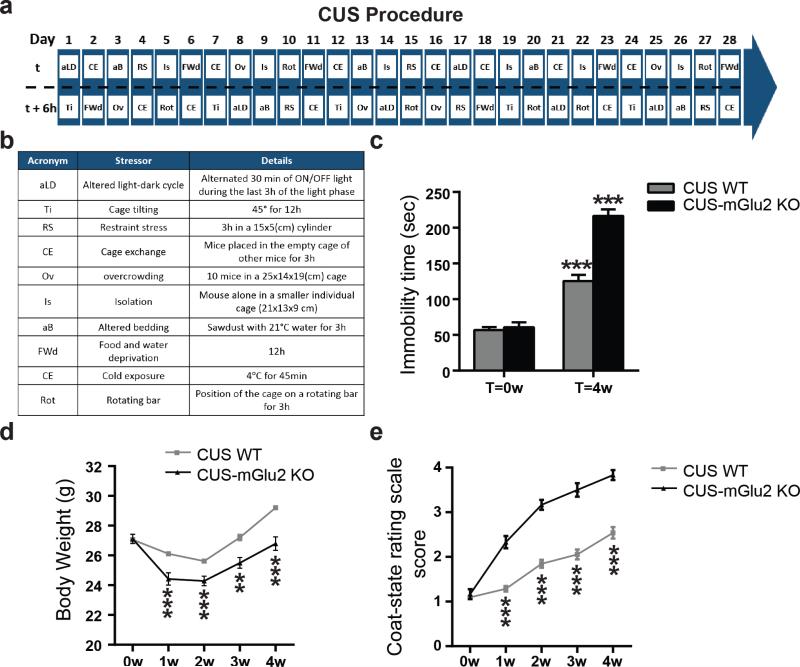
Chronic unpredictable stress was performed as detailed in figure 1A and 1B. Mice were subjected two times daily for 28 days to two different stressors accordingly to a random schedule to avoid any kind of habituation. Tissues were collected 12 hours after the last episode of stress. The different types of stressor used are detailed in the table in figure 1B. Control mice were left undisturbed in their home cages until euthanized, except for the body weight measurement and behavioral tests.
Figure 1. mGlu2 knockout mice show a severe susceptibility to stress.
a and b, Time course and design of the chronic unpredictable stress (CUS). c, CUS results in a higher immobility time at the FST in mGlu2 knockout mice compared to wild-type mice (F3,72=64.02). Bars represent mean + SEM, * indicate significant comparisons to all other values, ***p < 0.0001. d and e, Body weight and scores at the coat-rating scale over the 4 weeks of CUS in wild-type mice and mGlu2 knock-out mice. Data represent mean + SEM, * indicate significant comparisons to corresponding controls, **p < 0.01, ***p < 0.0001.
For the acute stress procedure, naïve C57bl mice were stressed for 2hrs followed by 2hr recovery in their home cages before the light-dark behavior test and euthanized 15 min later. The restraint device contained a 0.4 cm air hole and allowed mice to stretch their legs but not to move within the tube. Age-matched animals were used as control for each group. Control mice were left undisturbed in their home cage until the behavioral test followed by euthanization. For gene analysis by RT-PCR, after rapid decapitation, hippocampal and PFC tissues were dissected, flash frozen and stored at −80 °C until processing.
Additional naïve C57bl mice were not stressed and were screened using the LDT. Then, 15 minutes after the LDT, mice were euthanized to harvest brains for dissection of hippocampus and RT-PCR.
Behavioral tests
Forced swim test
The duration of immobility at the forced swim test was scored by blind observers, who were not aware of the groups. Mice were placed individually into a vertical glass cylinder (25 cm in height and 12 cm in diameter) filled with 12-cm-deep water at 23–24 °C. A 15-min pretest (habituation session) was carried out 24 h before the first test session. In each test session, animals were placed in the cylinder for 5 min and videotaped. Mice were considered “immobile” if they showed only minimal movements to keep the head above water or floating.
Sucrose preference test
The sucrose preference test has been performed as previously described (16). Mice were given a free choice between two bottles, one with 2% sucrose solution and another with water, for 24 hr (SI). To prevent possible effects of side preference in drinking behavior, the position of the bottles was switched after 12 h. No previous food or water deprivation was applied before the test. The consumption of water and sucrose solution was estimated in control and experimental groups by weighing the bottles.
Light dark test
The light dark test was performed as previously described (26). The apparatus consisted of a rectangular Plexiglas box (20×50×20 cm) with a black chamber comprising 1\3 of the total volume. The two chambers were separated by a Plexiglas septum with an open door (12×5 cm) that permitted the passage from the illuminated chamber (400 lx) to the enclosed dark chamber (4 lx). Mice were videotaped, and the time spent by each mouse in the light chamber was measured. Mice were considered to have entered a chamber when all four paws were positioned into the chamber. No pretest (habituation session) was carried out prior to the test session.
Molecular procedures
Gene expression, western blot and chromatin immunoprecipitation methods were performed as previously described (20). Details in SI.
Statistics
Statistical analyses were performed using the two-tailed unpaired Student's t tests (for comparison of two groups), one-way ANOVAs followed by Tukey's test for the post hoc analysis (for three groups) and one-way repeated-measure ANOVAs. Correlations between the behavioral tests were analyzed by Spearman's correlation.
Results
mGlu2 knock-out mice show a higher susceptibility to CUS
Previously, we showed that mGlu2 knock-out mice fail to respond to the newly recognized rapid antidepressant candidate, acetyl-L-carnitine (16, 21, 22). Here, we determined whether a genetic depletion in mGlu2 receptors may be involved in the behavioral response to stress and whether there are individual differences in wild type mice in response to chronic unpredictable stress (CUS, Fig.1a and b). This stress paradigm has face validity in its ability to model the symptoms of stress-related disorders (27). We subjected C57black and mGlu2 knock-out mice on the same background to CUS. Importantly, mGlu2 knock-out mice subjected to CUS show a higher immobility time at the forced swim test (Fig.1c) as well as a lower weight gain (Fig.1d) and a severe deterioration of the coat at the coat-state rating scale (Fig.1e) compared to wild-type CUS-mice.
Chronic unpredictable stress results in more (HS) and less (LS) susceptible endophenotypes
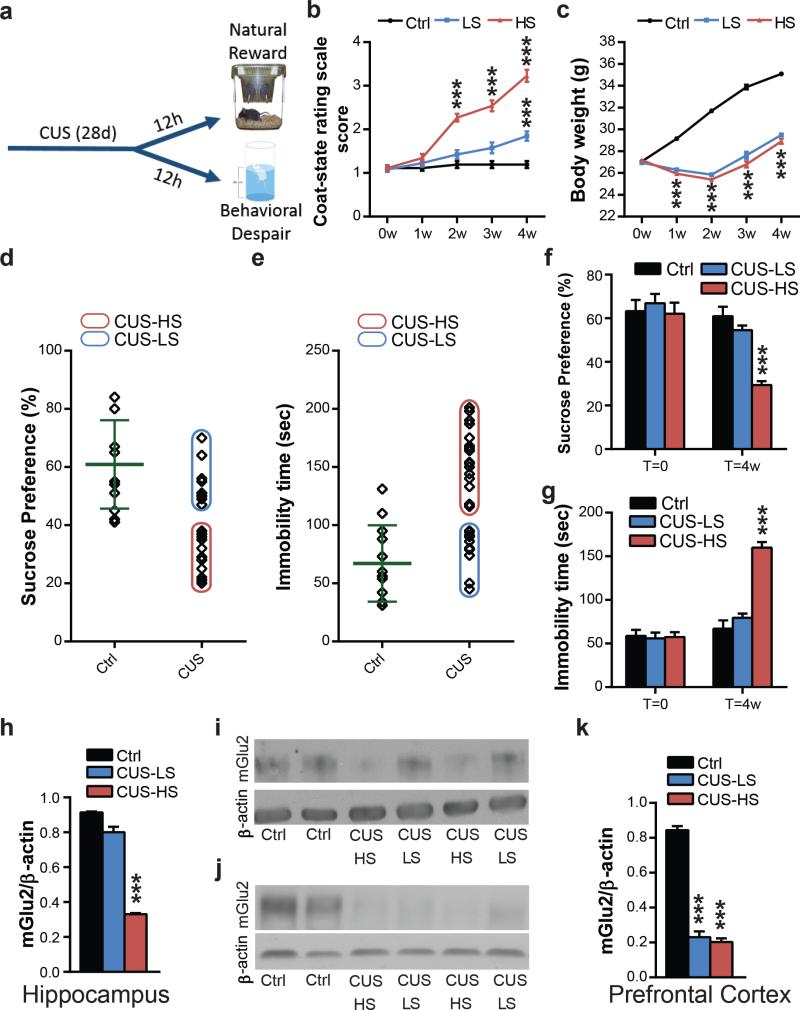
We next asked whether environmental stressors may lead to individual differences in wild-type mice in the response CUS upon mood-related behavioral traits. We found that CUS exposure resulted in significant differences in coat state deterioration at the coat-state rating scale (Fig.2b) between what we designated as high-susceptible (HS) and low-susceptible (LS) mice (Fig.2d and 2e). No differences in weight gain between HS and LS-mice were observed (Fig.2c).
Figure 2. Chronic unpredictable stress results in more (HS) and less (LS) susceptible endophenotypes and in individual difference in mGlu2 receptor expression: a molecular signature of susceptibility.
a, Time course of the CUS and behavioral outcome analyses b, CUS results in a clear separation in HS and LS-endophenotypes at the coat-state rating scale for the evaluation of the CUS-induced coat deterioration (F14,375=47.19). c, Animal body weight over the four weeks of CUS (F14,375=206.5; F25,350=3.62). d and e, Identification of high and low-susceptible subgroups based on the immobility time at the sucrose intake (d) and at the forced swim test (e) in CUS-mice. Green lines indicate the mean (60.92 in d; 67.08 in e) and the standard deviation (in 15.20; 32.82 in c) of the control group. f, HS-mice show a lower sucrose intake at the sucrose preference test compared to LS wild-type mice subjected to CUS and unstressed mice (F5,66=11.61). g, HS-mice show a higher immobility time at the FST compared to LS wild-type mice subjected to CUS and unstressed mice (F5,74=40.67). Bars represent mean + SEM, * indicate significant comparisons to corresponding controls, ***p < 0.0001. h and i, Western blot analysis and representative blots of HS-mice show lower mGlu2 hippocampal receptor expression compared to CUS-LS mice and unstressed mice (F2,15=264.7). j and k, Western blot analysis and representative blots of mGlu2 receptor expression in the prefrontal cortex (F2,15=185.9) shows a strong impairment in both CUS-HS and CUS-LS mice compared to unstressed mice. Bars represent mean + SEM, * indicate significant comparisons to respective control groups, ***p < 0.0001.
We then assessed natural reward preference and behavioral despair (Fig.2a) using, respectively, the sucrose preference test and the forced swim test. Anhedonia is a hallmark symptom of MDD that in rodents is evaluated by a decrease in the preference for a sucrose solution compared with water at the sucrose preference test (SPT). Remarkably, CUS-HS-mice showed a deficit in natural reward in the SPT and an increase in the immobility time at the forced swim test (FST) compared to CUS-LS-mice. In contrast, CUS-LS-mice showed no difference in sucrose intake and in immobility time at the FST compared to unstressed-mice (Fig.2f and g).
Hippocampal mGlu2 impairment in HS mice: a molecular signature of stress susceptibility
Next, we inquired whether stress-susceptible (HS) and resilient-mice (LS) differ in their expression of mGlu2 receptors within the hippocampus and PFC. Notably, CUS-HS-mice showed a significantly lower expression of mGlu2 receptor within the hippocampus compared to CUS-LS-mice (Fig.2h and i). No difference in hippocampal mGlu2 receptor expression was detected between CUS-LS-mice and unstressed-mice. Furthermore, both CUS-LS and CUS-HS-mice showed a substantial 82% lower mGlu2 receptor expression within the PFC compared to control mice (Fig.2j and k) and thus PFC mGlu2 expression does not distinguish between LS and HS mice.
Acute restraint stress results in behavioral and molecular individual differences that are reminiscent of the HS and LS-endophenotypes induced by CUS
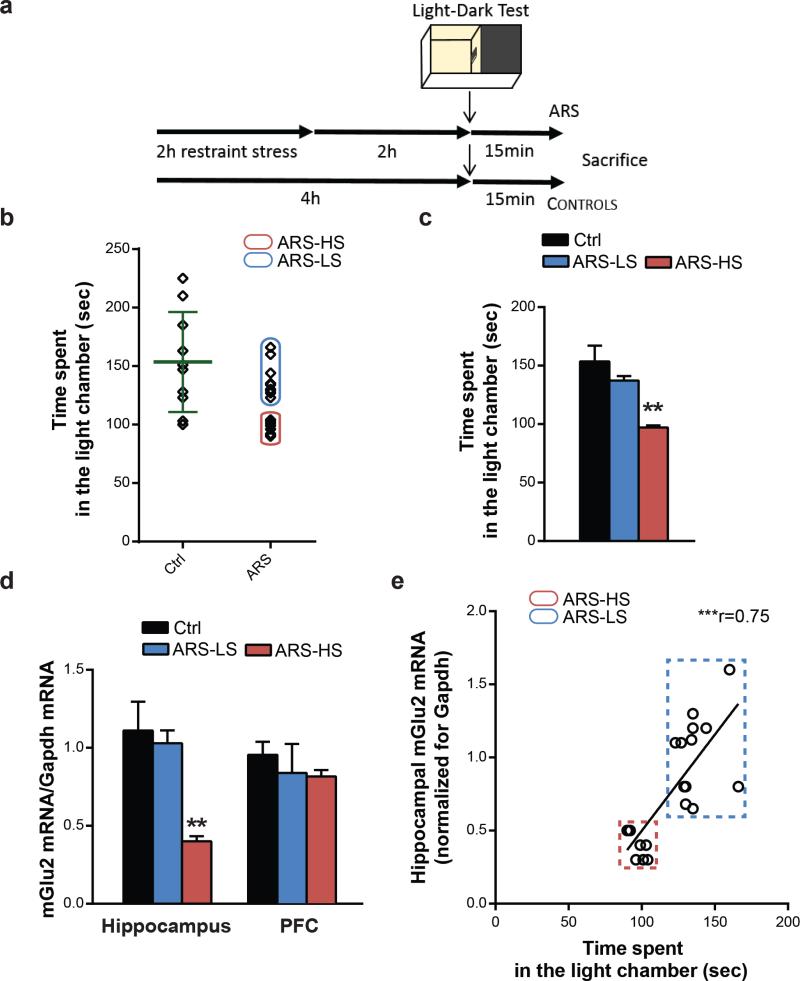
To determine whether individual differences could be detected also after acute stress of naïve mice, we evaluated the response to an acute restraint stress (ARS, Fig.3a) paradigm assessing anxiety-related behavior and examining resultant effects on mGlu2 transcript levels. After ARS, we were able to distinguish what appears to be HS-mice, which, as a group, showed a 37% decrease in the time spent in the light chamber at the light-dark test (LDT) (26) compared with another group of mice that we designated as LS because they showed only a 9% decrease (Fig.3b,c). Moreover, among these mice, acute stress behavioral effects correlated with a strong 64% and a mild 6% down-regulation in mGlu2 transcript levels within the hippocampus of HS and LS-mice, respectively, whereas no change was found in PFC after ARS (Fig.3d). A Spearman's correlation analysis showed a substantial positive correlation of hippocampal mGlu2 mRNA levels and time spent in the light chamber at the LDT in HS and LS-mice (Fig.3e).
Figure 3. A single episode of stress results in more and less susceptible endophenotypes reminiscent of the CUS-induced HS and LS animal clusters.
a, Time course and design of the acute stress and behavioral outcome. b, Identification of high and low-susceptible subgroups based on the time spent in the light chamber at the light dark test in acute restraint stressed-mice. Green lines indicate the mean (153.5) and the standard deviation (42.72) of the control group. c, Acute restraint stress results in behavioral and molecular individual differences that are reminiscent of the CUS HS and LS-endophenotypes. ARS-HS mice spent less time in the light chamber at the LDT compared to ARS-LS mice and unstressed mice (F2,27=10.75). d, ARS-HS mice show lower mGlu2 mRNA transcript levels compared to ARS-LS mice and unstressed mice within the hippocampus (F2,27=8.84) and no difference within the prefrontal cortex (F2,17=0.57). In a and c, bars represent mean + SEM, * indicate significant comparisons to all other values, **p < 0.01. e, Spearman's correlation analysis of hippocampal mGlu2 mRNA levels and time spent in the light chamber at the LDT in HS and LS-mice (r=0.75, p<0.0001).
Mineralocorticoid receptors (MR) regulate the stress-induced impairment in mGlu2 receptor through a p300-driven epigenetic mechanism
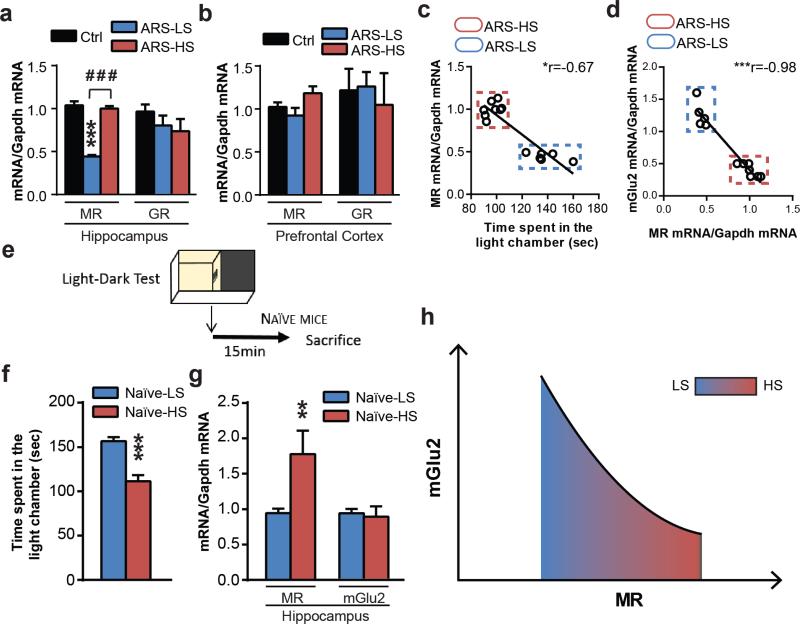
Since glucocorticoids play a pivotal role in regulating the tone of the stressed-glutamatergic synapse (15), we measured the levels of MR and GR transcripts in LS and HS mice. After ARS and the LDT, LS mice showed lower MR hippocampal transcripts compared to HS mice (Fig.4a) and no difference in hippocampal GR transcript levels (Fig.4a). No change in MR or GR levels was found between LS and HS mice within the PFC (Fig.4b). Spearman's correlation analyses show a large negative correlation of hippocampal MR mRNA levels and time spent in the light chamber at the LDT in HS and LS mice (Fig.4c) and a large negative correlation of hippocampal mGlu2 mRNA levels and MR mRNA levels (Fig.4d).
Figure 4. Glucocorticoids, via hippocampal down-regulation of MR receptors, lead to the loss of suppression of mGlu2 transcripts in resilient mice.
a, Acute restraint stress results in lower MR mRNA transcript levels in LS mice compared to HS mice and unstressed mice within the hippocampus (F2,25=44.55) and no difference in GR levels (F2,12=1.32). b, ARS-LS mice compared to ARS-HS mice and unstressed mice show no difference in either MR or GR transcript levels within the PFC (MR: F2,17=2.13; GR:F2,13=0.13). In a and b, bars represent mean + SEM, * indicate significant comparisons to to all other values, **p < 0.01. c, Spearman's correlation analysis of hippocampal MR mRNA levels and time spent in the light chamber at the LDT in HS and LS-mice (r=-0.67, p=0.01). d, Spearman's correlation analysis of hippocampal MR mRNA levels and hippocampal mGlu2 mRNA levels (r=-0.98, p<0.0001). e, Design of the behavioral outcome on naïve mice. f, Naïve mice show behavioral differences in the time spent in the light chamber at the LDT. g, Naïve mice show different MR mRNA transcript levels based on their baseline susceptibility and no difference in mGlu2 mRNA transcript levels. In e and f, bars represent mean + SEM, * indicate significant comparisons, **p < 0.01, ***p < 0.001. h, In the hippocampus, the relationship between MR and mGlu2 mRNA levels can be described by a quadratic curve that models the epigenetic allostasis concept described in the text.
Furthermore, we wondered whether reduced MR transcripts in resilient LS mice might be lower compared to HS mice because of an effect of earlier “non-shared” experiences (e.g., differential exposure to maternal care). Thus, we screened an inbred population of C57bl mice using the light-dark test screening method for HS vs LS mice without any prior acute stress (fig.4f) and we measured MR and mGlu2 transcripts in hippocampus. We sacrificed animals immediately after the end of the LDT to minimize any effects due to the minimal stress manipulation (fig.4e). Interestingly, the naïve mouse subgroup that we designed as naïve-LS mice, based upon lower scores in the light-dark test, show reduced MR transcripts in hippocampus compared to the naïve mouse subgroup that we designed as naïve-HS mice (fig.4g). No difference in mGlu2 transcripts was found in these stress-naive mice (fig.4g) suggesting that MR levels are markers of the anxiety level in the light-dark test and might mediate the stress-induced reduction of mGlu2 expression.
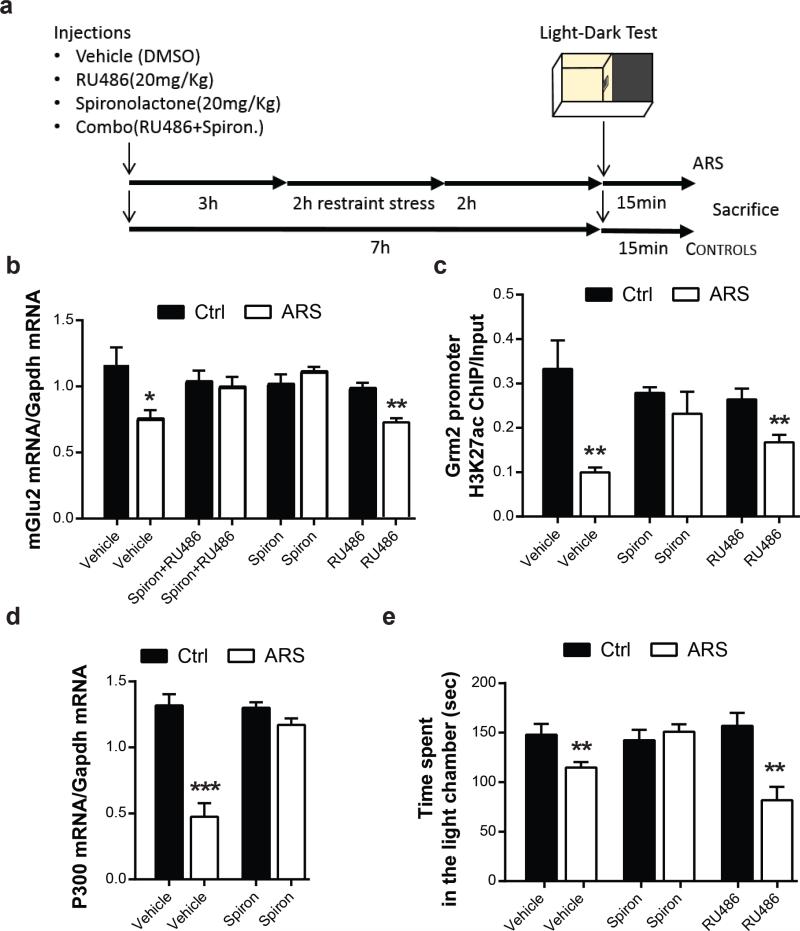
To establish the selective inhibitory role of MR activation on mGlu2 transcription we tested the effects of the selective MR-antagonist, spironolactone, as well as the selective GR-antagonist, RU486, which were administered 3 hours prior to an acute episode of restraint stress (Fig.5a). We did so on naïve C57bl mice without sorting into LS and HS animals, and we found that either the combination of GR- and MR-antagonists, or the injection of spironolactone alone, blocked the acute stress-induced down-regulation of mGlu2 receptors within the hippocampus (Fig.5b). As expected, because of the likely presence of both LS and HS mice, there was scatter in the data (SI Fig 1) but the overall spironolactone effect was highly significant and would undoubtedly have been higher had we used only HS mice. Thus, MR activation mediates an inhibition of mGlu2 transcription, whereas RU486 in a dose sufficient to alter spine synapse turnover in mouse cortex (25) was unable to block the rapid stress effect on mGlu2 transcript levels (Fig.5b).
Figure 5. Glucocorticoids, via MR receptors, link stressful experience to an epigenetic control of the glutamatergic synapse.
a, Time course and design of the treatments with the selective GR and MR antagonists. b, Spironolactone (20mg/kg, i.p.) alone or in combination with RU486 (also known as mifepristone, 20mg/kg, i.p.), but not RU486 alone, administered 3 hrs prior to the stress exposure, blocks the stress-induced decrease in mGlu2 mRNA transcript levels in the hippocampus of ARS mice c, Chromatin immunoprecipitation assay shows that spironolactone, but not RU486, blocks the stress-induced decrease in H3K27ac bound to the Grm2 promoter gene within the hippocampus. d, Spironolactone abolishes ARS reduction in the HAT p300 mRNA levels. e, Spironolactone, but not RU486, blocks the ARS-induced anxiety-related behavior at the light-dark test. Bars represent mean + SEM, * indicate significant comparisons to control groups, *p < 0.05, **p < 0.01, ***p <0.0001.
To explore the possible mechanistic link of stressors to an epigenetic control of the glutamatergic synapse, a second series of studies was carried out, again on naïve C57bl mice without selection for HS or LS endophenotype. Here, using chromatin immunoprecipitation assay (ChIP), we found that spironolactone, in contrast to RU486, blocked the stress-induced decrease in the level of H3K27ac, a transcriptional activity marker bound to the GRM2 promoter gene that regulates mGlu2 expression (Fig.5c). This occurs, at least in part, through the prevention by spironolactone of the stress-induced decrease in the acetyltransferase (HAT) p300 (Fig.5d), revealing a selective MR-mediated epigenetic control of the mGlu2 receptor. Moreover, in contrast to RU486, spironolactone also blocked the acute stress-induced anxiety behavior in the LDT (Fig.5e). Thus, a rapid epigenetic action of glucocorticoids via MR appears to be involved in the acute-stress effects on anxiety-related behavior and mGlu2 transcript down-regulation in hippocampus.
Discussion
A same event may be more or less stressful or have pathophysiological consequences for one individual compared to another depending on prior experiences even when the genotype is similar or identical, as for identical twins (1) or genetically homogeneous mice (2). Our findings show that both chronic unpredictable stress and acute restraint stress results in individual differences in an inbred population of wild-type mice that are more (HS) or less (LS) susceptible to stress-induced mood abnormalities as measured by tests of depression- and anxiety-related behaviors and coat appearance. HS mice, reflecting a more anxiety-prone trajectory of development, show a deficit in natural reward and a behavioral despair after CUS (depressive-like behaviors) that correlate with a substantial reduction of the subtype mGlu2 receptors in hippocampus. Indeed, mGlu2 knock-out mice mimic the behavioral responses shown by the HS-endophenotype. Similarly, wild-type mice subjected to ARS and subdivided in HS and LS mice using the rapid screening method introduced here, show individual differences in anxiety-related behavior in a light dark test and display reduced mGlu2 expression in hippocampus; this is reminiscent of the HS and LS-endophenotypes induced by the more prolonged CUS. Thus, after both ARS and CUS, stress susceptible HS mice are recognized by having reduced mGlu2 expression in hippocampus, while PFC mGlu2 expression does not distinguish between HS and LS subtypes.
Mechanistically, glucocorticoids enter the brain and bind to two subtypes of receptors, the higher-affinity mineralocorticoid receptor (MR) and the lower-affinity glucocorticoid receptor (GR), which are both highly expressed within the hippocampal formation (28, 29). There, they orchestrate physiological stress responses aimed at maintaining homeostasis through both delayed genomic and rapid non-genomic mechanisms (15, 30). We show here that acute stress effects on behavior and mGlu2 transcripts in hippocampus are blocked by spironolactone, a selective MR-antagonist, thus implicating rapid action of glucocorticoids via a receptor that is known to activate rapid glutamate release (31). Indeed, presynaptic mGlu2 receptors exert an inhibitory tone on glutamate release into the synaptic space. Perhaps one of the most striking aspects of our results is that the more susceptible HS mice display higher hippocampal MR transcripts compared to the resilient LS mice and, consequently, an increased effect of stress-induced suppression of hippocampal mGlu2 receptors.
Glutamatergic inputs to pyramidal neurons in the prefrontal cortex and hippocampus play a critical role in the maintenance of homeostasis in the face of stressful life events, which require adaptive responses involving changes in the central nervous and neuroendocrine systems. The hippocampus and PFC are interconnected and influence each other via direct and indirect neural activity in the control of stress-responses (32). The hippocampus mediates contextual and temporal aspects of stress-related memory as well as mood-related behaviors, whereas the PFC is mainly involved in decision making, self-regulatory behaviors and extinction processes (33, 34). Together with our previous data showing an epigenetic up-regulation of mGlu2 receptors induced by the fast-acting antidepressant acetyl-L-carnitine (16, 21, 22), the results of the present study lead us to hypothesize that a down-regulation of mGlu2 receptor in hippocampus of HS animals, but not the reduction that also occurs in PFC after CUS, is a critical event that increases susceptibility to stress to impair mood-related behaviors.
The reduction in mGlu2 inhibitory tone in hippocampus leads to an imbalance in relation to the role of the PFC and hippocampus in regulating of those behaviors. Indeed, CUS-resilient LS mice, which show selective mGlu2 receptor down-regulation within the PFC, but not in hippocampus, are better able to resist stressors. Hence, the ability of LS-mice to perceive stressful events in less threatening ways may be mediated by promoting adaptive coping strategies to sustain mGlu2 receptor expression within the hippocampus. Thus, such resilience may be an active strategy to assure normal excitatory input within the hippocampus through the presynaptic control of mGlu2 receptors on glutamate release. This active neurobiological process in resilient LS mice is reminiscent of previous findings that showed differential changes in gene expression in unsusceptible mice compared to susceptible mice after social defeat stress (35). One attractive hypothesis for future research on the role of hippocampus in stress responsivity is that there is a defect in a counter-regulatory mechanism in the crosstalk between glucocorticoids and glutamate, such as alterations in the endocannabinoid system that regulates glutamate release (15, 36).
How then does MR activation lead to reduced mGlu2 expression? Epigenetically, as shown by ChIP analysis and expression of the HAT p300 enzyme, the MR mediated effect in hippocampus of ARS mice involves inhibition of the HAT p300-driven acetylation of the H3K27, a transcriptional active mark bound to the GRM2 promoter gene, which regulates expression of the mGlu2 receptor. Thus glucocorticoids acting via mineralocorticoid receptors orchestrate neuronal excitability through a crosstalk with the mGlu2 receptor, which is the presynaptic metabotropic glutamate receptor that inhibits glutamate release into the synaptic space. Based upon the finding that early life stress alters methylation of CpG residues in the GR promoter resulting in a variable vulnerability to stress and anxious traits (29), one possibility to explain the present findings on MR would be an epigenetic modification of the MR promoter induced by early-life events. This is a topic for future investigation.
Recently, preclinical studies have shown that juvenile stress increases hippocampal MR mRNA levels and anxiety-like behavior in adulthood (37) and thus differential early life experiences may determine levels of MR expression. Here, we were able to show that higher baseline MR mRNA levels, obtained immediately after a light-dark anxiety test without any prior acute stress, characterize the difference between HS and LS mice. These individual differences are reminiscent of reports that show that individual differences in anxiety-related behavior in rats, found early in life, can predict lifespan (3) as well as cortical dendritic morphology (4). Also, our findings demand a better understanding of how “non-shared” experiences (2) determine individual behavioral responses via epigenetic mechanisms. Mother-infant interactions are key factors in emotional and cognitive development, and good or poor maternal care of newborn offspring is a well-known determinant of adult emotionality (38) via epigenetic modifications of specific sets of genes such as the glucocorticoid receptor (39). Yet, even among “good” and “poor” maternal care, there are variations in offspring outcome due to individual experiences of those offspring related to the consistency of that maternal care (‘attachment”), as well as the supply of nutrients and the exposure to a novelty (38, 40). Such “non-shared” experiences even among genetically identical offspring set each individual on a somewhat different trajectory of development, determining individual responses to subsequent novel experiences as potential stressors including exploratory behavior in the living environment (2).
Our findings provide a model at the molecular level to better understand the adaptive role of glucocorticoids and their receptors (41). Epigenetic Allostasis (Fig.4g) incorporates an epigenetic core into the Allostasis-Allostatic load model of stress and adaptation (41). In the case of MR and its regulation of mGlu2, the levels of MR and mGlu2 fit a quadratic MR-dependent curve reflecting the consequences of “non-shared” experiences that can program each individual via expression of MR to a somewhat different trajectory of development as far as responses (behavior, physiology and susceptibility to disease) to subsequent stressful life experiences. Epigenetic Allostasis implies that epigenetic changes induced by early life experiences bias the individual to responses to future stressful life events that may be adaptive under some circumstances (e.g., anxiety and vigilance in a dangerous environment with higher MR) or maladaptive (proneness to develop anxiety or depressive disorders with higher MR) under others.
Together, these results suggest that the individual responsivity to stress, perhaps as a result of developmentally-arising individual differences in neuronal hippocampal activity, may predict long-term consequences of social stress and susceptibility to mood-related behaviors. In particular, we have demonstrated individual differences in HS vs LS mice in the ability of stress to induce a decrease in hippocampal mGlu2 receptor expression. mGlu2 reductions appear to operate through a rapid (42, 31) MR-mediated epigenetic mechanism. Reductions in hippocampal mGlu2 levels may give rise to increased glutamate release and glutamatergic hyperactivity, supporting previous evidence that stress rapidly increases glutamate release in hippocampal pyramidal neurons through a non-genomic mechanism mediated by MR receptors (19). Such hyperactivity is also associated with dendritic shrinkage and suppressed dentate gyrus neurogenesis and dysregulated mood-related behaviors (15). Exploring the means, pharmacological or behavioral, to prevent the stress-induced decrease of mGlu2 (43, 44) in hippocampus is a potential new direction in managing negative effects of stressful experiences in susceptible individuals.
Supplementary Material
Acknowledgments
This work was supported by American Foundation for Suicide Prevention (AFSP) to CN, Hope for Depression Research Foundation (HDRF) to BSM and NIH Grant RO1 MH41256 to BSM. We thank the ACNP (American Congress of Neuropsychopharmacology) and ECNP (European Congress of Neuropsychopharmacology) for awarding the preliminary results of this research.
mGlu2 receptor knockout mice (mGlu2−/− mice) were kindly provided by S. Nakanishi, University of Kyoto, Kyoto, Japan. Authors declare no conflict of interest.
C.N. designed research and experiments, performed in vivo and in vitro experiments, analyzed data and wrote the manuscript. B.B. designed research, analyzed data and contributed to write the manuscript. D.Z. performed in vivo and in vitro experiments. F.N. analyzed data and supervised research. B.S.M. designed research, supervised research and wrote the manuscript.
Footnotes
Supplementary Information is available at Molecular Psychiatry's website.
References
- 1.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien S, Ballestar ML, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA. 2005;102:10604–9. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzlerm M, Kruger A. Emergence of individuality in genetically identical mice. Science. 2013;340:756–9. doi: 10.1126/science.1235294. [DOI] [PubMed] [Google Scholar]
- 3.Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc Natl Acad Sci USA. 2003;100:16131–6. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MM, Morrison JH, McEwen BS. Basal anxiety-like behavior predicts differences in dendritic morphology in the medial prefrontal cortex in two strains of rats. Behav Brain Res. 2012;229:280–8. doi: 10.1016/j.bbr.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Southwick SM, Charney DS. The Science of Resilience: Implications for the Prevention and Treatment of Depression. Science. 2012;338:79–82. doi: 10.1126/science.1222942. [DOI] [PubMed] [Google Scholar]
- 6.Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci U S A. 2012;109:17180–5. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machado-Vieira R, Soeiro-De-Souza MG, Richards EM, Teixeira AL, Zarate CA., Jr Multiple levels of impaired neural plasticity and cellular resilience in bipolar disorder: Developing treatments using an integrated translational approach. World J Biol Psychiatry. 2013;15:84–95. doi: 10.3109/15622975.2013.830775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zovkic IB, Meadows JP, Kaas GA, Sweatt JD. Interindividual variability in stress susceptibility: a role for epigenetic mechanisms in PTSD. Front Psychiatry. 2013;4:60. doi: 10.3389/fpsyt.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 11.Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci. 2006;1071:379–96. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- 12.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–57. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinnally EL, Mann JJ. Early Life Stress Programming and Suicide Risk. Psychiatric Annals. 2012;42:95–100. [Google Scholar]
- 14.Oquendo MA, Brent DA, Birmaher B, Greenhill L, Kolko D, Stanley B, et al. Posttraumatic stress disorder comorbid with major depression: factors mediating the association with suicidal behavior. Am J Psychiatry. 2005;162:560–6. doi: 10.1176/appi.ajp.162.3.560. [DOI] [PubMed] [Google Scholar]
- 15.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, Matrisciano F, et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci U S A. 2013;110:4804–9. doi: 10.1073/pnas.1216100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JS, Schmid-Burgk W, Claus D, Kornhuber HH. Increased serum glutamate in depressed patients. Arch Psychiatr Nervenkr. 1982;232:299–304. doi: 10.1007/BF00345492. [DOI] [PubMed] [Google Scholar]
- 18.Mitani H. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1155–8. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 19.Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur J Neurosci. 2007;11:2465–73. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- 20.Overstreet DH. The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev. 1993;17:51–68. doi: 10.1016/s0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- 21.Flight MH. Antidepressant epigenetic action. Nat Rev Neurosci. 2013;14:226. doi: 10.1038/nrn3466. [DOI] [PubMed] [Google Scholar]
- 22.Russo SJ, Charney DS. Next generation antidepressants. Proc Natl Acad Sci U S A. 2013;110:4441–2. doi: 10.1073/pnas.1301593110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–77. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A. 2011;38:16074–9. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasca C, Orlando R, Marchiafava M, Boldrini P, Battaglia G, Scaccianoce S, et al. Exposure to predator odor and resulting anxiety enhances the expression of the α2 δ subunit of voltage-sensitive calcium channels in the amygdala. J Neurochem. 2013;125:649–56. doi: 10.1111/j.1471-4159.2012.07895.x. [DOI] [PubMed] [Google Scholar]
- 27.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: Implications for the energetics of stress. Front Neuroendocrinol. 2013:S0091–3022(13)00073-3. doi: 10.1016/j.yfrne.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groeneweg FL, Karst H, de Kloet ER, Joëls M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J Endocrinol. 2011;2:153–67. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- 31.Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A. 2005;102:19204–7. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–84. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci. 2013;34:637–44. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brydges NM, Jin R, Seckl J, Holmes MC, Drake AJ, Hall J. Juvenile stress enhances anxiety and alters corticosteroid receptor expression in adulthood. Brain Behav. 2014;1:4–13. doi: 10.1002/brb3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang AC, Reeb-Sutherland BC, Romeo RD, McEwen BS. On the causes of early life experience effects: Evaluating the role of mom. Front Neuroendocrinol. 2013:S0091–3022(13)00068-X. doi: 10.1016/j.yfrne.2013.11.002. doi: 10.1016/j.yfrne.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–23. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress innoculation in young monkeys. Arch Gen Psychiat. 2004;61:933–41. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- 41.McEwen BS. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 42.Haller J, Mikics E, Makara GB. The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central neural system. A critical evaluation of findings. Front Neuroendocrinol. 2007;29:273–91. doi: 10.1016/j.yfrne.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–44. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 44.Timmermans W, Xiong H, Hoogenraad CC, Krugers HJ. Stress and excitatory synapses: from health to disease. Neuroscience. 2013;248:626–36. doi: 10.1016/j.neuroscience.2013.05.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.