Abstract
Peripheral nerves regenerate following injury due to the effective activation of the intrinsic growth capacity of the neurons and the formation of a permissive pathway for outgrowth due to Wallerian degeneration. Wallerian degeneration and subsequent regeneration are significantly influenced by various immune cells and the cytokines they secrete. Although macrophages have long been known to play a vital role in the degenerative process, recent work has pointed to their importance in influencing the regenerative capacity of peripheral neurons. In this review, we focus on the various immune cells, cytokines, and chemokines that make regeneration possible in the peripheral nervous system, with specific attention placed on the role macrophages play in this process.
Keywords: axotomy, chemokine, conditioning lesion, cytokine, dorsal root ganglion, macrophage
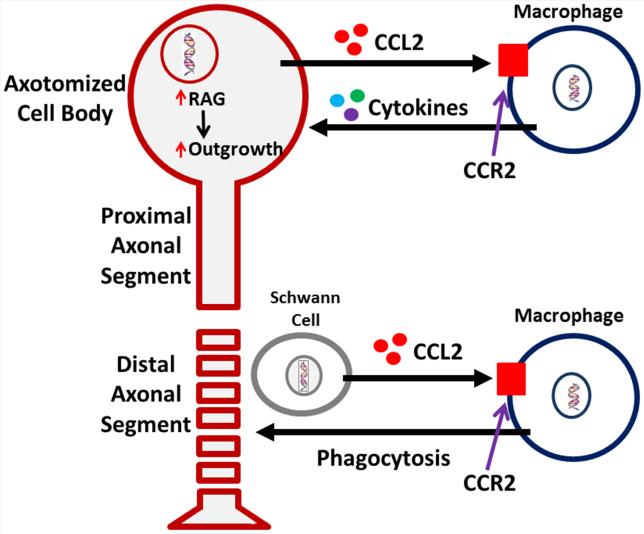
When an axon in the peripheral nervous system (PNS) is injured, a complex multicellular response occurs. The distal axonal segment degenerates, the cell body begins to express regeneration-associated genes (RAGs), and after a delay, the proximal segment forms a growth cone and begins to extend itself towards its denervated target. These processes of axonal degeneration and regeneration require changes not only in the injured neurons but also in non-neuronal cells including Schwann cells and immune cells. In this review, we have summarized recent advances in understanding these changes, focusing in particular on the role of chemokines, cytokines, and immune cells. One picture that will emerge is of macrophages playing two important roles in degeneration and regeneration by creating a pathway in the distal nerve segment conducive to axonal regeneration and by stimulating the axotomized neuronal cell bodies to switch to a regenerative phenotype (Fig. 1).
Figure 1.
A novel perspective on the response of macrophages to peripheral axotomy and their involvement in the regenerative response. Monocyte entry into a nerve, distal to the site of injury, is mediated through upregulation and release of the major monocyte chemokine, CCL2, by Schwann cells. Once the monocytes enter, beginning around 48 h post-injury, they phagocytose axonal and myelin debris resulting from the ongoing degenerative process. Removal of the debris, which is inhibitory to regenerating axons, is a known prerequisite for successful regeneration in vivo. Macrophages also enter into peripheral ganglia following nerve injury. Recent work has now suggested that macrophage presence around axotomized cell bodies helps mediate the conditioning lesion response. CCL2 is hypothesized to be the primary chemokine responsible for monocyte entry into the dorsal root ganglion. Under conditions in which macrophages do not enter the dorsal root ganglia, there is no enhanced growth after a conditioning lesion. Thus, there are two sites of action for macrophages in response to a peripheral nerve injury, in the distal nerve and around injured neuronal cell bodies.
THE BIOLOGY OF WALLERIAN DEGENERATION
In 1850, Augustus Waller described changes he observed in axons of cranial nerves after they are disconnected from their cell bodies (Waller, 1850; reprinted in Stoll et al., 2002). The phenomena he described have been collectively termed Wallerian degeneration (WD) and include among other phenomena the rapid disintegration of the distal axons and the subsequent influx of immune cells that rid the area of debris resulting from this breakdown. This process is thought to be necessary for successful regeneration to occur in the PNS(e.g., Gaudet et al., 2011). In the central nervous system (CNS), regeneration is inhibited perhaps in part because WD occurs much more slowly and myelin, which can inhibit regeneration, persists for years after axonal injury (for review see Vargas and Barres, 2007). The first sections of this review will focus on WD in the distal peripheral nerve segment in an effort to elucidate the role of macrophages in this process and the preparation for successful regeneration. The second half of the review will focus on changes in or surrounding the axotomized neuronal cell bodies and their impact on the conditioning lesion (CL) effect, a model of PNS regeneration. The studies cited throughout this review utilize rodent sciatic nerve and/or dorsal root ganglia (DRG) unless otherwise stated.
Successful WD relies on a number of cell types (for reviews see Gaudet et al., 2011; Rotshenker, 2011). Almost immediately after peripheral nerve injury, Schwann cells dissociate from axons, dedifferentiate, and, along with fibroblasts, secrete cytokines that promote infiltration of immune cells. Neutrophils, the first immunecells to infiltrate, accumulate in the distal stump within 8 h,buttheir presence is short-lived (Perkins and Tracey, 2000). Within days, hematogenous macrophages take over as the dominant leukocyte population and play a critical role in ensuring complete WD. Whereas many molecular and cellular mechanisms are involved in thecoordination of WD, the very first changes are in the axon itself.
Within minutes after damage to the axonal membrane, Ca2+-mediated proteolytic activity by the enzyme calpain initiates the breakdown of axons (George et al., 1995; for reviews see Coleman and Perry, 2002; and Wang et al., 2012). Zhai et al. (2003) found that removal of extracellular Ca2+ delayed axonal degeneration in explanted superior cervical ganglia (SCG) for 16 h or longer and observed the earliest change in axons undergoing WD to be the fragmentation of microtubules. They further hypothesized that there may be a Ca2+-dependent step in the ubiquitin proteasome system (UPS) in WD as inhibition of the UPS delays microtubule fragmentation both in vivo and in vitro. Recently, sterile alpha- and armadillo-motif-containing protein-1 (SARM-1), a member of the toll-like receptor (TLR) adaptor family, has been identified as important for injury-induced axon degeneration since a SARM knockdown was shown to protect axons in culture (Gerdts et al., 2013). SARM-1 may respond directly to an increase in axonal Ca2+ after axotomy as it is localized in the axonal compartment (Osterloh et al., 2012).
Changes in cytokine and chemokine secretion in peripheral nerves after axotomy
Although axon degeneration is generally complete by 48 h after injury, this is only the beginning of WD. Certain products of early axonal degeneration, referred to as danger-associated molecular patterns (DAMPs), stimulate Schwann cells through TLRs leading to the breakdown of myelin and recruitment of macrophages (Vargas and Barres, 2007; Martini et al., 2008; Pineau and Lacroix, 2009). In addition to myelin breakdown in WD, there is an inhibition in new myelin synthesis. Within 24 h after nerve transection, there aredecreases in the levels of mRNA for two myelin proteins, Po and myelin basic protein, and by 5 d these mRNAs are no longer detectable (Trapp et al., 1988).
JUN, an immediate early gene, is rapidly upregulated in both neuronal cell bodies (Guertin et al., 2005) and Schwann cells, and its expression is increased by elevated intracellular Ca2+ levels (De Felipe and Hunt, 1994). This upregulation of JUN triggers a change in Schwann cell phenotype from myelinating to non-myelinating/immature,a process known as Schwann cell dedifferentiation(reviewed by Jessen and Mirsky, 2008). Lee et al. (2009) found that proteasome inhibition prevented Schwann cells from expressing dedifferentiation markers such as glial fibrillary acidic protein (GFAP) in vitro and in vivo. Axonal injury also initiates rapid activation (within 10 min) of the receptor tyrosine kinase erbB2 (Guertin et al., 2005), and increased expression of Notch intracellular domain within Schwann cells that enwrap the injured nerves, both of which promote dedifferentiation of Schwann cells and demyelination (Jessen and Mirsky, 2008). This dedifferentiation is critical as it allows Schwann cells to clear myelin and express cytokines important for successful WD. Interestingly, Napoli et al. (2012) found that expression of an inducible Raf-kinase in myelinating Schwann cells by itself is sufficient to drive dedifferentiation and cause demyelination, breakdown of the blood-nerve barrier, and influx of immune cells in the absence of injury signals derived from axon degeneration.These striking findings point to the importance of the mitogen-activated protein kinase (MAPK) cascade in Schwann cells in coordinating the molecular and cellular events in WD.
Due to their loss of contact with the axon, these dedifferentiated Schwann cells upregulate synthesis and secretion of tumor necrosis factor alpha (TNF-α) and interleukin-1α (IL-1α) within 5-6 h after injury (Shamash et al., 2002). IL-1β begins to be expressed within 5-10 h after injury(Shamash et al., 2002)and contributes to macrophage recruitment (Perrin et al., 2005) and Schwann cell proliferation (Conti et al., 2002). Interestingly, blocking calpain in a chronically constricted sciatic nerve attenuated the normal TNF-α and IL-1β mRNA increases in response to nerve injury (Uceyler et al., 2007). The highest levels of TNF-α and IL-1β are detected at 1 d, before macrophage entry, raising the possibility of their involvement in macrophage recruitment. Chattopadhyay et al. (2007)showed that IL-1β and TNF-α induce the expression of matrix metalloproteinase-9 (MMP-9) which is up-regulated in Schwann cells and macrophages after sciatic nerve injury (La Fleur et al., 1996; Siebert et al., 2001). MMP-9 activates integrin-mediated signaling pathways that weaken the blood-nerve barrier(Hughes et al., 2002),contributes to monocyte extravasation and migration (Kessenbrock et al., 2011), and stimulates initial myelin breakdown by Schwann cells. Additionally, TNF-α induces production of the chemokines monocyte chemoattractant protein-1 (MCP-1, now referred to as CCL2, chemokine C-C motif ligand 2) and macrophage inflammatory protein-1α (MIP-1α, also known as CCL3), which show peak mRNA expression at 1 d after injury(Subang and Richardson, 2001; Perrin et al., 2005). Interestingly, Subang and Richardson (2001) also showed that in mice lacking both TNF-α receptors, CCL2 still increases in injured nerves, although not to the extent of that in WT mice, indicating that although TNF-α is important for CCL2 expression, there are other cytokines that can compensate in its absence.
Expression of these and other inflammatory cytokines is stimulated through DAMP-induced TLR signaling mediated by myeloid differentiation primary response gene 88 (MyD88) within Schwann cells (Takeda et al., 2003; for review see Piccinini and Midwood, 2010). Boivin et al. (2007) found that in the distal stump of the sciatic nerve from mice deficient in TLR signaling, early expression of IL-1β was abolished and expression of CCL2 was inhibited. In these same mice, they also found that macrophage recruitment and activation was inhibited, and myelin clearance, regeneration, and functional recovery were delayed. They also showed that direct injection of TLR2 and TLR4 ligands into the injury site enhanced the normal responses to injury in wild type (WT) mice. In line with these findings, considering that TLR2 forms a heterodimer with TLR1,Goethals et al. (2010) found that of the TLRs, TLR1 was upregulated in neurodegeneration, showing highest expression levels between 32 and 72 h after sciatic nerve axotomy. Karanth et al. (2006) found that induction of CCL2 in Schwann cell cultures was inhibited by an antibody to TLR4. This evidence elucidates the importance of TLR signaling (particularly TLR1/2 and TLR4) for cytokine production in WD.
TLRs signal through the transcription factor nuclear factor kappa B (NF-κB), which induces a variety of cytokines. In sciatic nerves from mutant mice in which the NF-κB pathway is conditionally inhibited in Schwann cells both CCL2 and CCR2 mRNA and protein expression are significantly lower after chronic constriction injury than in WT mice, whereas TNF-α mRNA expression is unchanged (Fu et al. 2010). In these animals, fewer macrophages accumulate in the sciatic nerve at 3 d after injury when compared to WT (Zhang et al. 2011).
Schwann cell-produced cytokines stimulate production by fibroblasts of IL-6(a gp130 cytokine family member) and granulocyte-macrophage colony-stimulating factor (GM-CSF within 4 h after axotomy (Reichert et al., 1996; Saada et al., 1996), with their mRNA levels peaking at 36 h (Cheepudomwit et al., 2008). During WD, GM-CSF was found by Saada et al. (1996)to stimulate cell surface expression of galectin 3 (gal3/MAC-2) in macrophages and Schwann cells participating in myelin phagocytosis. This same study suggested that injury-induced GM-CSF may be regulated by IL-1β since they found that addition of IL-1β to sciatic nerve explants upregulated GM-CSF production.
Schwann cell associated cytokines and chemokines initiate early breakdown of myelin by stimulating the expression of phospholipase A2 (PLA2) family members (Murakami et al., 1997). De et al. (2003)showed that PLA2 members are expressed in Schwann cells as early as 5 h after a sciatic nerve crush. PLA2 hydrolysis of phosphotidylcholine results in the production of lysophosphotidylcholine (LPC), which has potent and rapid myelinolytic activity (Hall and Gregson, 1971). Thus LPC in Schwann cells can initiate the earliest stages of myelin breakdown in WD. LPC activates the classical complement pathway by binding to C-reactive protein, which triggers the early influx of neutrophils through C5a (Hack et al., 1997) and, if bound to degenerated myelin, LPC can also act as an ‘eat me’ signal for macrophages (Martini et al., 2008).
The involvement of resident and hematogenous macrophages in WD
Prompt removal of myelin debris is critical in repair after injury for many reasons. Peripheral myelin contains molecules, such as myelin-associated glycoprotein (MAG), that are inhibitory to regeneration (McKerracher et al., 1994; Mukhopadhyay et al., 1994). Perry et al. (1995) suggested that some unidentified component helps macrophages distinguish between myelin to be phagocytosed versus myelin from Schwann cell membrane surrounding intact axons which is to be left alone, although they did not postulate what this molecule is. Indeed, degenerated myelin can activate formation of membrane attack complexes by the complement system that can damage nearby intact axons and myelin (Mead et al., 2002). CD47 on intact myelin and myelin-forming Schwann cells binds to signal regulatory protein α (SIRPα) on macrophages and thereby downregulates phagocytosis of undamaged myelin near the injury site (Gitik et al., 2011). Thus, Schwann cell dedifferentiation early after injury and later sustained macrophage accumulation are crucial for removal of myelin debris in order to prevent further damage and allow for regeneration.
One of the many factors mediating the recruitment of monocytes to injury sites and their subsequent phagocytosis of myelin during WD is the complement system. Dailey et al. (1998) showed that complement depletion by cobra venom factor inhibited macrophage recruitment and prevented macrophage activation allowing preservation of myelin. Complement receptor 3 (CR3) on the surface of macrophages recognizes C3 bound to degenerating myelin sheaths and thus participates in its uptake (Bruck, 1997). Interestingly, using macrophage and sciatic nerve co-cultures, Bruck et al. (1992) observed reductions in both the expression of CR3 on macrophages and the amount of myelin those macrophages ingested. Curiously, these reductions were a result of TNF-α treatment exposing perhaps a pleiotropic and somewhat dual nature of this cytokine. Vargas et al. (2010) showed that after peripheral nerve injury, endogenous antibodies activate complement, which plays a role in the influx of monocytes and is critical for opsonization of myelin by C3b as well asthe subsequent phagocytosis by macrophages. Thus it seems that complement present in the nerve is important for macrophage activation and myelin phagocytosis.
While the predominant thought is that infiltrating macrophages are the main responders to injury, there is evidence that resident macrophages are also involved in WD, such as the fact that they can induce inflammation through their expression of TLRs (for review see Davies et al., 2013). Resident macrophages comprise 2-9% of all cells in peripheral nerves, express CR3, and are known to be phagocytic (for review see Griffin et al., 1993). Ydens et al. (2012) found that IL-13 mRNA expression spiked in the nerve at 4 h after injury and suggested a possible source to be resident macrophages, since the infiltrating macrophages which typically express this cytokine have not yet entered the injured nerve. In chimeric rats that have received a bone marrow transplant that allowed identification of hematogenous macrophages, resident macrophages become similar morphologically to hematogenous macrophages, expressing ED1 and phagocytosing myelin (Mueller et al., 2001). This group also found evidence that some of these resident macrophages proliferate before they express ED1 and therefore concluded that the resident macrophages are not a homogenous population. Furthermore, IB4 positive resident macrophages exhibited a pro-inflammatory phenotype in the nerve 2 d after a partial ligation nerve injury, showing strong expression of IL-1β and weak expression of TNF-α (Schuh et al., 2014). Although the role of resident macrophages following injury is not yet fully understood, it appears that theyrespond in the injured nerve before the accumulation of hematogenous macrophages.
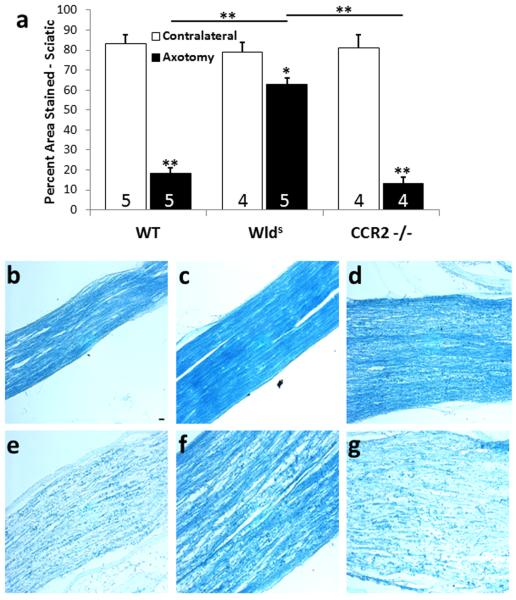
Circulating monocytes express the receptor for CCL2, C-C chemokine receptor type 2 (CCR2), and through CCL2/CCR2 signaling, these monocytes are recruited to the injured nerve where they differentiate into macrophages. As expected, macrophage accumulation in the sciatic nerve 7 d after injury is inhibited in CCR2 −/− mice (Siebert et al., 2000; Niemi et al., 2013). Barrette et al. (2008)concluded that, after sciatic nerve lesion, macrophages are critical for removal of myelin debris as well as neurotrophin synthesis. On the other hand, Niemi et al. (2013) found that in CCR2 −/− mice, WD progresses normally as indicated by the normal clearance of myelin 7 d after axotomy (Fig. 2). Additionally, injured nerves lacking hematogenous macrophages due to whole body irradiation exhibit normal loss of myelin basic protein at 5 d after injury, yet as time progresses this loss occurs more slowly than in control animals (Perry et al., 1995). These data may reflect the earlier mentioned evidence that Schwann cells are involved early on in the phagocytosis of myelin. Indeed, Stoll et al. (1989) indicated that Schwann cells associated with small axons may process their own myelin but that they might require the assistance of macrophages to phagocytose the myelin from larger axons.
Figure 2.
Efficiency of myelin clearance in the distal sciatic nerve of CCR2 −/− mice is comparable to that in WT mice despite the total absence of axotomy-induced macrophage infiltration into the distal nerve in the knockout animals (see Niemi et al., 2013). Seven days after the sciatic nerve was unilaterally transected, changes in reactivity for myelin proteins were determined in nerves from WT, Wlds, and CCR2 −/− mice by staining with LFB. The distal nerve segments from WT and CCR2 −/− mice showed significantly less myelin staining compared with contralateral nerves, whereas axotomized nerves from Wlds mice retained >80% of myelin reactivity compared with contralateral nerves (a). The micrographs represent sections from the ipsilateral (e-g) and contralateral (b-d) nerves from WT, Wlds, and CCR2 −/− mice, respectively. Infiltrating macrophages have been considered necessary for the phagocytosis and clearance of myelin debris after injury; however the reduction in this cell population at the distal sciatic nerve does not hinder the clearance mechanism. *p<0.05, **p<0.001. Scale bar, 20 μm. (Niemi et al., 2013)
Hematogenous macrophage accumulation in the distal nerve begins at 2-3 d after injury and peaks between 7 and 14 d depending on the degree of compromise to the BNB (Taskinen and Roytta, 1997; Mueller et al., 2003; Omura et al., 2005). In the transected sciatic nerve, IL-6 mRNA peaks at 36 h after injury, while LIF mRNA levels peak between 36 and 48 h, a timeframe that coincides with initial macrophage infiltration (Cheepudomwit et al., 2008). In the distal sciatic nerve after injury, CCL2 is localized to Schwann cells, and its expression peaks just before accumulation of hematogenous macrophages (Toews et al., 1998). Tofaris et al. (2002) showed that in Schwann cells, IL-6 induces LIF mRNA and LIF induces CCL2 mRNA. Indeed, LIF and CCL2 secreted by Schwann cells attract macrophages to the injured nerve (Sugiura et al., 2000). Perrin et al. (2005) showed that neutralizing CCL2, MIP-1α, or IL-1β using function blocking antibodies suppresses macrophage accumulation in the injured nerve and phagocytosis of myelin. Once macrophages enter the nerve they also express TNF-α, IL-1α, and IL-1β proteins (Shamash et al., 2002) and contribute to further recruitment of hematogenous macrophages and breakdown of myelin.
Delineating the macrophage phenotype in the distal nerve segment
Studies of the macrophage response to tissue injury have further delineated this population into activation states, namely M1 (classically activated) and M2 (alternatively activated). Interest in this issue in the context of nerve injury arises because M1 macrophages are generally proinflammatory and cytotoxic, whereas M2 macrophages generally participate in tissue repair and wound healing. Interconversion of macrophage phenotypes has been shown to occur in vitro in response to changes in the cytokine environment(Davis et al., 2013). M1 macrophages are produced by interferon-γ (IFN-γ) and lipopolysaccharide (LPS) whereas M2 macrophages result from stimulation by the cytokines IL-4 and/or IL-13(Anthony et al., 2007). Of particular interest in the context of this review, Mokarram et al. (2012) showed that, using IL-4 to phenotypically switch macrophages to the M2 phenotype, can promote regeneration in the transected tibial nerve.
It is not entirely clear in the literature as to the phenotype macrophages take in the distal nerve after transection. As indicated above, Ydens et al. (2012)detected IL-13at 4 h after injury and found M2 macrophages that did not express iNOS or IFN-γ but did express arginase 1.Similarly, it has been reported that macrophage in the distal nerve are of the M2 phenotype as they express high levels of IL-10, an anti-inflammatory cytokine (Rotshenker, 2011). On the other hand, Nadeau et al. (2011) found monocyte derived M1 macrophages present early after nerve injury but they were gone by 3-4 d, the same time point at which macrophages at the injury site begin to express anti-inflammatory, M2 associated markers such as arginase 1 and CD206. In addition, Komori et al. (2011) found that at 1 and 3 d after partial nerve ligation, the immune response was characterized byM1 macrophages in the nerve (i.e. iNOS positive and arginase 1 negative), whereas the DRG contained M2 macrophages.
Apolipoprotein-E is produced and secreted by resident fibroblasts and gal-3 by Schwann cells starting at day 2 of WD and later by macrophages (Aamar et al., 1992; Saada et al., 1995). Both apolipoprotein-E and gal-3 can polarize recruited macrophages toward an M2 phenotype in culture (MacKinnon et al., 2008; Baitsch et al., 2011). Recently it has been shown that CCL2/CCR2 signaling regulates macrophage polarization and drives macrophages toward an M2 state (Sierra-Filardi et al., 2014). Indeed, GM-CSF stimulated macrophages from CCR2 −/− mice display an M1 phenotype, increasing their expression of IL-6, CCL2, and TNF-α (Sierra-Filardi et al., 2014). Regardless of the differing hypotheses concerning the macrophage activation state after nerve injury, it may be that macrophages involved in tissue repair encompass a spectrum of activation states throughout the repair process (for review see Novak and Koh, 2013).
The inflammatory response after nerve injury must be carefully controlled in order to prevent consequent damage and allow for subsequent regeneration.M2 tissue repair macrophages likely mediate this effect, as M2 macrophages treated with IL-4 upregulate their expression of IL-10, an anti-inflammatory cytokine(Mosser and Edwards, 2008). IL-6 and IL-10 are two cytokines that help control the inflammatory response by regulating the synthesis and release of additional cytokines (for review see Opal and DePalo, 2000). The Rotshenker lab has shown that in sciatic nerves of rats, IL-6 upregulation after injury is detectable at 2 h and remains elevated for at least 21 d (Reichert et al., 1996). Upon analyzing this expression by non-neuronal cells they found that macrophages expressed the highest levels, fibroblasts expressed significant levels (though less than macrophages), and Schwann cells expressed little if any. No IL-6 was detectable in intact nerves. A similar study by this lab showed that IL-10 starts to increase considerably in the nerve 4 d after injury, peaking at day 7, and remaining elevated through day 14. (Be’eri et al., 1998). Indeed, the timing of this increase in IL-10 production coincides with the peak in macrophage accumulation. They found that the IL-10 expression pattern was similar to that of IL-6 expression with macrophages producing the highest levels of IL-10, fibroblasts producing significant amounts (although less than macrophages), and Schwann cells producing little if any. George et al. (2004)also found that infiltrating macrophages are the main cell type that expresses IL-10 during WD. Although IL-6 has been considered both pro-inflammatory and anti-inflammatory, like other gp130 cytokine family members, it functions mainly as an anti-inflammatory cytokine (Opal and DePalo, 2000). Indeed, a study of the immune response of IL-6 −/− mice showed that endogenous IL-6 plays a critical anti-inflammatory role by controlling circulating levels of pro-inflammatory cytokines such as TNF-α (Xing et al., 1998). IL-10 gradually downregulates production of proinflammatory cytokines through Janus kinase (JAK) / signal transducer and activator of transcription(STAT) signalingbringing WD to an end after clearance of degenerated myelin is complete, 2-3 wk after injury (Moore et al., 2001; Rotshenker, 2011). Additionally, IL-10 activation significantly down-regulates MMP-9 in macrophages, leading to less macrophage infiltration and breakdown of myelin(Kessenbrock et al., 2011).Overall, these data suggest that M2 macrophages may play a regulatory role in controlling inflammation after nerve injury and thereby allowing subsequent regeneration.
Other processes take place during WD besides clearance of myelin and axonal debris
Studies on WD have primarily focused on theclearance of myelin proteins and axonal debris, and it is widely believed that this is essential for regeneration to occur. Nevertheless, this proposition is not as easy to prove as has been assumed. The approach generally taken is to interfere with macrophage accumulation presumably in the distal nerve and then to assess regeneration. The problem is that macrophages not only accumulate in the distal nerve segment but they also accumulate in axotomized ganglia where it is highly likely that they alter the growth capacity of the neurons (see Fig. 1 and discussion below in sections on the CL response and Kwon et al., 2013; Niemi et al., 2013).
WD is generally consider to refer to “all of the events that occur distal to the site of axotomy” (Scherer and Salzer, 2001).One such event is the change that occurs in nonneuronal cell gene expression. An intriguing example of this is the increased expression of neurotrophic factors. For example, IL-1β secreted by hematogenous macrophages stimulates non-neuronal cells in the nerve to synthesize nerve growth factor (NGF; Heumann et al., 1987; Lindholm et al., 1987). Barrette et al. (2008) found that decreasing macrophage and neutrophil infiltration into the sciatic nerve decreased the normal axotomy-induced increase in all four neurotrophins [i.e., NGF, brain-derived neurotrophic factor (BDNF), and neurotrophins 3 and 4]. Nevertheless, the expression of BDNF, unlike that of NGF, is not upregulated by IL-1β (Meyer et al., 1992). Most availableevidence suggests that endogenous NGF does not promote regeneration (Diamond et al., 1987; Gloster and Diamond, 1992; Shoemaker et al., 2006; Lankford et al., 2013). On the other hand, heterozygote knockout animals indicate that endogenous BDNF and NT3 in non-neuronal cells do promote regeneration (Sahenk et al., 2008; Takemura et al., 2012).
Another such event occurring in the distal nerve segment after axotomy is Schwann cell proliferation (Scherer and Salzer, 2001). There is some evidence that macrophages are involved in this process although their exact role remains unclear(e.g., Baichwal et al., 1988; Fawcett and Keynes, 1990). Moreover, the function of Schwann cell proliferation is still unknown since blockade of this cell division response by knocking out cyclin D1 had no appreciable effects on the disappearance of neurofilament protein, the induction of JUN, or nerve regeneration(Kim et al., 2000).
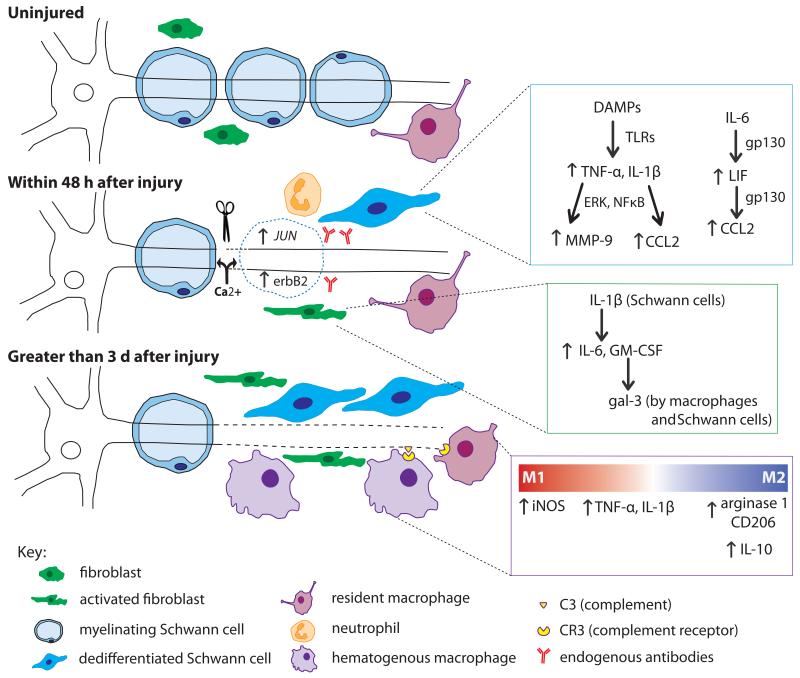
Clearly WD is a complex process that has been studied for many years, yet considering the plethora of molecules and cell types involved (Fig. 3), all of the intricacies are still not fully understood. The discovery in 1989 of a spontaneous mutant mouse provided a new model system in which to study this process, leading to a dramatic shift in our understanding of WD.
Figure 3.
The cells and molecules involved in WD in peripheral nerve. Within minutes of axonal injury, Ca2+ enters the proximal and distal nerve and initiates axonal breakdown. The resulting DAMPs stimulate the dedifferentiation of Schwann cells through the upregulation of JUN and erbB2. Through TLR signaling, dedifferentiated Schwann cells upregulate proinflammatory molecules (blue inset). Increased TNF-α and IL-1β activity leads to upregulation of both MMP-9 and CCL2. Schwann cells also respond to IL-6 from fibroblasts, which through gp130 signaling, triggers an autocrine cascade of LIF and CCL2 upregulation. Fibroblasts respond within hours by upregulating IL-6 and GM-CSF, the latter of which leads to gal-3 expression by macrophages and Schwann cells (green inset). Neutrophils infiltrate the injury site briefly within the first day after injury. Endogenous antibodies respond to nerve injury by activating complement. Three days post injury, macrophages have begun to infiltrate the nerve. Activated macrophages seem to play a dual role in WD evident by the factors they secrete (purple inset). Initially, they contribute to the inflammatory state by their production of TNF-α and IL-1β. CR3 on macrophages binds complement on degenerating myelin inducing phagocytosis. There is controversy over the subtype of macrophage involved; M1 markers have been shown at 1 and 3 d after injury, yet M2 markers have been found as early as 3 d and remain elevated for at least 14 d. It is thought that M2 macrophages are involved in regulating the inflammatory response in WD by upregulating the anti-inflammatory cytokine IL-10 leading to downregulation of pro-inflammatory cytokines once myelin degradation is complete.
The slow Wallerian degeneration mouse
Following axonal transection or crush, the axon distal to the injury site no longer receives proteins and mRNA from the cell body. Originally WD was considered to be a passive process induced by the lack of nutrients and cell-body derived factors following injury(Joseph, 1973). However, discovery of the slow WD mouse (Wlds) led to the realization that axons degenerate through an active process (Lunn et al., 1989). In this spontaneously arising mutant mouse, axons distal to the site of injury remain intact and are able to conduct action potentials, when stimulated, for up to 10 times longer than their wild-type (WT) counterparts (Lunn et al., 1989; Coleman and Freeman, 2010). Early studies on degeneration, following neurotrophin deprivation or injury,reported that WD occurs without activation of caspase-3, suggestingthat axons degenerate through a process separate from apoptosis (Buckmaster et al., 1995; Finn et al., 2000; Raff et al., 2002); however, more recent work on embryonic sensory neurons in vitroindicates that caspase-3 and -6 are involved in axon degeneration caused by trophic factor withdrawal though not by axotomy(Simon et al., 2012).
The Wlds mouse is characterized by a genetic mutation which brings two genes, E4 ubiquitin ligase (Ube4b) and nicotinamide mononucleotide adenylyl transferase (Nmnat1), together to form a fusion protein as a result of mRNA splicing (Lyon et al., 1993; Coleman et al., 1998). The mutation was identified as an 85kb tandem triplication likely involving nonhomologous recombination to form the genetic rearrangement (Coleman et al., 1998). Ube4b is an ubiquitin ligase involved in multi-ubiquitin chain assembly (Koegl et al., 1999). Nmnat1 is anenzyme necessary for the production of nicotinamide adenine dinucleotide (NAD+) (Schweiger et al., 2001).NAD+ and nicotinamide adenine dinucleotide phosphate (NADP) are best known for their roles in the electron transport chain and energy metabolism. Additionally, recent work has described a variety of functions for NAD+, including the ability to act as ligands for ion channels (Pollak et al., 2007; Klein et al., 2009).The resulting Wldsprotein contains the full length Nmnat1 protein with 70 amino acids from Ube4b on the N-terminal end, though only Nmnat1 is enzymatically active (Laser et al., 2003). An additional 18 amino acids from the 5′ UTR of Nmnat1 are also included in the protein (Samsam et al., 2003). The unique genomic rearrangement that led to the creation of the Wlds protein does not alter the normal expression levels of the normal Ube4b and Nmnat1 genes (Gillingwater et al., 2002). The key question surrounding the Wlds mouse has been how this fusion protein is able to protect axons from axonal degeneration.
A major observation in the Wlds mouse was a complete lack of early infiltrating macrophages into the nerve distal to the injury site, which was initially thought to be the cause of the slow degeneration (Brown et al., 1991a). However,bone marrow transplantation experiments that provided the mutant mouse with WT myeloid cells did not rescue the phenotype (Perry et al., 1990; Glass et al., 1993). This study provided evidence that the slow degeneration phenotype of the Wlds mouse was not the result of altered macrophages but instead was inherent to the neurons/axons themselves.
Much of the research on the Wlds mouse has focused on which part(s) of the fusion protein is critical for conveying its protective effects. Overexpression of the 70 amino acids from Ube4b or the sequence for Nmnat1 alone did not convey axonal protection when tested in vivo (Conforti et al., 2007), yet in vitro studies suggested Nmnat1 overexpression could protect severed neurites in primary culture (Araki et al., 2004; Wang et al., 2005). This discrepancy led to a model that suggested the two major domains of the protein are both necessary for the phenotypic protection. To further address this, a variant of the Wlds protein was created that lacked the enzymatic activity of Nmnat1 and resulted in a significant loss of axonal protection (Conforti et al., 2009; Yahata et al., 2009). Thus, Nmnat1 is necessary for the neuroprotective effects of the Wlds protein yet is not sufficient to convey this protection on its own, in vivo(Conforti et al., 2007).
Through the development of an antibody against the Wlds protein, it was shown that the protein localized almost entirely to the nucleus, with barely detectable levels in the cytoplasm or axon (Samsam et al., 2003; Gillingwater et al., 2006; Beirowski et al., 2009). With a strong localization in the nucleus, Araki and colleagues suggested that increased Nmnat1 activity in the nucleus led to increased activation of the NAD-sensitive sirtuin-1 (SIRT1). However, it was discovered that if the Wlds protein’s cytoplasmic localization was enhanced by mutating the nuclear localization signal (NLS) in Nmnat1 (Beirowski et al., 2009) or if a mutant Nmnat1 (cytNmnat1), engineered to localize specifically to the cytoplasm and axon (Sasaki et al., 2009a), was expressed, axonal protection was significantly enhanced beyond what is seen in the original Wlds mutant. This led to the realization that the Wlds protein was acting in the axon to delay WD.
Two other orthologs of Nmnat1 exist in mammals. While Nmnat1 is localized to the nucleus (Schweiger et al., 2001), Nmnat2 is cytoplasmically localized (Yalowitz et al., 2004), and Nmnat3 is found in mitochondria (Berger et al., 2005). Gilley and Coleman (2010) demonstrated that Nmnat2 acts as a cell-body derived axonal survival factor. Nmnat2 has a very short half-life and must be transported from the cell body down to the axon. Following injury, Nmnat2 levels are rapidly reduced in the distal axon, and this reduction coincides with axonal disintegration. Overexpression of Nmnat2in vitro can delay axonal degeneration (Gilley and Coleman, 2010; Milde et al., 2013). Nmnat1 has a much longer half-life and the Nmnat1 containing Wlds protein can substitute for the loss of endogenous Nmnat2 and delay axonal degeneration.
This suggests that the synthesis of NAD+ in the axon is critical for axonal survival;however, it is not clear that NAD+ itself is neuroprotective. Even though Nmnat activity is significantly increased in the Wlds mouse (Orsomando et al., 2012), no detectable increase in NAD+ levels is observed (Mack et al., 2001; Araki et al., 2004). In addition, inhibition of the rate-limiting enzyme in NAD+ synthesis, nicotinamide phosphoribosyltransferase, does not have significant effects on the Wlds phenotype (Conforti et al., 2009; Sasaki et al., 2009b).
In addition to an alteration in WD, regeneration of axons in the Wlds mouse has been shown to be delayed and incomplete (Bisby and Chen, 1990; Myers et al., 1996; Sommer and Schafers, 1998; Shin et al., 2014). The increase in NGF in the sciatic nerve after transection is severely blunted in the Wlds mouse, and this has been raised as a possible cause of the regeneration deficiency (Brown et al., 1991b). However, as already discussed, a number of studies have indicated that NGF does not promote regeneration in adult animals. More commonly, this regenerative deficiency is attributed to the delayed clearance of myelin and axonal debris (Bisby and Chen, 1990). The idea that the Wlds mouse would show normal regeneration given a suitable substrate for growth was supported by the finding that facial motor and DRG neurons in Wlds mice show normal increases in growth associated protein-43 (GAP-43) following injury even though regeneration is delayed in vivo(Bisby et al., 1995). However, recent work suggests that this view is incorrect. The conditioning lesion (CL) response, which is discussed further below, was examined in L5 DRGs after sciatic nerve transection, where neurite outgrowth was measured in explant cultures in Matrigel, which is largely composed of laminin. A major advantage of using the explant culture system is that neurite outgrowth is assayed without the influence of the potentially inhibitory distal nerve. A CL-dependent increase in outgrowth was seen in WT DRGs but not in Wlds DRGs (Niemi et al., 2013). The Wlds mice also showed a significant decrease in infiltrating monocytes into the DRG following sciatic nerve injury, likely resulting from a significant attenuation in CCL2 expression (Niemi et al., 2013).
Measurements of a variety of other cytokines and growth factors after axotomy have been shown to differ between WT andthe Wlds mice. The decrease in ciliary neurotrophic factor(CNTF) normally seen is slowed in these mutant mice (Subang et al., 1997). In addition, levels of TNF-α, IL-1α, and IL-1β are lower in Wlds mice (Shamash et al., 2002), as are levels of IL-6 (Reichert et al., 1996)together with GM-CSF and IL-10 (Be’eri et al., 1998). Finally, the upregulation of the extracellular matrix glycoprotein, tenascin C, is reduced in the mutant animals (Fruttiger et al., 1995)
With much still unknown about the mechanism driving WD, a large body of evidence built on the research conducted on the Wlds mouse suggests the importance of the NAD synthesis pathway in degeneration. The detailed mechanism of how the Wlds mutation conveys its axonal protection and its delayed regeneration remains unknown.
THE CELL BODY RESPONSE TO AXOTOMY
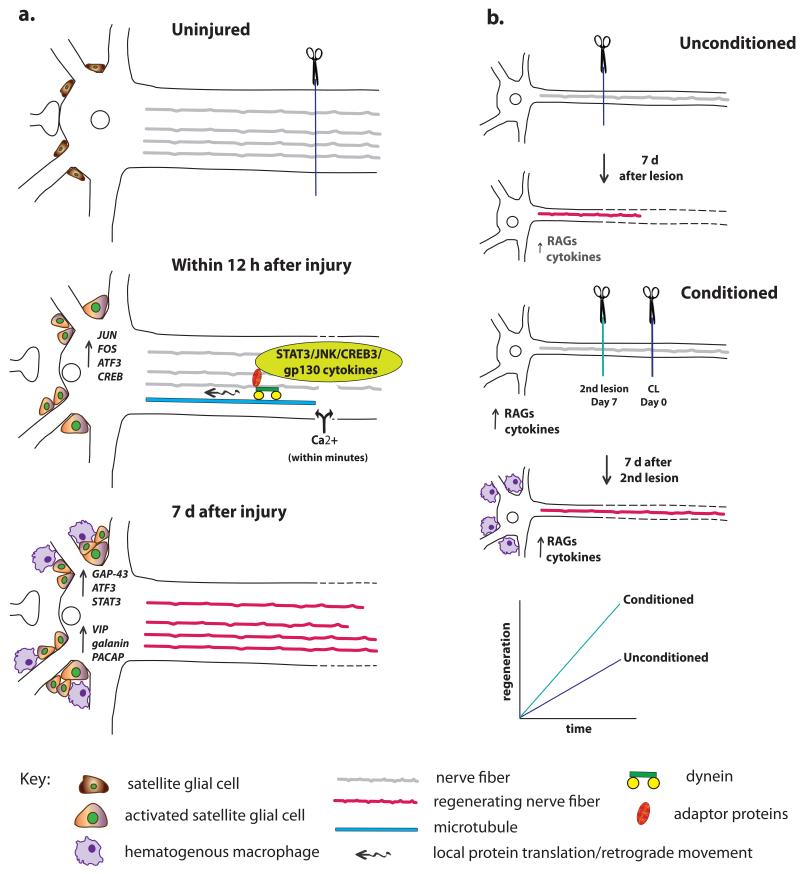
As noted previously, following axonal injury, peripheral neurons are able to regenerate their axons while central neurons cannot. The ability to regenerate in response to an injury is determined by many factors, including the presence or absence of a permissive environment into which axons can elongate, the influence of non-neuronal cells, and the intrinsic growth capacity of the neuron itself. The latter is dependent on the multitude of changes that occur to the neuronal cell body following injury, termed the “cell body response”(Fig. 5a; Grafstein, 1975). The hallmark of this response is the significant increase in de novo synthesis of mRNAs and proteins (Watson, 1974). These gene expression changes mark a phenotypic switch in the neuron from supporting synaptic transmission to supporting axonal regeneration(Harkonen, 1964; Zigmond, 1997).
Figure 5.
The cell body response and the CL response. (a) The cell body response describes the morphological and molecular changes that occur in neurons after axotomy. Two types of signals reach the cell body from the site of injury. First, there is a very rapid Ca2+ wave, and, later, there are signals that have been retrogradely transported along microtubules via dynein and adaptor proteins. These different signals lead to changes in RAG expression in the axotomized cell body. In addition (though not shown), the accumulation of macrophages and the activation of satellite glial cells near the cell bodies leads to the release of extrinsic signals that also alter neuronal gene expression, for example, the secretion of gp130 cytokines by satellite glial cells in the SCG triggers an increase in galanin expression (Sun et al., 1994; Habecker et al., 2009; Zigmond, 2012b). Finally, in addition to the cell body response there is an alteration of protein translation in the axons themselves. (b) When a CL is made prior and also distal to a test lesion and neurite outgrowth is measured from the test lesion either in vivo or in vitro, growth is accelerated compared to when only a test lesion is made. As discussed in the text, macrophage accumulation in the region of axotomized cell bodies is required for a normal CL response.
Following a peripheral nerve injury, neuronal cell bodies exhibit significant morphological changes that were first described over 100 years ago by Franz Nissl, including a shift in the localization of the nucleus within the cell body (Leinfelder, 1938). Neurons undergo chromatolysis, characterized by the disassociation of polyribosomes from the endoplasmic reticulum (Lieberman, 1971). Changes in the non-neuronal cell populations around peripheral cell bodies also occur.This includes significant increases in macrophages seen in the SCG and DRG following nerve injury beginning at 2 d and sustained for at least 2 wk (Lu and Richardson, 1993; Schreiber et al., 1995). In addition there is a proliferation of satellite cells in the ganglia (Humbertson Jr et al., 1969; Bachoo et al., 1992).
Axonal signaling to the cell body is the driving force behind the profound changes that occur in the cell body. Early work using Aplysia led to the idea that injury signaling from the axon to the cell body could be divided into early and late signals (Ambron et al., 1996). These early signals are mediated by Ca2+ influx and the later signals are dependent upon traditional retrograde transport.
Rapid Ca2+ signaling from the injury site to the axotomized cell body
Transection of an axon causes a breach in the membrane of the neuron (Fig. 5a). As noted earlier in this review, a significant increase in Ca2+ is seen immediately at the site of injury, and this signal is propagated towards the cell body(Ziv and Spira, 1995). Little is known about how this happens, but tetrodotoxin-sensitive sodium channels are involved (Iwata et al., 2004). Furthermore, release of intracellular Ca2+ stores may also help maintain the Ca2+ wave (Merianda et al., 2009). This intra-axonal increase in Ca2+ has many beneficial effects including membrane resealing and growth cone formation (Bradke et al., 2012). The Ca2+ wave is tightly regulated through, among many mechanisms, the activation of the Ca2+-sensitive protease, calpain (Gitler and Spira, 1998; 2002).
The Ca2+ wave could be responsible for the activation of immediate early genes JUN and FOS(West and Greenberg, 2011). However, transcriptional analysis in response to nerve injury shows that most changes occur at later time points that do not coincide with the timeline of early/fast injury-induced signals (Michaelevski et al., 2010b). Interestingly, a recent study showed that the Ca2+ wave causes the nuclear export of histone deacetylase 5 (HDAC5) which increases the overall histone acetylation (Cho and Cavalli, 2012; 2013; 2014). This epigenetic change would favor increased transcription and could prime the cell body for the significant gene expression changes to come.
The immediate early genes activated within hours of injury play a significant role in the regenerative capability of the neuron following injury(e.g., Patodia and Raivich, 2012). These include JUN, FOS, activating transcription factor 3 (ATF3), and cAMP response element binding protein (CREB). JUN, FOS, and ATF3 can form homo- and heteromeric complexes that contribute to the formation of activator protein-1 (AP-1) transcription factor complexes, which can regulate a multitude of genes (Jochum et al., 2001). JUNexpressed in axotomized neurons has been shown to play a significant role in regeneration. A conditional knock out of JUN in neurons suppressed regeneration and altered gene expression following facial motor nerve injury (Raivich et al., 2004). Subsequent studies also confirmed that overexpression of JUN in cortical neurons is able to induce neurite outgrowth (Lerch et al., 2014). Interestingly, Lerch and colleagues showed that this increase in regenerative ability did not coincide with increases in GAP-43 as it does in the PNS, suggesting that it may be acting through a different mechanism to promote regeneration in this context than it does in peripheral neurons. ATF3 is closely associated with regeneration in sensory, motor, and sympathetic neurons (Tsujino et al., 2000; Hyatt Sachs et al., 2007). In sensory neurons, injury to the peripheral but not the central branch is able to induce ATF3 expression (Tsujino et al., 2000; Seijffers et al., 2006).
The role of retrograde transport and nuclear uptake of signals from the injury site
The second, and more delayed injury-induced signaling phase is mediated by signaling complexes transported in a retrograde manner back to the cell body (Fig. 5a). Administration of colchicine, a drug which blocks axonal transport, significantly delays chromatolysis and RAG expression (Singer et al., 1982; Murphy et al., 1999). Proteomic analysis of these signaling complexes revealed a variety of proteins being trafficked including, JUN amino-terminal kinase (JNK), STAT3, dual leucine zipper kinase (DLK), and gp130 cytokines, among others (Michaelevski et al., 2010a).
A key aspect of the increased retrograde transport following peripheral nerve injury is the increased expression or activation of adaptor molecules that facilitate the association of transcription factors with the dynein motor proteins, which are important in retrograde transport. JNK-interacting protein 3 (JIP3) is a scaffolding protein which allows for anterograde transport of activated JNK3 down the axon in an intact neurons(Byrd et al., 2001; Cavalli et al., 2005). Following nerve injury, JIP3 shows increased expression and association with dyneinand dynactin (Cavalli et al., 2005; Drerup and Nechiporuk, 2013). Work in Drosophila demonstrated that DLK was also transported via JIP3 association (Klinedinst et al., 2013).
Another critical group of adaptor molecules, the importin family of proteins, has been linked to retrograde injury signaling. Importin-α is constitutively expressed in peripheral nerves; however, importin-β1 shows increased expression after injury through local translation in the axon (Hanz et al., 2003). It is the formation of heterodimers between importin-α and β which increase the association of NLS containing proteins and dynein motor complexes (Perry and Fainzilber, 2009). Interestingly, importin β1 and importin 7 have both been linked to vimentin-dependent retrograde transport of phosphorylated ERK(Perlson et al., 2005; Chuderland et al., 2008; Chuderland and Seger, 2008). Axon-specific knockout of importin-β1 affects over half of all genes changed as part of the cell body response to injury (Perry et al., 2012).
Work in Drosophila identified a MAP 3 kinase whose mammalian homologue is DLK as a retrograde injury signal (Yan et al., 2009; Xiong et al., 2010). In Drosophilia this protein is significantly increased in the axon after injury and is trafficked through interaction with the scaffolding protein, JIP-3 (Xiong et al., 2010; Klinedinst et al., 2013). A knockout of DLK in mice displayed a significant reduction in the elongation phase of regeneration while initiation remained normal in sensory neurons (Itoh et al., 2009). Interestingly, DLK knockouts show significant impairments in the retrograde transport and activation of STAT3 and JNK (Shin et al., 2012). Thus, DLK likely regulates regeneration through its influence on many other injury-derived retrograde signals.
Intra-axonal Luman/CREB3 was recently identified by Ying et al. as a novel retrograde injury signal(Ying et al., In Press). CREB3 is an endoplasmic-bound member of the bZIP transcription factor family, which is released from the endoplasmic reticulum in its active form through proteolysis in response to stressful events (Raggo et al., 2002). In response to a sciatic nerve crush, CREB3 is locally synthesized in the axon and is cleaved into its active form and retrogradely transported back to the cell body. Knockdown of CREB3 in dissociated culture, using siRNA, revealed a significant reduction in elongating neurite outgrowth, with no effect on branching(Ying et al., In Press). Interestingly, the use of an in vitro DRG explant compartmented culture, to better mimic in vivo conditions and to allow knockdown of CREB3 specifically in axons, exhibited a nearly 50% reduction in neurite length, suggesting that this novel injury signal plays an important role in peripheral nerve regeneration.
Another well documented retrograde injury signal is STAT3. Following injury to the sciatic nerve activation of STAT3 by phosphorylation of a specific tyrosine residue (Tyr 705) is seen in the nerve (Lee et al., 2004; Qiu et al., 2005). A correlative relationship between the activation of STAT3 and the ability for DRG neurons to regenerate following injury was demonstrated by showing that only an injury to the peripheral branch (sciatic nerve), and not the dorsal root, activated STAT3 and exhibited regeneration(Schwaiger et al., 2000). Furthermore, transcriptome analyses of injured DRG neurons have also identified STAT3, as an injury-induced locally translated axonal transcription factor (Michaelevski et al., 2010b; Smith et al., 2011; Ben-Yaakov et al., 2012). STAT3 is retrogradely transported back to the cell body through interaction with importin α and dynein (Ma and Cao, 2006; O’Brien and Nathanson, 2007; Ben-Yaakov et al., 2012). Prevention of this retrograde transport through blockade of STAT3’s NLS shows a significant reduction in the expression of the RAG,regenerating islet-derived protein 3 alpha, and reduced neuronal survival(Ben-Yaakov et al., 2012).
STAT3 is a common transcription factor used by multiple pathways such as the gp130 cytokines (Zigmond, 2012b). In addition to its role as a retrograde injury-induced signal, STAT3 could be pivotal in integrating cytokine and neurotrophin signals to help promote regeneration(Corness et al., 1998; Rajan et al., 1998; Shadiack et al., 1998; Ng et al., 2003). By examining sympathetic reinnervation of the heart following myocardial infarction, it was shown that NGF stimulates serine phosphorylation of STAT3 (Ser), in contrast to the tyrosine phosphorylation induced by cytokines (Pellegrino and Habecker, 2013). Additionally, transfection studies of STAT3-null neurons suggested that two pools of STAT3, phosphorylated on either serine or tyrosine, are necessary for a maximal regenerative response (Pellegrino and Habecker, 2013). Interestingly, an in vivo investigation into the importance of STAT3 in DRG regeneration revealed a phase specific role for STAT3 in the initiation, but not elongation of axonal regeneration. Using a viral-mediated Cre and floxed STAT3 mouse and in vivo imaging following injury to the saphenous nerve, Bareyre et al. found that neurons lacking STAT3 showed a significant reduction in growth rate at 48 to 72 h but a normal growth rate at 7 to 8 d following injury (Bareyre et al., 2011).
Extrinsic signals also produce changes in neuronal gene expression following injury (Ferguson and Son, 2011; Zigmond, 2012b). Inflammation and immune cell infiltration, dominated by hematogenous macrophages at the site of injury and around axotomized cell bodies comprise an important component of extrinsic signals associated with a peripheral nerve injury. Local inflammation in the DRG caused by injection of bacteria caused significant increases in GAP-43 and JUN (Lu and Richardson, 1995) in intact neurons. A recent study indicates that cytokines normally released by monocytes/macrophages can stimulate gene expression changes in neurons (Lisak et al., 2011). Rat cortical neurons incubated with combination of cytokines that can be secreted by macrophages, including IL-6 and IL-1β, showed marked changes in expression of genes for cell adhesion molecules, proteins related to apoptosis, various signaling pathways and others (Lisak et al., 2011). Taken together, these data suggest that macrophages, and other immune cells, may directly alter gene expression in neurons following nerve injury.
The damage signals discussed above are the driving force behind the massive changes in gene expression found to occur in regeneration competent neurons. The idea that there is a shift in the neurons’ phenotype promoting survival and regeneration and downregulating molecules involved in neurotransmission is supported by early studies in the sympathetic nervous system which showed increases in tubulin expression accompanied by a decrease in tyrosine hydroxylase expression (Cheah and Geffen, 1973; Koo et al., 1988; Sun and Zigmond, 1996a). Later studies showed the downregulation of a large number of synaptic transmission related molecules (Zhou et al., 1998; 2001; Boeshore et al., 2004). A number of array studies have been performed which provide more complete analysis of the overall gene expression changes following peripheral nerve injury in the DRG, SCG, and facial motor nucleus (Costigan et al., 2002; Boeshore et al., 2004; Zujovic et al., 2005; Kim et al., 2009; Michaelevski et al., 2010b; Ben-Yaakov et al., 2012).
A large portion of the identified RAGs encode proteins in one of several categories: cytoskeletal proteins and adaptors, metabolic enzymes, neuropeptides, cytokines and chemokines, neurotrophins, and transcription factors.. Adhesion molecules and enzymes upregulated following nerve injury include α7β1 integrin, a cell surface receptor for laminins (Yao et al., 1996; Ekstrom et al., 2003), and arginase1, a key enzyme in polyamine synthesis (Boeshore et al., 2004; Schreiber et al., 2004; Deng et al., 2009). Peripheral injury-induced neuropeptides include vasoactive intestinal peptide (VIP)(Villar et al., 1989; Hyatt-Sachs et al., 1993), galanin (Hokfelt et al., 1987; Mohney et al., 1994; Schreiber et al., 1994), and pituitary adenylate cyclase activating polypeptide (PACAP)(Moller et al., 1997; Armstrong et al., 2008; Habecker et al., 2009). Multiple transcription factors are involved in injury induced signaling, and many show significantly increased expression following injury, including ATF3 (Tsujino et al., 2000; Hyatt Sachs et al., 2007) and the immediate early gene, JUN(Jenkins et al., 1993). Immune molecules play a major role in the cell body response to injury, where cytokines such as LIF(Rao et al., 1993; Banner and Patterson, 1994; Sun et al., 1994; Gardiner et al., 2002), IL-6 (Habecker et al., 2009) and CCL2(Schreiber et al., 2001; Subang and Richardson, 2001; Tanaka et al., 2004; Niemi et al., 2013) are upregulated in response to axotomy. Gene expression for certain neurotrophic factors and their receptors are increased after nerve axotomy, as well. Different neuronal systems (e.g., sympathetic and sensory) show variations in trophic factor expression after injury (for review see Boyd and Gordon, 2003; Zigmond, 2012b; Harrington and Ginty, 2013).
The first RAG to be identified was GAP-43. Skene and Willard (1981) originally discovered GAP-43 as a rapidly transported axonal protein which is highly induced after sciatic nerve injury (Skene and Willard, 1981) and whose induction is correlated with regeneration (Skene and Willard, 1981; Katz and Black, 1986; Skene, 1989). Overexpression of GAP-43 led tospontaneous sprouting at the neuromuscular junctions as well as increased injury-induced terminal arborization of the gastrocnemius muscle following sciatic nerve crush (Aigner et al., 1995). Overexpression of GAP-43 and cytoskeleton-associated protein-23 (CAP-23) allows for CNS axon regeneration(Bomze et al., 2001).
RAGs that have been directly shown to promote regeneration
Out of the many genes whose expression is changed following peripheral nerve injury and are thus considered RAGs, only a handful have actually been directly implicated as being necessary or sufficient for regeneration. α7β1 integrin is localized to the growth cones of regenerating facial motor axons following injury. A knockout of the α7 subunit of this integrin displayed a significant reduction in whisker pad reinnervation following facial nerve crush (Werner et al., 2000). Another study using facial motor neurons detailed the necessity of PACAP in regeneration. A PACAP −/− mouse displayed significantly delayed regeneration following facial motor axotomy that coincided with near 10-fold increases in prominent inflammatory cytokines, IFN-γ, IL-6, and TNF-α, and a 90% reduction in the anti-inflammatory cytokine IL-4 (Armstrong et al., 2008).
Polyamine synthesis and arginase 1 have been tied to axonal regeneration in the PNS (Gilad et al., 1996; Lee and Wolfe, 2000; Schreiber et al., 2004). Overexpression of arginase 1 in cerebellar neurons was sufficient to overcome the inhibition of MAG and myelin and allow for axonal elongationin vitro, similar to the effects of administration of cAMP. Additionally, blocking polyamine synthesis prior to administration of cAMP to cultures inhibited their ability to extend axons on inhibitory substrates (Cai et al., 2002). ATF3, is a reliable neuronal marker of injury (Tsujino et al., 2000; Hyatt Sachs et al., 2007). Overexpression of ATF3 in uninjured DRG sensory neurons produced a significant increase in neurite outgrowth similar to the effect of a CL(Seijffers et al., 2006; Seijffers et al., 2007). This did not have an effect on central regeneration or growth on non-permissive substrates, such as MAG, suggesting its importance specifically in peripheral regeneration (Seijffers et al., 2007).
LIF, like IL-6 a member of the gp130 cytokine family, has been linked to regeneration in the SCG through activation of its signaling receptor gp130 (Habecker et al., 2009; Hyatt Sachs et al., 2010). In the DRG, a LIF −/− mouse shows a significant decrease in fibers positive for GAP-43,thus marking them as regenerating fibers, distal to the injury site 3 dafter a sciatic nerve crush as well as, a 50% reduction in growth in vitro following a CL(Cafferty et al., 2001). Small proline-rich repeat protein 1A (SPRR1A) shows a 60-fold increase in expression following peripheral nerve injury (Bonilla et al., 2002). Overexpression of SPRR1A in sensory neurons induces significant increases in neurite outgrowth in vitro, while knockdown of this gene inhibits regeneration (Bonilla et al., 2002).
As indicated above, the gene expression changes that occur as a result of peripheral injury also lead to a reduction in the machinery necessary for neurotransmission. Alteration of neuronal activity in injured neurons plays an important role in the regenerative process. Blinzinger and Kreutzberg (1968)demonstrated the displacement of synaptic boutons from the surface of regenerating motoneurons after axotomy. This phenomenon is now widely known as “synaptic stripping” (Graeber et al., 1993). Synaptic terminals are lost from the cell body during the first week post-axotomy (Matthews and Nelson, 1975; Purves, 1975; Sumner, 1975b; a). This process is reversible following regeneration and reinnervation of target tissue (Sumner, 1975a). In different studies, the removal of synapses has been hypothesized to be carried out by activated microglia (Blinzinger and Kreutzberg, 1968; Chen et al., 2014), astrocytes (Yamada et al., 2011), and satellite glial cells (Matthews and Nelson, 1975). In marked contrast, Perry and O’Connor (2010) have proposed that synaptic stripping is “a neuron autonomous event”. Results have also been presented that LIF and the absence of NGF might be signals leading to synaptic stripping.Nja and Purves (1978) presented evidence that synaptic stripping in sympathetic ganglia is triggered at least in part by the axotomy-induced blockadeof the retrograde transport of NGF.Guo et al. (1999) reported that LIF causes dendritic retraction of sympathetic neurons in culture, a phenomenon that might be part of synaptic stripping in vivo.
Interestingly, the expression of PSD-95, a major protein of the post-synaptic density (PSD) and known to be involved in the regulation of synaptic plasticity, is downregulated after injury (Che et al., 2000). Electrophysiological studies in which synaptic potentials were recorded have confirmed the occurrence of synaptic stripping following axotomy of facial or hypoglossal motor neurons (Yamada et al., 2008; Yamada et al., 2011). The impaired recovery of function fine muscle observed following facial nerve trauma in humans and in SCG axotomy in rats has been attributed to synaptic stripping and its incomplete reversibility after regeneration (Case and Matthews, 1986; Graeber et al., 1993). Along with synaptic stripping observed in the SCG following axotomy, it is interesting to note that a significant reduction in the mRNA of the α3, α5, α7, and β4 subunits of the postsynaptic nicotinic acetylcholine receptors is observed 48 h post-injury (Zhou et al., 1998).
The role of electrical activity and its effects on regeneration and axon growth has been debated over the past two decades. Findings in 2004 suggested that spontaneous activity was necessary for axon path finding during development (Hanson and Landmesser, 2004). Also, in vivostudies provided evidence that electrical stimulation increased outgrowth following peripheral nerve injury and repair in rodents(Brushart et al., 2002; Vivo et al., 2008; Geremia et al., 2010) and humans(Gordon et al., 2010). However, a recent study from the Bradke lab suggested that electrical activity and Ca2+ influx suppresses axon growth of adult sensory neurons (Enes et al., 2010). Interestingly, axotomy of the peripheral branch, but not the central branch, silences DRG neurons, and this correlates with the ability of the peripheral branch, but not the central branch, to regenerate.
In addition to neuronal cell bodies, axons contain the machinery necessary for protein synthesis. Looking at the axons of cultured adult DRG neurons following axonal injury, regenerating axons contained ribosomal proteins and translation initiation factors (Twiss et al., 2000; Zheng et al., 2001). An example of an axonally translated protein is the peptide calcitonin gene-related peptide (CGRP). Toth et al. (2009) demonstrated that CGRP mRNA and protein increased in the proximal sural nerve after a nerve crush. To determine whether local translation of CGRP had any effect on nerve regeneration, CGRP siRNA was delivered locally to the proximal segment of the sciatic axon following which a disruption of axonal outgrowth was seen. These studies and others indicate that axonally synthesized proteins can facilitate regeneration (Willis and Twiss, 2006).
THE CONDITIONING LESION RESPONSE
The cell body response is thought to underlie the increased regeneration that occurs after a CL. The effect of a CL was first described by McQuarrie and Grafstein (1973) in the context of regeneration in the PNS in their discovery that the growth rate of silver stained axons in the sciatic nerve increased following a test lesion if that nerve had been lesioned 1-2 wk earlier (Fig. 5b). Their research suggested that a prior CL primes the damaged neuron to initiate its intrinsic growth machinery so that after a subsequent test lesion the rate of regeneration is accelerated(McQuarrie and Grafstein, 1973). The cell body response—the upregulation in RAG expression mediated by several transcription factors and the consequent ability for peripheral axons to regenerate—is strongly enhanced if the axons first undergo a CL(Skene, 1989; Smith and Skene, 1997; Bosse et al., 2001). Included in this response, the CL induces changes in neuronal and non-neuronal cytokine expression and neuropeptide induction that facilitate nerve regeneration (Sachs et al., 2007; Gaudet et al., 2011; Zigmond, 2012b).
Later studies have revealed increased neurite outgrowthin vitro in sensory and sympathetic neurons in explant and dissociated cultures, after a CL in vivo. Several groups have confirmed marked increases in neurite outgrowth after a CL in explants of DRG and SCG (Edstrom et al., 1996; Shoemaker et al., 2005; Sachs et al., 2007; Niemi et al., 2013), and in dissociated neuron cultures of those ganglia (Hu-Tsai et al., 1994; White et al., 1996; Smith and Skene, 1997; Shoemaker et al., 2005; Sachs et al., 2007; Niemi et al., 2013). Additionally, in vivo regeneration studies established that a CL response occurs in motor, sensory and sympathetic neurons(McQuarrie et al., 1977; McQuarrie, 1978; Navarro and Kennedy, 1990). It is important to note thatinDRG neurons, which have both aperipheral and a central branch, a CL is produced only after a lesion of the peripheral branch(e.g. Smith and Skene, 1997; Seijffers et al., 2007).
Unlike in the PNS where damaged axons of DRG neurons can regenerate after a peripheral lesion, axons in the CNS are incapable of the same degree of regeneration after a dorsal columninjury(Ramon y Cajal, 1928). Crushed central branch axons can sprout and elongate to a limited extent(Oblinger and Lasek, 1984), but they fail to reinnervate their targets because their extensions become stunted after traversing a short distance into the dorsal root entry zone (Bignami et al., 1984; Liuzzi and Lasek, 1987). Central sensory axons projecting from the DRG and into the spinal cord that have received a prior CL are unable to produce regenerative neurite extensions after a subsequent lesion is made to the same central branch (Ramon y Cajal, 1928). The insertion of a peripheral nerve graft into the lesioned spinal cord, however, can generate a permissive environment within which DRG central processes can regenerate after a peripheral CL(Richardson and Issa, 1984). Moreover, if the peripheral branch of the DRG is lesioned prior to an injury made to the central branch, even in the absence of a graft, the central axons of the L4, L5 and L6 DRGs regenerate into and grow past the injured dorsal columns of the spinal cord (Neumann and Woolf, 1999). For the CL mechanism to have relevance for the clinical situation, it would be useful to elicit this type of effect after a CNS injury. In 2009, Ylera et al. showed that central branches of the rat DRG could regenerate if only a minor central lesion to the dorsal column occurred 3 d prior to a peripheral CL. These authors also reported that expression of RAGs such as neuropeptide Y, VIP, and SPRR1A increased when the peripheral branch was lesioned after a central injury. Importantly, these changes in RAG expression were comparable to those obtained in animals that were either peripherally conditioned prior to CNS injury or those animals that received a peripheral CL alone.(Ylera et al., 2009). However, a major dorsal column injury resulting in scar tissue abrogated the regenerative capacity of the CNS axons, despite a subsequent CL (Ylera et al., 2009). Accordingly, axons of the neurons that received a lesion to the central branch alone did not exhibit any regenerative response (Schreyer and Skene, 1993), including the upregulation of the three aforementioned RAGs (Ylera et al., 2009).
The disparate allowances for central and peripheral nerve regeneration have been partially attributed to the environment surrounding CNS and PNS lesions (Fawcett, 2006; Yiu and He, 2006). Glial cells, such as Schwann cells, play a significant role in nerve regeneration after injury (Gaudet et al., 2011). Schwann cells that myelinate axons in the PNS create a more accommodating atmosphere for regrowth by phagocytosing axonal and myelin debris together with infiltrating macrophages (Stoll et al., 1989; Gaudet et al., 2011), whereas oligodendrocytes, the Schwann cell’s myelinating counterpart in the CNS, either die by apoptosis or become quiescent after injury, leaving inhibitory myelin proteins to linger as an impediment to growing axons (Ozawa et al., 1994; Wolswijk, 1998). While MAG normally inhibits regrowth of sensory axons in vitro (Mukhopadhyay et al., 1994), a peripheral CL can reverse this result by stimulating axonal resistance to the inhibitory effect of myelin (Qiu et al., 2002). Nevertheless, in vivoexperiments on the effects of myelin proteins on axon regeneration have produced conflicting results. Triple-knockout mice of the myelin-derived inhibitor proteins, Nogo, MAG and oligodendrocyte myelin glycoprotein, showed no change in the regeneration of corticospinal or raphespinal serotonergic axons after spinal cord injury for one group (Lee et al., 2010), while another group reported enhanced regeneration and improved locomotion with these mice (Cafferty et al., 2010). These discrepancies require further study to establish the role that glial-derived proteins actually play in regeneration in vivo.
The inhibitory effect of these myelin proteins on in vitro and in vivoneurite outgrowth can be overcome by a CL-induced increase in neuronal cAMP levels (Neumann et al., 2002; Qiu et al., 2002). In fact, injection of a cAMP analog into sensory ganglia in lieu of a CL was sufficient to induce significant regeneration in vivo after a dorsal column lesion, and increased neurite outgrowth in vitro when plated on myelin (Neumann et al., 2002; Qiu et al., 2002). Aside from myelin proteins, chondroitin sulfate proteoglycans (CSPGs) play an inhibitory role in axonal regeneration after nerve injury (Snow et al., 1990; Zuo et al., 1998b; Davies et al., 1999; Moon et al., 2001). A large body of research has focused on the role of CSPGs in glial scar formation and regeneration in the CNS(Cregg et al., 2014), yet these inhibitory molecules have also been found to accumulate in endoneurial tissues around Schwann cells in the peripheral nerve after injury (Braunewell et al., 1995; Zuo et al., 1998a; Zuo et al., 2002). Transected sciatic nerves of rats that received injections near the injury site of chondroitinase, a bacterial enzyme that inactivates the growth inhibiting activity of CSPGs, displayed increased regeneration into the distal nerve stump compared to injured control nerves, suggesting that treatments that remove obstructing proteins could be a useful strategy to augment the regenerative capacity of axons after injury (Zuo et al., 2002).
Despite the presence of myelin proteins and CSPGs in both the PNS and CNS, nerve regeneration is much more successful in the PNS. This difference is due in part to Schwann cell clearance mechanisms that remove myelin protein from the path of regenerating axons, and the ability of these cells and macrophages to express MMPs after injury, which dampens inhibition by CSPGs(Zuo et al., 2002). Thus, clearly, there are a number of non-neuronal factors that influence the ability of axons to regenerate. As will be discussed below, there is now strong evidence thatthe CL effect, which is generally thoughtto represent an increase in the intrinsic growth capacity of a neuron, is in fact, dependent on stimulation by non-neuronal cells, i.e., macrophages.
The role of macrophages in the CL response
The CL effect has been thought to occur through the upregulation of RAGs (Smith and Skene, 1997);however, the underlying mechanisms that induce this transcriptional program remain poorly understood. Niemi et al. (2013) and Kwon et al. (2013) have identified a correlation between the CL induced increase in RAG expression and in neurite outgrowth, and an increased presence of macrophages surrounding DRG and SCG neuronal cell bodies. As already discussed, macrophages are widely believed to play a prominent role in WD of the distal nerve segment after injury through the facilitation of axonal and myelin clearance (Perry, 1994);however, studies that have attributed decreased nerve regeneration to abrogated macrophage accumulation at the distal nerve(e.g., Dailey et al., 1998; Barrette et al., 2008)have overlooked a second site of macrophage function. In addition to the nerve, macrophages also accumulate around the cell bodies of axotomized sensory and sympathetic ganglia (Lu and Richardson, 1993; Schreiber et al., 1995), though their role at this location has remained undefined.
To identify the macrophage function at this second site, Niemi et al. (2013) employed the CL model with two strains of mutant mice that display muted macrophage infiltration to the injured distal nerve—the Wlds mouse and the CCR2−/− mouse. Using explant and dissociated cultures of L5 DRG and SCG neurons to remove any effect of the inhibitory distal nerve on regeneration, Niemi et al. (2013) showed that, in Wlds and CCR2−/− mice, the absence of macrophages near the cell bodies of DRG neurons correlated with a complete loss of a CL effect (Fig. 4). In Wlds mice, neurite outgrowth in the SCG after a CL was reduced but not abolished, which interestingly correlated with the finding that while macrophage accumulation in the SCG was reduced it was also not abolished, as it was in the DRG (Niemi et al., 2013). In Wlds mice, mRNA levels for CCL2, the chemokine with the major responsibility for macrophage chemotaxis from the blood to peripheral tissues (Schreiber et al., 2001; Tanaka et al., 2004; Perrin et al., 2005), were attenuated comparably in both the DRG and SCG (Niemi et al., 2013).The finding that the injury-induced upregulation of CCL2 was lessened in the Wlds mouse in sensory and sympathetic neurons is the first demonstration of a change in expression of a RAG in these mutants(Niemi et al., 2013)and differs from the implications drawn by Bisby et al. (1995)who found no difference in the mRNA expression of GAP-43 and JUN. The fact that macrophages still accumulated in the SCG in the CCR2 −/− mice (though to a lesser extent than in WT mice) suggests that a second chemokine is involved in macrophage accumulation at this site (Surmi and Hasty, 2010; Ingersoll et al., 2011; Niemi et al., 2013).
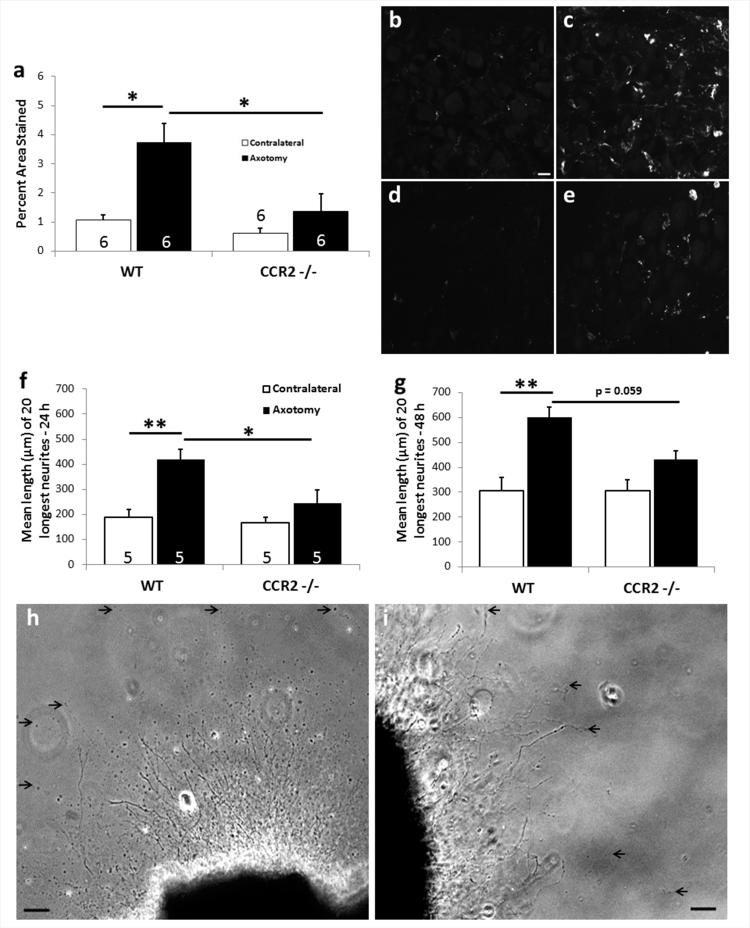
Figure 4.
Macrophage accumulation around axotomized DRG neurons is significantly reduced in CCR2 −/− mice leading to no effect of a CL. The sciatic nerve was unilaterally transected in WT and CCR2 −/− mice. Seven days post-injury, the ipsilateral and contralateral L5 DRGs were removed for analysis of macrophage accumulation using an antibody to CD11b (a-e) or placed in explant cultures for measuring neurite outgrowth in response to a CL (f-i). In response to nerve injury, A 4-fold increase in staining is evident in the axotomized WT L5 DRG (a,c). Axotomized DRGs from CCR2 −/− mice show significantly diminished macrophage accumulation compared to WT (a). Representative micrographs show very little CD11b+ staining in contralateral WT (b) or CCR2 −/− (d) ganglia, or in ipsilateral CCR2 −/− ganglia (e). DRGs from CCR2 −/− mice also show a complete lack of the CL response at 24 and 48 h in explant culture (f,g). Representative micrographs of neurite outgrowth at 48 h in vitro for WT (h) and CCR2 −/− (i). *p<0.05, **p<0.001. Scale bar, 20 μm (IHC); 100 μm (explant). (Modified from Niemi et al., 2013)
Concurrent with Niemi et al.’s publication, another lab observed that an accumulation of macrophages at the cell bodies of injured rat DRGs corresponded with an enhanced regenerative response that persisted for up to 28 d after a sciatic nerve CL (Kwon et al., 2013). Minocycline administered intrathecally at the level of the L5 DRG at the time of injury diminished the macrophage population in that ganglion by approximately half and resulted in subsequent decreased neurite outgrowth (Kwon et al., 2013). A similar study using clodronate liposomes to systemically reduce macrophage numbers have also described a loss of the CL effect of lesioned central processes of DRG neurons (Salegio et al., 2011). However, caveats for both of these studies must be considered. For example, how minocycline diminishes macrophage accumulation and function is unclear. (Herrmann et al., 2008)Kwon et al. refer to minocycline as a “macrophage deactivator”. Several studies have indicated that minocycline decreases the expression of CCL2 and MHC class IIin macrophages and microglia, though the exact mechanisms for these effects are not well understood(Zink et al., 2005; Nikodemova et al., 2007). More problematically, minocycline is known to have off-target effects that can influence the extravasation of macrophages into the injured tissue by inhibiting revascularization and downregulating MMP-9 and HIF-1α/VEGF (Keilhoff et al., 2008). Minocycline also protects Schwann cells from oxygen/glucose deprivation-induced death by stabilizing Bcl-2 expression (Keilhoff et al., 2008), reduces the activation of phagocytic Schwann cells and inhibits the opening of the blood-nerve barrier (Keilhoff et al., 2007), all of which raise questionsas to whether the results using minocycline are directly attributable to macrophage loss. Additional studies delineating the numerous effects of minocycline have been reported (Gabler and Creamer, 1991; Kloppenburg et al., 1995; Bilousova et al., 2009; Schmitz et al., 2012). Likewise, Salegio et al.’s (2011) modelused chlodronate liposomes, which systemically reduce macrophage numbers at both the CNS and PNS injury sites and within the DRGs, and therefore this approach precludes any definitive conclusions as to the importance of monocyte entry specifically into the DRG. On the other hand, injection of peritoneal macrophages directly into the DRG induced regeneration, though somewhat surprisingly only in the central branches of DRG neurons (Lu and Richardson, 1991), suggesting that macrophages can play an important role around axotomized neuronal cell bodies in nerve regeneration.
Kwon et al. supported previous findings showing that increased intracellular levels of cAMP in the DRG induced neurite outgrowth comparable to the effects of a CL (Neumann et al., 2002; Qiu et al., 2002) by demonstrating that injection of a cAMP analog into the DRG caused macrophage influx into the ganglia and that the resulting neurite outgrowth and macrophage numbers were both inhibited by injection of minocycline near the DRG (Kwon et al., 2013). Neuronal cultures with cAMP alone could not induce neurite outgrowth (Kwon et al., 2013), nor was conditioned medium taken from cAMP-stimulated macrophages and later applied to DRG neurons sufficient to induce neurite outgrowth. However, conditioned medium taken from macrophage-DRG neuron co-cultures treated with cAMP and applied to naïve neuron cultures resulted in a significant increase in neurite outgrowth (Kwon et al., 2013). The mechanism of this cAMP effect remains unknown.
Despite the mounting evidence that hematogenous macrophage action at the site of peripheral neuronal cell bodies plays a significant role in regeneration after a CL, significant neurite outgrowth can already be seen at 1 d post-injury before significant monocyte infiltration into the ganglion(Qiu et al., 2002; Zou et al., 2009; Kwon et al., 2013).Axonal injury causes dramatic changes in neuronal transcription and translation both at the cell body site and also locally near the site of injury, inducing the expression of RAGs in response to cytokine and neurotrophin production after injury (Caroni, 1998; Snider et al., 2002; Zigmond, 2012b).Whether macrophages play a role in stimulating RAG expression remains unknown. A small amount of increased neurite outgrowth was seen from neurons cultured one day after a CL, before macrophage accumulation is seen, and, based on this, Kwon et al, proposedthat macrophages may not be necessary to stimulate neurite outgrowth, but rather that they may be necessary for “the full manifestation of conditioning effects”(Kwon et al., 2013). Perhaps the axonal and cell body changes in protein expression that occur immediately following nerve injury are necessary to initiate a regenerative program in neurons, whereas macrophages are pivotal at later stages of regeneration and function to sustain neurite outgrowth. Possible cytokines that macrophages and other non-neuronal cells release in response to nerve injury which might be involved in maintaining this regenerative response will be discussed in the next section.
Which cytokines might be involved in the macrophage-dependent conditioning lesion response?
In the beginning of this review article, the roles of cytokines expressed in the distal sciatic nerve after a lesion on WD were discussed; however, these cytokines might also act locally to promote regeneration (e.g., Selvaraj et al., 2012)or at the level of the axotomized cell body following retrograde transport (e.g., LIF; Curtis et al., 1994; O’Brien and Nathanson, 2007) or following their release from neurons or from ganglionic non-neuronal cells (Sun et al., 1994; Murphy et al., 1995; Hyatt Sachs et al., 2010). Therefore, it would be interesting to determine the presence of these cytokines in ganglia after axotomy. The list in Table 1 is admittedly an incomplete summary of possible cytokines that might act in axotomized ganglia.
Table 1.
| Cytokine |
Cells that can produce
the cytokine |
Reference |
|---|---|---|
| CCL2 | Axotomized PNS neu- rons, Schwann cells |
(Flugel et al., 2001b; Schreiber et al., 2001; Tanaka et al., 2004(Toews et al., 1998)) |
| IL-1α | Macrophages, Schwann cells |
(Shamash et al., 2002) |
| IL-1β | Macrophages, Schwann cells |
(Murch, 1998; Shamash et al., 2002) |
| IL-6 | Axotomized PNS neu rons, satellite/Schwann cells, macrophages, fibroblasts, |
(Murphy et al., 1995; Reichert et al., 1996; Murch, 1998; Dubovy et al., 2010) |
| IL-10 | Macrophages | (Be’eri et al., 1998) |
| Fractalkine | DRG neurons | (Verge et al., 2004; Souza et al., 2013) |
| GM-CSF | Fibroblasts, Macrophages |
(Be’eri et al., 1998) |
| LIF | Macrophages, satel lite/Schwann cells |
(Anegon et al., 1990; Banner and Patterson, 1994; Sun et al., 1994) |
| Oncomodulin | Macrophages, neutro phils |
(Yin et al., 2006; Kurimoto et al., 2013; Kwon et al., 2013) |
| TNF-α | Macrophages, Schwann cells |
(Murch, 1998; Shamash et al., 2002) |
We will focus below on oncomodulin and cytokines of the gp130/IL-6 family as they have been studied most extensively as signaling molecules that alter gene transcription and axonal regeneration after axotomy. While the primary question in the context of this review is the identity of those cytokines released by macrophages that stimulate regeneration, the only molecule thus far that has been shown to have thisproperty is oncomodulin (Yin et al., 2003; Yin et al., 2006; Kwon et al., 2013)
Oncomodulin
In their study on the effects of minocycline on the CL effect, Kwon et al. (2013) reported an increase in oncomodulin mRNA in the DRG 7 d after sciatic nerve transection. Oncomodulin’s ability to stimulate nerve regeneration was first seen in the CNS and was proposed to be the mechanism by which inflammation in the eye might stimulate regeneration of lesioned retinal ganglion cells (RGCs) after lens damage or intravitreal injection of zymosan, a yeast cell wall preparation. In a search for a molecule secreted by infiltrating macrophages that might stimulate neurite outgrowth in cultured RGCs, this 11.7 kDa Ca2+-binding protein, was identified. Injection of oncomodulin into the vitreous together with a cAMP analogue or addition of the molecule to cultures together with forskolin and mannose resulted in a stimulation of neurite outgrowth by RGCs (Yin et al., 2003; Yin et al., 2006). More importantly, the effect on RGC axonal outgrowth after inflammation in vivo could be reduced with either an oncomodulin neutralizing antibody or a peptide that blocks oncomodulin interaction with its binding sites on the RGCs (Yin et al., 2009). While the blocking effects were substantial, they were not complete, leaving open the possibility of there being an additional mechanism(s) for inflammation induced axon outgrowth. Finally, the group also found higher molecular weight proteins (≥ 30 kDa) secreted by activated macrophages that inhibited RGCneurite outgrowth that might complicate studies with macrophage conditioned medium (Yin et al., 2006).
Other researchers looking at the same system found evidence for the involvement of CNTF and LIF, which are both upregulated in retinal astrocytes after an inflammatory stimulus (Leibinger et al., 2009b). The most impressive of their findings was the observation that CNTF knockout animals showed somewhat less than half as many RGC axons regenerating after lens injury and that stimulation of axon outgrowth was abolished in CNTF and LIF double knockout animals (Leibinger et al., 2009a).
These results on oncomodulin and CNTF and LIF using loss of function approaches are both impressive. At the moment, there has been no attempt to synthesize the two sets of findings. One possibility could be that there are two signaling pathways that impinge on the growth response after lens injury and that each of these three signaling molecules are required for the full effect.
Oncomodulin also stimulates neurite outgrowth in PNS neurons in the DRG, whether injected intraganglionically or added to neuronal cultures, and the effects found in vitro were potentiated by forskolin (Yin et al., 2006). A more recent study found that oncomodulin alone given either in vitro or intraganglionically produced no significant increase in DRG neurite outgrowth (Harel et al., 2012). On the other hand, administration of oncomodulin together with dibutyryl cAMP increased outgrowth, but the effect was modest when compared to that of intraganglionic administration of zymosan (Steinmetz et al., 2005; Harel et al., 2010), again suggesting additional macrophage mediators.
In addition to oncomodulin, Kwon et al. (2013) found increased expression of a number of cytokines in the axotomized DRG, including LIF, IL-6, IL-1β, and TNF-α. Intrathecal administration of minocycline completely blocked the expression of oncomodulin, LIF, and IL-6, though the mechanism underlying this suppression is unclear particularly given the varied cellular localizationswhere these cytokines can be synthesized. Oncomodulin is present in macrophages and neutrophils (Yin et al., 2006; Kurimoto et al., 2013) though at the time point examined, 7 d after sciatic nerve transection, there are very few neutrophils present in the DRG (Lindborg and Zigmond, unpublished observation). LIF, on the other hand, is present in satellite/Schwann cells in axotomized ganglia (Banner and Patterson, 1994; Sun et al., 1994; Matsuoka et al., 1997; Nagamoto-Combs et al., 1999) and in the sciatic nerve (Curtis et al., 1994; Sendtner et al., 1997). IL-6 is localized within the axotomized DRG to the neurons themselves (Murphy et al., 1995; Murphy et al., 1999) and to satellite glial cells (Dubovy et al., 2010). In the sciatic nerve distal to a lesion, IL-6 is present in Schwann cells (Bolin et al., 1995; Kurek et al., 1996)and in macrophages and fibroblasts (Reichert et al., 1996). Currently, there is no obvious explanation of how minocycline could completely inhibit the increased expression of LIF and IL-6.
When conditioned medium was obtained from co-cultures of DRG neurons and peritoneal macrophages treated with a cAMP analogue, the medium stimulated neurite outgrowth in neuronal cultures, and this effect was blocked by a neutralizing antibody to oncomodulin but not by “neutralizing” antibodies to either LIF or IL-6 (Kwon et al., 2013). Given the extensive literature on the effects of LIF and IL-6 on neurite outgrowth (see below), it is difficult to know whether these results might reflect the absence of these cytokines from the conditioned medium or the inability of the antibodies used to effectively neutralize them.
The mechanism of oncomodulin action is not known either in the retina or in the DRG. It is interesting to note, however, that the increase in the expression of the paradigmatic RAG, GAP-43, in the DRG following sciatic nerve transection is totally abolished by intrathecal administration of minocycline (Kwon et al., 2013).
gp130 cytokines
The gp130 cytokines are members of a family of proteins that do not share amino acid sequence homology but do share a three dimensional structure and act via the signaling receptor gp130 (Bazan, 1991; Ip et al., 1992; Bravo and Heath, 2000; Zigmond, 2012b). The first demonstration of an injury response of neurons to an endogenous gp130 cytokine came from studies on RAG expression in axotomized sympathetic neurons in theSCG using knockout mice or neutralizing antibodies (Rao et al., 1993; Sun et al., 1994; Sun and Zigmond, 1996a). These neurons express certain neuropeptides after injury that they do not normally express, changes that occur both at the mRNA and protein levels (Zigmond, 2012b). Very similar changes occur also in sensory and motor neurons. For example, galanin, VIP, and PACAP are upregulated after axotomy in all three classes of neurons (Zigmond et al., 1996; Zigmond, 2012b). These genes and proteins can be considered RAGs or GAPs, respectively (Zigmond, 2001). Experiments with LIF knockout animals demonstrated a reduction in galanin and VIP expression in SCG neurons after transection of the postganglionic internal and external carotid nerves (Rao et al., 1993)and a reduction of galanin in DRGs after sciatic nerve transection (Corness et al., 1996; Sun and Zigmond, 1996b). Interestingly, experiments with a galanin knockout mouse demonstrated a reduction in the CL effect in sensory neurons (Sachs et al., 2007).
In addition to LIF, IL-6 and IL-11 are also induced in axotomized sympathetic ganglia (Habecker et al., 2009). In the studies described above with the LIF knockout mouse, neuropeptide expression was significantly, but only partially, blocked. Therefore, experiments were performed in conditional knockouts in which the receptor gp130 was deleted in noradrenergic neurons with the aim of blocking the effects of all members of the gp130 family. In these animals, no significant induction of galanin, VIP, or PACAP mRNAs occurred in the axotomized SCG, indicating the involvement of more than one gp130 cytokine in this effect(Habecker et al., 2009). Interestingly, the axotomy-induced increase in mRNA for a fourth neuropeptide, cholecystokinin, was unaffected by the absence of gp130 signaling, suggesting a distinct mechanism was involved for that change in gene expression.
Thompson and colleagues, using LIF and IL-6 single knockout mice, showed an involvement of each of these cytokines in the increased neurite outgrowth by sensory neurons following a peripheral CL,both in dissociated cultures and within the spinal cord in vivo(Cafferty et al., 2001; Cafferty et al., 2004). The effect seen in each of these single knockouts was approximately 50% of the outgrowth in cultures seen in WT animals. A second group obtained different results, namely, that whereas IL-6 was sufficient to stimulate neurite outgrowth in vitro, knocking out IL-6 did not interfere with the CL response in the dorsal columns(Cao et al., 2006). The reason for the differences in these studies remains unclear. In addition, it has been reported that the increased outgrowth from DRG explants after a CL was similar in WT and LIF knockout animals; however, in the latter study data from ganglia that did not receive a CL was not presented making it difficult to evaluate the results (Ekstrom et al., 2000). Nevertheless, a similar lack of dependence of the CL response on LIF was shown in both explants and dissociated cultures of the SCG.(Shoemaker et al., 2005). One possibility to account for this variability is that multiple gp130 cytokines can play a role in the CL effect and that the actual cytokines involved may vary with the exact procedures used in a particular study. Consistent with this interpretation are findings with gp130 conditional knockouts. In SCG neurons from these animals, the CL was totally abolished (Fig. 6; Hyatt Sachs et al., 2010).
Figure 6.
The role of gp130 cytokines in the CL response in the mouse SCG. To remove the influence of all of the gp130 cytokines, a conditional knock out (CKO) of the receptor gp130 was produced in sympathetic neurons using a dopamine beta hydroxylase (DBH) Cre. Seven days following unilateral SCG axotomy in WT(C57) and gp130DBHcre CKO mice, ipsilateral (Ax) and contralateral sham-operated (Sh) SCGs were removed and maintained in explant culture. Neurite outgrowth from SCGs was imaged at 24 and 48 h, and the 20 longest neurites from each ganglion were measured. WT (C57) mice showed a significant CL response at both 24 (a) and 48 h (b) in culture. gp130 CKO mice showed no CL effect at either time point. *p<0.05. (Modified from Hyatt-Sachs et al., 2010)
Many of the effects of these cytokines are produced by the phosphorylation and activation of the enzyme JAK and the subsequent phosphorylation by JAK of STAT3 (for review see Taga, 1997). STAT3 phosphorylation by JAK can be blocked by the drug AG490. Liu and Snider (2001) reported that the increased neurite outgrowth of DRG neurons seen in culture after a CL was blocked by in vitro administration of AG490. Subsequently, it was shown that when this drug was given in vivo at the time of sciatic nerve transection, it blocked the growth of DRG fibers both in culture and in vivo into the dorsal columns following a sciatic nerve lesion(Qiu et al., 2005). While the JAK inhibitor only blocked neurite outgrowth in culture by about 50%, it only blocked STAT3 phosphorylation in the DRG to the same extent.
The exact mechanism by which gp130 cytokines and STAT3 affect regeneration is still not known. As already discussed, STAT3 is a transcription factor, and therefore it is natural that people have looked for changes in candidate gene expression. The injury-induced increase in expression in the DRG of GAP-43 protein was totally blocked in IL-6 knockout mice and blocked by about 50% in mice treated with AG490 (Cafferty et al., 2004; Qiu et al., 2005). In addition, however, two recent articles have indicated that STAT3 can produce effects on neurite outgrowth by acting directly on axons (Pellegrino and Habecker, 2013; Selvaraj and Sendtner, 2013).
EVIDENCE THAT MICROGLIA MIGHT PROMOTE REGENERATION AFTER AXOTOMY IN THE BRAIN AND SPINAL CORD
Nissl, who first described chromatolysis, also observed marked glial proliferation in the nucleus of origin following axotomy of cranial motor neurons (Nissl, 1984). Subsequently these cells were shown to be microglia, the CNS’s resident macrophages (Cammermeyer, 1965; Graeber et al., 1988b; Streit et al., 1988). In the facial motor nucleus, for example, microglial proliferation is seen after transection of the facial nerve in the periphery (Blinzinger and Kreutzberg, 1968; Graeber et al., 1988b). In addition to increased microglia numbers, changes occur in their expression of certain surface antigens [e.g., the complement receptor 3 (Graeber et al., 1988a)] and cytokines. Similar proliferation of microglia is seen around axotomized motor neurons after transection of the hypoglossal nerve (Reisert et al., 1984; Barron et al., 1990; Svensson and Aldskogius, 1993), the phrenic nerve (Gould and Goshgarian, 1997), and the sciatic nerve (Gehrmann et al., 1991; Zhang and De Koninck, 2006).
After a facial nerve lesion, CCL2 is expressed by the axotomized motoneurons, as it is in axotomized sympathetic and sensory neurons, and a small number of macrophages infiltrate into the facial motor nucleus (Flugel et al., 2001a; Flugel et al., 2001b). After sciatic nerve transection, CCL2 is expressed by lumbar motor neurons in the spinal cord, and it has been suggested that the chemokine is involved in microglial activation (Zhang and De Koninck, 2006).
All of the examples cited concern microgliosis around neurons that project out of the CNS and are capable of regeneration after a lesion. What happens to microglia in the vicinity of CNS neurons that do not project into the periphery and do not normally regenerate? No change in the number or activation of microglia surrounding projection neurons in the cortex was found after pyramidotomy (Leong et al., 1995). In the red nucleus variable results were observed in microglia after rubrospinal tract injury; however, even in the study in which microglial activation was reported, it was minimal compared to that seen for microglia surrounding cranial and spinal motor neurons (Barron et al., 1990; Shokouhi et al., 2010).
Shokouhi and colleagues asked the provocative question as to what would happen if these CNS axons were induced to regenerate (Shokouhi et al., 2010). For this purpose they placed a peripheral nerve graft near the transected axons of the rubrospinal tract. Axons grew into the graft, and microglial activation was seen surrounding the axotomized neurons in the red nucleus. Of course, it is not clear from this experiment whether microglia activation facilitates axonal regeneration or whether their activation is the consequence of axonal regeneration; however, the former hypothesis resembles the recent findings concerning macrophages and nerve regeneration of both sensory and sympathetic(Niemi et al., 2013).
Interestingly, interference with microglial proliferation in the hypoglossal nucleus after axotomy using the mitotic inhibitor cytosine arabinoside did not interfere with subsequent nerve regeneration (Svensson and Aldskogius, 1993). Nevertheless, this finding does not rule out the possibility that thestate of activation of the microglia rather than their increased numbers facilitates regeneration.
At least one study; however, suggests that microglia can have a negative effect on regeneration in the CNS. After optic nerve transection, a peripheral nerve bridge was placed in the eye and half of the animals were treated with a tripeptide macrophage inhibitory factor, which decreased the number of microglia in the retina. The treated retinas projected about twice as many retinal ganglion cells into the nerve graft as did control retinas (Thanos et al., 1993). Again this finding raises questions as to what the activation state of the microglia was in this study and whether their activation was similar to that in the motor neuron studies. Nevertheless, together these studies raise the possibility that activation of microglia, perhaps pharmacologically, might promote regeneration in the CNS.
SOME QUESTIONS FOR THE FUTURE
Current research on nerve regeneration can be divided roughly into two camps depending on the part of the nervous system being studied. Those who focus on the PNS seek- to understand the molecular and cellular mechanisms that underlie the degree of regeneration that does occur and how this regeneration might be enhanced. Those who focus on the CNS try to understand what prevents regeneration by most CNS neurons. While these two groups often work in isolation, much benefit could accrue from more interaction. For example, studies on the effects of gp130 cytokines on RAG expression and on regenerationbegan in the PNS (for review see Zigmond, 2012b). Subsequently, these studies were followed by the observation that depletion of suppressor of cytokine signaling 3 (SOCS3), which inhibits JAK/STAT signaling, significantly promotes regeneration of crushed retinal ganglion cell axons (Smith et al., 2009). Furthermore, addition of exogenous IL-6 into the injured spinal cord led to increased behavioral recovery after injury to the corticospinal tract(Yang et al., 2012; Zigmond, 2012a). In the case of the promotion of regeneration resulting from knocking out phosphatase and tensin homolog (PTEN), the flow of discovery went in the opposite direction. A conditional knockout ofPTEN in retinal ganglion cells was shown to promote regeneration of their axons (Park et al., 2008), and this led to the discovery that knocking out this protein in sensory neurons promotes their regeneration also, though possibly through a different signaling pathway (Christie et al., 2010).
With regard to the PNS, a number of groups have pointed out the limitations in regeneration that occur after injury, particularly in humans where the distances axons have to cross can be quite large(Fu and Gordon, 1997; Hoke, 2006). Fenrich and Gordon (2004), for example, pointed to the slowness of axons in crossing the lesion site, the progressive loss of regenerative capacity following chronic axotomy, and the progressive loss of support provided by Schwann cells after chronic denervation. Possible ways to overcome these obstacles in the future have been proposed, for example, by electrical stimulation of or pharmacological interventionswithin the axotomized neurons(Gordon et al., 2009) or genetic manipulations that speed up axonal elongation (Ma et al., 2011). Time will tell how effective these manipulations will be.
Of course elongated axon growth is only one aspect of effective regeneration. It is also important to establish effective synaptic connections with appropriate target cells. Recently, it was shown that even in a system that had previously been thought to show accurate regeneration, i.e., regenerating preganglionic axons of the sympathetic nervous system, only minimal recovery of normal function is reestablished (Lingappa and Zigmond, 2013).
During the course of this review a number of unresolved issues have been raised. For example, with regard to the stimulation of axonal regeneration of retinal ganglion cells due to local inflammation,how can the findings concerning oncomodulin and those concerning gp130 cytokines be reconciled?Also with regard to the effects of hematogenous macrophages on peripheral nerve outgrowth, how much can be explained by oncomodulin, and in both systems, how does oncomodulin produce its effects? In a number of instances, manipulations that decrease monocyte infiltration into the PNS also inhibit regeneration and this has been attributed to their impact on WD; however, given that these manipulations very likely also inhibited infiltration into peripheral ganglia, it needs to be established how much of theeffect on regeneration is due to changes at the level of the axon and how much to changes at the level of the neuronal cell body. Finally, for many years it has been widely believed that WD is primarily dependent on infiltrating monocytes. What is the explanation then for the fact that myelin clearance appears to be normal in CCR2 −/− mice in which monocyte infiltration into the nerve does not occur?
In the 86 years since the publication in 1928 of Ramon y Canal’s treatise “Degeneration and Regeneration of the Nervous System” much has been learned about the interactions between neurons and various non-neuronal cells in the context of axonal degeneration and regeneration, but much remains to be understood. Hopefully, further studies will lead to therapies that will allow better regeneration in the PNS and the possibility of regeneration in the CNS.
Highlights.
Assessment of the roles for non-neuronal cells and the cytokines they secrete in axonal degeneration and regeneration
After nerve injury, macrophages promote regeneration by acting bothin the distal nerve and at axotomized cell bodies
Hematogenous macrophages may not be essential for Wallerian degeneration
The regenerative effects of cytokines at axotomized ganglia requires further study
Acknowledgements
Work in the authors’ laboratory is supported by NIDDK 097223. We thank Rebecca Skerrett, Jared Cregg, and Teresa Evansfor critical reading of the manuscript and Mike McGraw for help with computer programs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aamar S, Saada A, Rotshenker S. Lesion-induced changes in the production of newly synthesized and secreted apo-E and other molecules are independent of the concomitant recruitment of blood-borne macrophages into injured peripheral nerves. J Neurochem. 1992;59:1287–1292. doi: 10.1111/j.1471-4159.1992.tb08439.x. [DOI] [PubMed] [Google Scholar]
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner H-R, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Ambron RT, Zhang XP, Gunstream JD, Povelones M, Walters ET. Intrinsic injury signals enhance growth, survival, and excitability of Aplysia neurons. J Neurosci. 1996;16:7469–7477. doi: 10.1523/JNEUROSCI.16-23-07469.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anegon I, Moreau JF, Godard A, Jacques Y, Peyrat MA, Hallet MM, Wong G, Soulillou JP. Production of human interleukin for DA cells (HILDA)/leukemia inhibitory factor (LIF) by activated monocytes. Cell Immunol. 1990;130:50–65. doi: 10.1016/0008-8749(90)90161-j. [DOI] [PubMed] [Google Scholar]
- Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Abad C, Chhith S, Cheung-Lau G, Hajji OE, Nobuta H, Waschek JA. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience. 2008;151:63–73. doi: 10.1016/j.neuroscience.2007.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachoo M, Hall A, Polosa C, Zigmond R. Postganglionic axotomy and 6- hydroxydopamine (6-HDA) produce a burst of satellite cell proliferation in adult rat superior cervical ganglion (SCG) Soc Neurosci Abstr. 1992;18:625. [Google Scholar]
- Baichwal RR, Bigbee JW, DeVries GH. Macrophage-mediated myelin-related mitogenic factor for cultured Schwann cells. Proc Natl Acad Sci U S A. 1988;85:1701–1705. doi: 10.1073/pnas.85.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baitsch D, Bock HH, Engel T, Telgmann R, Muller-Tidow C, Varga G, Bot M, Herz J, Robenek H, von Eckardstein A, Nofer JR. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1160–1168. doi: 10.1161/ATVBAHA.111.222745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner LR, Patterson PH. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc Natl Acad Sci U S A. 1994;91:7109–7113. doi: 10.1073/pnas.91.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Garzorz N, Lang C, Misgeld T, Buning H, Kerschensteiner M. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc Natl Acad Sci U S A. 2011;108:6282–6287. doi: 10.1073/pnas.1015239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette B, Hebert MA, Filali M, Lafortune K, Vallieres N, Gowing G, Julien JP, Lacroix S. Requirement of myeloid cells for axon regeneration. J Neurosci. 2008;28:9363–9376. doi: 10.1523/JNEUROSCI.1447-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron KD, Marciano FF, Amundson R, Mankes R. Perineuronal glial responses after axotomy of central and peripheral axons. A comparison. Brain Res. 1990;523:219–229. doi: 10.1016/0006-8993(90)91490-8. [DOI] [PubMed] [Google Scholar]
- Bazan JF. Neuropoietic cytokines in the hematopoietic fold. Neuron. 1991;7:197–208. doi: 10.1016/0896-6273(91)90258-2. [DOI] [PubMed] [Google Scholar]
- Be’eri H, Reichert F, Saada A, Rotshenker S. The cytokine network of wallerian degeneration: IL-10 and GM-CSF. Eur J Neurosci. 1998;10:2707–2713. [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Gilley J, Mazzola F, Conforti L, Janeckova L, Magni G, Ribchester RR, Coleman MP. Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. J Neurosci. 2009;29:653–668. doi: 10.1523/JNEUROSCI.3814-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, Blesch A, Pilpel Y, Twiss JL, Fainzilber M. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012 doi: 10.1038/emboj.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- Bignami A, Chi NH, Dahl D. Regenerating dorsal roots and the nerve entry zone: an immunofluorescence study with neurofilament and laminin antisera. Exp Neurol. 1984;85:426–436. doi: 10.1016/0014-4886(84)90152-3. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, Ethell IM. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- Bisby MA, Chen S. Delayed wallerian degeneration in sciatic nerves of C57BL/Ola mice is associated with impaired regeneration of sensory axons. Brain Res. 1990;530:117–120. doi: 10.1016/0006-8993(90)90666-y. [DOI] [PubMed] [Google Scholar]
- Bisby MA, Tetzlaff W, Brown MC. Cell body response to injury in motoneurons and primary sensory neurons of a mutant mouse, Ola (Wld), in which Wallerian degeneration is delayed. J Comp Neurol. 1995;359:653–662. doi: 10.1002/cne.903590411. [DOI] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85:145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Boeshore KL, Schreiber RC, Vaccariello SA, Sachs HH, Salazar R, Lee J, Ratan RR, Leahy P, Zigmond RE. Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J Neurobiol. 2004;59:216–235. doi: 10.1002/neu.10308. [DOI] [PubMed] [Google Scholar]
- Boivin A, Pineau I, Barrette B, Filali M, Vallieres N, Rivest S, Lacroix S. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci. 2007;27:12565–12576. doi: 10.1523/JNEUROSCI.3027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin LM, Verity AN, Silver JE, Shooter EM, Abrams JS. Interleukin-6 production by Schwann cells and induction in sciatic nerve injury. J Neurochem. 1995;64:850–858. doi: 10.1046/j.1471-4159.1995.64020850.x. [DOI] [PubMed] [Google Scholar]
- Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Skene JH. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci. 2001;4:38–43. doi: 10.1038/82881. [DOI] [PubMed] [Google Scholar]
- Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse F, Kury P, Muller HW. Gene expression profiling and molecular aspects in peripheral nerve regeneration. Restor Neurol Neurosci. 2001;19:5–18. [PubMed] [Google Scholar]
- Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nature reviews Neuroscience. 2012;13:183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- Braunewell KH, Martini R, LeBaron R, Kresse H, Faissner A, Schmitz B, Schachner M. Up-regulation of a chondroitin sulphate epitope during regeneration of mouse sciatic nerve: evidence that the immunoreactive molecules are related to the chondroitin sulphate proteoglycans decorin and versican. Eur J Neurosci. 1995;7:792–804. doi: 10.1111/j.1460-9568.1995.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Bravo J, Heath JK. Receptor recognition by gp130 cytokines. EMBO J. 2000;19:2399–2411. doi: 10.1093/emboj/19.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Lunn ER, Perry VH. Poor growth of mammalian motor and sensory axons into intact proximal nerve stumps. Eur J Neurosci. 1991a;3:1366–1369. doi: 10.1111/j.1460-9568.1991.tb00069.x. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perry VH, Lunn ER, Gordon S, Heumann R. Macrophage dependence of peripheral sensory nerve regeneration: possible involvement of nerve growth factor. Neuron. 1991b;6:359–370. doi: 10.1016/0896-6273(91)90245-u. [DOI] [PubMed] [Google Scholar]
- Bruck W. The role of macrophages in Wallerian degeneration. Brain Pathol. 1997;7:741–752. doi: 10.1111/j.1750-3639.1997.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck W, Bruck Y, Friede RL. TNF-alpha suppresses CR3-mediated myelin removal by macrophages. J Neuroimmunol. 1992;38:9–17. doi: 10.1016/0165-5728(92)90085-y. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22:6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster EA, Perry VH, Brown MC. The rate of Wallerian degeneration in cultured neurons from wild type and C57BL/WldS mice depends on time in culture and may be extended in the presence of elevated K+ levels. Eur J Neurosci. 1995;7:1596–1602. doi: 10.1111/j.1460-9568.1995.tb01155.x. [DOI] [PubMed] [Google Scholar]
- Byrd DT, Kawasaki M, Walcoff M, Hisamoto N, Matsumoto K, Jin Y. UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron. 2001;32:787–800. doi: 10.1016/s0896-6273(01)00532-3. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Gardiner NJ, Das P, Qiu J, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J Neurosci. 2004;24:4432–4443. doi: 10.1523/JNEUROSCI.2245-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Gardiner NJ, Gavazzi I, Powell J, McMahon SB, Heath JK, Munson J, Cohen J, Thompson SW. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J Neurosci. 2001;21:7161–7170. doi: 10.1523/JNEUROSCI.21-18-07161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Deng K, Mellado W, Lee J, Ratan RR, Filbin MT. Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron. 2002;35:711–719. doi: 10.1016/s0896-6273(02)00826-7. [DOI] [PubMed] [Google Scholar]
- Cammermeyer J. Juxtavascular karyokinesis and microglia cell proliferation during retrograde reaction in the mouse facial nucleus. Ergreb Ant Entwicklgs Gesch. 1965;38:1–22. [PubMed] [Google Scholar]
- Cao Z, Gao Y, Bryson JB, Hou J, Chaudhry N, Siddiq M, Martinez J, Spencer T, Carmel J, Hart RB, Filbin MT. The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J Neurosci. 2006;26:5565–5573. doi: 10.1523/JNEUROSCI.0815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P. Neuro-regeneration: plasticity for repair and adaptation. Essays Biochem. 1998;33:53–64. doi: 10.1042/bse0330053. [DOI] [PubMed] [Google Scholar]
- Case CP, Matthews MR. Incoming synapses and size of small granule-containing cells in a rat sympathetic ganglion after post-ganglionic axotomy. J Physiol. 1986;374:33–71. doi: 10.1113/jphysiol.1986.sp016065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli V, Kujala P, Klumperman J, Goldstein LS. Sunday Driver links axonal transport to damage signaling. J Cell Biol. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Myers RR, Janes J, Shubayev V. Cytokine regulation of MMP-9 in peripheral glia: implications for pathological processes and pain in injured nerve. Brain Behav Immun. 2007;21:561–568. doi: 10.1016/j.bbi.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che YH, Tamatani M, Tohyama M. Changes in mRNA for post-synaptic density-95 (PSD-95) and carboxy-terminal PDZ ligand of neuronal nitric oxide synthase following facial nerve transection. Brain Res Mol Brain Res. 2000;76:325–335. doi: 10.1016/s0169-328x(00)00013-9. [DOI] [PubMed] [Google Scholar]
- Cheah TB, Geffen LB. Effects of axonal injury on norepinephrine, tyrosine hydroxylase and monoamine oxidase levels in sympathetic ganglia. J Neurobiol. 1973;4:443–452. doi: 10.1002/neu.480040505. [DOI] [PubMed] [Google Scholar]
- Cheepudomwit T, Guzelsu E, Zhou C, Griffin JW, Hoke A. Comparison of cytokine expression profile during Wallerian degeneration of myelinated and unmyelinated peripheral axons. Neurosci Lett. 2008;430:230–235. doi: 10.1016/j.neulet.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Chen Z, Jalabi W, Hu W, Park HJ, Gale JT, Kidd GJ, Bernatowicz R, Gossman ZC, Chen JT, Dutta R, Trapp BD. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat Commun. 2014;5:4486. doi: 10.1038/ncomms5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 2012 doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Cavalli V. HDAC signaling in neuronal development and axon regeneration. Curr Opin Neurobiol. 2014;27C:118–126. doi: 10.1016/j.conb.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Sloutsky R, Naegle KM, Cavalli V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155:894–908. doi: 10.1016/j.cell.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie KJ, Webber CA, Martinez JA, Singh B, Zochodne DW. PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J Neurosci. 2010;30:9306–9315. doi: 10.1523/JNEUROSCI.6271-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuderland D, Konson A, Seger R. Identification and characterization of a general nuclear translocation signal in signaling proteins. Mol Cell. 2008;31:850–861. doi: 10.1016/j.molcel.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Chuderland D, Seger R. Calcium regulates ERK signaling by modulating its protein-protein interactions. Commun Integr Biol. 2008;1:4–5. doi: 10.4161/cib.1.1.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Conforti L, Buckmaster EA, Tarlton A, Ewing RM, Brown MC, Lyon MF, Perry VH. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc Natl Acad Sci U S A. 1998;95:9985–9990. doi: 10.1073/pnas.95.17.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- Conforti L, Fang G, Beirowski B, Wang MS, Sorci L, Asress S, Adalbert R, Silva A, Bridge K, Huang XP, Magni G, Glass JD, Coleman MP. NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell Death Differ. 2007;14:116–127. doi: 10.1038/sj.cdd.4401944. [DOI] [PubMed] [Google Scholar]
- Conforti L, Wilbrey A, Morreale G, Janeckova L, Beirowski B, Adalbert R, Mazzola F, Di Stefano M, Hartley R, Babetto E, Smith T, Gilley J, Billington RA, Genazzani AA, Ribchester RR, Magni G, Coleman M. Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J Cell Biol. 2009;184:491–500. doi: 10.1083/jcb.200807175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti G, De Pol A, Scarpini E, Vaccina F, De Riz M, Baron P, Tiriticco M, Scarlato G. Interleukin-1 beta and interferon-gamma induce proliferation and apoptosis in cultured Schwann cells. J Neuroimmunol. 2002;124:29–35. doi: 10.1016/s0165-5728(02)00003-6. [DOI] [PubMed] [Google Scholar]
- Corness J, Shi TJ, Xu ZQ, Brulet P, Hokfelt T. Influence of leukemia inhibitory factor on galanin/GMAP and neuropeptide Y expression in mouse primary sensory neurons after axotomy. Exp Brain Res. 1996;112:79–88. doi: 10.1007/BF00227180. [DOI] [PubMed] [Google Scholar]
- Corness J, Stevens B, Fields RD, Hokfelt T. NGF and LIF both regulate galanin gene expression in primary DRG cultures. Neuroreport. 1998;9:1533–1536. doi: 10.1097/00001756-199805110-00053. [DOI] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R, Scherer SS, Somogyi R, Adryan KM, Ip NY, Zhu Y, Lindsay RM, DiStefano PS. Retrograde axonal transport of LIF is increased by peripheral nerve injury: correlation with increased LIF expression in distal nerve. Neuron. 1994;12:191–204. doi: 10.1016/0896-6273(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Dailey AT, Avellino AM, Benthem L, Silver J, Kliot M. Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration. J Neurosci. 1998;18:6713–6722. doi: 10.1523/JNEUROSCI.18-17-06713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4:e00264–00213. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felipe C, Hunt SP. The differential control of c-jun expression in regenerating sensory neurons and their associated glial cells. J Neurosci. 1994;14:2911–2923. doi: 10.1523/JNEUROSCI.14-05-02911.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S, Trigueros MA, Kalyvas A, David S. Phospholipase A2 plays an important role in myelin breakdown and phagocytosis during Wallerian degeneration. Mol Cell Neurosci. 2003;24:753–765. doi: 10.1016/s1044-7431(03)00241-0. [DOI] [PubMed] [Google Scholar]
- Deng K, He H, Qiu J, Lorber B, Bryson JB, Filbin MT. Increased synthesis of spermidine as a result of upregulation of arginase I promotes axonal regeneration in culture and in vivo. J Neurosci. 2009;29:9545–9552. doi: 10.1523/JNEUROSCI.1175-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J, Coughlin M, Macintyre L, Holmes M, Visheau B. Evidence that endogenous beta nerve growth factor is responsible for the collateral sprouting, but not the regeneration, of nociceptive axons in adult rats. Proc Natl Acad Sci U S A. 1987;84:6596–6600. doi: 10.1073/pnas.84.18.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drerup CM, Nechiporuk AV. JNK-interacting protein 3 mediates the retrograde transport of activated c-Jun N-terminal kinase and lysosomes. PLoS Genet. 2013;9:e1003303. doi: 10.1371/journal.pgen.1003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovy P, Klusakova I, Svizenska I, Brazda V. Satellite glial cells express IL-6 and corresponding signal-transducing receptors in the dorsal root ganglia of rat neuropathic pain model. Neuron Glia Biol. 2010;6:73–83. doi: 10.1017/S1740925X10000074. [DOI] [PubMed] [Google Scholar]
- Edstrom A, Ekstrom PA, Tonge D. Axonal outgrowth and neuronal apoptosis in cultured adult mouse dorsal root ganglion preparations: effects of neurotrophins, of inhibition of neurotrophin actions and of prior axotomy. Neuroscience. 1996;75:1165–1174. doi: 10.1016/0306-4522(96)00324-7. [DOI] [PubMed] [Google Scholar]
- Ekstrom PA, Kerekes N, Hokfelt T. Leukemia inhibitory factor null mice: unhampered in vitro outgrowth of sensory axons but reduced stimulatory potential by nerve segments. Neurosci Lett. 2000;281:107–110. doi: 10.1016/s0304-3940(00)00816-8. [DOI] [PubMed] [Google Scholar]
- Ekstrom PA, Mayer U, Panjwani A, Pountney D, Pizzey J, Tonge DA. Involvement of alpha7beta1 integrin in the conditioning-lesion effect on sensory axon regeneration. Mol Cell Neurosci. 2003;22:383–395. doi: 10.1016/s1044-7431(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Enes J, Langwieser N, Ruschel J, Carballosa-Gonzalez MM, Klug A, Traut MH, Ylera B, Tahirovic S, Hofmann F, Stein V, Moosmang S, Hentall ID, Bradke F. Electrical activity suppresses axon growth through Ca(v)1.2 channels in adult primary sensory neurons. Curr Biol. 2010;20:1154–1164. doi: 10.1016/j.cub.2010.05.055. [DOI] [PubMed] [Google Scholar]
- Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23:371–383. doi: 10.1089/neu.2006.23.371. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Fenrich K, Gordon T. Canadian Association of Neuroscience review: axonal regeneration in the peripheral and central nervous systems--current issues and advances. Can J Neurol Sci. 2004;31:142–156. doi: 10.1017/s0317167100053798. [DOI] [PubMed] [Google Scholar]
- Ferguson TA, Son YJ. Extrinsic and intrinsic determinants of nerve regeneration. J Tissue Eng. 2011;2 doi: 10.1177/2041731411418392. 2041731411418392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn JT, Weil M, Archer F, Siman R, Srinivasan A, Raff MC. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J Neurosci. 2000;20:1333–1341. doi: 10.1523/JNEUROSCI.20-04-01333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugel A, Bradl M, Kreutzberg GW, Graeber MB. Transformation of donor-derived bone marrow precursors into host microglia during autoimmune CNS inflammation and during the retrograde response to axotomy. J Neurosci Res. 2001a;66:74–82. doi: 10.1002/jnr.1198. [DOI] [PubMed] [Google Scholar]
- Flugel A, Hager G, Horvat A, Spitzer C, Singer GM, Graeber MB, Kreutzberg GW, Schwaiger FW. Neuronal MCP-1 expression in response to remote nerve injury. J Cereb Blood Flow Metab. 2001b;21:69–76. doi: 10.1097/00004647-200101000-00009. [DOI] [PubMed] [Google Scholar]
- Fruttiger M, Schachner M, Martini R. Tenascin-C expression during wallerian degeneration in C57BL/Wlds mice: possible implications for axonal regeneration. J Neurocytol. 1995;24:1–14. doi: 10.1007/BF01370156. [DOI] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Gabler WL, Creamer HR. Suppression of human neutrophil functions by tetracyclines. J Periodontal Res. 1991;26:52–58. doi: 10.1111/j.1600-0765.1991.tb01626.x. [DOI] [PubMed] [Google Scholar]
- Gaete J, Kameid G, Alvarez J. Regenerating axons of the rat require a local source of proteins. Neurosci Lett. 1998;251:197–200. doi: 10.1016/s0304-3940(98)00538-2. [DOI] [PubMed] [Google Scholar]
- Gardiner EM, Pestonjamasp KN, Bohl BP, Chamberlain C, Hahn KM, Bokoch GM. Spatial and temporal analysis of Rac activation during live neutrophil chemotaxis. Curr Biol. 2002;12:2029–2034. doi: 10.1016/s0960-9822(02)01334-9. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann J, Monaco S, Kreutzberg GW. Spinal cord microglial cells and DRG satellite cells rapidly respond to transection of the rat sciatic nerve. Restor Neurol Neurosci. 1991;2:181–198. doi: 10.3233/RNN-1991-245605. [DOI] [PubMed] [Google Scholar]
- George A, Kleinschnitz C, Zelenka M, Brinkhoff J, Stoll G, Sommer C. Wallerian degeneration after crush or chronic constriction injury of rodent sciatic nerve is associated with a depletion of endoneurial interleukin-10 protein. Exp Neurol. 2004;188:187–191. doi: 10.1016/j.expneurol.2004.02.011. [DOI] [PubMed] [Google Scholar]
- George EB, Glass JD, Griffin JW. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J Neurosci. 1995;15:6445–6452. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Summers DW, Sasaki Y, DiAntonio A, Milbrandt J. Sarm1-mediated axon degeneration requires both SAM and TIR interactions. J Neurosci. 2013;33:13569–13580. doi: 10.1523/JNEUROSCI.1197-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia NM, Pettersson LM, Hasmatali JC, Hryciw T, Danielsen N, Schreyer DJ, Verge VM. Endogenous BDNF regulates induction of intrinsic neuronal growth programs in injured sensory neurons. Exp Neurol. 2010;223:128–142. doi: 10.1016/j.expneurol.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Gilad VH, Tetzlaff WG, Rabey JM, Gilad GM. Accelerated recovery following polyamines and aminoguanidine treatment after facial nerve injury in rats. Brain Res. 1996;724:141–144. doi: 10.1016/0006-8993(96)00287-9. [DOI] [PubMed] [Google Scholar]
- Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingwater TH, Ingham CA, Parry KE, Wright AK, Haley JE, Wishart TM, Arbuthnott GW, Ribchester RR. Delayed synaptic degeneration in the CNS of Wlds mice after cortical lesion. Brain. 2006;129:1546–1556. doi: 10.1093/brain/awl101. [DOI] [PubMed] [Google Scholar]
- Gillingwater TH, Thomson D, Mack TG, Soffin EM, Mattison RJ, Coleman MP, Ribchester RR. Age-dependent synapse withdrawal at axotomised neuromuscular junctions in Wld(s) mutant and Ube4b/Nmnat transgenic mice. J Physiol. 2002;543:739–755. doi: 10.1113/jphysiol.2002.022343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitik M, Liraz-Zaltsman S, Oldenborg PA, Reichert F, Rotshenker S. Myelin down-regulates myelin phagocytosis by microglia and macrophages through interactions between CD47 on myelin and SIRPalpha (signal regulatory protein-alpha) on phagocytes. J Neuroinflammation. 2011;8:24. doi: 10.1186/1742-2094-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Spira ME. Real time imaging of calcium-induced localized proteolytic activity after axotomy and its relation to growth cone formation. Neuron. 1998;20:1123–1135. doi: 10.1016/s0896-6273(00)80494-8. [DOI] [PubMed] [Google Scholar]
- Gitler D, Spira ME. Short window of opportunity for calpain induced growth cone formation after axotomy of Aplysia neurons. J Neurobiol. 2002;52:267–279. doi: 10.1002/neu.10084. [DOI] [PubMed] [Google Scholar]
- Glass JD, Brushart TM, George EB, Griffin JW. Prolonged survival of transected nerve fibres in C57BL/Ola mice is an intrinsic characteristic of the axon. J Neurocytol. 1993;22:311–321. doi: 10.1007/BF01195555. [DOI] [PubMed] [Google Scholar]
- Gloster A, Diamond J. Sympathetic nerves in adult rats regenerate normally and restore pilomotor function during an anti-NGF treatment that prevents their collateral sprouting. J Comp Neurol. 1992;326:363–374. doi: 10.1002/cne.903260305. [DOI] [PubMed] [Google Scholar]
- Goethals S, Ydens E, Timmerman V, Janssens S. Toll-like receptor expression in the peripheral nerve. Glia. 2010;58:1701–1709. doi: 10.1002/glia.21041. [DOI] [PubMed] [Google Scholar]
- Gordon T, Amirjani N, Edwards DC, Chan KM. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol. 2010;223:192–202. doi: 10.1016/j.expneurol.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Gordon T, Udina E, Verge VM, de Chaves EI. Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Motor Control. 2009;13:412–441. doi: 10.1123/mcj.13.4.412. [DOI] [PubMed] [Google Scholar]
- Gould DJ, Goshgarian HG. Glial changes in the phrenic nucleus following superimposed cervical spinal cord hemisection and peripheral chronic phrenicotomy injuries in adult rats. Exp Neurol. 1997;148:1–9. doi: 10.1006/exnr.1997.6556. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Bise K, Mehraein P. Synaptic stripping in the human facial nucleus. Acta Neuropathol. 1993;86:179–181. doi: 10.1007/BF00334886. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ, Kreutzberg GW. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J Neurosci Res. 1988a;21:18–24. doi: 10.1002/jnr.490210104. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Tetzlaff W, Streit WJ, Kreutzberg GW. Microglial cells but not astrocytes undergo mitosis following rat facial nerve axotomy. Neurosci Lett. 1988b;85:317–321. doi: 10.1016/0304-3940(88)90585-x. [DOI] [PubMed] [Google Scholar]
- Grafstein B. The nerve cell body response to axotomy. Exp Neurol. 1975;48:32–51. doi: 10.1016/0014-4886(75)90170-3. [DOI] [PubMed] [Google Scholar]
- Griffin JW, George R, Ho T. Macrophage systems in peripheral nerves. A review. J Neuropathol Exp Neurol. 1993;52:553–560. doi: 10.1097/00005072-199311000-00001. [DOI] [PubMed] [Google Scholar]
- Guertin AD, Zhang DP, Mak KS, Alberta JA, Kim HA. Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci. 2005;25:3478–3487. doi: 10.1523/JNEUROSCI.3766-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Chandrasekaran V, Lein P, Kaplan PL, Higgins D. Leukemia inhibitory factor and ciliary neurotrophic factor cause dendritic retraction in cultured rat sympathetic neurons. J Neurosci. 1999;19:2113–2121. doi: 10.1523/JNEUROSCI.19-06-02113.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habecker BA, Sachs HH, Rohrer H, Zigmond RE. The dependence on gp130 cytokines of axotomy induced neuropeptide expression in adult sympathetic neurons. Dev Neurobiol. 2009;69:392–400. doi: 10.1002/dneu.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack CE, Wolbink GJ, Schalkwijk C, Speijer H, Hermens WT, van den Bosch H. A role for secretory phospholipase A2 and C-reactive protein in the removal of injured cells. Immunol Today. 1997;18:111–115. doi: 10.1016/s0167-5699(97)01002-5. [DOI] [PubMed] [Google Scholar]
- Hall SM, Gregson NA. The in vivo and ultrastructural effects of injection of lysophosphatidyl choline into myelinated peripheral nerve fibres of the adult mouse. J Cell Sci. 1971;9:769–789. doi: 10.1242/jcs.9.3.769. [DOI] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43:687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- Harel L, Costa B, Fainzilber M. On the death Trk. Dev Neurobiol. 2010;70:298–303. doi: 10.1002/dneu.20769. [DOI] [PubMed] [Google Scholar]
- Harel R, Iannotti CA, Hoh D, Clark M, Silver J, Steinmetz MP. Oncomodulin affords limited regeneration to injured sensory axons in vitro and in vivo. Exp Neurol. 2012;233:708–716. doi: 10.1016/j.expneurol.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Harkonen M. Carboxylic esterases, oxidative enzymes and catecholamines in the superior cervical ganglion of the rat and the effect of pre- and post-ganglionic nerve division. Acta Physiol Scand. 1964;63(Suppl 273):1–94. [Google Scholar]
- Harrington AW, Ginty DD. Long-distance retrograde neurotrophic factor signalling in neurons. Nat Rev Neurosci. 2013;14:177–187. doi: 10.1038/nrn3253. [DOI] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R, Lindholm D, Bandtlow C, Meyer M, Radeke MJ, Misko TP, Shooter E, Thoenen H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci U S A. 1987;84:8735–8739. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke A. Mechanisms of disease: what factors limit the success of peripheral nerve regeneration in humans? Nat Clin Pract Neurol. 2006;2:448–454. doi: 10.1038/ncpneuro0262. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Wiesenfeld-Hallin Z, Villar M, Melander T. Increase of galanin-like immunoreactivity in rat dorsal root ganglion cells after peripheral axotomy. Neurosci Lett. 1987;83:217–220. doi: 10.1016/0304-3940(87)90088-7. [DOI] [PubMed] [Google Scholar]
- Hu-Tsai M, Winter J, Emson PC, Woolf CJ. Neurite outgrowth and GAP-43 mRNA expression in cultured adult rat dorsal root ganglion neurons: effects of NGF or prior peripheral axotomy. J Neurosci Res. 1994;39:634–645. doi: 10.1002/jnr.490390603. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Wells GM, Perry VH, Brown MC, Miller KM. Comparison of matrix metalloproteinase expression during Wallerian degeneration in the central and peripheral nervous systems. Neuroscience. 2002;113:273–287. doi: 10.1016/s0306-4522(02)00183-5. [DOI] [PubMed] [Google Scholar]
- Humbertson A, Jr, Zimmermann E, Leedy M. A chronological study of mitotic activity in satellite cell hyperplasia associated with chromatolytic neurons. Z Zellforsch Mikrosk Anat. 1969;100:507–515. doi: 10.1007/BF00344371. [DOI] [PubMed] [Google Scholar]
- Hyatt-Sachs H, Schreiber RC, Bennett TA, Zigmond RE. Phenotypic plasticity in adult sympathetic ganglia in vivo: effects of deafferentation and axotomy on the expression of vasoactive intestinal peptide. J Neurosci. 1993;13:1642–1653. doi: 10.1523/JNEUROSCI.13-04-01642.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt Sachs H, Rohrer H, Zigmond RE. The conditioning lesion effect on sympathetic neurite outgrowth is dependent on gp130 cytokines. Exp Neurol. 2010;223:516–522. doi: 10.1016/j.expneurol.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt Sachs H, Schreiber RC, Shoemaker SE, Sabe A, Reed E, Zigmond RE. Activating transcription factor 3 induction in sympathetic neurons after axotomy: response to decreased neurotrophin availability. Neuroscience. 2007;150:887–897. doi: 10.1016/j.neuroscience.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip NY, Nye SH, Boulton TG, Davis S, Taga T, Li Y, Birren SJ, Yasukawa K, Kishimoto T, Anderson DJ, et al. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992;69:1121–1132. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- Itoh A, Horiuchi M, Bannerman P, Pleasure D, Itoh T. Impaired regenerative response of primary sensory neurons in ZPK/DLK gene-trap mice. Biochem Biophys Res Commun. 2009;383:258–262. doi: 10.1016/j.bbrc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Iwata A, Stys PK, Wolf JA, Chen XH, Taylor AG, Meaney DF, Smith DH. Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J Neurosci. 2004;24:4605–4613. doi: 10.1523/JNEUROSCI.0515-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R, McMahon S, Bond A, Hunt S. Expression of c-Jun as a response to dorsal root and peripheral nerve section in damaged and adjacent intact primary sensory neurons in the rat. Eur J Neurosci. 1993;5:751–759. doi: 10.1111/j.1460-9568.1993.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- Jochum W, Passegue E, Wagner EF. AP-1 in mouse development and tumorigenesis. Oncogene. 2001;20:2401–2412. doi: 10.1038/sj.onc.1204389. [DOI] [PubMed] [Google Scholar]
- Joseph BS. Somatofugal events in Wallerian degeneration: a conceptual overview. Brain Res. 1973;59:1–18. doi: 10.1016/0006-8993(73)90250-3. [DOI] [PubMed] [Google Scholar]
- Karanth S, Yang G, Yeh J, Richardson PM. Nature of signals that initiate the immune response during Wallerian degeneration of peripheral nerves. Exp Neurol. 2006;202:161–166. doi: 10.1016/j.expneurol.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Katz DM, Black IB. Expression and regulation of catecholaminergic traits in primary sensory neurons: relationship to target innervation in vivo. J Neurosci. 1986;6:983–989. doi: 10.1523/JNEUROSCI.06-04-00983.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhoff G, Langnaese K, Wolf G, Fansa H. Inhibiting effect of minocycline on the regeneration of peripheral nerves. Dev Neurobiol. 2007;67:1382–1395. doi: 10.1002/dneu.20384. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Schild L, Fansa H. Minocycline protects Schwann cells from ischemia-like injury and promotes axonal outgrowth in bioartificial nerve grafts lacking Wallerian degeneration. Exp Neurol. 2008;212:189–200. doi: 10.1016/j.expneurol.2008.03.028. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Brown M, Werb Z. Measuring matrix metalloproteinase activity in macrophages and polymorphonuclear leukocytes. Curr Protoc Immunol. 2011 doi: 10.1002/0471142735.im1424s93. Chapter 14:Unit14.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Figueroa KW, Li KW, Boroujerdi A, Yolo T, Luo ZD. Profiling of dynamically changed gene expression in dorsal root ganglia post peripheral nerve injury and a critical role of injury-induced glial fibrillary acidic protein in maintenance of pain behaviors [corrected] Pain. 2009;143:114–122. doi: 10.1016/j.pain.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, Pomeroy SL, Whoriskey W, Pawlitzky I, Benowitz LI, Sicinski P, Stiles CD, Roberts TM. A developmentally regulated switch directs regenerative growth of Schwann cells through cyclin D1. Neuron. 2000;26:405–416. doi: 10.1016/s0896-6273(00)81173-3. [DOI] [PubMed] [Google Scholar]
- Klein C, Grahnert A, Abdelrahman A, Muller CE, Hauschildt S. Extracellular NAD(+) induces a rise in [Ca(2+)](i) in activated human monocytes via engagement of P2Y(1) and P2Y(11) receptors. Cell Calcium. 2009;46:263–272. doi: 10.1016/j.ceca.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Klinedinst S, Wang X, Xiong X, Haenfler JM, Collins CA. Independent pathways downstream of the Wnd/DLK MAPKKK regulate synaptic structure, axonal transport, and injury signaling. J Neurosci. 2013;33:12764–12778. doi: 10.1523/JNEUROSCI.5160-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenburg M, Verweij CL, Miltenburg AM, Verhoeven AJ, Daha MR, Dijkmans BA, Breedveld FC. The influence of tetracyclines on T cell activation. Clin Exp Immunol. 1995;102:635–641. doi: 10.1111/j.1365-2249.1995.tb03864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- Komori T, Morikawa Y, Inada T, Hisaoka T, Senba E. Site-specific subtypes of macrophages recruited after peripheral nerve injury. Neuroreport. 2011;22:911–917. doi: 10.1097/WNR.0b013e32834cd76a. [DOI] [PubMed] [Google Scholar]
- Koo EH, Hoffman PN, Price DL. Levels of neurotransmitter and cytoskeletal protein mRNAs during nerve regeneration in sympathetic ganglia. Brain Res. 1988;449:361–363. doi: 10.1016/0006-8993(88)91054-2. [DOI] [PubMed] [Google Scholar]
- Kurek JB, Austin L, Cheema SS, Bartlett PF, Murphy M. Up-regulation of leukaemia inhibitory factor and interleukin-6 in transected sciatic nerve and muscle following denervation. Neuromuscul Disord. 1996;6:105–114. doi: 10.1016/0960-8966(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Kurimoto T, Yin Y, Habboub G, Gilbert HY, Li Y, Nakao S, Hafezi-Moghadam A, Benowitz LI. Neutrophils express oncomodulin and promote optic nerve regeneration. J Neurosci. 2013;33:14816–14824. doi: 10.1523/JNEUROSCI.5511-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MJ, Kim J, Shin H, Jeong SR, Kang YM, Choi JY, Hwang DH, Kim BG. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J Neurosci. 2013;33:15095–15108. doi: 10.1523/JNEUROSCI.0278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fleur M, Underwood JL, Rappolee DA, Werb Z. Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J Exp Med. 1996;184:2311–2326. doi: 10.1084/jem.184.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankford KL, Arroyo EJ, Liu CN, Somps CJ, Zorbas MA, Shelton DL, Evans MG, Hurst SI, Kocsis JD. Sciatic nerve regeneration is not inhibited by anti-NGF antibody treatment in the adult rat. Neuroscience. 2013;241C:157–169. doi: 10.1016/j.neuroscience.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Laser H, Mack TG, Wagner D, Coleman MP. Proteasome inhibition arrests neurite outgrowth and causes “dying-back” degeneration in primary culture. J Neurosci Res. 2003;74:906–916. doi: 10.1002/jnr.10806. [DOI] [PubMed] [Google Scholar]
- Lee HK, Shin YK, Jung J, Seo SY, Baek SY, Park HT. Proteasome inhibition suppresses Schwann cell dedifferentiation in vitro and in vivo. Glia. 2009;57:1825–1834. doi: 10.1002/glia.20894. [DOI] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Neitzel KL, Devlin BK, MacLennan AJ. STAT3 phosphorylation in injured axons before sensory and motor neuron nuclei: potential role for STAT3 as a retrograde signaling transcription factor. J Comp Neurol. 2004;474:535–545. doi: 10.1002/cne.20140. [DOI] [PubMed] [Google Scholar]
- Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–252. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci. 2009a;29:14334–14341. doi: 10.1523/JNEUROSCI.2770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci. 2009b;29:14334–14341. doi: 10.1523/JNEUROSCI.2770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinfelder PJ. Retrograde degeneration in the optic nerves and retinal ganglion cells. Trans Am Ophthalmol Soc. 1938;36:307–315. [PMC free article] [PubMed] [Google Scholar]
- Leong SK, Ling EA, Fan DP. Glial reaction after pyramidotomy in mice and rats. Neurodegeneration. 1995;4:403–413. doi: 10.1006/neur.1995.0049. [DOI] [PubMed] [Google Scholar]
- Lerch JK, Martinez-Ondaro YR, Bixby JL, Lemmon VP. cJun promotes CNS axon growth. Mol Cell Neurosci. 2014;59:97–105. doi: 10.1016/j.mcn.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman AR. The axon reaction: a review of the principle features of perikaryal responses to axon injury. In: Pfeiffer CC, Smythies JR, editors. Int Rev Neurobiol. Vol. 14. Academic Press; New York: 1971. pp. 49–124. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987;330:658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Lingappa JR, Zigmond RE. Limited recovery of pineal function after regeneration of preganglionic sympathetic axons: evidence for loss of ganglionic synaptic specificity. J Neurosci. 2013;33:4867–4874. doi: 10.1523/JNEUROSCI.3829-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisak RP, Nedelkoska L, Studzinski D, Bealmear B, Xu W, Benjamins JA. Cytokines regulate neuronal gene expression: differential effects of Th1, Th2 and monocyte/macrophage cytokines. J Neuroimmunol. 2011;238:19–33. doi: 10.1016/j.jneuroim.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Liu RY, Snider WD. Different signaling pathways mediate regenerative versus developmental sensory axon growth. J Neurosci. 2001;21:RC164. doi: 10.1523/JNEUROSCI.21-17-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi FJ, Lasek RJ. Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science. 1987;237:642–645. doi: 10.1126/science.3603044. [DOI] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Inflammation near the nerve cell body enhances axonal regeneration. J Neurosci. 1991;11:972–978. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Responses of macrophages in rat dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1993;22:334–341. doi: 10.1007/BF01195557. [DOI] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Changes in neuronal mRNAs induced by a local inflammatory reaction. J Neurosci Res. 1995;41:8–14. doi: 10.1002/jnr.490410103. [DOI] [PubMed] [Google Scholar]
- Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of Wallerian degeneration does not hinder regeneration in peripheral nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Ogunkolade BW, Brown MC, Atherton DJ, Perry VH. A gene affecting Wallerian nerve degeneration maps distally on mouse chromosome 4. Proc Natl Acad Sci U S A. 1993;90:9717–9720. doi: 10.1073/pnas.90.20.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CH, Omura T, Cobos EJ, Latremoliere A, Ghasemlou N, Brenner GJ, van Veen E, Barrett L, Sawada T, Gao F, Coppola G, Gertler F, Costigan M, Geschwind D, Woolf CJ. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest. 2011;121:4332–4347. doi: 10.1172/JCI58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Cao X. Regulation of Stat3 nuclear import by importin alpha5 and importin alpha7 via two different functional sequence elements. Cell Signal. 2006;18:1117–1126. doi: 10.1016/j.cellsig.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T, Court F, Conforti L, Fernando FS, Tarlton A, Andressen C, Addicks K, Magni G, Ribchester RR, Perry VH, Coleman MP. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- Martini R, Fischer S, Lopez-Vales R, David S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia. 2008;56:1566–1577. doi: 10.1002/glia.20766. [DOI] [PubMed] [Google Scholar]
- Matsuoka I, Nakane A, Kurihara K. Induction of LIF-mRNA by TGF-beta 1 in Schwann cells. Brain Res. 1997;776:170–180. doi: 10.1016/s0006-8993(97)01015-9. [DOI] [PubMed] [Google Scholar]
- Matthews MR, Nelson VH. Detachment of structurally intact nerve endings from chromatolytic neurones of rat superior cervical ganglion during the depression of synaptic transmission induced by post-ganglionic axotomy. J Physiol. 1975;245:91–135. doi: 10.1113/jphysiol.1975.sp010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG. The effect of a conditioning lesion on the regeneration of motor axons. Brain Res. 1978;152:597–602. doi: 10.1016/0006-8993(78)91116-2. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B. Axon outgrowth enhanced by a previous nerve injury. Arch Neurol. 1973;29:53–55. doi: 10.1001/archneur.1973.00490250071008. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B, Gershon MD. Axonal regeneration in the rat sciatic nerve: effect of a conditioning lesion and of dbcAMP. Brain Res. 1977;132:443–453. doi: 10.1016/0006-8993(77)90193-7. [DOI] [PubMed] [Google Scholar]
- Mead RJ, Singhrao SK, Neal JW, Lassmann H, Morgan BP. The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol. 2002;168:458–465. doi: 10.4049/jimmunol.168.1.458. [DOI] [PubMed] [Google Scholar]
- Merianda TT, Lin AC, Lam JS, Vuppalanchi D, Willis DE, Karin N, Holt CE, Twiss JL. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol Cell Neurosci. 2009;40:128–142. doi: 10.1016/j.mcn.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992;119:45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelevski I, Medzihradszky KF, Lynn A, Burlingame AL, Fainzilber M. Axonal transport proteomics reveals mobilization of translation machinery to the lesion site in injured sciatic nerve. Mol Cell Proteomics. 2010a;9:976–987. doi: 10.1074/mcp.M900369-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelevski I, Segal-Ruder Y, Rozenbaum M, Medzihradszky KF, Shalem O, Coppola G, Horn-Saban S, Ben-Yaakov K, Dagan SY, Rishal I, Geschwind DH, Pilpel Y, Burlingame AL, Fainzilber M. Signaling to transcription networks in the neuronal retrograde injury response. Science Signaling. 2010b;3:ra53. doi: 10.1126/scisignal.2000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde S, Gilley J, Coleman MP. Subcellular localization determines the stability and axon protective capacity of axon survival factor Nmnat2. PLoS Biol. 2013;11:e1001539. doi: 10.1371/journal.pbio.1001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohney RP, Siegel RE, Zigmond RE. Galanin and vasoactive intestinal peptide messenger RNAs increase following axotomy of adult sympathetic neurons. J Neurobiol. 1994;25:108–118. doi: 10.1002/neu.480250203. [DOI] [PubMed] [Google Scholar]
- Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33:8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller K, Reimer M, Hannibal J, Fahrenkrug J, Sundler F, Kanje M. Pituitary adenylate cyclase-activating peptide (PACAP) and PACAP type 1 receptor expression in regenerating adult mouse and rat superior cervical ganglia in vitro. Brain Res. 1997;775:156–165. doi: 10.1016/s0006-8993(97)00937-2. [DOI] [PubMed] [Google Scholar]
- Moon LD, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Leonhard C, Wacker K, Ringelstein EB, Okabe M, Hickey WF, Kiefer R. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab Invest. 2003;83:175–185. doi: 10.1097/01.lab.0000056993.28149.bf. [DOI] [PubMed] [Google Scholar]
- Mueller M, Wacker K, Ringelstein EB, Hickey WF, Imai Y, Kiefer R. Rapid response of identified resident endoneurial macrophages to nerve injury. Am J Pathol. 2001;159:2187–2197. doi: 10.1016/S0002-9440(10)63070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Regulatory functions of phospholipase A2. Crit Rev Immunol. 1997;17:225–283. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- Murch SH. Local and systemic effects of macrophage cytokines in intestinal inflammation. Nutrition. 1998;14:780–783. doi: 10.1016/s0899-9007(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Murphy PG, Borthwick LS, Johnston RS, Kuchel G, Richardson PM. Nature of the retrograde signal from injured nerves that induces interleukin-6 mRNA in neurons. J Neurosci. 1999;19:3791–3800. doi: 10.1523/JNEUROSCI.19-10-03791.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PG, Grondin J, Altares M, Richardson PM. Induction of interleukin-6 in axotomized sensory neurons. J Neurosci. 1995;15:5130–5138. doi: 10.1523/JNEUROSCI.15-07-05130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RR, Heckman HM, Rodriguez M. Reduced hyperalgesia in nerve-injured WLD mice: relationship to nerve fiber phagocytosis, axonal degeneration, and regeneration in normal mice. Exp Neurol. 1996;141:94–101. doi: 10.1006/exnr.1996.0142. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, Iwakura Y, de Rivero Vaccari JP, Keane RW, Lacroix S. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1beta and TNF: implications for neuropathic pain. J Neurosci. 2011;31:12533–12542. doi: 10.1523/JNEUROSCI.2840-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamoto-Combs K, Vaccariello SA, Zigmond RE. The levels of leukemia inhibitory factor mRNA in a Schwann cell line are regulated by multiple second messenger pathways. J Neurochem. 1999;72:1871–1881. doi: 10.1046/j.1471-4159.1999.0721871.x. [DOI] [PubMed] [Google Scholar]
- Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Navarro X, Kennedy WR. The effect of a conditioning lesion on sudomotor axon regeneration. Brain Res. 1990;509:232–236. doi: 10.1016/0006-8993(90)90547-o. [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Ng YP, He W, Ip NY. Leukemia inhibitory factor receptor signaling negatively modulates nerve growth factor-induced neurite outgrowth in PC12 cells and sympathetic neurons. J Biol Chem. 2003;278:38731–38739. doi: 10.1074/jbc.M304623200. [DOI] [PubMed] [Google Scholar]
- Niemi JP, DeFrancesco-Lisowitz A, Roldan-Hernandez L, Lindborg JA, Mandell D, Zigmond RE. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J Neurosci. 2013;33:16236–16248. doi: 10.1523/JNEUROSCI.3319-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem. 2007;282:15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- Nissl F. Uber eine neue untersuchungsmethode des zentral orans speziell zur festellung der lokalisation der nervenzellen. Zentralbl Nervenheilk Psychiatr. 1984;17:337–344. [Google Scholar]
- Nja A, Purves D. The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J Physiol. 1978;277:53–75. [PMC free article] [PubMed] [Google Scholar]
- Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JJ, Nathanson NM. Retrograde activation of STAT3 by leukemia inhibitory factor in sympathetic neurons. J Neurochem. 2007;103:288–302. doi: 10.1111/j.1471-4159.2007.04736.x. [DOI] [PubMed] [Google Scholar]
- Oblinger MM, Lasek RJ. A conditioning lesion of the peripheral axons of dorsal root ganglion cells accelerates regeneration of only their peripheral axons. J Neurosci. 1984;4:1736–1744. doi: 10.1523/JNEUROSCI.04-07-01736.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T, Omura K, Sano M, Sawada T, Hasegawa T, Nagano A. Spatiotemporal quantification of recruit and resident macrophages after crush nerve injury utilizing immunohistochemistry. Brain Res. 2005;1057:29–36. doi: 10.1016/j.brainres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- Orsomando G, Cialabrini L, Amici A, Mazzola F, Ruggieri S, Conforti L, Janeckova L, Coleman MP, Magni G. Simultaneous Single-Sample Determination of NMNAT Isozyme Activities in Mouse Tissues. PLoS One. 2012;7:e53271. doi: 10.1371/journal.pone.0053271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou YJ, Nathan C, Ding A, Brown RH, Jr., Conforti L, Coleman M, Tessier-Lavigne M, Zuchner S, Freeman MR. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Suchanek G, Breitschopf H, Bruck W, Budka H, Jellinger K, Lassmann H. Patterns of oligodendroglia pathology in multiple sclerosis. Brain. 1994;117(Pt 6):1311–1322. doi: 10.1093/brain/117.6.1311. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patodia S, Raivich G. Role of transcription factors in peripheral nerve regeneration. Front Mol Neurosci. 2012;5:8. doi: 10.3389/fnmol.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino MJ, Habecker BA. STAT3 integrates cytokine and neurotrophin signals to promote sympathetic axon regeneration. Mol Cell Neurosci. 2013;56:272–282. doi: 10.1016/j.mcn.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins NM, Tracey DJ. Hyperalgesia due to nerve injury: role of neutrophils. Neuroscience. 2000;101:745–757. doi: 10.1016/s0306-4522(00)00396-1. [DOI] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Perrin FE, Lacroix S, Aviles-Trigueros M, David S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain. 2005;128:854–866. doi: 10.1093/brain/awh407. [DOI] [PubMed] [Google Scholar]
- Perry RB, Doron-Mandel E, Iavnilovitch E, Rishal I, Dagan SY, Tsoory M, Coppola G, McDonald MK, Gomes C, Geschwind DH, Twiss JL, Yaron A, Fainzilber M. Subcellular knockout of importin beta1 perturbs axonal retrograde signaling. Neuron. 2012;75:294–305. doi: 10.1016/j.neuron.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RB, Fainzilber M. Nuclear transport factors in neuronal function. Semin Cell Dev Biol. 2009;20:600–606. doi: 10.1016/j.semcdb.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Perry VH. Macrophages and the Nervous System. R. G. Landes; Austin, TX: 1994. [Google Scholar]
- Perry VH, Brown MC, Lunn ER, Tree P, Gordon S. Evidence that very slow wallerian degeneration in C57BL/Ola mice is an intrinsic property of the peripheral nerve. Eur J Neurosci. 1990;2:802–808. doi: 10.1111/j.1460-9568.1990.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Perry VH, O’Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro. 2010;2:e00047. doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Tsao JW, Fearn S, Brown MC. Radiation-induced reductions in macrophage recruitment have only slight effects on myelin degeneration in sectioned peripheral nerves of mice. Eur J Neurosci. 1995;7:271–280. doi: 10.1111/j.1460-9568.1995.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010 doi: 10.1155/2010/672395. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau I, Lacroix S. Endogenous signals initiating inflammation in the injured nervous system. Glia. 2009;57:351–361. doi: 10.1002/glia.20763. [DOI] [PubMed] [Google Scholar]
- Pollak N, Dolle C, Ziegler M. The power to reduce: pyridine nucleotides--small molecules with a multitude of functions. Biochem J. 2007;402:205–218. doi: 10.1042/BJ20061638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975;252:429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Raggo C, Rapin N, Stirling J, Gobeil P, Smith-Windsor E, O’Hare P, Misra V. Luman, the cellular counterpart of herpes simplex virus VP16, is processed by regulated intramembrane proteolysis. Mol Cell Biol. 2002;22:5639–5649. doi: 10.1128/MCB.22.16.5639-5649.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, Lipp HP, Aguzzi A, Wagner EF, Behrens A. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Rajan P, Gearan T, Fink JS. Leukemia inhibitory factor and NGF regulate signal transducers and activators of transcription activation in sympathetic ganglia: convergence of cytokine- and neurotrophin-signaling pathways. Brain Res. 1998;802:198–204. doi: 10.1016/s0006-8993(98)00611-8. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Degeneration and Regeneration of the Nervous System. Oxford University Press; New York: 1928. [Google Scholar]
- Rao MS, Sun Y, Escary JL, Perreau J, Tresser S, Patterson PH, Zigmond RE, Brulet P, Landis SC. Leukemia inhibitory factor mediates an injury response but not a target-directed developmental transmitter switch in sympathetic neurons. Neuron. 1993;11:1175–1185. doi: 10.1016/0896-6273(93)90229-k. [DOI] [PubMed] [Google Scholar]
- Reichert F, Levitzky R, Rotshenker S. Interleukin 6 in intact and injured mouse peripheral nerves. Eur J Neurosci. 1996;8:530–535. doi: 10.1111/j.1460-9568.1996.tb01237.x. [DOI] [PubMed] [Google Scholar]
- Reisert I, Wildemann G, Grab D, Pilgrim C. The glial reaction in the course of axon regeneration: a stereological study of the rat hypoglossal nucleus. J Comp Neurol. 1984;229:121–128. doi: 10.1002/cne.902290109. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroinflammation. 2011;8:109. doi: 10.1186/1742-2094-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saada A, Dunaevsky-Hutt A, Aamar A, Reichert F, Rotshenker S. Fibroblasts that reside in mouse and frog injured peripheral nerves produce apolipoproteins. J Neurochem. 1995;64:1996–2003. doi: 10.1046/j.1471-4159.1995.64051996.x. [DOI] [PubMed] [Google Scholar]
- Saada A, Reichert F, Rotshenker S. Granulocyte macrophage colony stimulating factor produced in lesioned peripheral nerves induces the up-regulation of cell surface expression of MAC-2 by macrophages and Schwann cells. J Cell Biol. 1996;133:159–167. doi: 10.1083/jcb.133.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs HH, Wynick D, Zigmond RE. Galanin plays a role in the conditioning lesion effect in sensory neurons. Neuroreport. 2007;18:1729–1733. doi: 10.1097/WNR.0b013e3282f0d3f4. [DOI] [PubMed] [Google Scholar]
- Sahenk Z, Oblinger J, Edwards C. Neurotrophin-3 deficient Schwann cells impair nerve regeneration. Exp Neurol. 2008;212:552–556. doi: 10.1016/j.expneurol.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Salegio EA, Pollard AN, Smith M, Zhou XF. Macrophage presence is essential for the regeneration of ascending afferent fibres following a conditioning sciatic nerve lesion in adult rats. BMC Neurosci. 2011;12:11. doi: 10.1186/1471-2202-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsam M, Mi W, Wessig C, Zielasek J, Toyka KV, Coleman MP, Martini R. The Wlds mutation delays robust loss of motor and sensory axons in a genetic model for myelin-related axonopathy. J Neurosci. 2003;23:2833–2839. doi: 10.1523/JNEUROSCI.23-07-02833.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Baloh RH, Milbrandt J. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J Neurosci. 2009a;29:6526–6534. doi: 10.1523/JNEUROSCI.1429-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Lund FE, Milbrandt J. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci. 2009b;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Salzer JL. Axon-Schwann cell interactions during peripheral nerve degeneration and regeneration. Oxford University Press; Oxford: 2001. [Google Scholar]
- Schmitz T, Endesfelder S, Chew LJ, Zaak I, Buhrer C. Minocycline protects oligodendroglial precursor cells against injury caused by oxygen-glucose deprivation. J Neurosci Res. 2012;90:933–944. doi: 10.1002/jnr.22824. [DOI] [PubMed] [Google Scholar]
- Schreiber RC, Boeshore KL, Laube G, Veh RW, Zigmond RE. Polyamines increase in sympathetic neurons and non-neuronal cells after axotomy and enhance neurite outgrowth in nerve growth factor-primed PC12 cells. Neuroscience. 2004;128:741–749. doi: 10.1016/j.neuroscience.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Schreiber RC, Hyatt-Sachs H, Bennett TA, Zigmond RE. Galanin expression increases in adult rat sympathetic neurons after axotomy. Neuroscience. 1994;60:17–27. doi: 10.1016/0306-4522(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Schreiber RC, Krivacic K, Kirby B, Vaccariello SA, Wei T, Ransohoff RM, Zigmond RE. Monocyte chemoattractant protein (MCP)-1 is rapidly expressed by sympathetic ganglion neurons following axonal injury. Neuroreport. 2001;12:601–606. doi: 10.1097/00001756-200103050-00034. [DOI] [PubMed] [Google Scholar]
- Schreiber RC, Shadiack AM, Bennett TA, Sedwick CE, Zigmond RE. Changes in the macrophage population of the rat superior cervical ganglion after postganglionic nerve injury. J Neurobiol. 1995;27:141–153. doi: 10.1002/neu.480270203. [DOI] [PubMed] [Google Scholar]
- Schreyer DJ, Skene JH. Injury-associated induction of GAP-43 expression displays axon branch specificity in rat dorsal root ganglion neurons. J Neurobiol. 1993;24:959–970. doi: 10.1002/neu.480240709. [DOI] [PubMed] [Google Scholar]
- Schuh CD, Pierre S, Weigert A, Weichand B, Altenrath K, Schreiber Y, Ferreiros N, Zhang DD, Suo J, Treutlein EM, Henke M, Kunkel H, Grez M, Nusing R, Brune B, Geisslinger G, Scholich K. Prostacyclin mediates neuropathic pain through interleukin 1beta-expressing resident macrophages. Pain. 2014;155:545–555. doi: 10.1016/j.pain.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Schwaiger FW, Hager G, Schmitt AB, Horvat A, Hager G, Streif R, Spitzer C, Gamal S, Breuer S, Brook GA, Nacimiento W, Kreutzberg GW. Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT) Eur J Neurosci. 2000;12:1165–1176. doi: 10.1046/j.1460-9568.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- Schweiger M, Hennig K, Lerner F, Niere M, Hirsch-Kauffmann M, Specht T, Weise C, Oei SL, Ziegler M. Characterization of recombinant human nicotinamide mononucleotide adenylyl transferase (NMNAT), a nuclear enzyme essential for NAD synthesis. FEBS Lett. 2001;492:95–100. doi: 10.1016/s0014-5793(01)02180-9. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Allchorne AJ, Woolf CJ. The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci. 2006;32:143–154. doi: 10.1016/j.mcn.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj BT, Frank N, Bender FL, Asan E, Sendtner M. Local axonal function of STAT3 rescues axon degeneration in the pmn model of motoneuron disease. J Cell Biol. 2012;199:437–451. doi: 10.1083/jcb.201203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj BT, Sendtner M. CNTF, STAT3 and new therapies for axonal degeneration: what are they and what can they do? Expert Rev Neurother. 2013;13:239–241. doi: 10.1586/ern.13.9. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Gotz R, Holtmann B, Thoenen H. Endogenous ciliary neurotrophic factor is a lesion factor for axotomized motoneurons in adult mice. J Neurosci. 1997;17:6999–7006. doi: 10.1523/JNEUROSCI.17-18-06999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadiack AM, Vaccariello SA, Sun Y, Zigmond RE. Nerve growth factor inhibits sympathetic neurons’ response to an injury cytokine. Proc Natl Acad Sci U S A. 1998;95:7727–7730. doi: 10.1073/pnas.95.13.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci. 2002;22:3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 2012;74:1015–1022. doi: 10.1016/j.neuron.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Geisler S, DiAntonio A. Dynamic regulation of SCG10 in regenerating axons after injury. Exp Neurol. 2014;252:1–11. doi: 10.1016/j.expneurol.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker SE, Sachs HH, Vaccariello SA, Zigmond RE. A conditioning lesion enhances sympathetic neurite outgrowth. Exp Neurol. 2005;194:432–443. doi: 10.1016/j.expneurol.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Shoemaker SE, Sachs HH, Vaccariello SA, Zigmond RE. Reduction in nerve growth factor availability leads to a conditioning lesion-like effect in sympathetic neurons. J Neurobiol. 2006;66:1322–1337. doi: 10.1002/neu.20297. [DOI] [PubMed] [Google Scholar]
- Shokouhi BN, Wong BZ, Siddiqui S, Lieberman AR, Campbell G, Tohyama K, Anderson PN. Microglial responses around intrinsic CNS neurons are correlated with axonal regeneration. BMC Neurosci. 2010;11:13. doi: 10.1186/1471-2202-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert H, Dippel N, Mader M, Weber F, Bruck W. Matrix metalloproteinase expression and inhibition after sciatic nerve axotomy. J Neuropathol Exp Neurol. 2001;60:85–93. doi: 10.1093/jnen/60.1.85. [DOI] [PubMed] [Google Scholar]
- Siebert H, Sachse A, Kuziel WA, Maeda N, Bruck W. The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J Neuroimmunol. 2000;110:177–185. doi: 10.1016/s0165-5728(00)00343-x. [DOI] [PubMed] [Google Scholar]
- Sierra-Filardi E, Nieto C, Dominguez-Soto A, Barroso R, Sanchez-Mateos P, Puig-Kroger A, Lopez-Bravo M, Joven J, Ardavin C, Rodriguez-Fernandez JL, Sanchez-Torres C, Mellado M, Corbi AL. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol. 2014;192:3858–3867. doi: 10.4049/jimmunol.1302821. [DOI] [PubMed] [Google Scholar]
- Simon DJ, Weimer RM, McLaughlin T, Kallop D, Stanger K, Yang J, O’Leary DD, Hannoush RN, Tessier-Lavigne M. A caspase cascade regulating developmental axon degeneration. J Neurosci. 2012;32:17540–17553. doi: 10.1523/JNEUROSCI.3012-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer PA, Mehler S, Fernandez HL. Blockade of retrograde axonal transport delays the onset of metabolic and morphologic changes induced by axotomy. J Neurosci. 1982;2:1299–1306. doi: 10.1523/JNEUROSCI.02-09-01299.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene JH. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Skene JH, Willard M. Characteristics of growth-associated polypeptides in regenerating toad retinal ganglion cell axons. J Neurosci. 1981;1:419–426. doi: 10.1523/JNEUROSCI.01-04-00419.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RP, Lerch-Haner JK, Pardinas JR, Buchser WJ, Bixby JL, Lemmon VP. Transcriptional profiling of intrinsic PNS factors in the postnatal mouse. Mol Cell Neurosci. 2011;46:32–44. doi: 10.1016/j.mcn.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, Zhou FQ, Zhong J, Markus A. Signaling the pathway to regeneration. Neuron. 2002;35:13–16. doi: 10.1016/s0896-6273(02)00762-6. [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schafers M. Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998;784:154–162. doi: 10.1016/s0006-8993(97)01327-9. [DOI] [PubMed] [Google Scholar]
- Souza GR, Talbot J, Lotufo CM, Cunha FQ, Cunha TM, Ferreira SH. Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc Natl Acad Sci U S A. 2013;110:11193–11198. doi: 10.1073/pnas.1307445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz MP, Horn KP, Tom VJ, Miller JH, Busch SA, Nair D, Silver DJ, Silver J. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci. 2005;25:8066–8076. doi: 10.1523/JNEUROSCI.2111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Griffin JW, Li CY, Trapp BD. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol. 1989;18:671–683. doi: 10.1007/BF01187086. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller’s observations to neuroinflammation. J Peripher Nerv Syst. 2002;7:13–27. doi: 10.1046/j.1529-8027.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Subang MC, Bisby MA, Richardson PM. Delay of CNTF decrease following peripheral nerve injury in C57BL/Wld mice. J Neurosci Res. 1997;49:563–568. doi: 10.1002/(SICI)1097-4547(19970901)49:5<563::AID-JNR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Subang MC, Richardson PM. Influence of injury and cytokines on synthesis of monocyte chemoattractant protein-1 mRNA in peripheral nervous tissue. Eur J Neurosci. 2001;13:521–528. doi: 10.1046/j.1460-9568.2001.01425.x. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Lahav R, Han J, Kou SY, Banner LR, de Pablo F, Patterson PH. Leukaemia inhibitory factor is required for normal inflammatory responses to injury in the peripheral and central nervous systems in vivo and is chemotactic for macrophages in vitro. Eur J Neurosci. 2000;12:457–466. doi: 10.1046/j.1460-9568.2000.00922.x. [DOI] [PubMed] [Google Scholar]
- Sumner BE. A quantitative analysis of boutons with different types of synapse in normal and injured hypoglossal nuclei. Exp Neurol. 1975a;49:406–417. doi: 10.1016/0014-4886(75)90097-7. [DOI] [PubMed] [Google Scholar]
- Sumner BE. A quantitative analysis of the response of presynaptic boutons to postsynaptic motor neuron axotomy. Exp Neurol. 1975b;46:605–615. doi: 10.1016/0014-4886(75)90129-6. [DOI] [PubMed] [Google Scholar]
- Sun Y, Rao MS, Zigmond RE, Landis SC. Regulation of vasoactive intestinal peptide expression in sympathetic neurons in culture and after axotomy: the role of cholinergic differentiation factor/leukemia inhibitory factor. J Neurobiol. 1994;25:415–430. doi: 10.1002/neu.480250407. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zigmond RE. Involvement of leukemia inhibitory factor in the increases in galanin and vasoactive intestinal peptide mRNA and the decreases in neuropeptide Y and tyrosine hydroxylase mRNA in sympathetic neurons after axotomy. J Neurochem. 1996a;67:1751–1760. doi: 10.1046/j.1471-4159.1996.67041751.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zigmond RE. Leukaemia inhibitory factor induced in the sciatic nerve after axotomy is involved in the induction of galanin in sensory neurons. Eur J Neurosci. 1996b;8:2213–2220. doi: 10.1111/j.1460-9568.1996.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Surmi BK, Hasty AH. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul Pharmacol. 2010;52:27–36. doi: 10.1016/j.vph.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M, Aldskogius H. Regeneration of hypoglossal nerve axons following blockade of the axotomy-induced microglial cell reaction in the rat. Eur J Neurosci. 1993;5:85–94. doi: 10.1111/j.1460-9568.1993.tb00208.x. [DOI] [PubMed] [Google Scholar]
- Taga T. The signal transducer gp130 is shared by interleukin-6 family of haematopoietic and neurotrophic cytokines. Ann Med. 1997;29:63–72. doi: 10.3109/07853899708998744. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Takemura Y, Imai S, Kojima H, Katagi M, Yamakawa I, Kasahara T, Urabe H, Terashima T, Yasuda H, Chan L, Kimura H, Matsusue Y. Brain-derived neurotrophic factor from bone marrow-derived cells promotes post-injury repair of peripheral nerve. PLoS One. 2012;7:e44592. doi: 10.1371/journal.pone.0044592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48:463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Taskinen HS, Roytta M. The dynamics of macrophage recruitment after nerve transection. Acta Neuropathol. 1997;93:252–259. doi: 10.1007/s004010050611. [DOI] [PubMed] [Google Scholar]
- Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci. 1993;13:455–466. doi: 10.1523/JNEUROSCI.13-02-00455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews AD, Barrett C, Morell P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J Neurosci Res. 1998;53:260–267. doi: 10.1002/(SICI)1097-4547(19980715)53:2<260::AID-JNR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002;22:6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth CC, Willis D, Twiss JL, Walsh S, Martinez JA, Liu WQ, Midha R, Zochodne DW. Locally synthesized calcitonin gene-related peptide has a critical role in peripheral nerve regeneration. J Neuropathol Exp Neurol. 2009;68:326–337. doi: 10.1097/NEN.0b013e31819ac71b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Hauer P, Lemke G. Axonal regulation of myelin protein mRNA levels in actively myelinating Schwann cells. J Neurosci. 1988;8:3515–3521. doi: 10.1523/JNEUROSCI.08-09-03515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- Twiss JL, Smith DS, Chang B, Shooter EM. Translational control of ribosomal protein L4 mRNA is required for rapid neurite regeneration. Neurobiol Dis. 2000;7:416–428. doi: 10.1006/nbdi.2000.0293. [DOI] [PubMed] [Google Scholar]
- Uceyler N, Tscharke A, Sommer C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immun. 2007;21:553–560. doi: 10.1016/j.bbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Vargas ME, Watanabe J, Singh SJ, Robinson WH, Barres BA. Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proc Natl Acad Sci U S A. 2010;107:11993–11998. doi: 10.1073/pnas.1001948107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar MJ, Roa M, Huchet M, Hokfelt T, Changeux JP, Fahrenkrug J, Brown JC, Epstein M, Hersh L. Immunoreactive calcitonin gene-related peptide, vasoactive intestinal polypeptide, and somatostatin in developing chicken spinal cord motoneurons. Eur J Neurosci. 1989;1:269–287. doi: 10.1111/j.1460-9568.1989.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Vivo M, Puigdemasa A, Casals L, Asensio E, Udina E, Navarro X. Immediate electrical stimulation enhances regeneration and reinnervation and modulates spinal plastic changes after sciatic nerve injury and repair. Exp Neurol. 2008;211:180–193. doi: 10.1016/j.expneurol.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Waller AV. Experiments on the section of glossopharyngeal and hypoglossal nerves of the frog and observations of the alternatives produced thereby in the structure of their primitive fibres. Philos Trans R Soc Lond B Biol Sci. 1850;140:423–429. [Google Scholar]
- Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 2005;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Medress ZA, Barres BA. Axon degeneration: Molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012;196:7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson WE. Cellular responses to axotomy and to related procedures. Br Med Bull. 1974;30:112–115. doi: 10.1093/oxfordjournals.bmb.a071179. [DOI] [PubMed] [Google Scholar]
- Werner A, Willem M, Jones LL, Kreutzberg GW, Mayer U, Raivich G. Impaired axonal regeneration in alpha7 integrin-deficient mice. J Neurosci. 2000;20:1822–1830. doi: 10.1523/JNEUROSCI.20-05-01822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Greenberg ME. Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DM, Mansfield K, Kelleher K. Increased neurite outgrowth of cultured rat dorsal root ganglion cells following transection or inhibition of axonal transport of the sciatic nerve. Neurosci Lett. 1996;208:93–96. doi: 10.1016/0304-3940(96)12554-4. [DOI] [PubMed] [Google Scholar]
- Willis DE, Twiss JL. The evolving roles of axonally synthesized proteins in regeneration. Curr Opin Neurobiol. 2006;16:111–118. doi: 10.1016/j.conb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Wang X, Ewanek R, Bhat P, DiAntonio A, Collins CA. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol. 2010;191:211–223. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata N, Yuasa S, Araki T. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J Neurosci. 2009;29:6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalowitz JA, Xiao S, Biju MP, Antony AC, Cummings OW, Deeg MA, Jayaram HN. Characterization of human brain nicotinamide 5′-mononucleotide adenylyltransferase-2 and expression in human pancreas. Biochem J. 2004;377:317–326. doi: 10.1042/BJ20030518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Hayashi Y, Jinno S, Wu Z, Inoue K, Kohsaka S, Nakanishi H. Reduced synaptic activity precedes synaptic stripping in vagal motoneurons after axotomy. Glia. 2008;56:1448–1462. doi: 10.1002/glia.20711. [DOI] [PubMed] [Google Scholar]
- Yamada J, Nakanishi H, Jinno S. Differential involvement of perineuronal astrocytes and microglia in synaptic stripping after hypoglossal axotomy. Neuroscience. 2011;182:1–10. doi: 10.1016/j.neuroscience.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Yan D, Wu Z, Chisholm AD, Jin Y. The DLK-1 kinase promotes mRNA stability and local translation in c. elegans synapses and axon regeneration. Cell. 2009;138:1005–1018. doi: 10.1016/j.cell.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wen H, Ou S, Cui J, Fan D. IL-6 promotes regeneration and functional recovery after cortical spinal tract injury by reactivating intrinsic growth program of neurons and enhancing synapse formation. Exp Neurol. 2012;236:19–27. doi: 10.1016/j.expneurol.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Yao CC, Ziober BL, Squillace RM, Kramer RH. Alpha7 integrin mediates cell adhesion and migration on specific laminin isoforms. J Biol Chem. 1996;271:25598–25603. doi: 10.1074/jbc.271.41.25598. [DOI] [PubMed] [Google Scholar]
- Ydens E, Cauwels A, Asselbergh B, Goethals S, Peeraer L, Lornet G, Almeida-Souza L, Van Ginderachter JA, Timmerman V, Janssens S. Acute injury in the peripheral nervous system triggers an alternative macrophage response. J Neuroinflammation. 2012;9:176. doi: 10.1186/1742-2094-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Gilbert HY, Yang Y, Yang Z, Berlinicke C, Li Z, Zaverucha-do-Valle C, He H, Petkova V, Zack DJ, Benowitz LI. Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci U S A. 2009;106:19587–19592. doi: 10.1073/pnas.0907085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- Ying Z, Misra V, Verge VMK. Sensing nerve injury at the axonal ER--activated Luman/CREB3 serves as a novel axonally synthesized retrograde regeneration signal. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1407462111. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylera B, Erturk A, Hellal F, Nadrigny F, Hurtado A, Tahirovic S, Oudega M, Kirchhoff F, Bradke F. Chronically CNS-injured adult sensory neurons gain regenerative competence upon a lesion of their peripheral axon. Curr Biol. 2009;19:930–936. doi: 10.1016/j.cub.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Wang J, Kim A, Liu Q, Watts R, Hoopfer E, Mitchison T, Luo L, He Z. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39:217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, Martin KC, Twiss JL. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci. 2001;21:9291–9303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Deneris E, Zigmond RE. Differential regulation of levels of nicotinic receptor subunit transcripts in adult sympathetic neurons after axotomy. J Neurobiol. 1998;34:164–178. doi: 10.1002/(sici)1097-4695(19980205)34:2<164::aid-neu6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Deneris E, Zigmond RE. Nicotinic acetylcholine receptor subunit proteins alpha7 and beta4 decrease in the superior cervical ganglion after axotomy. J Neurobiol. 2001;46:178–192. doi: 10.1002/1097-4695(20010215)46:3<178::aid-neu1001>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Zigmond RE. LIF, NGF, and the cell body response to axotomy. Neuroscientist. 1997;3:176–185. [Google Scholar]
- Zigmond RE. Can galanin also be considered as growth-associated protein 3.2? Trends Neurosci. 2001;24:494–496. doi: 10.1016/s0166-2236(00)01914-7. [DOI] [PubMed] [Google Scholar]
- Zigmond RE. Cytokines that promote nerve regeneration. Exp Neurol. 2012a;238:101–106. doi: 10.1016/j.expneurol.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond RE. gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury. Front Mol Neurosci. 2012b;4:1–18. doi: 10.3389/fnmol.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond RE, Hyatt-Sachs H, Mohney RP, Schreiber RC, Shadiack AM, Sun Y, Vaccariello SA. Changes in neuropeptide phenotype after axotomy of adult peripheral neurons and the role of leukemia inhibitory factor. Perspect Dev Neurobiol. 1996;4:75–90. [PubMed] [Google Scholar]
- Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, Tarwater P, Clements J, Barber S. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA. 2005;293:2003–2011. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Spira ME. Axotomy induces a transient and localized elevation of the free intracellular calcium concentration to the millimolar range. J Neurophysiol. 1995;74:2625–2637. doi: 10.1152/jn.1995.74.6.2625. [DOI] [PubMed] [Google Scholar]
- Zou H, Ho C, Wong K, Tessier-Lavigne M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci. 2009;29:7116–7123. doi: 10.1523/JNEUROSCI.5397-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zujovic V, Luo D, Baker HV, Lopez MC, Miller KR, Streit WJ, Harrison JK. The facial motor nucleus transcriptional program in response to peripheral nerve injury identifies Hn1 as a regeneration-associated gene. J Neurosci Res. 2005;82:581–591. doi: 10.1002/jnr.20676. [DOI] [PubMed] [Google Scholar]
- Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. J Neurobiol. 1998a;34:41–54. [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Dyess K, Ferguson TA, Muir D. Degradation of chondroitin sulfate proteoglycan enhances the neurite-promoting potential of spinal cord tissue. Exp Neurol. 1998b;154:654–662. doi: 10.1006/exnr.1998.6951. [DOI] [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Graham J, Krekoski CA, Ferguson TA, Muir D. Regeneration of axons after nerve transection repair is enhanced by degradation of chondroitin sulfate proteoglycan. Exp Neurol. 2002;176:221–228. doi: 10.1006/exnr.2002.7922. [DOI] [PubMed] [Google Scholar]