Abstract
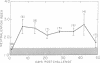
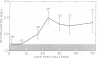
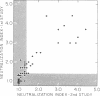
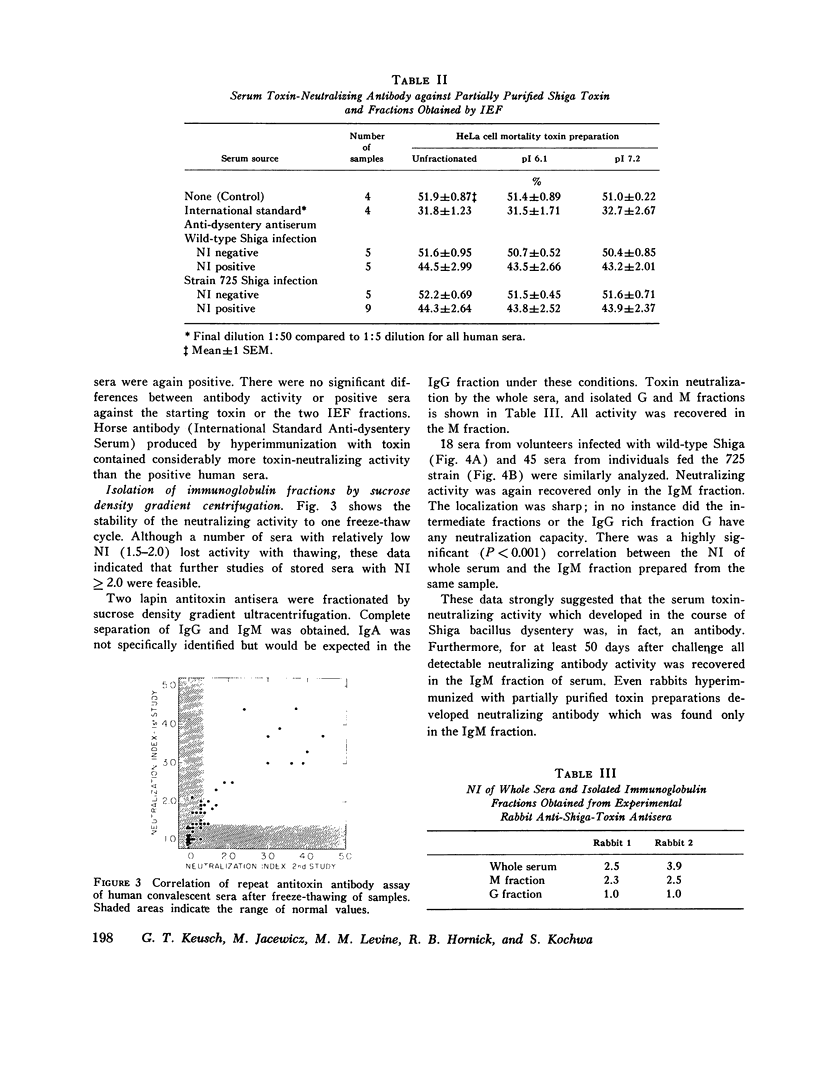
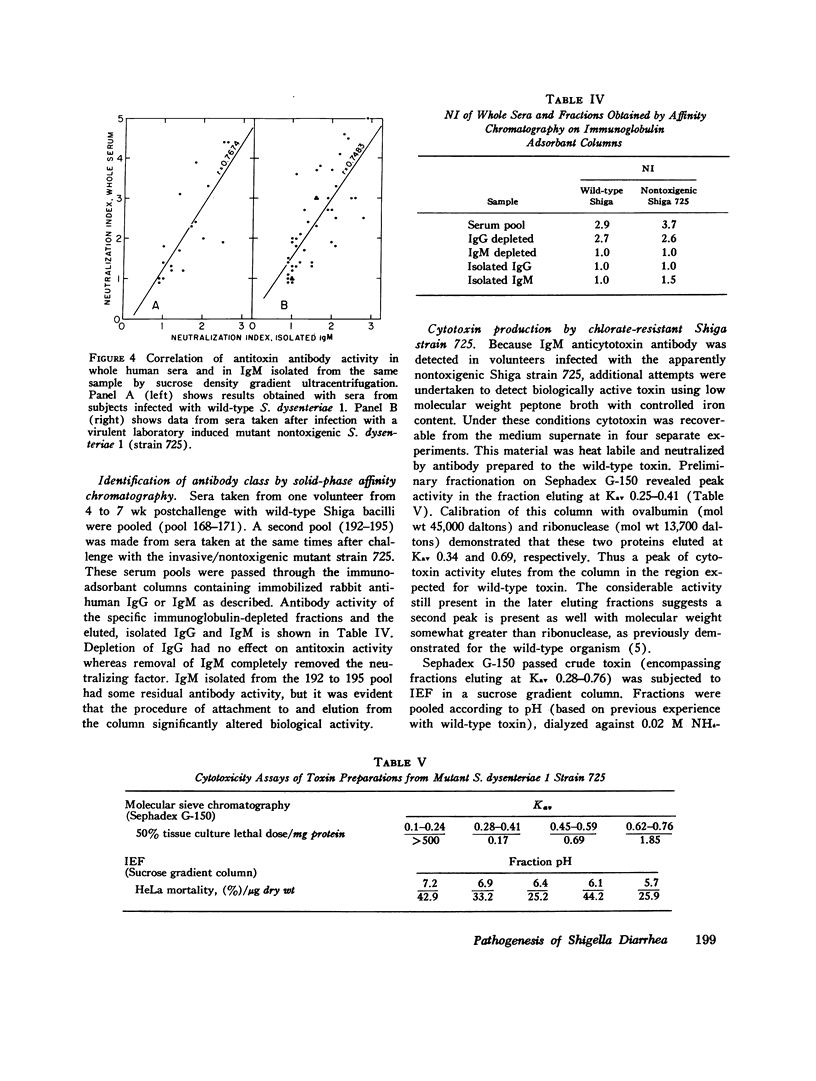
The serum antitoxin response to the cytotoxin contained in preparations of Shigella dysenteriae 1 (Shiga's bacillus) exotoxin was studied in natural and experimental infections of man. Natural infection resulted in the rapid appearance of toxin-neutralizing antibody, which disappeared some time between 9 and 18 mo after infection. Experimental infection of human volunteers provided the opportunity to study immunoglobulin class of the antibody in sera obtained serially from 7 to 50 days after infection. Neutralizing antibody was present only in the IgM fraction isolated by sucrose density gradient ultracentrifugation. This was confirmed by the use of solid-phase immunoaffinity chromatography. Even though the time-course and immunoglobulin class of the antitoxin antibody response was similar to that previously observed for anti-O polysaccharide antibody, the biologically active cytotoxin was shown to be highly susceptible to destruction by proteolytic enzymes. Sera from subjects infected with a virulent invasive chlorate-resistant Shiga mutant thought to be "nontoxigenic" also contained antibody which was similarly restricted to the IgM fraction. Biologically active cytotoxin was recovered when this mutant organism was grown in liquid media with controlled ion concentration. The mutant cytotoxin was heat labile, neutralized by antiwild-type cytotoxin antibody, and was separable by isoelectric focusing into two fractions with pI 7.2 and 6.1 like the wild-type toxin. These studies show that cytotoxin antigen is produced during in vivo infection with Shiga bacilli, resulting in a serum antitoxin antibody response. Without explanation is the restriction of the antibody to the IgM class and lack of evidence for an IgG antibody to the protein cytotoxin. Finally, mutant strain 725, previously designated "nontoxigenic," was shown to produce biologically active cytotoxin in vitro and, in experimentally infected volunteers, to result in a serum IgM antibody similar to that observed during infection with the wild-type strain.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cáceres A., Mata L. J. Serologic response of patients with shiga dysentery. J Infect Dis. 1974 Apr;129(4):439–443. doi: 10.1093/infdis/129.4.439. [DOI] [PubMed] [Google Scholar]
- Gemski P., Jr, Takeuchi A., Washington O., Formal S. B. Shigellosis due to Shigella dysenteriae. 1. Relative importance of mucosal invasion versus toxin production in pathogenesis. J Infect Dis. 1972 Nov;126(5):523–530. doi: 10.1093/infdis/126.5.523. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Donta S. T. Classification of enterotoxins on the basis of activity in cell culture. J Infect Dis. 1975 Jan;131(1):58–63. doi: 10.1093/infdis/131.1.58. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Grady G. F., Mata L. J., McIver J. The pathogenesis of Shigella diarrhea. I. Enterotoxin production by Shigella dysenteriae I. J Clin Invest. 1972 May;51(5):1212–1218. doi: 10.1172/JCI106915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M., Hirschman S. Z. Quantitative microassay in cell culture for enterotoxin of Shigella dysenteriae. J Infect Dis. 1972 May;125(5):539–541. doi: 10.1093/infdis/125.5.539. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M. Serum enterotoxin-neutralizing antibody in human shigellosis. Nat New Biol. 1973 Jan 3;241(105):31–32. doi: 10.1038/newbio241031a0. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M. The pathogenesis of Shigella diarrhea. V. Relationship of shiga enterotoxin, neurotoxin, and cytotoxin. J Infect Dis. 1975 May;131 (Suppl):S33–S39. doi: 10.1093/infdis/131.supplement.s33. [DOI] [PubMed] [Google Scholar]
- Keusch G. T. Pathogenesis of Shigella diarrhea. 3. Effects of shigella enterotoxin in cell culture. Trans N Y Acad Sci. 1973 Jan;35(1):51–58. doi: 10.1111/j.2164-0947.1973.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Levine M. M., DuPont H. L., Formal S. B., Hornick R. B., Takeuchi A., Gangarosa E. J., Snyder M. J., Libonati J. P. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis. 1973 Mar;127(3):261–270. doi: 10.1093/infdis/127.3.261. [DOI] [PubMed] [Google Scholar]
- McIver J., Grady G. F., Keusch G. T. Production and characterization of exotoxin(s) of Shigella dysenteriae type 1. J Infect Dis. 1975 May;131(5):559–566. doi: 10.1093/infdis/131.5.559. [DOI] [PubMed] [Google Scholar]
- Newcomb R. W., Ishizaka K. Human diphtheria antitoxin in immunoglobulin classes IgG and IgA. J Immunol. 1967 Jul;99(1):40–48. [PubMed] [Google Scholar]
- Ourth D. D., Edsall G. Antibody activities of 19S and 7S globulin fractions from rabbit antisera to tetanus toxoid. Br J Exp Pathol. 1972 Oct;53(5):492–500. [PMC free article] [PubMed] [Google Scholar]
- Ourth D. D. Neutralization of diphtheria toxin by human immunoglobulin classes and subunits. Immunochemistry. 1974 May;11(5):223–225. doi: 10.1016/0019-2791(74)90199-2. [DOI] [PubMed] [Google Scholar]
- VAN HEYNINGEN W. E., GLADSTONE G. P. The neurotoxin of Shigella shigae. I. Production, purification and properties of the toxin. Br J Exp Pathol. 1953 Apr;34(2):202–216. [PMC free article] [PubMed] [Google Scholar]