Background
Neisseria gonorrhoeae is the second most common bacterial cause of sexually transmitted infections (STIs) in North America, following Chlamydia trachomatis.1-3 Globally, gonococcal infections are now an urgent problem because N. gonorrhoeae is capable of rapidly developing resistance to multiple antibiotic classes.4-8 Over time, N. gonorrhoeae has become less susceptible to numerous antibiotics, including the sulfonamides, penicillins, tetracyclines and fluoroquinolones. More recently, cases of resistance to cephalosporins, the current first-line treatment, have been reported.
According to the Public Health Agency of Canada (PHAC), the incidence of gonorrhea has more than doubled, from approximately 15 cases per 100,000 in 1997 to up to 33 cases per 100,000 in 2009.5,9 In the United States in 2011, the reported rate was even higher, at 104.2 cases per 100,000.10 In both Canada and the United States, gonorrhea is more common in young adults (women aged 15-24 and men aged 20-24).5,10 In the United States, the occurrence in this age group is about 5 times that of the national average.10 The incidence of this infection, however, is confounded by factors such as changes in both reporting practices and screening, as well as the use of diagnostic tests with different sensitivities.
Risk factors for gonorrhea include sexual contact with an infected person or someone from an endemic area; previous gonorrhea, STIs or human immunodeficiency virus (HIV); being a sexually active youth; having multiple partners; and being a sex worker, street youth and/or man who has sex with men (MSM).1,5 Geographic clustering of gonococcal infections is associated with minority ethnic groups, low socioeconomic status and lack of education.1
Gonorrhea is often asymptomatic in females and symptomatic in males.1,5,11 When symptomatic, the clinical presentation in females includes vaginal discharge, dysuria, dyspareunia, abnormal uterine bleeding, lower abdominal and/or rectal pain.5,11 In males, symptoms include urethral discharge and/or itch, dysuria and testicular or rectal pain. The urethra and cervix are the most frequently affected anatomical sites, followed by anal and pharyngeal areas.1,5,11
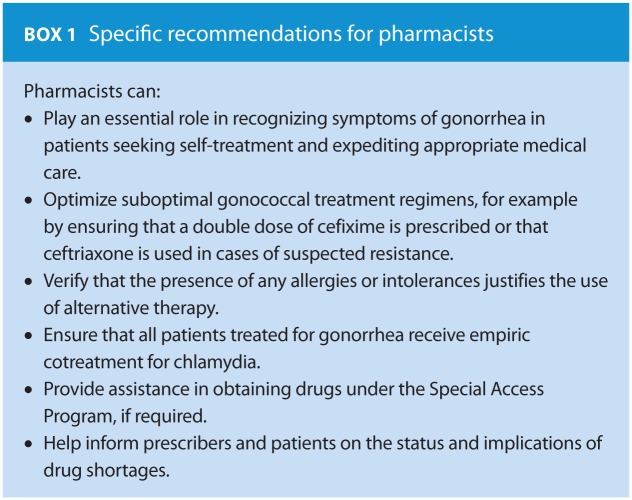
Gonococcal infections are considered uncomplicated in the absence of bacteremia or pathogen spread to extragenital sites.12 However, left untreated, this infection can have serious sequelae. These include pelvic inflammatory disease, infertility, ectopic pregnancy and chronic pain in women and epididymo-orchitis, reactive arthritis and, rarely, infertility in men.5,11 In both genders, the infection can progress to disseminated disease. N. gonorrhoeae infection can also facilitate the transmission of HIV. Pharmacists can help their patients by being familiar with symptoms of gonorrhea infections and being aware of new guidelines and treatment regimens (Box 1).
Treatment
Considerations for selection of antimicrobial therapy
The ideal treatment regimen for gonorrhea should cure at least 95% of infections.4,12 In other words, an antibiotic to which more than 5% of N. gonorrhoeae strains exhibit resistance should not be used, given the close correlation between in vitro susceptibility testing and clinical failure. When treatment failures are minimized, the potential spread of resistant disease is also reduced. Also, antibiotic therapy should be effective at all anatomical sites, well tolerated and easy to comply with (preferably single-dose therapy at the point of care, i.e., directly observed therapy).4
Summary of treatment guidelines
New recommendations for the treatment of gonorrhea have been published to address the increased prevalence of N. gonorrhoeae resistance. The Alberta Treatment Guidelines for STIs13 were updated in 2012, while the PHAC,5 Public Health Ontario11 and the Quebec Institut national d’excellence en santé et en services sociaux14 updated their guidelines in 2013. Updated 2014 guidelines are also available from British Columbia and Saskatchewan.15,16 Other provincial guidelines either have not yet been updated17 or are referring clinicians to the new Canadian guidelines.18
Tables 1 and 2 present the latest guidelines from the PHAC,5 Public Health Ontario,11 the US Centers for Disease Control (CDC)2 and 2 European agencies.19,20
Table 1.
| Guidelines | Anogenital infection* | Pharyngeal infection |
|---|---|---|
| Public Health Agency of Canada: Recommendations for Gonorrhea Treatment 2013† | Preferred‡:
|
Preferred:
|
| Alternatives: | Alternatives:
|
|
| Public Health Ontario: Recommendations for Gonorrhea Treatment 2013 | Preferred:
|
Preferred:
|
Alternatives:
|
Alternatives:
|
|
| Quebec (INESSS): Infection à Neisseria gonorrhoeae 2013 | Preferred:
|
Preferred:
|
| Update to CDC Guidelines 20122 | Preferred:
|
Preferred:
|
Alternatives (if ceftriaxone not available):
|
||
| European Guidelines 2012 | Preferred:
|
Preferred:
|
Alternatives:
Cefixime 400 mg PO as a single dose plus azithromycin 2 g PO as a single dose
|
Alternatives:
Ceftriaxone 500 mg IM as a single dose
|
|
Ceftriaxone 500 mg IM as a single dose
Spectinomycin 2 g IM as a single dose plus azithromycin 2 g PO as a single dose |
Ciprofloxacin 500 mg PO as a single dose or ofloxacin 400 mg PO as a single dose
Azithromycin 2 g PO as a single dose |
|
| UK National Guidelines (BASHH) 2011 | Preferred:
|
Preferred: Ceftriaxone 500 mg IM as a single dose plus azithromycin 1 g PO as a single dose
Ciprofloxacin 500 mg PO as a single dose or ofloxacin 400 mg PO as a single dose Note: Spectinomycin has poor efficacy in pharyngeal infections. |
Alternatives (if IM contraindicated or patient refuses):
Ciprofloxacin 500 mg PO as a single dose or ofloxacin 400 mg PO as a single dose
|
Anogenital sites include urethral, rectal, vaginal and endocervical.
The Public Health Agency of Canada’s guidelines are for adults and youth 9 years of age and older.
First-line recommendations are listed as preferred regimens and second-line recommendations are listed as alternatives. The order of appearance does not suggest a preference for one particular regimen over another.
Azithromycin is preferred over doxycycline due to high rates of resistance to tetracyclines and concern about compliance.
Spectinomycin is available only through Health Canada’s Special Access Program.
Azithromycin at a dose of 2 g is associated with significant gastrointestinal adverse effects that can be minimized if taken with food or with use of antiemetic prophylaxis. If vomiting occurs within 1 hour of administration, the dose of azithromycin should be repeated.
Table 2.
|
Choices include:
or
|
The guidelines address the increasing resistance to cephalosporins, with recommendations varying based on the geographic region. Canadian guidelines recommend either intramuscular (IM) ceftriaxone or oral cefixime as the preferred antibiotic of choice. In contrast, the CDC and organizations in other countries advocate only parenteral cephalosporins as first-line therapy. Similar to the international guidelines, Ontario also recommends parenteral cephalosporins as the preferred regimen, given local reports of resistance to oral cefixime.21 The recommended dose of ceftriaxone IM varies between North America and other countries. Both Canada and the United States recommend the lower 250 mg dose, while Europe and the UK propose a higher dose of 500 mg.
All guidelines currently recommend co-treatment with azithromycin for C. trachomatis, regardless of chlamydia test results. The reasons for co-treatment include the high rate of coinfection and antigonococcal activity of azithromycin and doxycycline.5 The use of combination treatment for gonorrhea aims to improve treatment efficacy and delay emergence and spread of resistance to the cephalosporins.
Resistance patterns and mechanisms in the treatment of gonorrhea
Over the last 2 decades, new antimicrobial susceptibility surveillance programs such as Canada’s National N. gonorrhoeae Surveillance Program were developed in response to the rapid rise of resistance to N. gonorrhoeae.22 This program is coordinated by the National Microbiology Laboratory from the PHAC. Each year the program reports rising numbers of gonorrhea cases, with an increasing proportion resistant to at least 1 antibiotic. Between 2000 and 2009, no reported isolates were resistant to either ceftriaxone or cefixime. However, a shift occurred in the modal minimum inhibitory concentration (MIC) for both drugs, including a combined total of 208 isolates with decreased susceptibility. Among these isolates, more exhibited reduced susceptibility to cefixime than to ceftriaxone. The mechanism for resistance was largely due to alterations in the penA, porB1b and mtrR genes,23 which diminish b-lactam binding to the cell wall, decrease permeability of cephalosporins and increase drug efflux from the cell, respectively.
In the United States, the Gonococcal Isolate Surveillance Project (GISP) monitors trends in antimicrobial susceptibilities of N. gonorrhoeae in order to guide treatment recommendations.24 GISP defines decreased susceptibility of N. gonorrhoeae to cefixime and ceftriaxone as an MIC of ≥0.5 mcg/mL. From 2008 to 2012, GISP reported a small increase in the percentage of N. gonorrhoeae isolates with an MIC ≥0.125 mcg/mL for ceftriaxone, from 0.1% to 0.3%. One isolate with an MIC ≥0.5 mcg/mL was found. A total of 4 isolates with decreased susceptibility (MIC of 0.5 mcg/mL) were reported between 1992 and 1997. For cefixime, the percentage of isolates ≥0.25 mcg/mL increased from 0.1% in 2006 to 1.0% in 2012. In 2012, 3 isolates resistant to cefixime were reported, 2 with MICs of 0.5 mcg/mL and 1 with an MIC of 1 mcg/mL.
Clinical treatment failures
Clinical cases of cephalosporin treatment failures in Canada are only recent. A retrospective cohort study published in 2013 from a Toronto sexual health clinic described treatment failure with cefixime.21 N. gonorrhoeae (with identical molecular typing to baseline) was isolated at the follow-up test-of-cure visit in 13 of 133 individuals. Nine individuals were reported to have failed cefixime in the treatment of urethral, rectal or pharyngeal gonococcal infections. The other 4 were not considered treatment failures as their records did not include information as to possible sexual reexposure. The clinical failure rate was 25% for those with a cefixime MIC of 0.12 mcg/mL or greater, compared with 1.9% for those with a cefixime MIC of less than 0.12 mcg/mL. In contrast, an Alberta study reported treatment failures in pharyngeal infections with cefixime 400 mg in 13.1% of patients, although failures were not related to elevated cefixime MICs but rather attributed to reduced drug concentrations of oral cephalosporins in the pharynx compared with other sites.25
Prior to this study, most clinical failures were reported internationally.26-30 In Japan, a case of ceftriaxone-resistant N. gonorrhoeae (MIC of 2 mcg/mL) was reported in 2011 in a female commercial sex worker.26 In Sweden in 2010, a heterosexual male cultured positive more than once for ceftriaxone-resistant strains (MIC of 0.125 or 0.25 mcg/mL) requiring a 1 g dose of ceftriaxone for treatment success.27 In 2010, 3 cases of cefixime failure were reported, 2 in Norway28 and 1 in England,29 and all were in heterosexual men. In Austria in 2011, 1 case of cefixime failure was reported in an MSM.30 In all of these cases, the initial treatment was oral cefixime 400 mg. In most cases, ceftriaxone was prescribed following identification of the treatment failure.
Cefixime dosing
The use of the cephalosporins in the treatment of gonorrhea is a concern, regardless of whether ceftriaxone or cefixime at an elevated dose of 800 mg is used as first-line therapy. Note only does the lack of alternative therapies limit clinicians in tailoring therapies based on safety considerations such as drug allergies, pregnancy and adverse effects, but the focus on one class for therapy has historically led to a rapid rise in resistance. The newest guidelines address the issue of increasing resistance to the cephalosporins with the knowledge that choices are limited once this class is no longer effective.
The Canadian guidelines are unique, as they continue to recommend oral cefixime with an increased dose to overcome rising MICs. The 800 mg dose of cefixime is off-label use; however, the Canadian guidelines state that it is safe and effective and provides a prolonged time above the MIC when compared with the 400 mg dose.5 Data for the 800 mg dose of cefixime in patients with gonorrhea are limited to a few older trials.31,32
A 1992 study compared both the 400 mg and 800 mg doses of cefixime with IM ceftriaxone 250 mg in patients with uncomplicated N. gonorrhoeae urethritis or cervicitis.31 Among the 155 evaluable patients, efficacy was comparable between the 3 groups, with bacterial eradication rates of 99%, 95% and 100%, respectively. In the 3 cases of treatment failure (1 case with cefixime 400 mg and 2 cases with cefixime 800 mg), the cefixime MIC ranged from 0.004 to 0.008 mcg/mL, while the geometric mean MIC for all 187 isolates was 0.005 mcg/mL. Three patients reported adverse effects after taking the 800 mg cefixime dose compared with 10 in the 400 mg group and none in the ceftriaxone group. The most frequent side effect reported with cefixime was diarrhea/loose stools (3% of the total group). A 1991 randomized unblinded study of 333 patients similarly evaluated oral cefixime 400 mg or 800 mg and ceftriaxone 250 mg IM.32 All 3 regimens again demonstrated similar rates of bacterial eradication (96%, 98% and 98%, respectively). All regimens were well tolerated, with 13% of patients reporting mild to moderate side effects. Gastrointestinal side effects (e.g., nausea, diarrhea and epigastric pain) occurred more frequently in the group taking 800 mg of cefixime compared with 400 mg of cefixime (18% vs. 8%). The 6 patients with persistent infection following cefixime therapy had baseline MICs of 0.004 mcg/mL (n = 3) and 0.015 mcg/mL (n = 3). The 2 patients with persistent infection following ceftriaxone therapy had baseline MICs of 0.001 and 0.004 mcg/mL.
Approach to treatment failures/prevention of spread and resistance
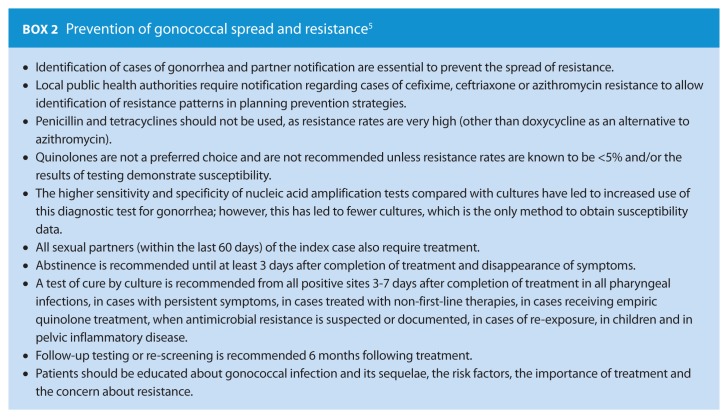
The PHAC recommends consultation with infectious disease specialists along with culture and sensitivity testing for cases of treatment failure. Therapy includes higher doses of ceftriaxone ranging from 250 mg to 1 g IM given in combination with azithromycin 1 g orally. Second-line therapy and treatment failure therapy all require a test of cure (see Table 3). It is essential that the development of resistance to N. gonorrhoeae be minimized by optimizing treatment options based on geographical resistance patterns. Patient education and partner notification are of utmost importance in improving public awareness and reducing the spread of this resistant organism (Box 2).
Table 3.
| Public Health Agency of Canada | Treatment to be guided by antimicrobial susceptibility testing in consultation with infectious disease specialists and local public health authorities, with test of cure by culture collected 3 to 7 days following completion of treatment. |
| Public Health Ontario | A higher dose of ceftriaxone should be used with azithromycin, e.g., ceftriaxone 1 g IM plus azithromycin 2 g PO each as a single dose (first-line treatment recommended if not initially used). |
| US Centers for Disease Control | Ceftriaxone 250 mg IM plus azithromycin 2 g PO each as a single dose. |
| UK, Europe | Alternative regimens as outlined in Table 1, with review of local and national resistance trends. |
Conclusion
The growing resistance of N. gonorrhoeae to cephalosporins has led to the use of increased doses of ceftriaxone and cefixime to ensure effective treatment. The PHAC continues to recommend oral cefixime but at an increased dose of 800 mg, or IM ceftriaxone, with only the latter recommended for MSM and pharyngeal infections. These recommendations are based on geographical differences of MICs across the country, while allowing for provincial/territorial differences in guidelines. Guidelines from the province of Ontario, the United States and Europe endorse IM ceftriaxone as the sole first-line agent for all gonococcal infections. Of note, Sanofi-Pasteur has recently announced a nationwide shortage of cefixime predicted to last until October 2015. Although provincial public health agencies are reserving cefixime specifically for the treatment of gonococcal infections, supplies may not last until the end of the back order. N. gonorrhoeae has demonstrated rapid changes in its susceptibility patterns over the years. In response, the public health agencies remain vigilant in monitoring the evolution of this organism and maintaining up-to-date guidelines. Pharmacists can play a role by educating their patients with respect to compliance, follow-up, partner notification and strategies for prevention of transmission of this ever-adaptable organism. ■
Acknowledgments
The authors wish to thank the following individuals for their valuable comments and advice during the preparation of the manuscript: Mirella Giudice and Linda Ahmad, drug information pharmacists at The Ottawa Hospital.
Footnotes
Author Contributions:J. Piszczek, R. St. Jean and Y. Khaliq contributed substantially to the conception and design, acquisition of data, analysis and interpretation of data for this manuscript and approved the final version. J. Piszczek prepared the initial draft of the article, and R. St. Jean and Y. Khaliq revised the article for critically important intellectual content.
Declaration of Conflicting Interests:The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding:The authors received no financial support for the research, authorship and/or publication of this article.
References
- 1. Leone PA. Epidemiology, pathogenesis and clinical manifestations of Neisseria gonorrhoeae infection. April 2013. Available: www.uptodate.com/contents/epidemiology-pathogenesis-and-clinical-manifestations-of-neisseria-gonorrhoeae-infection?source=see_link#H4 (accessed Nov. 8, 2013).
- 2. Centers for Disease Control and Prevention. Update to CDC’s Sexually Transmitted Diseases Treatment Guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep 2012;61(31):590-4. [PubMed] [Google Scholar]
- 3. Public Health Agency of Canada. Canadian guidelines on sexually transmitted infections. 2013 Update. Available: www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/section-5-6-eng.php (accessed Jan. 29, 2015).
- 4. World Health Organization. Sexually transmitted bacterial pathogen for which there are increasing antimicrobial resistance concerns: Neisseria gonorrhea. Available: www.who.int/csr/resources/publications/drugresist/IIAMRmanual.pdf (accessed Nov. 8, 2013).
- 5. Public Health Agency of Canada. Canadian guidelines on sexually transmitted infections. July 2013. Available: www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/section-5-6-eng.php#footnote-t1 (accessed Dec. 3, 2013).
- 6. MacDonald NE, Stanbrook MB, Flegel K, et al. Gonorrhea: what goes around comes around. CMAJ 2011;183:1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kondro W. Untreatable gonorrhea rampant. CMAJ 2012;184:E591. [Google Scholar]
- 8. Ison CA. Antimicrobial resistance in sexually transmitted infections in the developed world: implications for rational treatment. Curr Opin Infect Dis 2012;25:73-8. [DOI] [PubMed] [Google Scholar]
- 9. Public Health Agency of Canada. Executive summary—report on sexually transmitted infections in Canada: 2009. Available: www.phac-aspc.gc.ca/sti-its-surv-epi/sum-som-eng.php (accessed Nov. 11, 2013).
- 10. Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Available: www.cdc.gov/std/stats10/gonorrhea.htm (accessed Nov. 8, 2013).
- 11. Ontario Agency for Health Protection and Promotion (Public Health Ontario). Guidelines for testing and treatment of gonorrhea in Ontario. Toronto (ON): Queen’s Printer for Ontario; 2013. Available: www.publichealthontario.ca/en/eRepository/Guidelines_Gonorrhea_Ontario_2013.pdf (accessed Oct. 25, 2013). [Google Scholar]
- 12. Swygard H, Sena AC, Cohen MS. Treatment of uncomplicated gonococcal infections. Available: www.uptodate.com/contents/treatment-of-uncomplicated-gonococcal-infections?detectedLanguage=en&source=search_result&search=gonorrhea+treatment&selectedTitle=1%7E150&provider=noProvider (accessed Nov. 9, 2013).
- 13. Alberta Health. Public Health notifiable disease management guidelines. July 2012. Available: www.health.alberta.ca/documents/Guidelines-Gonococcal-Infections-2012.pdf (accessed Oct. 25, 2013).
- 14. Institut national d’excellence en santé et en services sociaux Québec. Infection à Chlamydia trachomatis et infection à Neisseria gonorrhoeae. Available: www.inesss.qc.ca/fileadmin/doc/INESSS/Outils/Guides_ITSS/Guide_ITSS-Chlamydia_gonorrhoeae_majaout2013_.pdf (accessed Oct. 25, 2013).
- 15. BC Centre for Disease Control. Sexually transmitted infections in adolescents and adults 2014. Available: www.bccdc.ca/NR/rdonlyres/46AC4AC5-96CA-4063-A563-0BA9F4A0A6E9/0/CPS_BC_STI_Treatment_Guidelines_27082014.pdf (accessed Oct. 15, 2014).
- 16. Saskatchewan Ministry of Health. Guidelines for testing and treatment of gonorrhea in Saskatchewan, 2014. Available: www.health.gov.sk.ca/adx/aspx/adxGetMedia.aspx?DocID=fb8126c0-30ee-4a51-adfd-af986da1b106&MediaID=8614&Filename=FAQs-Gonorrhea-GuidelinesforTesting-Treatment.pdf&l=English (accessed Oct. 15, 2014).
- 17. Yukon Health and Social Services. Yukon treatment guidelines for sexually transmitted infections (STIs) in adolescents and adults 2010. Available: www.hss.gov.yk.ca/pdf/STI_TreatmentGuidelines2012_singles.pdf (accessed Oct. 25, 2013).
- 18. Manitoba Health. Public Health and Primary Health Care. Communicable disease management protocol—gonorrhea. January 2012. Available: www.gov.mb.ca/health/publichealth/cdc/protocol/gonorrhea.pdf (accessed Oct. 25, 2013).
- 19. Bignell C, FitzGerald M, for the Guideline Development Group. U.K. national guideline for the management of gonorrhoea in adults. Int J STD AIDS 2011;22:541-7. Available: www.bashh.org/documents/3920.pdf (accessed June 3, 2013). [DOI] [PubMed] [Google Scholar]
- 20. Bignell C, Unemo M. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 2013;24:85-92. [DOI] [PubMed] [Google Scholar]
- 21. Allen VG, Mitterni L, Seah C, et al. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA 2013;309:163-70. [DOI] [PubMed] [Google Scholar]
- 22. Martin I, Jayaraman G, Wong T, et al. Trends in antimicrobial resistance in Neisseria gonorrhoeae isolated in Canada: 2000-2009. Sex Transm Dis 2011;38:892-8. [DOI] [PubMed] [Google Scholar]
- 23. Martin I, Sawatzky P, Allen V, et al. Emergence and characterization of Neisseria gonorrhoeae isolates with decreased susceptibilities to ceftriaxone and cefixime in Canada: 2001-2010. Sex Transm Dis 2012;39:316-23. [DOI] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. Gonococcal Isolate Surveillance Project. Available: www.cdc.gov/std/gisp/default.htm (accessed March 24, 2014).
- 25. Gratrix J, Bergman J, Egan C, et al. Retrospective review of pharyngeal gonorrhea treatment failures in Alberta, Canada. Sex Transm Dis 2013;40:877-9. [DOI] [PubMed] [Google Scholar]
- 26. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis 2011;17:148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Unemo M, Golparian D, Hestner A. Ceftriaxone treatment failure of pharyngeal gonorrhea verified by international recommendations, Sweden, July 10, 2010. Euro Surveill 2011;16:19792. [PubMed] [Google Scholar]
- 28. Unemo M, Golparian D, Syversen G, et al. Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro Surveill 2010;15:19721. [DOI] [PubMed] [Google Scholar]
- 29. Ison CA, Hussey J, Sankar KN, et al. Gonorrhoea treatment failures to cefixime and azithromycin in England. Euro Surveill 2011;16:19833. [PubMed] [Google Scholar]
- 30. Unemo M, Golparian D, Stary A, et al. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveill 2011;16:19998. [PubMed] [Google Scholar]
- 31. Portilla I, Lutz B, Montalvo M, et al. Oral cefixime versus intramuscular ceftriaxone in patients with uncomplicated gonococcal infections. Sex Transm Dis 1992;19:94-8. [PubMed] [Google Scholar]
- 32. Handsfield HH, McCormack W, Hook EW, et al. A comparison of single-dose cefixime with ceftriaxone in the treatment of uncomplicated gonorrhea. N Engl J Med 1991;325:1337-41. [DOI] [PubMed] [Google Scholar]