Abstract
According to the long-standing definition, septic and aseptic total joint replacement loosening are two distinct conditions with little in common. Septic joint replacement loosening is driven by bacterial infection whereas aseptic loosening is caused by biomaterial wear debris released from the bearing surfaces. However, recently it has been recognized that the mechanisms that drive macrophage activation in septic and aseptic total joint replacement loosening resemble each other. In particular, accumulating evidence indicates that in addition to mediating bacterial recognition and the subsequent inflammatory reaction, toll-like receptors (TLRs) and their ligands, pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPS), play a key role in wear debris-induced inflammation and macrophage activation. In addition, subclinical bacterial biofilms have been identified from some cases of seemingly aseptic implant loosening. Furthermore, metal ions released from some total joint replacements can activate TLR signaling similar to bacterial derived PAMPs. Likewise, metal ions can function as haptens activating the adaptive immune system similar to bacterial derived antigens. Thus, it appears that aseptic and septic joint replacement loosening share similar underlying pathomechanisms and that this strict dichotomy to sterile aseptic and bacterial-caused septic implant loosening is somewhat questionable. Indeed, rather than being two, well-defined clinical entities, peri-implant osteolysis is, in fact, a spectrum of conditions in which the specific clinical picture is determined by complex interactions of multiple local and systemic factors.
Keywords: total joint replacement, aseptic loosening, septic loosening, toll-like receptors, PAMPs, DAMPs
I. SEPTIC AND ASEPTIC LOOSENING OF TOTAL JOINT REPLACEMENT
According to the long-standing definition, septic and aseptic loosening of total joint replacements (TJRs) are two distinct conditions that, excluding the accompanying peri-implant bone loss, have little in common. Septic loosening of TJRs may present as a rapidly developing acute condition that is driven by a fulminant infection of the artificial components by a highly virulent bacteria such as Staphylococcus aureus.1–3 Alternatively, septic loosening may develop as a slow indolent process in which bacterial-induced inflammation causes periprosthetic bone loss and mechanical dislodgement of the prosthesis from the underlying bone bed. The bacteria gain access to the implant either directly during primary surgery causing early postoperative infection developing within some days or a few weeks after the initial surgery. In addition, bacteria can reach the implant via hematogenous or contiguous spread causing late infections that sometimes occur years after the primary surgery. Patients suffering from infection of a TJR may present with local symptoms and signs (pain, poor function, redness, purulent drainage from the wound, etc.) and, in the more fulminant cases, with systemic symptoms of an acute inflammation (general malaise, fever, chills). The more fulminant TJR infections rarely possess a diagnostic challenge whereas the diagnosis of chronic low-grade prosthesis infection might be difficult and occasionally even indistinguishable from aseptic loosening of TJR.
Aseptic loosening of TJRs is a slowly advancing process that typically takes years to develop. In the early phase, symptoms are absent or mild due to wear particle-induced synovitis. In these cases, problems with the implant are only evident on routine follow-up radiographs that reveal progressive wear of the bearing surfaces and developing osteolytic lesions. Later on, as the condition progresses, these osteolytic lesions progress and may lead to migration of the artificial components due to undermining of the underlying supporting bony bed. The condition is primarily driven by low-grade chronic inflammation caused by biomaterial wear debris released either from joint replacement bearing surfaces and/or from the interfaces between the bone, bone cement (if present), and the implant surface.4,5
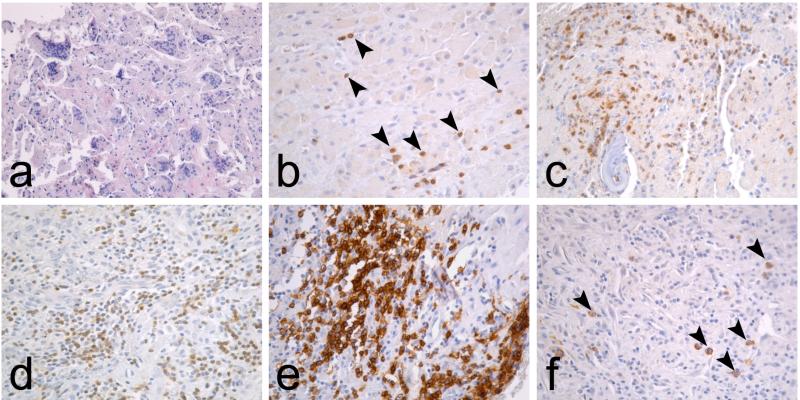
The dissimilarity of these two conditions is reflected also in the peri-implant histopathology.6 The interface tissue developing around aseptically loose metal-on-conventional ultrahigh molecular weight polyethylene (UHMWPE) type of TJRs is characterized by macrophage infiltrates and foreign body giant cell formation on a background of fibrous tissue stroma (Table 1, Fig. 1). Occasional CD3+ T lymphocytes are also visible but neutrophils and other lymphocyte subtypes such as B cells and plasma cells are absent. In contrast, the interface tissue surrounding septic implants is characterized by infiltration of various inflammatory cell populations including neutrophils and lymphocyte subsets (Table 1, Fig. 1).
TABLE 1.
Cell populations in aseptic and septic interface tisues.
| Marker | OA | Aseptic | Septic |
|---|---|---|---|
| HSP47 | ++ | + | ++ |
| NE | - | - | ++ |
| CD68 | + | +++ | ++ |
| CD3 | ± | + | ++ |
| CD20 | + | - | ++ |
| CD138 | - | - | + |
Cell populations in osteoarthritic control synovial membrane, aseptic, and septic interface tissues were evaluated by immunohistochemistry with cell type-specific antibodies. Fibroblasts were identified by heat shock protein (HSP) 47, neutrophils by neutrophil elastase (NE), macrophages by CD68, T lymphocytes by CD3, B lymphocytes by CD20, and plasma cells by CD138 immunostainings. The number of positive cells in each tissue was evaluated on a semiquantitative scale, with no positive staining (-), occasional positive cells (±), some positive cells (+), moderate numbers of positive cells (++), and large numbers of positive cells (+++). Data from Ref. 6.
FIG. 1.
Typical interface tissue histopathology in aseptic and septic loosening. (a) Interface tissue developing around aseptically loose TJRs is characterized with macrophage infiltrates and foreign body giant cells. (b) Occasional T lymphocytes (arrowheads), identified by CD3 immunostaining, are scattered among macrophages but neutrophils and other lymphocyte subpopulations are absent. In contrast, the septic interface is characterized by (c) NE+ neutrophil infiltrates, (d) diffuse CD3+ T lymphocyte infiltrates, (e) nodular CD20+ B lymphocyte infiltrates, and (f) occasional CD138+ plasma cells (arrowheads).
Although the classical dichotomy between septic and aseptic loosening of TJRs thus seems clear cut and well grounded, research addressing the cellular and molecular biology of aseptic loosening of TJRs during past 15 years has somewhat complicated this picture. Indeed, accumulating evidence indicates that these two seemingly distinct conditions have significant overlap.
II. PATHOGENESIS OF ASEPTIC LOOSENING
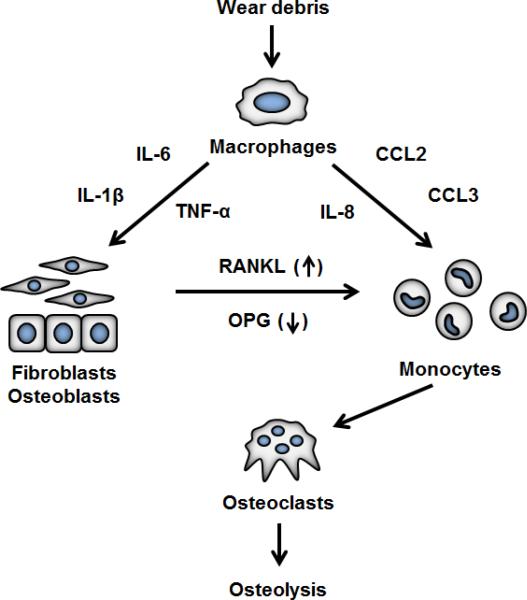
The standing paradigm of aseptic joint replacement loosening states that peri-implant osteolysis is primarily driven by chronic, low-grade inflammation caused by UHMWPE wear particles released from the bearing surfaces of TJRs or by wear of polymethyl methacrylate (PMMA, bone cement) that is sometimes used to fix the metal components of the joint replacement to the surrounding bone. Macrophages are considered as the key mediators of this wear debris-induced inflammation.4,5,7 Macrophages activated by UHMWPE or PMMA wear debris secrete chemokines and pro-inflammatory cytokines that lead to further macrophage recruitment, increased osteoclasto-genesis, and suppression of osteoblast formation and function.8–10 Together, these changes create a microenvironment that favors bone resorption over bone formation, thus ultimately leading to peri-implant osteolysis and implant loosening (Fig. 2). Although this sequence of events leading from the generation of wear debris to the formation of osteoclasts has been well characterized, the key question in the basic research of aseptic osteolysis has been how macrophages interact with wear debris and how exactly this interaction leads to macrophage activation and an inflamma-tory phenotype.
FIG. 2.
The “particle disease” theory in brief. Macrophages are activated by wear particles to produce chemokines and inflammatory mediators. Chemokines recruit additional monocytes into interface tissue, and inflammatory cytokines lead to increased ostoclastogenesis and bone resorption, primarily by increasing receptor activator of Nf-κB ligand (RANKL) and decresing osteoprotegeing (OPG) production from local fibroblasts and osteoblast. The exact mechanisms by which wear particles are recognized by macrophages and thus cause macrophage activation is still incompletely understood.
III. TOLL-LIKE RECEPTORS AND THE CONCEPT OF MACROPHAGE POLARIZATION
At least a partial answer to this important question is toll-like receptors (TLRs). TLRs are a family of pattern recognition receptors that recognize a broad range of molecules derived from bacteria, viruses, and fungi.11–13 The TLR ligands are evolutionary well-conserved structural components of these pathogens and display a repeating molecular structure; examples of typical TLR ligands include lipopolysaccharide (the primary cell wall constituent of Gram-negative bacteria), lipoteichoic acid (the primary cell wall constituent of Gram-positive bacteria) and single- and double-stranded RNA (the genome of RNA viruses).
In addition to the pathogen-derived molecules, or pathogen-associated molecular patterns (PAMPs), TLRs also recognize various host-derived ligands that are released from necrotic cells and fragmented extracellular matrix during times of tissue damage.13–16 Some examples of such endogenous TLR ligands include heat shock proteins, uric acid, high-mobility group box 1 (HMGB1), fibrinogen, and hyaluronan. Collectively these exo- and endogenous TLR ligands are known as danger-associated molecular patterns (DAMPs). The array of TLRs and other pattern recognition receptors enables the innate immune system to recognize and react to danger caused by pathogen invasion as well as sterile tissue damage, and mount an inflamma-tory response ultimately aimed to clear the tissue insult and initiate tissue regeneration17.
Signaling via some TLRs, especially TLR4, leads to macrophage activation into a pro-inflammatory phenotype.18,19 The phenomenon is known as classical macrophage activation or, in reference to the role that these types of macrophages play in T helper (Th) cell type 1 inflammatory response, M1 macrophage polarization.19–22 M1 macrophages are effector cells of the cell-mediated immunity; they are effective in antigen presentation and killing of phagocytosed pathogens via production of oxygen radicals, and produce inflammatory cytokines and chemokines. Additional signals that induce M1-like macrophage phenotypes include GM-CSF and the Th1 signature cytokine IFN-γ; indeed, the M1 macrophage activation induced by TLR4 signaling is thought to be due to autocrine and paracrine type 1 interferon signaling.19,23
In addition to this M1 macrophage polarization, macrophages can assume various other functional phenotypes collectively known as alternative macrophage activation.19,20,22,24 Macrophages exposed to the Th2 signature cytokines interleukin-4 (IL-4), IL-13, or to macrophage colony stimulating factor (M-CSF) assume an anti-inflammatory phenotype known as alternative macrophage activation or M2 polarization. These cells produce anti- rather than pro-inflammatory cytokines, and participate in tissue regeneration and angiogenesis by secreting various growth factors and extracellular matrix precursors.19,25 Indeed, the physiological switch from inflammation to tissue regeneration and healing is thought to be mediated in part by a local switch in macrophage phenotype from M1 to various forms of M2 macrophage activation.25,26
IV. TLRS IN WEAR DEBRIS RECOGNITION
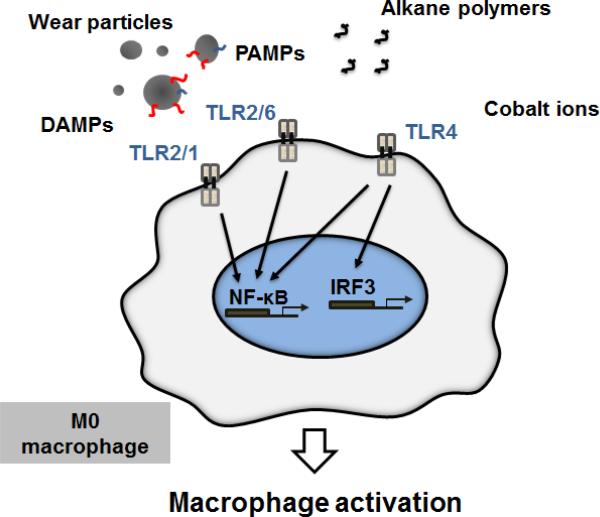
TLRs have been shown to play a critical role in the activation of macrophages and the innate immune system in general in the context of various infections and sterile tissue damage, but what is their relevance to aseptic osteolysis? In addition to recognizing exo- and endogenous danger signal molecules, there is some evidence that inorganic wear debris released from joint replacement implants can be recognized directly by certain TLRs (Fig. 3). Maitra et al. reported that alkane polymers released from UHMWPE and subsequently oxidized by interface tissue macrophages can directly bind to TLR2 and TLR2/1 dimers and activate proinflammatory signaling.27 Similarly, cobalt ions occasionally released in large amount form TJRs with metal-on-metal bearing surfaces can activate TLR4 signaling.28–30
FIG. 3.
Toll-like receptors (TLRs) in wear debris-induced macrophage activation. Wear particles accumulate and concentrate both exo- and endogenous danger signal molecules on their surfaces. These DAMPs and PAMPs are recognized primarily by TLR2/1, TLR2/6, and TLR4, which induce macrophage activation and production of inflammatory cytokines and chemokines by activating NF-κB and IRF3. Additionally, oxidized alkane polymers and cobalt ions can directly induce TLR signaling and macrophage activation.
In addition, various PAMPs can adhere firmly to UHMWPE wear particles, thus opsonizing them for recognition by TLRs.31–34 Potential PAMP sources include minor infections in the skin, gastrointestinal tract, and periodontal tissue from which bacterial structural components are released systemically to the circulation, eventually arriving to the peri-implant tissue.35–38 In addition, the presence of subclinical, low-grade, and biofilm-hidden bacterial growth on the implant surfaces is one possible source of wear debris opsonizing PAMPs that has also been extensively discussed in the literature.39–44 Strong support for the role of subclinical bacterial infection in pathogenesis of aseptic loosening comes from clinical observations that occurrence of aseptic TJR loosening is reduced by the combined use of antibiotic prophylaxis and antibiotic-loaded bone cement.45,46 The possibility that endogenous TLR ligands could also opsonize wear debris for TLR recognition is intriguing and currently largely unexplored.
Recently, Pearl et al. provided evidence that one or some combination of TLRs is involved in wear debris-induced inflammation and osteolysis using the mouse calvarial model.47 In mice deficient in the adaptor protein intermediary factor MyD88, which is required for the initiation of the intracellular signaling cascades of TLRs, PMMA particle-induced osteolysis was significantly reduced compared to wild-type mice. Similar results were obtained in an in vitro model in which macrophages derived from MyD88-deficient mice showed significantly reduced TNF-α production compared to macrophages derived from wild-type mice stimulated with PMMA particles. Thus, the results directly support the hypothesis that TLRs are involved in the recognition of PMMA debris; however, the results should be interpreted cautiously as MyD88 mediates signaling also from receptors other than TLRs.
Greenfield et al. demonstrated, using TLR2–/–, TLR4–/–, and TLR2–/–/TLR4–/– knockout mice and both in vitro and in vivo model systems, that titanium particle-induced inflammation and osteolysis were partially dependent on TLR2 and TLR4 but only if titanium particles were contaminated with TLR2 or TLR4 ligands. Inflammation and osteolysis caused by titanium particles without PAMPs developed similarly in both TLR knockout and wild-type mice, suggesting that TLR2 or TLR4 do not mediate recognition of PAMP-free titanium particles.48 Although titanium particle-induced inflammation and osteolysis was enhanced by PAMP binding to particles, pure titanium particles were enough to cause these reactions. The results of Pearl et al. and Greenfield et al. thus seem to lead to different conclusions about the role of TLRs in debris recognition; one possible explanation for this discrepancy between the studies is the different nature of wear particles (PMMA versus titanium) used in the experiments.
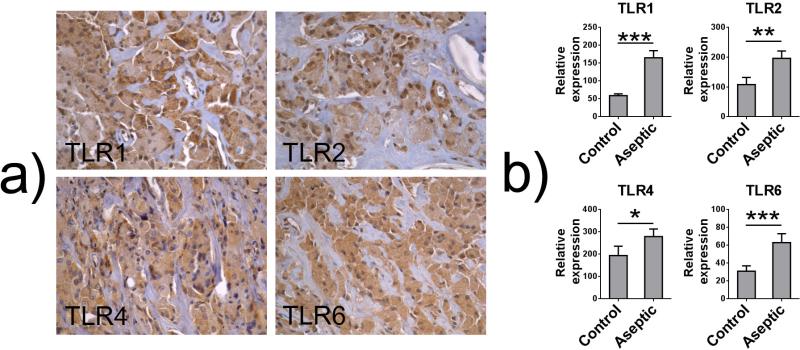
In animal model systems, wear debris has generally led to increased local expression of some TLRs although rapid downregulation of TLR system after intramedullary titanium particle injection has also been reported.49–51 Finally, retrieval studies investigating the interface tissue developing around aseptically loose TJRs have consistently shown that the interface tissue macrophages and foreign body giant cells express a spectrum of TLRs as evaluated by immunohistochemical stainings6,52–54 [Fig. 4(a)]. Furthermore, in qRT PCR analysis, the expression of all TLRs except TLR3 and TLR7 (that recognize virus-derived PAMPs) was significantly increased in aseptic interface tissue compared to osteoarthritic synovial tissue55 [Fig. 4(b)].
FIG. 4.
Toll-like receptors (TLRs) in aseptic interface tissue. (a) Interface tissue macrophages and foreign body giant cells express a spectrum of TLRs including TLR1, TLR2, TLR4, and TLR6 as evaluated by immunohistochemical staining. (b) The relative expression of several TLRs including TLR1, TLR2, TLR4, and TLR6 is significantly increased in aseptic interface tissue compared to osteoarthritic synovial tissue as evaluated by qRT PCR. (*) p < 0.05; (**) p < 0.01; (***) p < 0.001 as evaluated using Mann-Whitney U test. Data from Ref. 55.
V. MACROPHAGE POLARIZATION IN ASEPTIC LOOSENING
TLR signaling is one of the cues that induces M1 macrophage polarization. Thus, if TLR signaling is indeed involved in wear debris recognition, it is reasonable to assume that M1 macrophages are found in tissues surrounding aseptically loose TJRs. Although few retrieval studies have directly applied the relatively novel concept of macrophage polarization to the research of aseptic loosening, previous retrieval studies have consistently reported the production of M1-related mediators including iNOS, TNF-α, IL-1β IL-6, PGE-2, IL-8, CCL2, and CCL3 in the interface tissue.10,56–67 Of the few currently existing studies that have specifically aimed to characterize the macrophage polarization state in the interface tissue, Rao et al. found an increased proportion of M1 to M2 macrophages from aseptic interface tissue compared to controls and concluded that M1 activation predominates in the aseptic interface tissue.68 However, Koulouvaris et al. found increased expression of M2-related markers and arrived at the opposite conclusion.69
To shed further light on this currently somewhat controversial matter, we recently performed genome-wide expression profiling of the interface tissue surrounding tissues from loose revised implants using microarray technology. In an attempt to determine the macrophage phenotype in the interface tissue, this genome-wide expression profile of the peri-implant tissue was compared to the expression profile of cultured M1 and M2 macrophages. Several M1-related genes such as STAT1, CCL2, CCL3, IL-8, and CD86 were found to be upregulated and several M2-associated genes such as mannose receptor (CD206) and CCL16 were downregulated in the interface tissue (manuscript in preparation).
Taken together, these reports would seem to support the hypothesis that M1 macrophage activation predominates in the peri-implant tissues, thus providing further support to the role of TLRs in wear debris-induced macrophage activation. However, TLR signaling is not the only cue that can induce M1 polarization and in fact IFN-γ and GM-CSF are commonly used in vitro to generate M1 macrophages. A retrieval study by Jämsen et al. profiled the expression of macrophage activating and polarizing cytokines from peri-implant tissues and osteoarthritic control tissues using qRT PCR and immunohistochemistry.70 Although high expression of several chemokines and osteoclast-related products was found in the peri-implant tissues, no significant production of macrophage polarizing cytokines IFN-γ, GM-CSF, IL-4, IL-13, or IL-17 could be detected. It thus seems that other macrophage activating and polarizing signals, such as wear particles per se or DAMPs adhering to particles surfaces, rather than classical macrophage polarizing cytokines are responsible for the peri-implant tissue macrophage phenotype.
VI. METAL WEAR AND THE ROLE OF ADAPTIVE IMMUNE SYSTEM
Adverse local tissue response caused by various metals released from total joint replacements forms a potentially interesting subclass of aseptic loosening and biomaterial-induced inflammation. This adverse host response is clinically and histopathologically distinct from the one caused by UHMWPE or PMMA wear particles, and is characterized by large osteolytic areas as well as formation of cystic or solid tissue masses commonly known as pseudotumours. Peri-implant histopathology is characterized by large areas of necrosis and mononuclear cell infiltrates consisting of not only macrophages but also of various lymphocyte sub-populations ranging from T and B lymphocytes to mature plasma cells.71–74 Peri-vascular T lymphocyte infiltrates, or aseptic lymphocyte-dominated vasculitis-associated lesions (ALVALs), have been suggested to be typical for the condition, although recent reports challenge this assumption.75–77 Occasionally extensive peri-implant osteolysis or pseudotumour formation is not seen but the patient still presents with continuous pain in the implant area accompanied by synovitis and accumulation of lymphocytes in the peri-implant tissues.74
These types of local adverse reactions have been attributed to the release of large amounts of nanosized metal particles and metal ions from the implant, specifically cobalt and chromium, with smaller amounts of other metals such as nickel. Considerable metal release is a particularly common problem for total joint replacements with metal-on-metal (MoM) bearings or modular components and occurs due to the combined effect of mechanical wear of articulating surfaces and subsequent corrosion of nanosized wear particles as well as scratched implant surfaces. Indeed, highly elevated cobalt and chromium ion levels can be measured around MoM implants and even from the circulation of MoM implant recipients, and the poor clinical performance of these implants has been attributed to the considerable metal release that can complicate these types of implants.78,79
For a time it has been recognized that larger, about 1μm sized, metal particles evoke inflammatory responses from macrophages presumably via similar mechanisms that have been described for UHMWPE and PMMA wear particles of similar sizes.80–82 The mechanisms by which smaller metal nanoparticles and ions lead to local adverse host response are, however, relatively poorly understood. In vitro cobalt and chromium ions are geno- and cytotoxic at the clinically relevant concentrations, possibly explaining the wide areas of necrosis that are associated to metal release.83–85 Metal ions also suppress osteoblast function and activate endothelium to recruit mononuclear cells.86–88 Following the seminal discovery that nickel ions activate TLR4 signaling, it was recently demonstrated that cobalt ions can also induce TLR4 signaling by binding to and cross-linking the receptor protein, thus providing a direct mechanism by which also cobalt ions could activate macrophages and other cells directly.28–30
In contrast to the adverse host reaction associated with UHMWPE and PMMA wear that is considered to be mediated solely by the innate immunity,89,90 it has been long speculated that adaptive immune response could play a role in the adverse tissue response to implant-derived metal debris. This assumption is based on the observation of lymphocyte subpopulations in the peri-implant tissues surrounding MoM implants and on the well-characterized ability of metal ions to activate adaptive immunity by acting as haptens with host proteins.91 The best characterized example of this type IV hypersensitivity reaction is dermal allergy to nickel.92 Despite the fact that a similar dermal allergy can develop against cobalt and chromium, the extent to which type IV hypersensitivity contributes to the reaction against implant-derived metals is still somewhat controversial.93.
The activation of the adaptive immune system is a complex process that is initiated by activation of dendritic cells via recognition of a danger signal molecule by TLRs.94 This recognition of danger signal is followed by internalization of the antigen and migration of dendritic cell to local lymphatic tissue. If the initial activation of the dendritic cell was sufficient, the antigen is presented to the lymphocyte population with a repertoire of co-stimulatory molecules and the lymphocytes that recognize the foreign antigen with their T-cell receptor become activated. These cells clonally expand, migrate to the inflamed tissue, and regulate the functions of such cells as macrophages by secreting, e.g., IFN-γ and support M1 polarization.
It seems unlikely that UHMWPE or PMMA wear debris, even though sufficient to activate innate immunity either directly or indirectly by accumulation of PAMPs and DAMPs on particles surfaces, could activate adaptive immunity mainly due to lack of foreign antigen that could be presented to the lymphocyte population. In contrast, the metal ions have, at least theoretically, potential to activating both innate and adaptive immunity by providing both the necessary danger signal to initiate innate immune activation, and the foreign antigen to activate adaptive immunity by forming haptens with host proteins. It however seems likely, however, that the tendency to react against metal haptens is determined by individual hereditary factors, with some patients being genetically more susceptible to developing type IV hypersensitivity against metals than others. This might be the reason for the varied reactions caused by metal wear, with some patients developing symptoms and lymphocyte-dominated synovitis with relatively minor metal wear while others present with extensive peri-implant osteolysis, necrosis, and pseudotumors with more considerable metal wear.
Finally, in contrast to aseptic loosening with or without metal wear, there probably is little question that the adaptive immune system is involved to the host response against bacterial growth in fulminant septic TJR loosening. Indeed, it would seem intuitive that bacterial infection of implant components does not lack danger signals that can stimulate innate immunity and plentitude of foreign antigens that can activate adaptive immunity. Indeed, the peri-implant tissue of septic loosening is typically infiltrated by various lymphocyte subpopulations.6 It has even been suggested that in addition to presence of neutrophils in the peri-implant tissues, lymphocyte subpopulations, especially B and plasma cells, might serve as useful diagnostic markers of implant-related infection.6,55 Although this hypothesis is attractive, the currently existing clinical studies have not found additional benefit of analyzing lymphocyte subpopulations in the diagnosis of implant-related infection to that achieved with analyzing neutrophils.95–97 The current literature in the field is however limited and further studies on the matter are warranted.
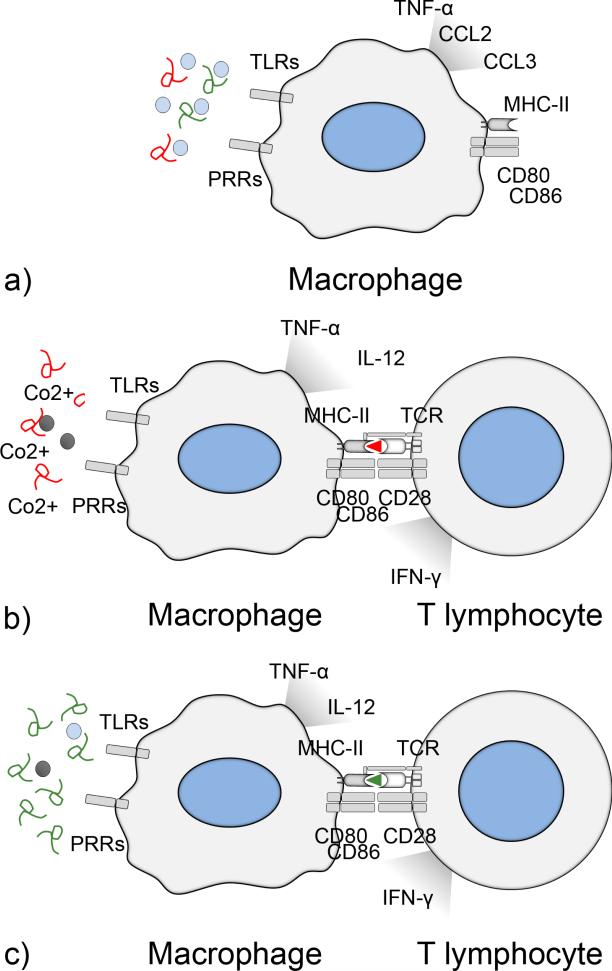
Taken together, it would seem that the local adverse tissue reaction against total joint replacement wear by-products is a spectrum of conditions that share considerable mechanistic overlap but might be distinguished by their ability to activate adaptive immune responses (Fig. 5); aseptic loosening due to UHMWPE or PMMA wear is mainly mediated by the innate immune system reacting against the polymers themselves or DAMPs sticking to polymer surfaces but lacks presentable antigen; byproducts of metal wear have the ability to stimulate both arms of the immune systems at least in genetically susceptible individuals, while the fulminant septic loosening is characterized by activation of both innate and adaptive immune systems
FIG. 5.
Adaptive immune system in aseptic and septic loosening. (a) UHMWPE and PMMA wear particles accumulate PAMPs and DAMPs on their surfaces and are sufficient to activate macrophages via TLRs and other pattern recognition receptors (PRRs), leading to production of pro-inflammatory cytokines and chemokines ultimately leading to aseptic osteolysis. Due to the lack of presentable antigen, the adaptive immune system is not activated and lymphocyte subpopulations are rare in the peri-implant tissues. (b) In the case of metal wear, cobalt ions can activate macrophages directly via TLR activation and indirectly by inducing cell necrosis and release of large amounts of DAMPs. In addition, at least in genetically susceptible individuals, cobalt ions can activate adaptive immune system by forming haptens with host proteins. T helper type 1 lymphocytes enhance macrophages inflammatory responses by secreting interferon-γ that induce M1 polarization. As a marker of this lymphocyte activation, various lymphocyte subpopulations are characteristic for the adverse host reaction against metals. (c) In fulminant septic loosening, dividing bacteria provide both the danger signal and a plentitude of various antigens to activate the adaptive immune system.
VII. CONCLUSIONS
Septic and aseptic loosening of TJRs have traditionally been considered as two distinct and well-defined conditions with the former being caused by implant infection and the latter by implant-derived biomaterial wear debris. However, recent evidence indicates that in addition to mediating bacterial recognition and the subsequent inflammatory reaction, TLRs and their ligands play a key role in wear debris-induced inflammation and macrophage activation. It thus seems that these two conditions share similar underlying pathomechanisms and that the strict dichotomy between sterile aseptic and bacterial septic implant loosening is somewhat questionable. Indeed, it can be speculated that rather than being two, well-defined clinical entities, peri-implant osteolysis is, in fact, a spectrum of conditions in which the specific clinical picture is determined by complex interactions of multiple factors including type and load of wear debris, PAMP accumulation, extent of bacterial biofilm formation, DAMPs associated with tissue destruction, the extent to which adaptive immune system is activated, and individual hereditary factors; “pure” UHMWPE and PMMA wear debris-induced aseptic loosening and fulminant septic loosening represent the ends of the spectrum, while adverse local host response against metals falls somewhere in between having characteristics of both conditions. In addition to these factors, is has been reported that macrophage phenotype is an important factor that regulates the mode in which macrophages respond to wear debris; in M1 macrophages the inflamma-tory response to wear debris is enhanced and in M2 macrophages it is effectively suppressed.98–101 These observations highlight the fact that the properties of the local and systemic cytokine environment, which in turn determines the balance of M1 versus M2 macrophage polarization, might be additional factors that regulate the local biological reaction to wear debris from joint replacements.
ACKNOWLEDGMENTS
This work has been funded in part by NIH Grants No. 2R01AR055650-05 and No. 1R01AR063717, the Ellenburg Chair in Surgery at Stanford University, The Stanford Medical Scholars Program, and the Jane and Aatos Erkko Foundation.
REFERENCES
- 1.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–54. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 2.Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19:349–56. doi: 10.1097/01.qco.0000235161.85925.e8. [DOI] [PubMed] [Google Scholar]
- 3.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–63. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 4.Gallo J, Goodman SB, Konttinen YT, Raska M. Particle disease: biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013;19:213–24. doi: 10.1177/1753425912451779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman SB, Gibon E, Yao Z. The basic science of periprosthetic osteolysis. Instr Course Lect. 2013;62:201–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Pajarinen J, Cenni E, Savarino L, Gomez-Barrena E, Tamaki Y, Takagi M, Salo J, Konttinen YT. Profile of toll-like receptor-positive cells in septic and aseptic loosening of total hip arthroplasty implants. J Biomed Mater Res A. 2010;94:84–92. doi: 10.1002/jbm.a.32674. [DOI] [PubMed] [Google Scholar]
- 7.Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251–61. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- 8.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–86. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Nich C, Takakubo Y, Pajarinen J, Ainola M, Salem A, Sillat T, Rao AJ, Raska M, Tamaki Y, Takagi M, Konttinen YT, Goodman SB, Gallo J. Macrophages-Key cells in the response to wear debris from joint replacements. J Biomed Mater Res A. 2013;101:3033–45. doi: 10.1002/jbm.a.34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman SB, Ma T. Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials. 2010;31:5045–50. doi: 10.1016/j.biomaterials.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–5. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 15.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 18.Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–24. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 21.Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen M, Ma GF, Konttinen YT. Regulation of macrophage activation. Cell Mol Life Sci. 2003;60:2334–46. doi: 10.1007/s00018-003-3020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–61. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 24.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 25.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37.. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 229:176–85. doi: 10.1002/path.4133. 201. [DOI] [PubMed] [Google Scholar]
- 27.Maitra R, Clement CC, Crisi GM, Cobelli N, Santambrogio L. Immunogenecity of modified alkane polymers is mediated through TLR1/2 activation. PLoS One. 2008;3:e2438. doi: 10.1371/journal.pone.0002438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyson-Capper AJ, Lawrence H, Holland JP, Deehan DJ, Kirby JA. Metal-on-metal hips: cobalt can induce an endotoxin-like response. Ann Rheum Dis. 2013;72:460–1. doi: 10.1136/annrheumdis-2012-202468. [DOI] [PubMed] [Google Scholar]
- 29.Rachmawati D, Bontkes HJ, Verstege MI, Muris J, von Blomberg BM, Scheper RJ, van Hoogstraten IM. Transition metal sensing by Toll-like receptor-4: next to nickel, cobalt and palladium are potent human dendritic cell stimulators. Contact Dermatitis. 2013;68:331–8. doi: 10.1111/cod.12042. [DOI] [PubMed] [Google Scholar]
- 30.Konttinen YT, Pajarinen J. Adverse reactions to metal-on-metal implants. Nat Rev Rheumatol. 2013;9:5–6. doi: 10.1038/nrrheum.2012.218. [DOI] [PubMed] [Google Scholar]
- 31.Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001;16:2082–91. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- 32.Bi Y, Collier TO, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin mediates biological responses of titanium particles without stimulating their phagocytosis. J Orthop Res. 2002;20:696–703. doi: 10.1016/S0736-0266(01)00176-0. [DOI] [PubMed] [Google Scholar]
- 33.Brooks RA, Wimhurst JA, Rushton N. Endotoxin contamination of particles produces misleading inflammatory cytokine responses from macrophages in vitro. J Bone Joint Surg Br. 2002;84:295–9. doi: 10.1302/0301-620x.84b2.12061. [DOI] [PubMed] [Google Scholar]
- 34.Cho DR, Shanbhag AS, Hong CY, Baran GR, Goldring SR. The role of adsorbed endotoxin in particle-induced stimulation of cytokine release. J Orthop Res. 2002;20:704–13. doi: 10.1016/S0736-0266(01)00179-6. [DOI] [PubMed] [Google Scholar]
- 35.Greenfield EM, Bi Y, Ragab AA, Goldberg VM, Nalepka JL, Seabold JM. Does endotoxin contribute to aseptic loosening of orthopedic implants? J Biomed Mater Res B Appl Biomater. 2005;72:179–85. doi: 10.1002/jbm.b.30150. [DOI] [PubMed] [Google Scholar]
- 36.Nalepka JL, Lee MJ, Kraay MJ, Marcus RE, Goldberg VM, Chen X, Greenfield EM. Lipopolysaccharide found in aseptic loosening of patients with inflammatory arthritis. Clin Orthop Relat Res. 2006;451:229–35. doi: 10.1097/01.blo.0000224050.94248.38. [DOI] [PubMed] [Google Scholar]
- 37.Xing Z, Pabst MJ, Hasty KA, Smith RA. Accumulation of LPS by polyethylene particles decreases bone attachment to implants. J Orthop Res. 2006;24:959–66. doi: 10.1002/jor.20038. [DOI] [PubMed] [Google Scholar]
- 38.Tatro JM, Taki N, Islam AS, Goldberg VM, Rimnac CM, Doerschuk CM, Stewart MC, Greenfield EM. The balance between endotoxin accumulation and clearance during particle-induced osteolysis in murine calvaria. J Orthop Res. 2007;25:361–9. doi: 10.1002/jor.20289. [DOI] [PubMed] [Google Scholar]
- 39.Tunney MM, Patrick S, Gorman SP, Nixon JR, Anderson N, Davis RI, Hanna D, Ramage G. Improved detection of infection in hip replacements: a currently underestimated problem. J Bone Joint Surg Br. 1998;80:568–72. doi: 10.1302/0301-620x.80b4.8473. [DOI] [PubMed] [Google Scholar]
- 40.Clarke MT, Roberts CP, Lee PT, Gray J, Keene GS, Rushton N. Polymerase chain reaction can detect bacterial DNA in aseptically loose total hip arthroplasties. Clin Orthop Relat Res. 2004;(427):132–7. doi: 10.1097/01.blo.0000136839.90734.b7. [DOI] [PubMed] [Google Scholar]
- 41.Nelson CL, McLaren AC, McLaren SG, Johnson JW, Smeltzer MS. Is aseptic loosening truly aseptic? Clin Orthop Relat Res. 2005;(437):25–30. doi: 10.1097/01.blo.0000175715.68624.3d. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi N, Procop GW, Krebs V, Kobayashi H, Bauer TW. Molecular identification of bacteria from aseptically loose implants. Clin Orthop Relat Res. 2008;466:1716–25. doi: 10.1007/s11999-008-0263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47:1403–9. doi: 10.1086/592973. [DOI] [PubMed] [Google Scholar]
- 44.Sierra JM, García S, Martínez-Pastor JC, Tomás X, Gallart X, Vila J, Bori G, Maculé F, Mensa J, Riba J, Soriano A. Relationship between the degree of osteolysis and cultures obtained by sonication of the prostheses in patients with aseptic loosening of a hip or knee arthroplasty. Arch Orthop Trauma Surg. 2011;131:1357–61. doi: 10.1007/s00402-011-1307-4. [DOI] [PubMed] [Google Scholar]
- 45.Espehaug B, Engesaeter LB, Vollset SE, Havelin LI, Langeland N. Antibiotic prophylaxis in total hip arthroplasty. Review of 10,905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg Br. 1997;79:590–5. doi: 10.1302/0301-620x.79b4.7420. [DOI] [PubMed] [Google Scholar]
- 46.Engesaeter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74:644–51. doi: 10.1080/00016470310018135. [DOI] [PubMed] [Google Scholar]
- 47.Pearl JI, Ma T, Irani AR, Huang Z, Robinson WH, Smith RL, Goodman SB. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials. 2011;32:5535–42. doi: 10.1016/j.biomaterials.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenfield EM, Beidelschies MA, Tatro JM, Goldberg VM, Hise AG. Bacterial pathogen-associated molecular patterns stimulate biological activity of orthopaedic wear particles by activating cognate Toll-like receptors. J Biol Chem. 2010;285:32378–84. doi: 10.1074/jbc.M110.136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pajarinen J, Mackiewicz Z, Pöllänen R, Takagi M, Epstein NJ, Ma T, Goodman SB, Konttinen YT. Titanium particles modulate expression of Toll-like receptor proteins. J Biomed Mater Res A. 2010;92:1528–37. doi: 10.1002/jbm.a.32495. [DOI] [PubMed] [Google Scholar]
- 50.Valladares RD, Nich C, Zwingenberger S, Li C, Swank KR, Gibon E, Rao AJ, Yao Z, Goodman SB. Toll-like receptors-2 and 4 are overexpressed in an experimental model of particle-induced osteolysis. J Biomed Mater Res A. 2014;102:3004–11. doi: 10.1002/jbm.a.34972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulus AC, Frenzel J, Ficklscherer A, Roßbach BP, Melcher C, Jansson V, Utzschneider S. Polyethylene wear particles induce TLR 2 upregulation in the synovial layer of mice. J Mater Sci Mater Med. 2014;25:507–13. doi: 10.1007/s10856-013-5095-y. [DOI] [PubMed] [Google Scholar]
- 52.Takagi M, Tamaki Y, Hasegawa H, Takakubo Y, Konttinen L, Tiainen VM, Lappalainen R, Konttinen YT, Salo J. Toll-like receptors in the interface membrane around loosening total hip replacement implants. J Biomed Mater Res A. 2007;81:1017–26. doi: 10.1002/jbm.a.31235. [DOI] [PubMed] [Google Scholar]
- 53.Lähdeoja T, Pajarinen J, Kouri VP, Sillat T, Salo J, Konttinen YT. Toll-like receptors and aseptic loosening of hip endoprosthesis-a potential to respond against danger signals? J Orthop Res. 2010;28:184–90.. doi: 10.1002/jor.20979. [DOI] [PubMed] [Google Scholar]
- 54.Tamaki Y, Takakubo Y, Goto K, Hirayama T, Sasaki K, Konttinen YT, Goodman SB, Takagi M. Increased expression of toll-like receptors in aseptic loose periprosthetic tissues and septic synovial membranes around total hip implants. J Rheumatol. 2009;36:598–608. doi: 10.3899/jrheum.080390. [DOI] [PubMed] [Google Scholar]
- 55.Pajarinen J. Toll-like receptors and macrophage polarization in the loosening of total hip replacements [dissertation] University of Helsinki; Helsinki: 2012. [cited n n n]. Available from: http://urn.fi/ URN:ISBN:978-952-9657-66-7. [Google Scholar]
- 56.Kim KJ, Rubash HE, Wilson SC, D'Antonio JA, McClain EJ. A histologic and biochemical comparison of the interface tissues in cementless and cemented hip prostheses. Clin Orthop Relat Res. 1993;(287):142–52.. [PubMed] [Google Scholar]
- 57.Chiba J, Rubash HE, Kim KJ, Iwaki Y. The characterization of cytokines in the interface tissue obtained from failed cementless total hip arthroplasty with and without femoral osteolysis. Clin Orthop Relat Res. 1994 Mar;(300):304–12. [PubMed] [Google Scholar]
- 58.Xu JW, Konttinen YT, Lassus J, Natah S, Ceponis A, Solovieva S, Aspenberg P, Santavirta S. Tumor necrosis factor-alpha (TNF-alpha) in loosening of total hip replacement (THR). Clin Exp Rheumatol. 1996;14:643–8. [PubMed] [Google Scholar]
- 59.Hukkanen M, Corbett SA, Batten J, Konttinen YT, McCarthy ID, Maclouf J, Santavirta S, Hughes SP, Polak JM. Aseptic loosening of total hip replacement. Macrophage expression of inducible nitric oxide synthase and cyclo-oxygenase-2, together with peroxynitrite formation, as a possible mechanism for early prosthesis failure. J Bone Joint Surg Br. 1997;79:467–74. doi: 10.1302/0301-620x.79b3.7469. [DOI] [PubMed] [Google Scholar]
- 60.Ishiguro N, Kojima T, Ito T, Saga S, Anma H, Kurokouchi K, Iwahori Y, Iwase T, Iwata H. Macrophage activation and migration in interface tissue around loosening total hip arthroplasty components. J Biomed Mater Res. 1997;35:399–406. doi: 10.1002/(sici)1097-4636(19970605)35:3<399::aid-jbm14>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 61.Xu JW, Konttinen YT, Waris V, Pätiälä H, Sorsa T, Santavirta S. Macrophage-colony stimulating factor (M-CSF) is increased in the synovial-like membrane of the periprosthetic tissues in the aseptic loosening of total hip replacement (THR). Clin Rheumatol. 1997;16:243–8. doi: 10.1007/BF02238958. [DOI] [PubMed] [Google Scholar]
- 62.Watkins SC, Macaulay W, Turner D, Kang R, Rubash HE, Evans CH. Identification of inducible nitric oxide synthase in human macrophages surrounding loosened hip prostheses. Am J Pathol. 1997;150:1199–206. [PMC free article] [PubMed] [Google Scholar]
- 63.Lassus J, Waris V, Xu JW, Li TF, Hao J, Nietosvaara Y, Santavirta S, Konttinen YT. Increased interleukin-8 (IL-8) expression is related to aseptic loosening of total hip replacement. Arch Orthop Trauma Surg. 2000;120:328–32. doi: 10.1007/s004020050475. [DOI] [PubMed] [Google Scholar]
- 64.Stea S, Visentin M, Granchi D, Ciapetti G, Donati ME, Sudanese A, Zanotti C, Toni A. Cytokines and osteolysis around total hip prostheses. Cytokine. 2000;12:1575–9. doi: 10.1006/cyto.2000.0753. [DOI] [PubMed] [Google Scholar]
- 65.Konttinen YT, Xu JW, Waris E, Li TF, Gómez-Barrena E, Nordsletten L, Santavirta S. Interleukin-6 in aseptic loosening of total hip replacement prostheses. Clin Exp Rheumatol. 2002;20:485–90. [PubMed] [Google Scholar]
- 66.Suh KT, Chang JW, Jung JS. The role of inducible nitric oxide synthase in aseptic loosening after total hip arthroplasty. J Bone Joint Surg Br. 2002;84:753–7. doi: 10.1302/0301-620x.84b5.12314. [DOI] [PubMed] [Google Scholar]
- 67.Wang CT, Lin YT, Chiang BL, Lee SS, Hou SM. Over-expression of receptor activator of nuclear factor-kappaB ligand (RANKL), inflammatory cytokines, and chemokines in periprosthetic osteolysis of loosened total hip arthroplasty. Biomaterials. 2010;31:77–82. doi: 10.1016/j.biomaterials.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 68.Rao AJ, Gibon E, Ma T, Yao Z, Smith RL, Goodman SB. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012;8:2815–23. doi: 10.1016/j.actbio.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koulouvaris P, Ly K, Ivashkiv LB, Bostrom MP, Nestor BJ, Sculco TP, Purdue PE. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. J Orthop Res. 2008;26:106–16. doi: 10.1002/jor.20486. [DOI] [PubMed] [Google Scholar]
- 70.Jämsen E, Kouri VP, Olkkonen J, Cör A, Goodman SB, Konttinen YT, Pajarinen J. Characterization of macrophage polarizing cytokines in the aseptic loosening of total hip replacements. J Orthop Res. 2014;32:1241–6. doi: 10.1002/jor.22658. [DOI] [PubMed] [Google Scholar]
- 71.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 72.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 73.Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine-year follow-up. J Bone Joint Surg Am. 2006;88:1183–91. doi: 10.2106/JBJS.D.02916. [DOI] [PubMed] [Google Scholar]
- 74.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321–7. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujishiro T, Moojen DJ, Kobayashi N, Dhert WJ, Bauer TW. Perivascular and diffuse lymphocytic inflammation are not specific for failed metal-on-metal hip implants. Clin Orthop Relat Res. 2011;469:1127–33. doi: 10.1007/s11999-010-1649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ng VY, Lombardi AV, Jr, Berend KR, Skeels MD, Adams JB. Perivascular lymphocytic infiltration is not limited to metal-on-metal bearings. Clin Orthop Relat Res. 2011;469:523–9. doi: 10.1007/s11999-010-1570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watters TS, Cardona DM, Menon KS, Vinson EN, Bolognesi MP, Dodd LG. Aseptic lymphocyte-dominated vasculitis-associated lesion: a clinicopathologic review of an underrecognized cause of prosthetic failure. Am J Clin Pathol. 2010;134:886–93. doi: 10.1309/AJCPLTNEUAH8XI4W. [DOI] [PubMed] [Google Scholar]
- 78.De Smet K, De Haan R, Calistri A, Campbell PA, Ebramzadeh E, Pattyn C, Gill HS. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Joint Surg Am. 2008;90(Suppl 4):202–8. doi: 10.2106/JBJS.H.00672. [DOI] [PubMed] [Google Scholar]
- 79.Hart AJ, Sabah SA, Bandi AS, Maggiore P, Tarassoli P, Sampson B, A Skinner J. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J Bone Joint Surg Br. 2011;93:1308–13. doi: 10.1302/0301-620X.93B10.26249. [DOI] [PubMed] [Google Scholar]
- 80.Nakashima Y, Sun DH, Trindade MC, Maloney WJ, Goodman SB, Schurman DJ, Smith RL. Signaling pathways for tumor necrosis factor-alpha and inter-leukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. J Bone Joint Surg Am. 1999;81:603–15. doi: 10.2106/00004623-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Taki N, Tatro JM, Nalepka JL, Togawa D, Goldberg VM, Rimnac CM, Greenfield EM. Polyethylene and titanium particles induce osteolysis by similar, lymphocyte-independent, mechanisms. J Orthop Res. 2005;23:376–83. doi: 10.1016/j.orthres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 82.Caicedo MS, Desai R, McAllister K, Reddy A, Jacobs JJ, Hallab NJ. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: a novel mechanism for implant debris reactivity. J Orthop Res. 2009;27:847–54. doi: 10.1002/jor.20826. [DOI] [PubMed] [Google Scholar]
- 83.Catelas I, Petit A, Vali H, Fragiskatos C, Meilleur R, Zukor DJ, Antoniou J, Huk OL. Quantitative analysis of macrophage apoptosis vs. necrosis induced by cobalt and chromium ions in vitro. Biomaterials. 2005;26:2441–53. doi: 10.1016/j.biomaterials.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 84.Billi F, Campbell P. Nanotoxicology of metal wear particles in total joint arthroplasty: a review of current concepts. J Appl Biomater Biomech. 2010;8:1–6. [PubMed] [Google Scholar]
- 85.Gill HS, Grammatopoulos G, Adshead S, Tsialogiannis E, Tsiridis E. Molecular and immune toxicity of CoCr nanoparticles in MoM hip arthroplasty. Trends Mol Med. 2012;18:145–55. doi: 10.1016/j.molmed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 86.Andrews RE, Shah KM, Wilkinson JM, Gartland A. Effects of cobalt and chromium ions at clinically equivalent concentrations after metal-on-metal hip replacement on human osteoblasts and osteoclasts: implications for skeletal health. Bone. 2011;49:717–23. doi: 10.1016/j.bone.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 87.Zijlstra WP, Bulstra SK, van Raay JJ, van Leeuwen BM, Kuijer R. Cobalt and chromium ions reduce human osteoblast-like cell activity in vitro, reduce the OPG to RANKL ratio, and induce oxidative stress. J Orthop Res. 2012;30:740–7. doi: 10.1002/jor.21581. [DOI] [PubMed] [Google Scholar]
- 88.Ninomiya JT, Kuzma SA, Schnettler TJ, Krolikowski JG, Struve JA, Weihrauch D. Metal ions activate vascular endothelial cells and increase lymphocyte chemotaxis and binding. J Orthop Res. 2013;31:1484–91. doi: 10.1002/jor.22377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007;28:5044–8. doi: 10.1016/j.biomaterials.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiranek W, Jasty M, Wang JT, Bragdon C, Wolfe H, Goldberg M, Harris W. Tissue response to particulate polymethylmethacrylate in mice with various immune deficiencies. J Bone Joint Surg Am. 1995;77:1650–61. doi: 10.2106/00004623-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, Dai S. Structural basis of metal hypersensitivity. Immunol Res. 2013;55:83–90. doi: 10.1007/s12026-012-8351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt M, Goebeler M. Nickel allergies: paying the Toll for innate immunity. J Mol Med (Berl) 2011;89:961–70. doi: 10.1007/s00109-011-0780-0. [DOI] [PubMed] [Google Scholar]
- 93.Granchi D, Cenni E, Giunti A, Baldini N. Metal hypersensitivity testing in patients undergoing joint replacement: a systematic review. J Bone Joint Surg Br. 2012;94:1126–34. doi: 10.1302/0301-620X.94B8.28135. [DOI] [PubMed] [Google Scholar]
- 94.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 95.Pandey R, Drakoulakis E, Athanasou NA. An assessment of the histological criteria used to diagnose infection in hip revision arthroplasty tissues. J Clin Pathol. 1999;52:118–23. doi: 10.1136/jcp.52.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Athanasou NA, Pandey R, de Steiger R, Crook D, Smith PM. Diagnosis of infection by frozen section during revision arthroplasty. J Bone Joint Surg Br. 1995;77:28–33. [PubMed] [Google Scholar]
- 97.Niki Y, Matsumoto H, Otani T, Yatabe T, Funayama A, Maeno S, Tomatsu T, Toyama Y. Phenotypic characteristics of joint fluid cells from patients with continuous joint effusion after total knee arthroplasty. Biomaterials. 2006;27:1558–65. doi: 10.1016/j.biomaterials.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 98.Trindade MC, Lind M, Goodman SB, Maloney WJ, Schurman DJ, Smith RL. Interferon-gamma exacerbates polymethylmethacrylate particle-induced interleukin-6 release by human monocyte/macrophages in vitro. J Biomed Mater Res. 1999;47:1–7. doi: 10.1002/(sici)1097-4636(199910)47:1<1::aid-jbm1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 99.Trindade MC, Nakashima Y, Lind M, Sun DH, Goodman SB, Maloney WJ, Schurman DJ, Smith RL. Interleukin-4 inhibits granulocyte-macrophage colony-stimulating factor, interleukin-6, and tumor necrosis factor-alpha expression by human monocytes in response to polymethylmethacrylate particle challenge in vitro. J Orthop Res. 1999;17:797–802. doi: 10.1002/jor.1100170602. [DOI] [PubMed] [Google Scholar]
- 100.Pajarinen J, Kouri VP, Jämsen E, Li TF, Mandelin J, Konttinen YT. The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomater. 2013;9:9229–40. doi: 10.1016/j.actbio.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 101.Rao AJ, Nich C, Dhulipala LS, Gibon E, Valladares R, Zwingenberger S, Smith RL, Goodman SB. Local effect of IL-4 delivery on polyethylene particle induced osteolysis in the murine calvarium. J Biomed Mater Res A. 2013;101:1926–34. doi: 10.1002/jbm.a.34486. [DOI] [PMC free article] [PubMed] [Google Scholar]