Abstract
Genetic predisposition and environmental challenges interact to determine individual vulnerability to obesity and type 2 diabetes. We previously established a mouse model of chronic subordination stress-induced hyperphagia, obesity, metabolic like-syndrome and insulin resistance in the presence of a high-fat diet. However, it remains to be established if social stress could also aggravate glucose intolerance in subjects genetically predisposed to develop obesity and type 2 diabetes. To answer this question, we subjected genetically obese mice due to deficiency of the leptin receptor (db/db strain) to chronic subordination stress. Over five weeks, subordination stress in db/db mice led to persistent hyperphagia, hyperglycemia and exacerbated glucose intolerance altogether suggestive of an aggravated disorder when compared to controls. On the contrary, body weight and fat mass were similarly affected in stressed and control mice likely due to the hyperactivity shown by subordinate mice. Stressed db/db mice also showed increased plasma inflammatory markers. Altogether our results suggest that chronic stress can aggravate glucose intolerance but not obesity in genetically predisposed subjects on the basis of a disrupted leptin circuitry.
Keywords: Social defeat, Subordination, Corticosterone, Metabolic function
Introduction
In the past 20 years, obesity has reached epidemic proportions. The pervasiveness of obesity largely explains the dramatic increase of type 2 diabetes (T2D) and associated life-threatening complications (WHO 2006; Eckel et al. 2011). However, the mechanisms of obesity-related comorbidities remain unclear, as does our understanding of individual susceptibility. While the majority of individuals with obesity develop insulin resistance, T2D, dyslipidemia, gout, hypertension and cardiovascular disease, approximately 30 % of obese individuals are metabolically healthy most likely due to preserved insulin sensitivity (Blüher 2012).
Leptin is a 16-kDa protein mainly produced and secreted by white adipose tissue with the crucial role of informing various brain centers via leptin receptors about the amount of body fat (Zhang et al. 1994). Leptin deficiency can be caused by mutations in the leptin gene as in rare autosomal recessive disease associated with marked obesity and impaired gonadal function (Montague et al. 1997; Strobel et al. 1998; Farooqi et al. 1999). In contrast to congenital leptin deficiency, the majority of obese individuals have higher leptin concentrations than lean individuals and are leptin resistant (Considine et al. 1996a; Moon et al. 2011). Hyperleptinemia in humans can be due to mutations at highly conserved positions of the extracellular domain of the leptin receptor gene (Chung et al. 1997; Francke et al. 1997; Thompson et al. 1997; Clément et al. 1998). In 1966, Hummel et al. described a spontaneous mutation in a C57BL/Ks mouse colony that provoked hyperphagia, decreased energy expenditure and obesity, fasting hyperglycemia increasing with age and increase in plasma insulin and leptin concentration (Hummel et al. 1966; Coleman and Hummel 1967; Frederich et al. 1995; Chen et al. 1996; Chua et al. 1996; Lee et al. 1996; Madiehe et al. 2002). Later the mouse leptin receptor gene, originally named diabetes (db/db) and currently referred to as Lepr, was cloned, and the mutation that determines the above-described phenotype was identified. Although it was determined that the exact defect in the leptin receptor present in db/db mice is not present in obese humans (Tartaglia et al. 1995, Considine et al. 1996b), the db/db mouse has been used to study the leptin-dependent mechanism of obesity and T2D for over 40 years. More recently, abnormalities in cognitive and emotional responses have also been characterized in db/db mice, making them available to investigate neurobehavioral co-morbidities associated with metabolic dysfunction (Dinel et al. 2011; Ernst et al. 2013; Li et al. 2002; Sharma et al. 2012). Psychosocial stress is an important risk factor for to the development of diabetes, obesity and associated co-morbid disorders (Block et al. 2009; Torres and Nowson 2007; Trento et al. 2010). It can be modeled in mice using the chronic subordination stress (CSS) that induces vulnerability to obesity, metabolic syndrome and insulin resistance further aggravated by hypercaloric diet (e.g., Bartolomucci et al. 2004, 2009; Dadomo et al. 2011; Sanghez et al. 2013; Razzoli et al. 2014, 2015a, b; Patterson et al. 2013). In this study, we aimed at establishing whether obese and diabetic prone db/db mice could develop an aggravated metabolic phenotype due to the exposure to the psychological challenge of CSS impinging upon their abnormal behavioral reactivity. Collectively our data demonstrate that CSS can worsen glucose intolerance but not obesity in db/db mice.
Materials and methods
Animals and general procedure
In these experiments, a 5-day baseline phase was followed by 4 weeks of CSS. During baseline and the first week of stress, all mice were fed a standard diet, while during the second, third and fourth week of stress, mice were fed a high-fat diet (HFD). During the last week of stress, animals underwent a glucose tolerance test (GTT) and body composition analysis. Random-fed glucose level was monitored weekly always at the same time.
Animals and diet
Experimental subjects were 3-month-old male db/db mice (B6.BKS(D)-Leprdb/J) purchased from Jackson Laboratories USA. Three- to four-month-old male CD-1 mice were obtained from Charles River USA to be used as dominant animals. All mice were maintained on a 12:12 h light/dark cycle (lights on at 05:00 am) at 22 ± 2 °C. Mice were fed a standard (2018 Tecklad, Harlan; 3.1 kcal/g, 18 % kcal from fat) or a HFD (D12451 Research Diet, 4.73 kcal/g, 45 % kcal from fat).
Food intake (expressed in kcal/g) was quantified every day for the entire duration of the experiment by measuring the amount of food available in the food hopper every 24 h (measured between 08:00 am and 08:30 am). To limit spillage and protect against measurement inaccuracy, each mouse was given daily only 3–4 whole food pellets, and the bottom of the cage was carefully searched for any food crumbs that, if found, were weighed together with the food found in the hopper.
All animal experiments were approved by the University of Minnesota Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Animals.
Chronic subordination stress protocol
The protocol used has been described in detail in Sanghez et al. (2013) and is a modified version of our standard procedure (Bartolomucci et al. 2001, 2004, 2009). Briefly, stable resident/intruder pairs of adult male mice were established after a baseline period lasting 5 days. At the beginning of the 4-week stress phase, each CD1 male resident mouse received a db/db intruder mouse and the two animals were allowed to freely interact for a maximum of 10 min. Invariably the interaction led to the social subordination of the db/db subject, as determined by direct observation by a trained observer on the basis of the display of upright posture, flight behavior and squeaking vocalization by the subordinate individual (Bartolomucci et al. 2001, 2009; Dadomo et al. 2011). After the interaction that was carried out between 0830 and 0930 hours, dominant and subordinate mice were separated by a perforated partition, which allowed continuous visual, auditory and olfactory contact but no physical interaction. Age- and weight-matched db/db mice housed in groups of three were included as the control group according to our standard protocol and received daily gentle handling at the same time of the social defeat procedure (Bartolomucci et al. 2001, 2004, 2009; Sanghez et al. 2013).
Home-cage locomotor activity
Locomotor activity was determined throughout the experiments in subordinate mice by means of an automated system that used small passive infrared sensors positioned on the top of each cage (ActiMeter, TechnoSmart, Rome, Italy) (Bartolomucci et al. 2009). This system allowed a continuous monitoring of mice locomotor activity, except during the aggressive interaction. Average daily activity was measured during the dark phase and during the light phase of both the baseline and the stress periods. The period of the activity rhythm was analyzed using the ActogramJ software package (Department of Neurobiology and Genetics, University of Wuerzburg, Germany).
Random-fed glucose and glucose tolerance test (GTT)
Random-fed glucose levels were assessed between 0830 and 0930 hours weekly immediately prior the social defeat interaction. GTT was performed following an overnight fast starting at 0800 hours. A small blood droplet was sampled at 0, 30, 60 and 120 min after an i.p. injection of 1 g/kg d-glucose dissolved in sterile saline (10 ml/kg body weight) by nicking the mouse tail with a sharp razor blade, and blood glucose levels were measured with an Accucheck Aviva glucometer (Roche Diagnostics, Indianapolis, IN).
Body composition analysis
Body composition parameters such as total body fat and total body fat free mass were determined by quantitative nuclear magnetic resonance using EchoMRI-100 (QNMR Systems, Houston, TX).
Plasma analysis
Starting at 0800 hours, after overnight fasting mice were euthanized by decapitation following brief CO2 exposure (<1 min). Trunk blood was collected in EDTA-coated tubes (Sarstedt, Nümbrecht, Germany). Corticosterone was determined by a RIA kit (MP Biomedical, Solon, OH, USA). Insulin, leptin, total ghrelin, GLP-1, amylin, MCP-1, TNF-alpha, IL-6, resistin, and glucagon were determined using MILLIPLEX MAP Mouse Metabolic Hormone Magnetic Bead Panel (Millipore, Billerica, MA, USA) and detected using the Bio-Plex 200 system (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data are represented as group mean ± standard error of the mean and were analyzed with one-way ANOVA or two-way ANOVA for repeated measures (Statsoft, Inc. Tulsa, OK, USA). Post hoc analysis was conducted with Duncan`s post hoc tests (1 way ANOVAs and significant main effect) or Tukey’s HSD [ANOVA for repeated measure or nonsignificant main effect (Wilcox 1987)]. The level of statistical significance was set for p ≤ 0.05.
Results
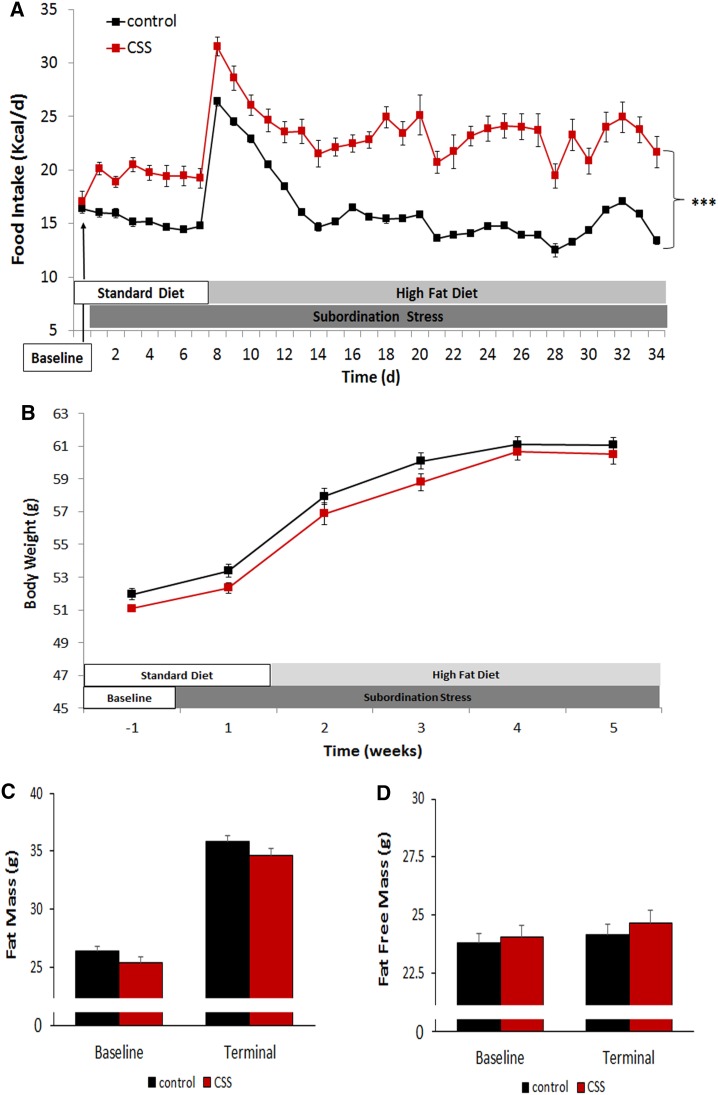
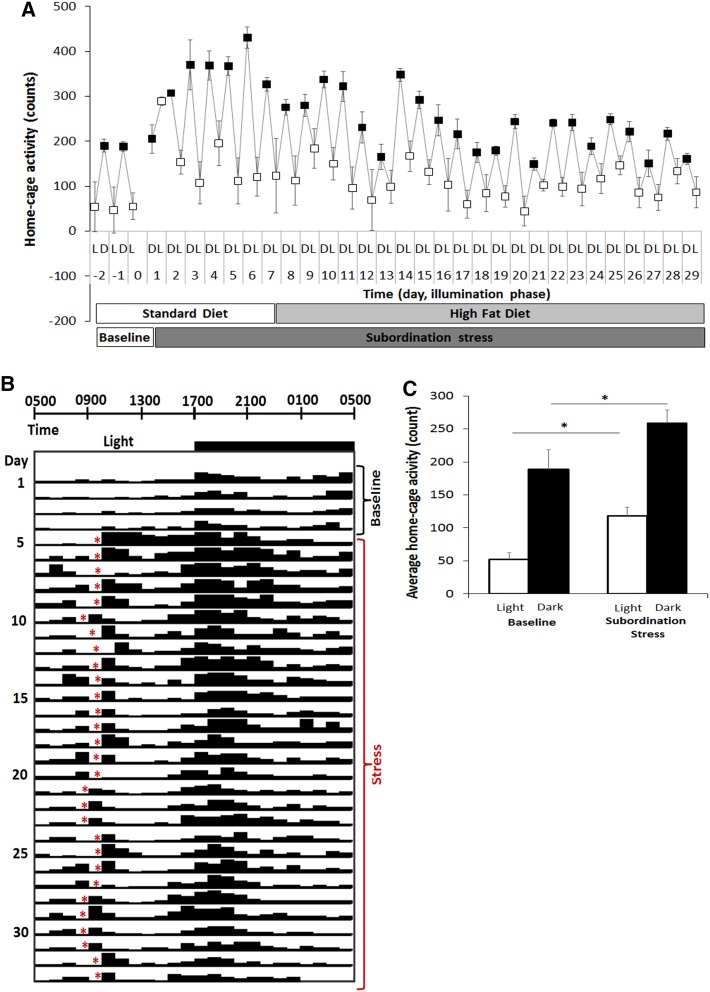
Subordination stress induced a significant hyperphagia compared to control mice throughout the experimental phase [stress F(1,26) = 33.39, p < 0.001; time: F(4,104) = 104.41, p < 0.001; stress × time interaction F(4,104) = 15.99, p < 0001] (Fig. 1a). Nevertheless, hyperphagia did not lead to differences in body weight (Fig. 1b) or body composition (Fig. 1c, d). However, the increased caloric intake in the subordinate db/db mice occurred in parallel with a significant increase in activity corresponding to a disruption of the daily rhythmicity of mouse spontaneous behaviors and suggesting increased energy expenditure (Fig. 2a–c) [stress: F(1,10) = 10.66, p < 0.01; dark/light phase F(1,10) = 116.58, p < 0.0001].
Fig. 1.
Metabolic consequences of chronic psychosocial stress in subordinate db/db mice. a Food intake expressed as kcal/day. b Body weight. Body composition presented as fat mass (c) and fat free mass (d). N = 17 control, N = 12 stress. Data are shown as mean and SEM. Symbols refers to post hoc test. ***p < 0.001 versus control
Fig. 2.
CSS-induced increase of locomotor activity in subordinate db/db mice. a Increased average daily dark (D, filled circles) and light (L, empty circles) phase values during the stress phase vs baseline. b Animals experiencing the social defeat encounter (indicated by the red asterisks) daily between ZT3.5 (0830 hours) and ZT4.5 (0930 hours) exhibited a disrupted pattern of activity compared to baseline. c Quantification of CSS-induced increase in subordinate mice activity during both the light and the dark phase of the stress compared to baseline values. (N = 11). *p < 0.05 versus corresponding phase in baseline
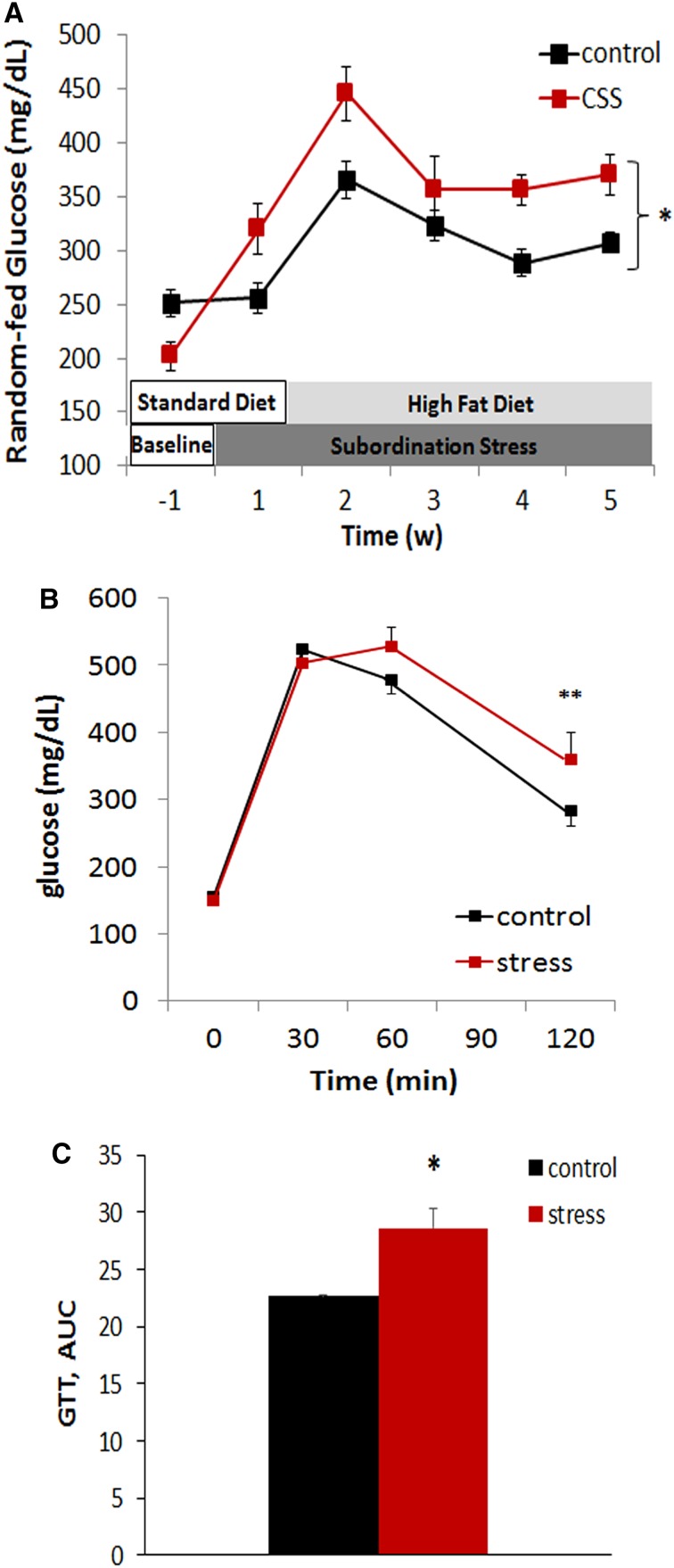
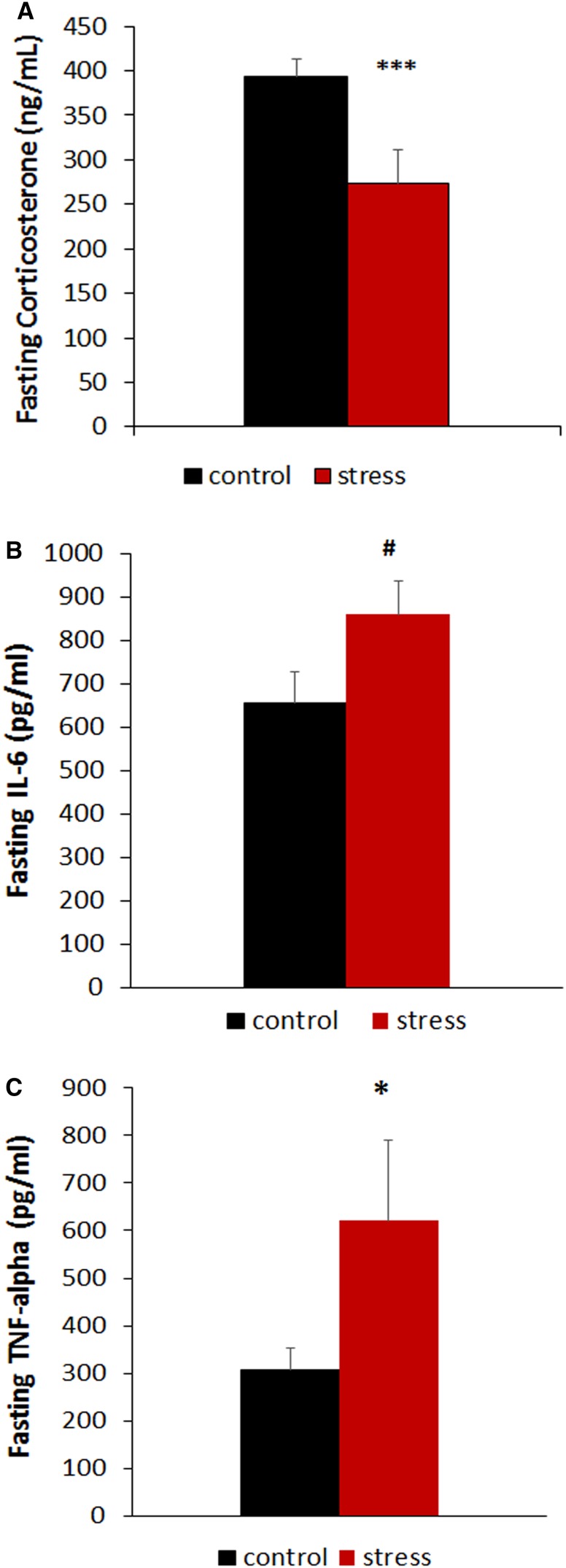
Remarkably, a time-dependent increase in random-fed glycaemia became apparent in subordinated mice both during the first week of stress in which mice are fed a low fat diet and in the subsequent weeks in which mice are switched to HFD diet, with glucose levels recorded at the end of the last week of stress on the HFD corresponding to 307.12 ± 9.78 mg/dl in control and 370.0 ± 18.54 mg/dl in subordinate db/db mice [stress F(1,26) = 19.059, p < 0.001; time: F(4,104) = 17.52, p < 0.001] (Fig. 3a). Subordinate db/db mice also showed an aggravated glucose intolerance in the GTT when compared to controls [time: F(3,75) = 174.57, p < 0.001; stress × time interaction: F(3,75) = 3.45, p < 0.05; AUC F(1,25) = 2.2, p < 0.05] (Fig. 3b, c). Furthermore, stressed db/db mice showed lower fasting corticosterone levels [F(1,21) = 9.05, p < 0.01] (Fig. 4a) in the presence of similar glucose and glucagon levels (Table 1).
Fig. 3.
Glucose homeostasis is worsened in subordinate db/db mice. a Random-fed glucose levels were assessed between 08:30 am and 09:30 am weekly. b Glucose tolerance test (GTT). c Area under the curve (AUC) data for GTT were calculated and expressed as (mg/dl × 120 min, ×103). N = 17 control, N = 12 stress. Data are shown as mean and SEM. Symbols refers to post hoc test. **p < 0.01, *p < 0.05 versus control
Fig. 4.
CSS-induced dysregulation of endocrine responses to fasting in subordinate db/db mice. a Plasma corticosterone. b Plasma IL-6. c Plasma TNF-alpha. N = 17 control, N = 12 stress. Data are shown as mean and SEM. Symbols refers to post hoc test. ***p < 0.001, *p < 0.05, # p = 0.07, versus control
Table 1.
Metabolic responses to fasting in subordinate db/db mice
| Control | Stress | F(1,26) | p | |
|---|---|---|---|---|
| Total ghrelin (pg/ml) | 31.6 ± 3.6 | 42.0 ± 4.1 | 3.46 | 0.074 |
| GLP-1 (pg/ml) | 578.9 ± 38.9 | 684.8 ± 41.8 | 3.21 | 0.085 |
| Glucagon (pg/ml) | 255.7 ± 33.3 | 285.5 ± 16.7 | 0.46 | 0.501 |
| Insulin (ng/ml) | 13.2 ± 0.6 | 13.4 ± 1.1 | 0.03 | 0.858 |
| Leptin (ng/ml) | 30.5 ± 5.0 | 31.3 ± 4.4 | 0.01 | 0.919 |
| Resistin (ng/ml) | 18.9 ± 1.8 | 18. 2 ± 1.9 | 0.08 | 0.782 |
| Amylin (pg/ml) | 599.7 ± 94.5 | 615.4 ± 200.1 | 0.01 | 0.937 |
| Glucose (mg/dl) | 154.4 ± 6.9 | 163.5 ± 10.4 | 0.57 | 0.454 |
| MCP-1 (pg/ml) | 395.4 ± 12.1 | 1066.3 ± 681.1 | 1.53 | 0.227 |
N = 17 control, N = 12 stress. Data are shown as mean and SEM
Finally, subordinate db/db mice showed higher plasma TNF-alpha [F(1,26) = 4.61, p < 0.05] and a trend for increased IL-6 [F(1,24) = 3.55, p = 0.071] (Fig. 4b, c), suggesting increased systemic inflammation which is often associated with leptin or leptin receptor deficiency associated obesity and T2D (Loffreda et al. 1998). Plasma GLP-1 (p = 0.07) and ghrelin (p = 0.08) were slightly increased in CSS mice, while other endocrine markers were not significantly affected by chronic stress (Table 1).
Discussion
Here we demonstrate that exposure to chronic social stress is able to aggravate glucose intolerance in individuals having a genetic predisposition to develop obesity and T2D. Indeed we showed that leptin receptor-deficient mice exposed to social subordination stress manifested persistent hyperphagia, hyperglycemia and aggravated glucose intolerance altogether suggestive of impaired glucose homeostasis. While obesity could not be worsened by stress in db/db mice, probably due to the concurrent increase in locomotor activity induced by stress, they also showed a greater elevation of pro-inflammatory cytokine plasma levels.
Similar to previously published data in strains not genetically predisposed to obesity (Patterson et al. 2013; Sanghez et al. 2013; Table 2), the current results further support the multistage and polygenic nature of T2D and suggest that the disease develops in the presence of multiple risk factors. In mouse strains of different genetic backgrounds, we and others showed that CSS aggravates diet-induced obesity and caused hypoactivity and hyperphagia (Table 2; Bartolomucci et al. 2005, 2009; Dadomo et al. 2011; Patterson et al. 2013; Razzoli et al. 2015a, b). In addition, stress in wild-type subordinate mice fed HFD caused a metabolic syndrome, glucose intolerance and insulin resistance; furthermore, they exhibit hormonal changes (i.e., higher leptin and decreased adiponectin) and excessive free fatty acids levels, both of which are recognized risk factors for the development of T2D. Therefore, we aimed at testing whether genetically obese mice, i.e., the db/db strain (Boquist et al. 1974), would be more vulnerable to the stress-induced dysregulation of glucose tolerance. Subordinate db/db mice developed a sustained hyperphagic response as it is normally observed in our model (see above), but surprisingly they did not differ in body weight and fat mass, as well as leptin and insulin levels from controls. One explanation for this outcome could be due to the increased overall activity induced in subordinate db/db mice never before observed in this model using different mouse strains (Fig. 2; Table 1). It is worth to point out that db/db mice are known to exhibit behavioral abnormalities, such as depression/anxiety and psychosis-like behaviors (Li et al. 2002; Sharma et al. 2010) and impaired ability to anticipate negative events in a fear potentiated startle paradigm (Sharma et al. 2010). It is also worth pointing out that different models of CSS, known to elicit a similar depression-like phenotype, often induce opposite metabolic consequences, thus suggestive of different underlying molecular mechanisms (See Razzoli et al. 2015a, b, for a review).
Table 2.
Metabolic and neuroendocrine effect induced by chronic subordination stress in db/db and wild-type mice fed high-fat diet
| Wild type* | db/db | |
|---|---|---|
| Physiology and Behavior | ||
| Food intake | ↑ | ↑ |
| Body weight gain | ↑ | = |
| Fat mass | ↑ | = |
| Lean mass | Na | = |
| Stress-induced home cage activity | ↓ | ↑ |
| Glucose homeostasis | ||
| Random-fed glucose | = | ↑ |
| Fasting Glucose | ↑ | = |
| Glucose intolerance in the GTT | ↑ | ↑ |
| Hormones # | ||
| Corticosterone–fed state | ↑ | Na |
| Corticosterone | = | ↓ |
| Insulin | ↑ | = |
| TNF-alpha | Na | ↑ |
| Il-6 | ↑ | ↑ |
| Total ghrelin | ↓ | = |
| GLP-1 | Na | = |
| Glucagon | Na | = |
| Leptin | ↑ | = |
| Resistin | Na | = |
| Adiponectin | ↓ | Na |
| Amylin | Na | = |
| MCP-1 | Na | = |
db/db mice are known to develop obesity due to hyperphagia that is sustained by the defective leptin circuitry (Bates et al. 2003) and compensate for the obesity-associated insulin resistance by pancreatic beta-cell hyperplasia, thereby maintaining only mildly elevated blood glucose levels (Davis et al. 2010). Under subordination stress, db/db mice became even more hyperphagic thus likely contributing to persistent increase in plasma glycaemia. Overall these findings extend previous results in wild-type mice and support a link between stress and altered leptin signaling in the development of stress-induced T2D in vulnerable obese subjects (Könner et al. 2009; Sanghez et al. 2013).
Remarkably, subordinate db/db mice in spite of their sustained hyperglycemia and glucose intolerance were able to return to control glycemic levels after overnight fasting suggestive of increased glucose clearance possibly mediated by muscle metabolism associated with hyperactivity (e.g., Ikemoto et al. 1995). The observed glucose intolerance could thus be explained more as a stress-induced failure to suppress fasting-induced hepatic gluconeogenesis rather than impaired glucose clearance (Chan et al. 1975). On the other hand, the dampened response of corticosterone to fasting of subordinate db/db mice apparently contrasts with the observed stress-induced elevation in corticosterone levels and hyperglycemia commonly induced by this model in different mouse strains (Sanghez et al. 2013; Bartolomucci et al. 2009; Patterson et al. 2013). However, it must be noted that corticosterone level has mostly been investigated in the fed state (e.g., Bartolomucci et al. 2004; Razzoli et al. 2014) when no hypoglycemia-induced counter-regulatory hormone secretion is present. In the only study in which corticosterone has been measured in fasting conditions (Sanghez et al. 2013), subordinate wild-type mice showed similar corticosterone levels but increased glucose compared to controls. Conversely, subordinate db/db mice showed a lower corticosterone concentration than controls in the presence of fasting glucose levels similar to non-stressed subjects. Our data are in line with previous data (Oishi et al. 2007) showing that overnight fasting induced a drop of glucose level from ~300 to ~150 mg/dl, with a parallel increase in plasma corticosterone in db/db mice. Conversely wild-type mice showed an increase in corticosterone only after ~24 h of fasting and a glucose level of ~67 mg/dl (Oishi et al. 2007). It seems plausible to hypothesize that in our experiment control db/db mice activated the HPA axis to contrast a rapidly lowering glucose level, while stressed db/db remained hyperglycemic and did not showed the counter-regulatory increase in corticosterone likely because of increased activity-associated glucose clearance.
Finally, subordinate db/db mice showed a systemic low-grade pro-inflammatory profile, as suggested by elevation of TNF-alpha levels and mild increase in IL-6 levels, in line with results in wild-type mice (Patterson et al. 2013). Adipose tissue in obese and diabetic individuals might develop an inflammatory milieu which ultimately leads to insulin resistance (Patel et al. 2013). Macrophages, immune-inflammatory cells and natural killer cells in innate immunity are significantly activated in leptin receptor-deficient mice (Lee et al. 2010). The present data could support the link between a dysregulated immune and metabolic function since leptin is also known to modulate lymphocyte proliferation, apoptosis and cytokine secretion (Fantuzzi and Faggioni 2000; Farooqi et al. 2002). Furthermore these data are in line with new epidemiological studies suggesting a major role for inflammation markers in the adult onset of diabetes in obese individuals (Luft et al. 2013).
Genetic factors are estimated to account for 40–70 % of variance in human obesity, while the aetiology of the most common forms of obesity is considered to stem from the complex interaction of multiple genes and the environment (Barsh et al. 2000; O’Rahilly 2009). Nevertheless, monogenic models of obesity contribute to the understanding of the mechanisms at the basis of the regulation of adiposity, since the existing human counterparts present strikingly similar phenotypes (reviewed in Lutz and Woods 2012). The db/db mouse is analogous to human homozygotes for a mutation corresponding to a truncated form of the leptin receptor; these patients present early onset obesity associated with hyperleptinemia, impulsive eating behavior and emotional liability (Clément et al. 1998). Similarly, other monogenic forms of human obesity have been associated with mutations of genes involved in the melanocortin signaling pathway such as: proopiomelanocortin (Krude et al. 1998), prohormone convertase 1 (Jackson et al. 1997) and melanocortin 4 receptor (Farooqi et al. 2000). All these forms of non-syndromic monogenic obesity share an early onset obesity that is largely dependent on excessive caloric consumption, suggesting a potential therapeutic approach based on the control the food intake (Meyre and Froguel 2010). The present data on db/db mice add to this picture, in that one of our model most robust features is the induction of hyperphagia in subordinate mice, which likely worsened the metabolic phenotype of db/db subordinate mice (present data; Sanghez et al. 2013; Razzoli et al. 2015a, b). Specifically, the hyperphagic response of subordinate db/db mice suggests that stress can be a major environmental factor able to impair or aggravate glucose intolerance and eventually lead to T2D even in already obese individuals due to a deficient leptin receptor signaling pathway. Remarkably, it is well accepted that in the human population only a subgroup of obese patients develop T2D (Blüher 2012) and that stress and low socioeconomic status are major risk factors for obesity and metabolic disorders (Mackenbach et al. 1997; Mooy et al. 2000; McEwen and Mirsky 2002; Chandola et al. 2006; Adler and Rehkopf 2008). It then seems reasonable to suggest that preventing or limiting chronic stress in the obese population might limit development of comorbid disease.
Acknowledgments
The authors wish to thank Marina Yoder for technical assistance in the conduct of the corticosterone radio-immuno-assay. This work was supported by U of MN Medical School to AB and by NSF IOS1025199 to WCE.
Conflict of interest
Author MR, Author JM, Author AG, Author WCE, and Author AB declare that they have no conflict of interest.
Human and animal rights
All procedures performed in studies involving animals were in accordance with the ethical standards of the University of Minnesota Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Animals.
Contributor Information
Maria Razzoli, Phone: +1-612-301-7694, Email: mrazzoli@umn.edu.
Alessandro Bartolomucci, Phone: +1-612-626-7006, Email: abartolo@umn.edu.
References
- Adler NE, Rehkopf DH. US disparities in health: descriptions, causes, and mechanisms. Ann Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- Barsh GS, Farooqi IS, O’Rahilly S. Genetics of body-weight regulation. Nature. 2000;404:644–651. doi: 10.1038/35007519. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Gaspani L, Limiroli E, Panerai AE, Ceresini G, Poli MD, Parmigiani S. Social status in mice: behavioral, endocrine and immune changes are context dependent. Phys Behav. 2001;73:401–410. doi: 10.1016/S0031-9384(01)00453-X. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology. 2004;29:899–910. doi: 10.1016/j.psyneuen.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Sacerdote P, Panerai AE, Sgoifo A, Dantzer R, Parmigiani S. Social factors and individual vulnerability to chronic stress exposure. Neurosci Biobehav Rev. 2005;29(1):67–81. doi: 10.1016/j.neubiorev.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, Dadomo H, Franceschini P, Dell’Omo G, Parmigiani S, et al. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS ONE. 2009;4(1):e4331. doi: 10.1371/journal.pone.0004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, et al. STAT3 signaling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421(6925):856–959. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial stress and change in weight among US adults. Am J Epidemiol. 2009;170(2):181–192. doi: 10.1093/aje/kwp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüher M. Are there still healthy obese patients? Curr Opin Endocrinol Diabetes Obes. 2012;19:341–346. doi: 10.1097/MED.0b013e328357f0a3. [DOI] [PubMed] [Google Scholar]
- Boquist L, Hellman B, Lernmark A, Täljedal IB. Influence of the mutation “diabetes” on insulin release and islet morphology in mice of different genetic backgrounds. J Cell Biol. 1974;62(1):77–89. doi: 10.1083/jcb.62.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TM, Young KM, Hutson NJ, Brumley FT, Exton JH. Hepatic metabolism of genetically diabetic (db/db) mice. I. Carbohydrate metabolism. Am J Physiol. 1975;229(6):1702–1712. doi: 10.1152/ajplegacy.1975.229.6.1702. [DOI] [PubMed] [Google Scholar]
- Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332:521–525. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/S0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271(5251):994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- Chung WK, Power-Kehoe L, Chua M, Chu F, Aronne L, Huma Z, Sothern M, Udall JN, Kahle B, Leibel RL. Exonic and intronic sequence variation in the human leptin receptor gene (LEPR) Diabetes. 1997;46(9):1509–1511. doi: 10.2337/diab.46.9.1509. [DOI] [PubMed] [Google Scholar]
- Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougnères P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- Coleman DL, Hummel KP. Studies with the mutation, diabetes, in the mouse. Diabetologia. 1967;3(2):238–248. doi: 10.1007/BF01222201. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Considine RV, Considine EL, Williams CJ, Hyde TM, Caro JF. The hypothalamic leptin receptor in humans: identification of incidental sequence polymorphisms and absence of the db/db mouse and fa/fa rat mutations. Diabetes. 1996;45:992–994. doi: 10.2337/diab.45.7.992. [DOI] [PubMed] [Google Scholar]
- Dadomo H, Sanghez V, Di Cristo L, Lori A, Ceresini G, Malinge I, Parmigiani S, Palanza P, Sheardown M, Bartolomucci A. Vulnerability to chronic subordination stress-induced depression-like disorders in adult 129SvEv male mice. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(6):1461–1471. doi: 10.1016/j.pnpbp.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Davis RC, Castellani LW, Hosseini M, Ben-Zeev O, Mao HZ, Weinstein MM, Jung DY, Jun JY, Kim JK, Lusis AJ, et al. Early hepatic insulin resistance precedes the onset of diabetes in obese C57BLKS-db/db mice. Diabetes. 2010;59:1616–1625. doi: 10.2337/db09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinel AL, André C, Aubert A, Ferreira G, Layé S, Castanon N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS ONE. 2011;6(9):e24325. doi: 10.1371/journal.pone.0024325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Kahn SE, Ferrannini E, Goldfine AB, Nathan DM, Schwartz MW, Smith RJ, Smith SR. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? JCEM. 2011;96(6):1654–1663. doi: 10.1210/jc.2011-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A, Sharma AN, Elased KM, Guest PC, Rahmoune H, Bahn S. Diabetic db/db mice exhibit central nervous system and peripheral molecular alterations as seen in neurological disorders. Transl Psychiatry. 2013;3:e263. doi: 10.1038/tp.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Yeo GS, Keogh JM, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106(2):271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI0215693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke S, Clement K, Dina C, Inoue H, Behn P, Vatin V, Basdevant A, Guy-Grand B, Permutt MA, Froguel P, et al. Genetic studies of the leptin receptor gene in morbidly obese French Caucasian families. Hum Genet. 1997;100(5–6):491–496. doi: 10.1007/s004390050540. [DOI] [PubMed] [Google Scholar]
- Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Thompson KS, Itakura H, Lane MD, Ezaki O. Expression of an insulin-responsive glucose transporter (GLUT4) minigene in transgenic mice: effect of exercise and role in glucose homeostasis. Proc Natl Acad Sci USA. 1995;92(3):865–869. doi: 10.1073/pnas.92.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RS, Creemers JW, Ohagi S, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16(3):303–306. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- Könner AC, Klöckener T, Brüning JC. Control of energy homeostasis by insulin and leptin: targeting the arcuate nucleus and beyond. Phys Behav. 2009;97:632–638. doi: 10.1016/j.physbeh.2009.03.027. [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- Lahlou N, Issad T, Lebouc Y, Carel JC, Camoin L, Roger M, Girard J. Mutations in the human leptin and leptin receptor genes as models of serum leptin receptor regulation. Diabetes. 2002;51:1980–1985. doi: 10.2337/diabetes.51.6.1980. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Lee S, Jang I, Park J, Lee J, Lee S, Baek S, Lee S, Lee H. Systemic immunity of obese-diabetes model (db/db) mice. Mol Cell Toxicol. 2010;6(2):143–149. doi: 10.1007/s13273-010-0021-6. [DOI] [Google Scholar]
- Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/S0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12(1):57–65. [PubMed] [Google Scholar]
- Luft VC, Schmidt MI, Pankow JS, Couper D, Ballantyne CM, Young JH, Duncan BB. Chronic inflammation role in the obesity-diabetes association: a case–cohort study. Diabetol Metabol Syndr. 2013;5(1):31. doi: 10.1186/1758-5996-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz TA, Woods SC (2012) Overview of animal models of obesity. Curr Protoc Pharmacol. Chapter 5: Unit 5.61. doi:10.1002/0471141755.ph0561s58 [DOI] [PMC free article] [PubMed]
- Mackenbach JP, Kunst AE, Cavelaars AE, Groenhof F, Geurts JJ. Socioeconomic inequalities in morbidity and mortality in Western Europe. Lancet. 1997;349:1655–1659. doi: 10.1016/S0140-6736(96)07226-1. [DOI] [PubMed] [Google Scholar]
- Madiehe AM, Hebert S, Mitchell TD, Harris RB. Strain-dependent stimulation of growth in leptin-treated obese db/db mice. Endocrinology. 2002;143(10):3875–3883. doi: 10.1210/en.2002-220362. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Mirsky AE. How socioeconomic status may “get under the skin” and affect the heart. Eur Heart J. 2002;23:1727–1728. doi: 10.1053/euhj.2002.3283. [DOI] [PubMed] [Google Scholar]
- Meyre D, Froguel P. Monogenic Obesity. In: Freemark M, editor. Contemporary endocrinology: pediatric obesity: etiology, pathogenesis, and treatment. New York: Humana Press; 2010. pp. 35–45. [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Moon HS, Chamberland JP, Diakopoulos KN, Fiorenza CG, Ziemke F, Schneider B, Mantzoros CS. Leptin and amylin act in an additive manner to activate overlapping signaling pathways in peripheral tissues: in vitro and ex vivo studies in humans. Diabetes Care. 2011;34:132–138. doi: 10.2337/dc10-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooy JM, De Vries H, Grootenhuis PA, Bouter LM, Heine RJ. Major stressful life events in relation to prevalence of undetected type 2 diabetes: the Hoorn study. Diabetes Care. 2000;23:197–201. doi: 10.2337/diacare.23.2.197. [DOI] [PubMed] [Google Scholar]
- Oishi K, Ohkura N, Matsuda J, Ishida N. Food deprivation induces adipose plasminogen activator inhibitor-1 (PAI-1) expression without accumulation of plasma PAI-1 in genetically obese and diabetic db/db mice. Thromb Haemost. 2007;98(4):864–870. [PubMed] [Google Scholar]
- O’Rahilly S. Human genetics illuminates the paths to metabolic disease. Nature. 2009;462(7271):307–314. doi: 10.1038/nature08532. [DOI] [PubMed] [Google Scholar]
- Patel PS, Buras ED, Balasubramanyam A (2013) The role of the immune system in obesity and insulin resistance. J Obes 2013: Art ID 616193, 9. doi:10.1155/2013/616193 [DOI] [PMC free article] [PubMed]
- Patterson ZR, Khazall R, Mackay H, Anisman H, Abizaid A. Central ghrelin signaling mediates the metabolic response of C57BL/6 male mice to chronic social defeat stress. Endocrinology. 2013;154(3):1080–1091. doi: 10.1210/en.2012-1834. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Karsten C, Yoder JM, Bartolomucci A, Engeland WC. Chronic subordination stress phase advances adrenal and anterior pituitary clock gene rhythms. Am J Physiol Regul Integr Comp Physiol. 2014;07(2):R198–R205. doi: 10.1152/ajpregu.00101.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Sanghez V, Bartolomucci A (2015) Chronic subordination stress induces hyperphagia and disrupts eating behavior in mice modeling binge-eating-like disorder. Front Nutr 1(30) [DOI] [PMC free article] [PubMed]
- Razzoli M, Cero C, Bartolomucci A. How does stress affects energy balance? Recent advances in physiology and neurobehavioral genetics. In: Tucci V, editor. Handbook of neurobehavioral genetics and phenotyping. London: Wiley; 2015. [Google Scholar]
- Sanghez V, Razzoli M, Carobbio S, Campbell M, McCallum J, Cero C, Ceresini G, Cabassi A, Govoni P, Franceschini P, et al. Psychosocial stress induces hyperphagia and exacerbates diet-induced insulin resistance and the manifestations of the metabolic syndrome. Psychoneuroendocrinology. 2013;38(12):2933–2942. doi: 10.1016/j.psyneuen.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav. 2010;101:381–388. doi: 10.1016/j.physbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AN, Elased KM, Lucot JB. Rosiglitazone treatment reversed depression- but not psychosis-like behavior of db/db diabetic mice. J Psychopharmacol. 2012;26(5):724–732. doi: 10.1177/0269881111434620. [DOI] [PubMed] [Google Scholar]
- Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18:213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Thompson DB, Sutherland J, Apel W, Ossowski V. A physical map at 1p31 encompassing the acute insulin response locus and the leptin receptor. Genomics. 1997;39(2):227–230. doi: 10.1006/geno.1996.4504. [DOI] [PubMed] [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11–12):887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Trento M, Kucich C, Tibaldi P, et al. A study of central serotoninergic activity in healthy subjects and patients with type 2 diabetes treated by traditional one-to-one care or group care. J Endocrinol Invest. 2010;33(9):624–628. doi: 10.1007/BF03346660. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. New designs in analysis of variance. Ann Rev Psychol. 1987;38:29–60. doi: 10.1146/annurev.ps.38.020187.000333. [DOI] [Google Scholar]
- World Health Organization (2006) Overweight and obesity. Factsheet no. 311. World Health Organization, Geneva
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]