Abstract
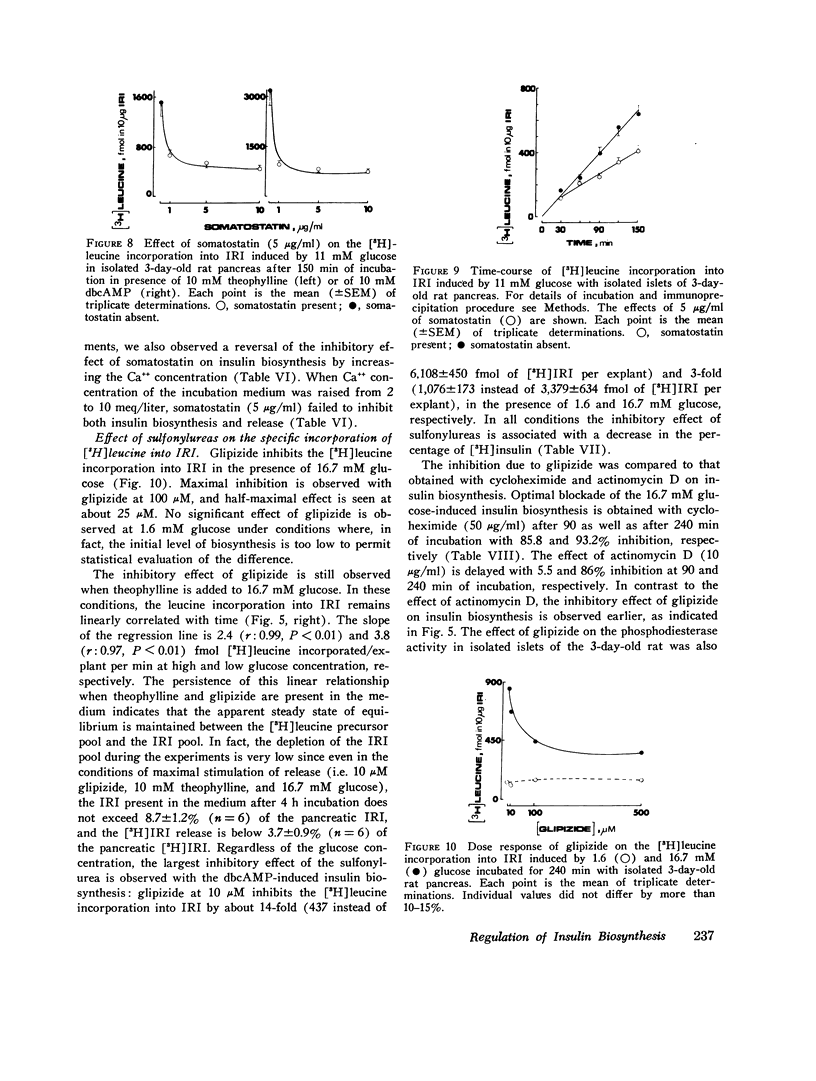
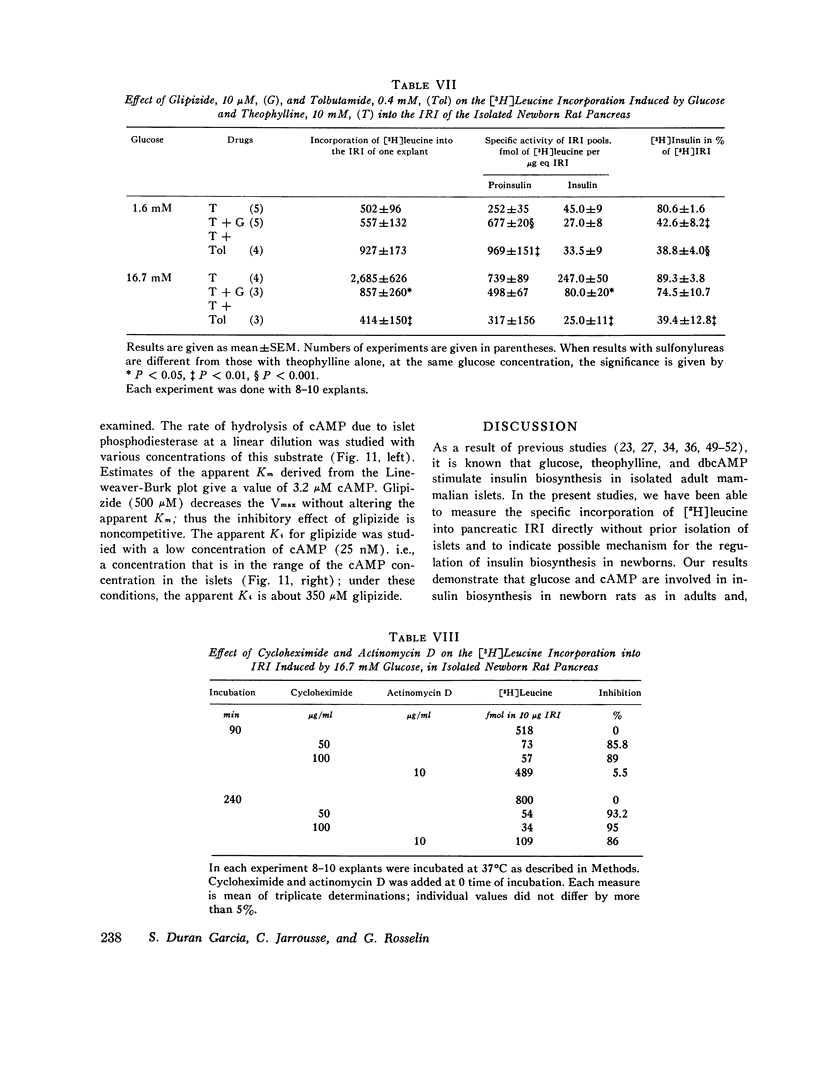
The purpose of the present study was to investigate the regulation of insulin biosynthesis during the perinatal period. The incorporation of [3H]leucine into total immunoreactive insulin (IRI) and into IRI fractions was measured by a specific immunoprecipitation procedure after incubation, extraction, and gel filtration in isolated 3-day-old rat pancreases without prior isolation of islets. IRI fractions were identified by their elution profile, their immunological properties, and their ability to compete with the binding of 125 I-insulin in rat liver plasma membranes. No specific incorporation of [3H]leucine was found in the IRI eluted in the void volume, making it unlikely that this fraction behaves as a precursor of (pro) insulin in this system. In all conditions tested, the incorporation of [3H]leucine was linearly correlated with time. Optimal concentration of glucose (11 mM) activated six- to sevenfold the [3H]leucine incorporation into IRI. Theophylline or N6O2-dibutyryl- (db) cAMP at 1.6 mM glucose significantly increased the [3H]leucine incorporation. Glucose at 16.7 mM further enhanced the effect of both drugs. Contrarily, somatostatin (1-10 mug/ml) inhibits the rate of [3H]leucine incorporation into IRI in the presence of 11 mM glucose; this effect was observed at 5.5 mM glucose and was not modified by any further increase in glucose concentrations up to 27.5 mM. Theophylline or dbcAMP at 10 mM concentration did not reverse the somatostatin inhibitory effect on either insulin biosynthesis or release. Somatostatin also inhibited both processes in isolated islets from the 3-day-old rat pancreas. High Ca++ concentration in the incubation medium reversed the inhibitory effect of somatostatin on glucose-induced insulin biosynthesis as well as release. In both systems the inhibitory effect of somatostatin on insulin biosynthesis and release correlated well. Glipizide (10-100 muM) AND TOLBUTAMIDE (400 MUM) inhibited the stimulatory effect of glucose, dbcAMP, and theophylline on [3H]leucine incorporation into IRI. The concentrations of glipizide that were effective in inhibiting [3H]leucine incorporation into IRI were smaller than those required to inhibit the phosphodiesterase activity in isolated islets of 3-day-old rat pancreas. These data suggest the following conclusions: (a) the role of the cAMP-phosphodiesterase system on insulin biosynthesis is likely to be greater in newborns than in adults; (b) the greater effectiveness of glucose and the cAMP system on insulin biosynthesis than on insulin release might possibly be related to the rapid accumulation of pancreatic IRI which is observed in the perinatal period; (c) somatostatin, by direct interaction with the endocrine tissue, can inhibit glucose and cAMP-induced insulin biosynthesis as well as release; calcium reverses this inhibition; (d) sulfonylureas inhibit insulin biosynthesis in newborn rat pancreas an effect which has to be considered in the use of these agents in human disease.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J.H., Randle P. J., Täljedal I. -B. Cyclic nucleotide phosphodiesterase activity in normal mouse pancreatic islets. FEBS Lett. 1972 Feb 15;20(3):263–266. doi: 10.1016/0014-5793(72)80082-6. [DOI] [PubMed] [Google Scholar]
- Asplund K. Effects of glucose on insulin biosynthesis in foetal and newborn rats. Horm Metab Res. 1973 Nov;5(6):410–415. doi: 10.1055/s-0028-1093914. [DOI] [PubMed] [Google Scholar]
- Balodimos M. C., Camerini-Dávalos R. A., Marble A. Nine years' experience with tolbutamide in the treatment of diabetes. Metabolism. 1966 Nov;15(11):957–970. doi: 10.1016/0026-0495(66)90044-8. [DOI] [PubMed] [Google Scholar]
- Bowen V., Lazaus N. R. Glucose-mediated insulin release: 3',5' cAMP phosphodiesterase. Diabetes. 1973 Oct;22(10):738–743. doi: 10.2337/diab.22.10.738. [DOI] [PubMed] [Google Scholar]
- Chideckel E. W., Palmer J., Koerker D. J., Ensinck J., Davidson M. B., Goodner C. J. Somatostatin blockade of acute and chronic stimuli of the endocrine pancreas and the consequences of this blockade on glucose homeostasis. J Clin Invest. 1975 Apr;55(4):754–762. doi: 10.1172/JCI107986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. L., Steiner D. F. Insulin biosynthesis in the rat: demonstration of two proinsulins. Proc Natl Acad Sci U S A. 1969 Jan;62(1):278–285. doi: 10.1073/pnas.62.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. R., Rutter W. J. Synthesis and accumulation of insulin in the fetal rat pancreas. Dev Biol. 1972 Dec;29(4):468–481. doi: 10.1016/0012-1606(72)90084-x. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., Ashcroft S. J., Randle P. J. Concentration of adenosine 3':5'-cyclic monophosphate in mouse pancreatic islets measured by a protein-binding radioassay. Biochem J. 1973 Jun;134(2):599–605. doi: 10.1042/bj1340599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Requirement for calcium ion in insulin secretion by the perfused rat pancreas. Am J Physiol. 1968 Jan;214(1):174–178. doi: 10.1152/ajplegacy.1968.214.1.174. [DOI] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L. Reversal of somatostatin inhibition of insulin secretion by calcium. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1015–1019. doi: 10.1016/0006-291x(74)90414-8. [DOI] [PubMed] [Google Scholar]
- Dunbar J. C., Foà P. P. An inhibitory effect of tolbutamide and glibenclamide (glyburide) on the pancreatic islets of normal animals. Diabetologia. 1974 Feb;10(1):27–35. doi: 10.1007/BF00421411. [DOI] [PubMed] [Google Scholar]
- Efendic S., Luft R., Grill V. Effect of somatostatin on glucose induced insulin release in isolated perfused rat pancreas and isolated rat pancreatic islets. FEBS Lett. 1974 Jun 1;42(2):169–172. doi: 10.1016/0014-5793(74)80778-7. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Insulin receptors in the liver: specific binding of ( 125 I)insulin to the plasma membrane and its relation to insulin bioactivity. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1833–1837. doi: 10.1073/pnas.68.8.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Freychet P. The interactions of proinsulin with insulin receptors on the plasma membrane of the liver. J Clin Invest. 1974 Nov;54(5):1020–1031. doi: 10.1172/JCI107845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Hane S., Gustafson G., Guillemin R., Forsham P. H. Evidence for a physiologic role of pancreatic glucagon in human glucose homeostasis: studies with somatostatin. Metabolism. 1975 Feb;24(2):175–182. doi: 10.1016/0026-0495(75)90018-9. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Schneider V., Forsham P. H. Effect of somatostatin on plasma glucose and insulin responses to glucagon and tolbutamide in man. J Clin Endocrinol Metab. 1974 Dec;39(6):1057–1060. doi: 10.1210/jcem-39-6-1057. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lovinger R., Grodsky G. M. Inhibition by somatostatin of glucagon and insulin release from the perfused rat pancreas in response to arginine, isoproterenol and theophylline: evidence for a preferential effect on glucagon secretion. Endocrinology. 1975 Mar;96(3):749–754. doi: 10.1210/endo-96-3-749. [DOI] [PubMed] [Google Scholar]
- Grill V., Cerasi E. Stimulation by D-glucose of cyclic adenosine 3':5'-monophosphate accumulation and insulin release in isolated pancreatic islets of the rat. J Biol Chem. 1974 Jul 10;249(13):4196–4201. [PubMed] [Google Scholar]
- Heinze E., Schatz H., Nierle C., Pfeiffer E. F. Insulin biosynthesis in isolated pancreatic islets of fetal and newborn rats. Diabetes. 1975 Apr;24(4):373–377. doi: 10.2337/diab.24.4.373. [DOI] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Täljedal I. B. The pancreatic beta-cell recognition of insulin secretagogues: does cyclic AMP mediate the effect of glucose? Proc Natl Acad Sci U S A. 1974 Sep;71(9):3405–3409. doi: 10.1073/pnas.71.9.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Effects of glucose concentration on incorporation of [3H]leucine into insulin using isolated mammalian islets of Langerhans. Biochim Biophys Acta. 1966 Dec 28;130(2):519–521. doi: 10.1016/0304-4165(66)90250-9. [DOI] [PubMed] [Google Scholar]
- Jarrousse C., Rosselin G. Interaction of amino acids and cyclic AMP on the release of insulin and glucagon by newborn rat pancreas. Endocrinology. 1975 Jan;96(1):168–177. doi: 10.1210/endo-96-1-168. [DOI] [PubMed] [Google Scholar]
- KENNY A. J. Extractable glucagon of the human pancreas. J Clin Endocrinol Metab. 1955 Sep;15(9):1089–1105. doi: 10.1210/jcem-15-9-1089. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Oka H., Munemure M., Suzuki S., Yasuda H. Stimulation of guanosine 3',5'-cyclic monophosphate accumulation in rat anterior pituitary gland in vitro by synthetic somatostatin. Biochem Biophys Res Commun. 1974 Nov 6;61(1):53–57. doi: 10.1016/0006-291x(74)90532-4. [DOI] [PubMed] [Google Scholar]
- Kuo W. N., Hodgins D. S., Kuo J. F. Adenylate cyclase in islets of Langerhans. Isolation of islets and regulation of adenylate cyclase activity by various hormones and agents. J Biol Chem. 1973 Apr 25;248(8):2705–2711. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarow A., Wells L. J., Carpenter A. M., Hegre O. D., Leonard R. J., McEvoy R. C. The Banting Memorial Lecture 1973: Islet differentiation, organ culture, and transplantation. Diabetes. 1973 Dec;22(12):877–912. doi: 10.2337/diab.22.12.877. [DOI] [PubMed] [Google Scholar]
- Lernmark A. Specificity of cyclic AMP potentiation of glucose-stimulated insulin release. Horm Res. 1974;5(4):227–233. doi: 10.1159/000178635. [DOI] [PubMed] [Google Scholar]
- Levy J., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release-XVII. Effects of sulfonylureas and diazoxide on insular biosynthetic activity. Biochem Pharmacol. 1975 Jan 15;24(2):235–239. doi: 10.1016/0006-2952(75)90282-8. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Mayhew D. A possible role for the adenylcyclase system in insulin secretion. J Clin Invest. 1967 Nov;46(11):1724–1734. doi: 10.1172/JCI105663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert C. L., Knatterud G. L., Prout T. E., Klimt C. R. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes. 1970;19(Suppl):789–830. [PubMed] [Google Scholar]
- Mintz D. H., Levey G. S., Schenk A. Adenosine 3',5'-cyclic monophosphate and phosphodiesterase activities in isolated fetal and neonatal rat pancreatic islets. Endocrinology. 1973 Feb;92(2):614–617. doi: 10.1210/endo-92-2-614. [DOI] [PubMed] [Google Scholar]
- Moiel R. H., Ryan J. R. Tolbutamide orinase in human breast milk. Clin Pediatr (Phila) 1967 Aug;6(8):480–480. doi: 10.1177/000992286700600813. [DOI] [PubMed] [Google Scholar]
- Montague W., Cook J. R. The role of adenosine 3':5'-cyclic monophosphate in the regulation of insulin release by isolated rat islets of Langerhans. Biochem J. 1971 Mar;122(1):115–120. doi: 10.1042/bj1220115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. E., Korner A. The effect of glucose on insulin biosynthesis by isolated islets of Langerhans of the rat. Biochim Biophys Acta. 1970 Jun;208(3):404–413. doi: 10.1016/0304-4165(70)90213-8. [DOI] [PubMed] [Google Scholar]
- Mortimer C. H., Tunbridge W. M., Carr D., Yeomans L., Lind T., Coy D. H., Bloom S. R., Kastin A., Mallinson C. N., Besser G. M. Effects of growth-hormone release-inhibiting hormone on circulating glucagon, insulin, and growth hormone in normal, diabetic, acromegalic, and hypopituitary patients. Lancet. 1974 Apr 20;1(7860):697–701. doi: 10.1016/s0140-6736(74)92903-1. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Noto Y., Miyamoto S., Mabuchi H., Takeda R. Inhibition by somatostatin of insulin release from isolated pancreatic islets. FEBS Lett. 1975 Jun 1;54(1):103–105. doi: 10.1016/0014-5793(75)81080-5. [DOI] [PubMed] [Google Scholar]
- Oliver J. R., Wagle S. R. Studies on the inhibition of insulin release, glycogenolysis and gluconeogenesis by somatostatin in the rat islets of langerhans and isolated hepatocytes. Biochem Biophys Res Commun. 1975 Feb 3;62(3):772–777. doi: 10.1016/0006-291x(75)90466-0. [DOI] [PubMed] [Google Scholar]
- Permutt M. A., Kipnis D. M. Insulin biosynthesis. I. On the mechanism of glucose stimulation. J Biol Chem. 1972 Feb 25;247(4):1194–1199. [PubMed] [Google Scholar]
- Pipeleers D. G., Levy J., Malaisse-Lagae F., Malaisse W. J. In vitro biosynthesis and release of three immuno-reactive insulin-like components by a human insulinoma. Diabete Metab. 1975 Mar;1:7–11. [PubMed] [Google Scholar]
- Pipeleers D. G., Marichal M., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. XIV. Glucose regulation of insular biosynthetic activity. Endocrinology. 1973 Nov;93(5):1001–1011. doi: 10.1210/endo-93-5-1001. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. G., Marichal M., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. XV. Participation of cations in the recognition of glucose by the beta-cell. Endocrinology. 1973 Nov;93(5):1012–1018. doi: 10.1210/endo-93-5-1012. [DOI] [PubMed] [Google Scholar]
- Portha B., Levacher C., Picon L., Rosselin G. Diabetogenic effect of streptozotocin in the rat during the perinatal period. Diabetes. 1974 Nov;23(11):889–895. doi: 10.2337/diab.23.11.889. [DOI] [PubMed] [Google Scholar]
- Rançon F., Rosselin G. Etude comparative de l'immuno-réactivité de l'insuline, du C peptide et de la proinsuline de porc avec différents immunsérums anti-proinsuline de porc. C R Acad Sci Hebd Seances Acad Sci D. 1972 Apr 5;274(14):2112–2115. [PubMed] [Google Scholar]
- Rosselin E. G., Drouet J., Drouet A. Activité spécifique de l'iode 131 I.S.3 et de l'iode 125 mesurée par marquage de la molécule d'insuline. Rev Fr Etud Clin Biol. 1968 Oct;13(8):812–814. [PubMed] [Google Scholar]
- Rosselin G., Assan R., Yalow R. S., Berson S. A. Separation of antibody-bound and unbound peptide hormones labelled with iodine-131 by talcum powder and precipitated silica. Nature. 1966 Oct 22;212(5060):355–357. doi: 10.1038/212355a0. [DOI] [PubMed] [Google Scholar]
- Sakurai H., Dobbs R., Unger R. H. Somatostatin-induced changes in insulin and glucagon secretion in normal and diabetic dogs. J Clin Invest. 1974 Dec;54(6):1395–1402. doi: 10.1172/JCI107886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams D. J., Montague W. The role of adenosine 3':5'-cyclic monophosphate in the regulation of insulin release. Properties of islet-cell adenosine 3':5'-cyclic monophosphate phosphodiesterase. Biochem J. 1972 Oct;129(4):945–952. doi: 10.1042/bj1290945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz H., Maier V., Hinz M., Nierle C., Pfeiffer E. F. Stimulation of H-3-leucine incorporation into the proinsulin and insulin fraction of isolated pancreatic mouse islets in the presence of glucagon, theophylline and cyclic AMP. Diabetes. 1973 Jun;22(6):433–441. doi: 10.2337/diab.22.6.433. [DOI] [PubMed] [Google Scholar]
- Schatz H., Maier V., Hinz M., Nierle C., Pfeiffer E. F. The effect of tolbutamide and glibenclamide on the incorporation of ( 3 H)leucine and on the conversion of proinsulin to insulin in isolated pancreatic islets. FEBS Lett. 1972 Oct 1;26(1):237–240. doi: 10.1016/0014-5793(72)80581-7. [DOI] [PubMed] [Google Scholar]
- Sodoyez-Goffaux F., Sodoyez J. C., Foà P. P. Effects of gestational age, birth and feeding on the insulinogenic response to glucose and tolbutamide by fetal and newborn rat pancreas. Diabetes. 1971 Sep;20(9):586–591. doi: 10.2337/diab.20.9.586. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L., Nolan C., Rubenstein A. H., Margoliash E., Aten B., Oyer P. E. Proinsulin and the biosynthesis of insulin. Recent Prog Horm Res. 1969;25:207–282. doi: 10.1016/b978-0-12-571125-8.50008-9. [DOI] [PubMed] [Google Scholar]
- Tanese T., Lazarus N. R., Devrim S., Recant L. Synthesis and release of proinsulin and insulin by isolated rat islets of Langerhans. J Clin Invest. 1970 Jul;49(7):1394–1404. doi: 10.1172/JCI106357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Turtle J. R., Kipnis D. M. An adrenergic receptor mechanism for the control of cyclic 3'5' adenosine monophosphate synthesis in tissues. Biochem Biophys Res Commun. 1967 Sep 7;28(5):797–802. doi: 10.1016/0006-291x(67)90388-9. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalow T. S., Berson S. A. "Big, big insulin". Metabolism. 1973 May;22(5):703–713. doi: 10.1016/0026-0495(73)90242-4. [DOI] [PubMed] [Google Scholar]
- Zucker P., Logothetopoulos J. Persisting enhanced proinsulin-insulin and protein biosynthesis (3H-leucine incorporation) by pancreatic islets of the rat after glucose exposure. Diabetes. 1975 Feb;24(2):194–200. doi: 10.2337/diab.24.2.194. [DOI] [PubMed] [Google Scholar]



