Abstract
Pneumonia is initiated by microbes in the lung, but physiological processes integrating responses across diverse cell types and organ systems dictate the outcome of respiratory infection. Resistance, or actions of the host to eradicate living microbes, in the lungs involves a combination of innate and adaptive immune responses triggered by air-space infection. Resilience, or the ability of the host tissues to withstand the physiologically damaging effects of microbial and immune activities, is equally complex, precisely regulated, and determinative. Both immune resistance and tissue resilience are dynamic and change throughout the lifetime, but we are only beginning to understand such remodeling and how it contributes to the incidence of severe pneumonias, which diminishes as childhood progresses and then increases again among the elderly. Here, we review the concepts of resistance, resilience, and remodeling as they apply to pneumonia, highlighting recent advances and current significant knowledge gaps.
Keywords: respiratory infection, innate immunity, cytokines, lung, STAT3, NF-κB
Introduction
Pneumonia is the second-greatest burden of disease worldwide, behind only ischemic heart disease (1). It is the leading cause of death from infection in the United States (2). More children die of pneumonia than of other causes across the globe (3), and it is the most common reason for American children to become hospitalized (4). For older Americans, approximately half of all infectious disease hospitalizations and deaths are from pneumonia (5), which confers a greater risk of death than any of the other 10 most common reasons that the US elderly get hospitalized (6). As concerning as these statistics are, they underestimate pneumonia's impact. Infections of the respiratory tract complicate many diseases [e.g., HIV/AIDS, chronic obstructive pulmonary disease (COPD), cystic fibrosis, and more], which are unaccounted for in these assessments (4, 7). Furthermore, less immediate sequelae of pneumonia, such as cardiovascular disease and myocardial infarction (8), long-term defects in pulmonary function (9), and impaired cognitive abilities (10), are not factored into these disease burdens.
This review highlights biological responses elicited by lung infection and how they confer immune resistance and tissue resilience. By immune resistance, we mean host pathways that, driven by innate and adaptive immunity, diminish the number of living pathogens during infection. Immune resistance effector functions directly kill or remove microbes, such as the generation of hypochlorite by neutrophils. Affector mechanisms of immune resistance contribute indirectly, by the coordination of diverse activities to enhance effector efficacy and the consequent reduction of microbial numbers, like neutrophil recruitment elicited by chemokines. By tissue resilience, we mean the ability of the host to withstand, endure, or tolerate the stress of a given microbial challenge (11, 12). Tissue resilience pathways do not alter microbial burdens, but they diminish the pathophysiology caused (directly and indirectly) by those microbes. Tissue resilience is essential for combating damage elicited by pathogens or by immune resistance pathways. Tissue resilience includes effector plus affector mechanisms, such as antiproteases that prevent digestion of the extracellular matrix plus the cytokines that stimulate antiprotease expression, respectively. We prefer the term resilience to the term tolerance when discussing mammalian infections, because immune functions are such a critical component of infection and because the common term tolerance has a very specific different meaning in relation to immune functions.
We focus on bacterial pneumonias because they are especially common and severe (13, 14). Viral and fungal pneumonias are also important, and we sometimes extrapolate from those to resistance and resilience processes that may apply to bacterial pneumonia, identifying etiologic agents involved when doing so. We include animal studies as well as observations and experiments in humans. Animal models have strengths and limitations for elucidating the biology of pneumonia (15, 16). Our focus on the changes elicited by bacteria in the respiratory tract, and on how those changes then influence immune resistance and tissue resilience, precludes extensive discussion of pathways functioning at baseline to help prevent lung infection and injury. Transcriptional regulation is involved in changes mediating resistance and resilience. Two different transcription factors, NF-κB and STAT3, are especially implicated, and we propose that these factors facilitate distinct resistance or resilience processes (Figure 1). In most but not all cases, NF-κB drives immune resistance programs that are essential to microbial elimination but that can lead to lung injury and other pathophysiological manifestations. Conversely, we consider STAT3 primarily as a mediator of tissue resilience programs, although roles in resistance are also observed. STAT3 and other resilience pathways are essential for preventing injury, but excess can favor acute infection and promote chronic lung diseases. These central paradigms are expanded below. Unfortunately, editorial constraints mean that many outstanding and relevant studies cannot be included. Rather than summarizing the field, we emphasize a few areas that we consider to be particularly topical and timely.
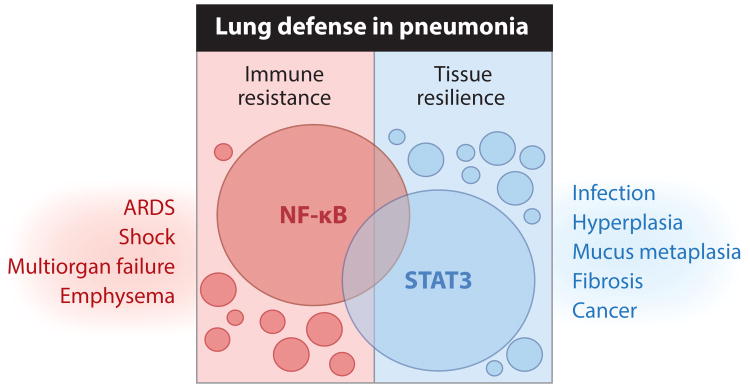
Figure 1.
Lung defense requires a careful balance between immune resistance and tissue resilience pathways, shaped largely by NF-κB and STAT3, respectively. In response to lung infection, multiple signals are initiated to limit the number of living pathogens in the respiratory tract, some of which are more broadly applicable than others (small versus large circles). NF-κB represents a prominent signaling node for achieving such immune resistance, driving the expression of numerous factors eliciting innate immunity and antimicrobial defense. Correspondingly, pathways must be initiated that enable pneumonic lung tissue to tolerate the net pathological consequences of both the infection and the resulting immune response. Gene programs controlled by the STAT3 transcription factor promote such resilience by preserving tissue integrity and preventing excessive inflammation. Although the known functions of NF-κB and STAT3 are skewed toward resistance and resilience, respectively, these pathways are capable of functioning in an opposite manner through distinct and overlapping mechanisms, represented by intersections. Regardless of their dependence on NF-κB or STAT3, overexuberant resistance or resilience pathways are sufficient to drive lung diseases such as those indicated, demanding a controlled and balanced response. ARDS denotes acute respiratory distress syndrome.
Resistance: Cell-Specific Roles that Initiate, Direct, and Amplify Antibacterial Innate Immunity
NF-κB and Innate Immunity Across Diverse Cell Types During Pneumonia
Without exception, initial interactions of microbes with the respiratory tract involve innate immunity, the speed, amplitude, and direction of which are critical to resistance (17). A pivotal mediator of innate immune responses is canonical signaling of NF-κB, whose transcriptional activity requires the RelA protein, also known as p65 (18). Targeted mutation of RelA in mice causes remarkable susceptibility to infection, with death due to infection within days to weeks of birth, even for mice in specific pathogen-free environments and on maintenance antibiotics (19, 20). Complementing these loss-of-function studies, increasing NF-κB activity in the lungs causes inflammatory injury (18), consistent with roles in antimicrobial resistance pathways that are tissue damaging. Mutations of upstream signaling intermediates that activate RelA, including MyD88, IRAK-4, and IKKβ, predispose to infections with bacterial respiratory pathogens in humans and in mice (21, 22). In short, whole-organism deficiency of canonical NF-κB signaling is profoundly immunosuppressive. RelA mutation in subsets of cells rather than in the whole organism can cause significant phenotypes during pneumonia, as discussed below, revealing unique cell roles in resistance. The phenotypes of cell-specific RelA (or IKKβ or MyD88) mutations are milder than when NF-κB signaling is targeted throughout the organism. One reason may be that NF-κB contributes to expression of the same protein from diverse cell types, continuing even when select cells cannot express it. In addition, there are cell-specific roles that depend upon NF-κB; a well-established example is E-selectin from endothelial cells. However, no cell-specific roles are essential to all innate immunity. For these reasons, widespread deletion of canonical NF-κB signaling is consistently far more detrimental than its targeted removal during pneumonia. Optimal immune resistance against bacteria in the lung requires coordinated innate immune activities from a great number of very different cell types, involving pathways of cellular redundancy and cellular specialization.
Alveolar Macrophage Roles in Pneumonia Resistance
Alveolar macrophages are professional phagocytes on the apical epithelial surface in the distal lung. They patrol these surfaces and ingest debris or other materials that are inhaled or otherwise deposited there. Alveolar macrophages avidly consume even unopsonized materials, which they dispose of by internal dissolution and/or physical removal after accessing the mucociliary escalator. The ingestion of inert particles does not trigger inflammatory responses from these cells, due to homeostatic mechanisms curbing baseline responsiveness (23). However, when these cells recognize pathogens, they produce alarm cytokines such as TNF-α, IL-1α, and IL-1β. The first burst of these cytokines during pneumonia is from alveolar macrophages and subsequently triggers rapid and coordinated responses to local infection (24). These alveolar macrophage cytokines enhance immune resistance by alerting epithelial cells, endothelial cells, neutrophils, hepatocytes, and other cells to pathogen presence in the lung (20, 25–28). When macrophages are deficient in RelA, cytokine expression and neutrophil recruitment are delayed, and pneumococcal growth is sustained (24). These functions of alveolar macrophages as sentinel cells are essential to rapid and effective immune resistance during lung infection (Figure 2).
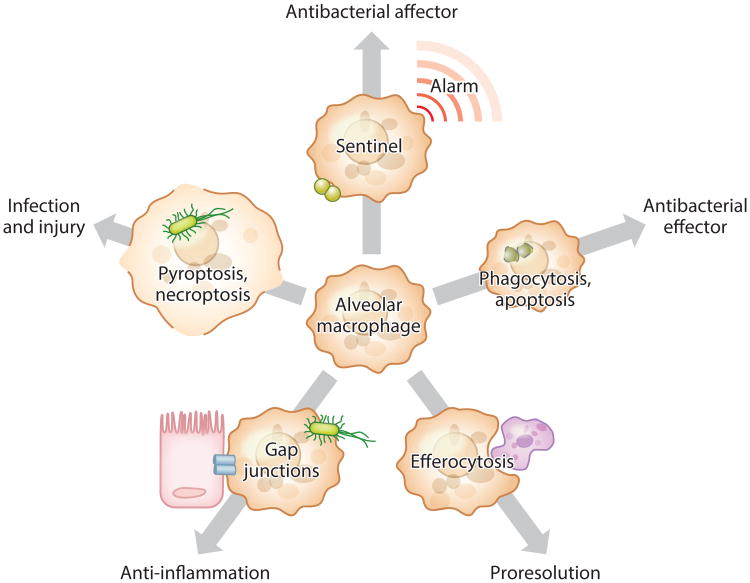
Figure 2.
Roles of alveolar macrophages in resistance and resilience during pneumonia. As sentinel cells, alveolar macrophages sound the initial alarm and are essential to the rapid activation of other cells, the recruitment of neutrophils, and the killing of bacteria. In addition, alveolar macrophages are effectors of antibacterial resistance, capable of killing ingested bacteria, especially when undergoing apoptosis. The apoptosis of other cells in the lung stimulates alveolar macrophage efferocytosis, which eliminates dying leukocytes, prevents their release of danger signals in the lungs, and switches macrophage phenotypes to producing anti-inflammatory and proresolving signals. Select subsets of alveolar macrophages become nonmotile and adhere to the epithelium via gap junctions, through which these macrophages transmit Ca2+ waves that dampen inflammatory signaling. However, when nonapoptotic pathways of cell death are triggered by microbial virulence factors, alveolar macrophages become poor bacteria killers and release powerful proinflammatory signals that can exacerbate lung injury.
Typically, alveolar macrophages develop neonatally, proliferate locally, and live for many years as a stable population that does not rely on replacement by blood-borne precursors (29, 30). Alveolar macrophages can survive the stresses of acute inflammation and remain present following resolution; whereas newly recruited phagocytes are eliminated, the original alveolar macrophages persist (31). However, there are clear examples of alveolar macrophages dying during lung infection, which has significant consequences for immune resistance.
In murine models of pneumococcal pneumonia as well as in interactions of cultured cells with pneumococcus, alveolar macrophages can be driven to die by apoptosis (32). This outcome is influenced by bacterial burden per macrophage, host factors such as opsonins and macrophage Mcl-1, and bacterial factors such as toxins and capsular polysaccharides (33). Apoptotic alveolar macrophage death facilitates the killing of internalized pneumococci (32, 33). Consistent with this postulated pathway of protection, TRAIL is a cytokine that induces macrophage apoptosis, and TRAIL-deficient mice have decreased macrophage apoptosis and increased bacterial burdens during pneumococcal pneumonia (34). Thus, the apoptotic death of alveolar macrophages enhances immune resistance during pneumococcal pneumonia (Figure 2).
In contrast, alveolar macrophage death by other pathways enhances microbial growth. For example, during Pseudomonas aeruginosa pneumonia, pyroptotic pathways are activated, and diminishing pyroptotic pathways by diverse mechanisms consistently decreases bacterial burdens and diminishes inflammatory injury (35). Although roles of alveolar macrophages per se and direct connections between pyroptosis and bacterial killing were not investigated, these studies support the emerging concept that alveolar macrophage death by pyroptosis may be detrimental to immune resistance of the lungs. Similarly, during pneumococcal pneumonia, chitinase 3 -like 1 (Chi3l1) is essential (36). Chi3l1 prevents macrophage pyroptosis and enhances the killing of internalized bacteria, which may be causally related and applicable to alveolar macrophages during pneumonia (36). Necroptosis is another pathway of cell death and (to our knowledge) has yet to be examined in the context of pneumonia. Microbial factors that directly induce alveolar macrophage necrosis, such as pneumolysin from pneumococcus or PB1-F2 from influenza virus, can promote respiratory infection, perhaps via that mechanism (37, 38). Autophagy pathways are involved in lung infections (39) and may help direct these coordinated macrophage resistance processes during pneumonia. Autophagy diminishes pyroptotic cell death in Legionella-infected macrophages (40) and enhances macrophage killing of ingested P. aeruginosa (41) and Candida albicans (42). Lines of evidence converge to suggest that alveolar macrophage death by nonapoptotic routes compromises immune resistance.
Lung Epithelial Cell Roles in Pneumonia Resistance
Forming the cellular surface of the respiratory tract, the epithelium helps combat respiratory infection. Constitutive functions of epithelial cells have well-described resistance functions, such as those involving the mucociliary escalator (43, 44) and surfactant proteins A and D (45). However, inducible epithelial responses that contribute to immune resistance have been more difficult to discern. Multiple studies demonstrate epithelial cell responses as important, including investigations showing diminished pulmonary innate immunity after epithelial cell-specific interruptions of canonical NF-κB signaling (17). These loss-of-function studies are complemented by a gain-of-function approach involving a CC10-driven transgene; whereas P. aeruginosa grows inexorably in lungs of MyD88−/− mice, P. aeruginosa is effectively killed in lungs of MyD88-deficient mice with MyD88 transgenically expressed in airway epithelial cells (46). Thus, MyD88 only in epithelial cells confers host defense to otherwise highly susceptible MyD88-deficient mice. Although epithelial cell responses are clearly crucial to pneumonia outcome, their precise roles in resistance are unknown.
Epithelial cells respond to respiratory infection with the production of diverse bactericidal or bacteriostatic compounds. In our opinions, none of these have been demonstrated to individually exert especially profound effects during infection. β-Defensin antimicrobial proteins can be induced in cultured respiratory epithelial cells, but there is scant evidence for β-defensin induction during pneumonia or for contributions of β-defensins to integrated pulmonary resistance. The deficiency of murine β-defensin 1 was demonstrated to significantly exacerbate Haemophilus influenzae infection (47), but little else beyond this single decade-old study implicates this family of antimicrobial proteins in integrated resistance during pneumonia. In contrast to defensins, the chemokine CCL20 is consistently and strongly induced in epithelial cells of infected lungs (48, 49), and CCL20 has β-defensin-like antimicrobial activities in vitro (50). This finding suggests that epithelial cell-derived CCL20 may be an antimicrobial protein in the air spaces, but there is no direct evidence for this possibility to our knowledge. The chemical demands for CCL20's antibacterial activity (including very low salt concentrations) temper enthusiasm for this otherwise intriguing concept. Antimicrobial proteins in the cathelicidin, lysozyme, and lipocalin families increase and function in infected lungs, but their sources may more likely be leukocytes (51–54). RegIIIγ is a C-type lectin that is induced severalfold in lung infection, particularly prominently in epithelial cells, and antibodies against RegIIIγ increase living Staphylococcus aureus ∼10-fold (55). However, the majority (>99.9%) of S. aureus are effectively eliminated within less than a day even with RegIIIγ blockade, suggesting a modest scope. Splunc1, related to bactericidal/permeability-increasing protein, is an epithelium-derived protein whose expression increases severalfold during pneumonia, and its deficiency impairs resistance (56, 57). This resistance defect may be due to Splunc1 surface tension modulation that limits biofilm formation by P. aeruginosa and Klebsiella pneumoniae (56, 57). Whether its induction (or instead its high basal levels) is critical to immune resistance is uncertain. Analogous to neutrophils killing microbes with myeloperoxidase-derived hypochlorite, epithelial cells can kill microbes via lactoperoxidase generation of hypothiocyanate and hypoiodous acid (58, 59). This role may be important to airway defense, but data so far are based largely on cell culture and gain-of-function approaches, leaving the net significance of epithelial cell–derived hypohalous acids in natural defenses unsettled. The activities of epithelial cells in driving hypohalous acid defense in vivo, including the dynamics of lactoperoxidase and its substrates (e.g., H2O2, SCN−, and I−) during infection, require further study.
Does the absence of evidence demonstrating individually vital roles for select epithelium-derived products suggest modest effector roles for this cell type? We think not. Airway host defense can be pharmacologically activated by bacterial products or their mimetics, which is astonishingly protective against a very wide variety of even highly pathogenic microbes (60). Activation of the epithelium is required for this protection, as demonstrated by the abrogation of this immune resistance by the epithelium-specific, surfactant protein C–driven targeting of MyD88 (61). That these improved defenses can be modeled in epithelial cell cultures (61) suggests that this extraordinary protection involves antimicrobial effector functions of these cells, perhaps a combination of those described above and others yet to be discovered. In addition, the extraordinary defenses downstream of epithelial activation may also involve epithelial affector roles in immune resistance.
Epithelial cells make a great many immune mediators during pneumonia, but very few inducible immune mediators have been demonstrated to be uniquely or nearly uniquely derived from these cells. During pneumococcal pneumonia, CXCL5, GM-CSF, and CCL20 are specifically epithelial in origin (48, 49), which may apply more broadly to other lung infections. CXCL5 is an ELR-positive CXC chemokine with complex roles during pneumonia. It can be essential to neutrophil recruitment in settings of modest stimulation, but it conversely limits neutrophil recruitment during severe infection (62). GM-CSF is a growth factor with pleiotropic effects in myeloid cell dynamics and is essential to maximal neutrophil recruitment in inflamed lungs (63). During pneumococcal pneumonia, the interruption of canonical NF-κB signaling throughout the epithelium compromises neutrophil recruitment, and this result can be reversed by increasing either CXCL5 or GM-CSF, suggesting that lung concentrations for these two cytokines are limiting, NF-κB dependent, epithelium derived, and essential to rapid neutrophil recruitment (48). In addition, GM-CSF from epithelial cells enhances the recruitment and function of CD103+ dendritic cells during influenza infection, facilitating the accumulation of influenza-specific, IFNγ-secreting CD4+ and CD8+ T cells in the infected lung (64) and thus providing evidence for epithelial responses orchestrating adaptive immunity. During respiratory syncytial virus (RSV) infection, CCL20 and its receptor CCR6 contribute to the recruitment of conventional dendritic cells to the lung, driving immunopathology (65). In neutropenic mice, CCR6 enhances dendritic cell recruitment and antifungal lung defense (66), which may be downstream of CCL20. Potentially productive roles of CCL20 generation by epithelial cells during lung infection remain uncertain. A fundamental underlying question is why epithelial cells should be specific sources for these or other immune signals. Furthermore, the few epithelium-specific signals so far identified are likely not the only (or most important) infection-induced products of this interface between the lungs and the external environment, indicating that a more comprehensive description of epithelium-specific responses in the pneumonic lung is needed.
Although the above studies emphasize NF-κB signaling, epithelial STAT3 functions in immune resistance as well. Mice with Stat3 targeted in their lung epithelium have increased microbial burdens during diverse lung infections (18, 55, 67, 68). Signaling to epithelial STAT3 that drives pulmonary immune resistance comes from IL-6, IL-22, HVEM, and likely others (55, 63, 69, 70). Pathways downstream of epithelial STAT3 and driving immune resistance may constitute both effector and affector roles. Although demonstrably significant, the epithelial products and pathways contributing to immune resistance downstream of STAT3 have yet to be compellingly defined.
Neutrophil Roles in Pneumonia Resistance
Neutropenia, whether purposefully induced in experiments or arising naturally from genetic or environmental causes, consistently predisposes to lung infection. Thus, neutrophils are an essential component of immune resistance to respiratory pathogens. Neutrophils are typically portrayed as professional antimicrobial effector cells, involving phagocytosis, degranulation, the respiratory burst, antimicrobial proteins, and neutrophil extracellular traps (NETs). The loss of these antimicrobial effector functions is almost certainly a major reason that neutropenic subjects are prone to pneumonia.
Emerging evidence suggests that, in addition to their effector roles, neutrophils are also affector cells. Neutrophils are important sources of immune mediators like cytokines and eicosanoids, with neutrophil-derived signals influencing innate and adaptive immune responses (71). The concept of affector neutrophils is only beginning to be applied to the lung. In bioinformatics analyses of severe influenza infections, a transcriptional signature associated with lethality emerges, and profiling of distinct cell types within these lungs demonstrates that neutrophils are the source of the signature (72). The neutrophil program involves a positive feedback loop by which neutrophils in the infected lungs recruit and activate more neutrophils, with diminishing neutrophil recruitment capable of preventing mortality (72). There are likely many components to this positive feedback, including an unexpected loop in which neutrophil-derived CXCL10 recruits and activates CXCR3+ neutrophils in lung injury settings, including influenza infection (73). Whereas those cases suggest that neutrophil-driven neutrophil accumulation exacerbates immunopathology during severe lung infection, other settings reveal protective resistance roles for affector neutrophils. For example, during pneumococcal pneumonia in mice, IFNγ is essential to maximal immune resistance, and the sole source of IFNγ in these lungs is recruited neutrophils (74). This IFNγ promotes antibacterial NETs (74), again emphasizing neutrophil-to-neutrophil signaling. Neutrophils also signal to other cells during pneumonia. As mentioned above, TRAIL stimulates alveolar macrophage apoptosis and is essential to effective host defense against pneumococcus (34). The primary source of TRAIL during pneumonia is neutrophils, and depletion of neutrophils results in decreased TRAIL and less alveolar macrophage apoptosis (34). Because alveolar macrophage apoptosis is an antibacterial effector pathway (Figure 2), this neutrophil-derived paracrine signal enhances resistance in the lung. Finally, affector roles of neutrophils outside the lungs may also help fight respiratory pathogens. Neutrophils in the marginal zone of the spleen function as B helper cells by elaborating cytokines like BAFF, APRIL, and IL-21 that facilitate the development of antibodies against T cell–independent antigens (75). Consequently, neutropenic patients have decreased antibodies against respiratory pathogens such as pneumococcus, P. aeruginosa, and Escherichia coli (75). Thus, affector neutrophils influence lung infection in diverse fashions. Little is known about transcriptional regulation in neutrophils; where, when, and how neutrophil affector functions influence resistance to respiratory pathogens must be better defined.
Hepatocytes as a Source of Endocrine Immune Resistance
Not all cells mediating resistance against respiratory infection are in the lung. The immune system is integrated across organs, and innate immune resistance against respiratory pathogens requires coordinated efforts of cells in the bone marrow, spleen, lymph nodes, gastrointestinal tract, adipose tissue, brain, and more. The liver mobilizes an impressive armament of immunomodulatory agents, which it secretes directly into the blood to serve endocrine innate immune functions. These rapid changes in blood plasma constituents form the acute phase response, first discovered in pneumonia patients (76) and now recognized to follow virtually any form of physiological duress. Dozens of acute phase proteins are linked to a variety of immunomodulatory and tissue-protective functions (77). Changes in acute phase proteins are biomarkers in diseases such as pneumonia. However, the net functional significance of these acute changes in plasma components remains remarkably speculative.
Intrapulmonary delivery of bacteria or bacterial products elicits a rapid and robust acute phase response (25, 37, 78, 79). The liver alters expression of more than a thousand genes during pneumococcal pneumonia (79). Hence, lung infection and host signals of pulmonary origin recalibrate the hepatic transcriptome in a lung-liver axis. During bacterial pneumonia, these liver-activating signals include IL-6, TNF-α, IL-1α, and IL-1β (25). Genetic deletion of IL-6 impairs liver acute phase protein expression and ablates Stat3 activation (25). RelA is also activated in the liver during pneumonia, but activation of this transcription factor depends instead on TNF-α and IL-1 (25, 80). As in the case of IL-6, the interruption of TNF and IL-1 signaling diminishes hepatic acute phase protein induction (25). These cytokines are critical during pneumonia (17), and the liver is a conduit through which these cytokines exert roles in resistance.
Determining the direct influence of hepatocyte activation on pneumonia outcomes requires an effective and precise way of severing the lung-liver axis. Because both Stat3 and RelA were implicated in acute phase biology, we targeted them in hepatocytes (79). Simultaneous mutation of both Stat3 and Rela, but not mutation of either alone, ablates the hepatic transcriptional response to pneumococcal pneumonia (79). This hepatocyte-specific deficiency of both Stat3 and RelA prevents all changes in the plasma proteins that were measured (79), revealing that liver transcription is responsible for these humoral events, with similar effects on hepatic acute phase protein expression observed for gram-negative pneumonia and diverse other inflammatory challenges (79). Mice with no hepatic acute phase responses due to targeting of Stat3 plus RelA have more severe infections, including worse bacteremia during pneumococcal pneumonia (79). Acute phase remodeling of blood plasma enhances opsonization of blood-borne pneumococcus and phagocytosis by macrophages (79), demonstrating that one function of the hepatic acute phase response is to provide a vascular shield against disseminated infection.
Although it is clear that the lung-liver axis is both present and relevant, the overall impact of hepatocyte activity during pneumonia is poorly defined. A prevailing question centers on the identity of liver-derived products influencing pneumonia outcomes. Changes in short pentraxins such as serum amyloid P (particularly dynamicin mice) or C-reactive protein (particularly dynamic in humans) may contribute, as these are in effect functional equivalents to antibody (81) and are required for bacterial opsonization and resistance during pneumococcal pneumonia in mice (82). Other known acute phase proteins, such as lipopolysaccharide (LPS)-binding protein, serum amyloid A, and complement, affect immune resistance and pulmonary inflammation. Yet, for most of these proteins, the primary cellular sources (hepatocyte or other) and dynamics (roles of basal levels or changes in expression) are yet to be discriminated during pneumonia. The net contribution of these and other acute phase proteins is likely attributable to integrated changes in a wide array of mediators such that aiming to identify the most important factor(s) may be of limited significance, if not impossible. An additional but tremendously important layer of complexity related to the lung-liver axis is the fact that secreted acute phase proteins represent only a fraction of the many transcriptional changes observed in livers during pneumonia; other gene expression changes play additional important roles (37, 78, 79). For instance, liver responses during pneumococcal pneumonia include enrichment for genes linked to cholesterol biosynthesis (37), and the plasma increases in cholesterol during the acute phase response lead to its increase in the infected air spaces, to inactivation of the cytotoxin pneumolysin, and to diminished alveolar macrophage necrosis (37). Many questions remain about how hepatic transcriptional activities influence immune resistance, including effects on leukocytes and lung cells and their effector and affector roles during infection.
Resilience: Tissue-Protective Pathways Countering Lung Injury
Immune resistance can be as dangerous as the microbes activating it. Tissue resilience balances the physiological damage from microbes and immune resistance pathways (11, 12). The signals governing resilience in mammals are more obscure than those promoting resistance, representing an important direction for researchers interested in the physiology of infection. During pneumonia, inadequate tissue resilience promotes lung injury. Pneumonia is the most common cause of acute respiratory distress syndrome (ARDS), with current mortality levels of 20–30% accounting for approximately 200,000 deaths annually in the United States (83). A better understanding of resilience mechanisms during pneumonia will help elucidate pathways that break down in patients with or at risk for ARDS and that are potentially targetable for therapeutic, prognostic, and/or diagnostic purposes.
Inducible Tissue Resilience Mediated by STAT3
Essential roles for STAT3 are evident in patients with hyper-IgE syndrome (HIES), formerly known as Job's syndrome (84). The disease is caused by a dominant negative mutation in the STAT3 gene, resulting in approximately 25% of normal STAT3 activity in the cells of patients heterozygous for the mutation. HIES patients suffer from recurrent lung infections, beginning in infancy and continuing throughout their lives. Most HIES patients die prematurely from pneumonia and its consequences (85), likely due to myriad essential roles of STAT3 in both resistance and resilience (Figure 3). As noted above, STAT3 in lung epithelial cells and in hepatocytes contributes to immune resistance. In addition, HIES patients have defective development of Th17 cells (86), discussed below as an important resistance mechanism involved in immunological remodeling. Roles of STAT3 in tissue resilience are evident as well. Cells from these patients have impaired responses to the anti-inflammatory cytokine IL-10 (87). HIES patients universally develop large pneumatocoeles, often requiring surgery, reflecting an abrogated lung repair process and extraordinarily dysfunctional tissue resilience (84). STAT3 contributes to tissue resilience in many ways, including the promotion of multipotency, proliferation, and migration as well as the prevention of apoptosis and other cell death pathways (18).
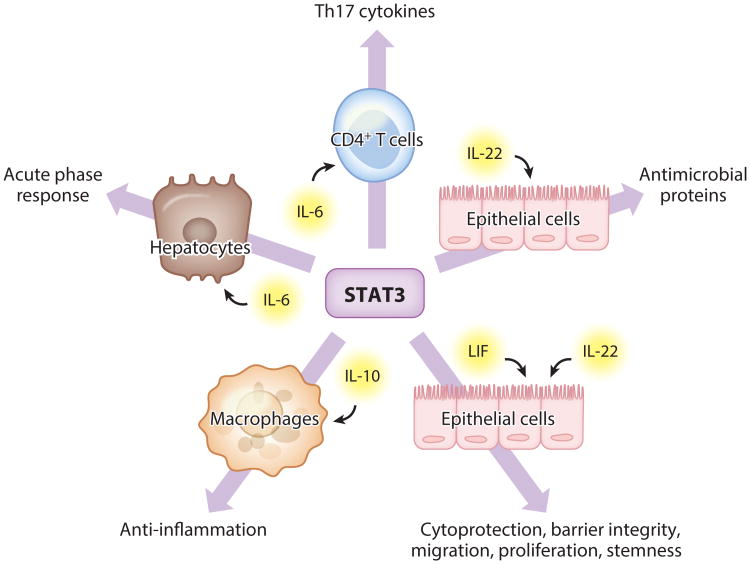
Figure 3.
The STAT3 transcription factor facilitates diverse processes paramount to pneumonia biology both inside and outside of the lungs. The indicated outcomes represent consequences of STAT3 activity downstream of IL-6, leukemia inhibitory factor (LIF), IL-22, and IL-10 in select cell types during pneumonia. These outcomes are critical determinants of immune resistance (antimicrobial proteins, acute phase response, Th17 cytokines), tissue resilience (anti-inflammatory signals, epithelial preservation, acute phase response), and immune remodeling (Th17 cytokines). Pathways shown are those highlighted in the review and reflect only a fraction of the known roles of STAT3 that may be relevant to pneumonia.
Lung Stat3 activation is characteristic of the acute pulmonary inflammation elicited by hyperoxia, ozone, immune complexes, LPS, viruses, or bacteria (18). Lung epithelium–specific targeting consistently supports a role for Stat3 in tissue resilience. Stat3 mutation in alveolar and/or airway epithelium exacerbates lung injury across a wide variety of stimuli tested, including LPS, hyperoxia, naphthalene, and adenovirus (67, 88–90). In some cases, lung injury has been linked to defective expression of antiapoptotic gene programs due to Stat3 deficiency, although mechanisms downstream of Stat3 are complex, multifactorial, and typically not well-defined (67, 88–90). During E. coli pneumonia, epithelial Stat3 is required for tissue resilience, as demonstrated by severe alveolar edema in infected mice with Stat3 mutations in those cells (68). This tissue resilience defect seems the likely cause of excess pathophysiology in these mice because bacteria get effectively cleared despite compromised immune resistance (68). Complementing the STAT3 loss of function in mouse studies and HIES patients, transgenic Stat3 gain of function in lung epithelial cells can protect against acute lung injury (91) but also results in chronic lung diseases such as adenocarcinoma (92).
Many factors may activate STAT3 in pneumonic lungs, and IL-6 is only partly responsible (93). Using a bioassay comparing air-space cytokine activities, we found that leukemia inhibitory factor (LIF) is even more prominent than IL-6 or any other cytokine in the IL-6 family in activating epithelial Stat3 (68). LIF is a pleiotropic cytokine most widely appreciated for its capacity to preserve the totipotency of embryonic stem cells, suggesting potential roles in tissue regeneration and repair. Other known functions of LIF involve fertility, development, and hematopoiesis. LIF is present at elevated concentrations in pleural effusions (94) and bronchoalveolar lavage fluid collected from ARDS patients (95). A rare but severe disease, Stuve-Wiedemann syndrome, is caused by null mutations in the LIF receptor (96). Respiratory distress represents one of several defining characteristics of Stuve-Wiedemann syndrome, which typically results in mortality within the first few months of life. During E. coli pneumonia in mice, LIF blockade results in devastating lung injury that is independent of resistance signals like neutrophilia, proinflammatory cytokines, and bacterial clearance (97). LIF potently activates Stat3 in airway and alveolar epithelial cells of mice, and transcriptional profiling of pneumonic lungs with LIF blockade reveals an enrichment of genes belonging to many functional categories related to tissue resilience (e.g., cell death, cell cycle, and energy production) (97). These results suggest that LIF activation of Stat3 in epithelial cells helps preserve barrier integrity and function during infection (97). Constructing mechanistic pathways will require further knowledge about the sources, targets, and direct consequences of LIF and STAT3 signaling during pneumonia.
Lung Epithelial Cell Roles in Pneumonia Resilience
Epithelial cells have multiple functions providing tissue resilience during pneumonia. Epithelial barrier integrity limits lung injury by preventing fluid access to the air spaces, which is dependent upon regulated epithelial cell tight junctions (98). During pneumonia, IL-22 stimulates resilience by enhancing this barrier integrity (99). Epithelial cells further counter air-space edema accumulation by effectively pumping fluid out of the lumen via Na+-dependent transport, which is enhanced by endogenous epinephrine and glucocorticoids (98). As discussed above, Stat3 activation in the epithelium, by LIF and other factors, is cytoprotective and critical to limiting lung injury (67, 68, 88–90, 97). When epithelial cells die in the infected lung, tissue resilience demands their rapid replacement via carefully coordinated proliferation and differentiation activities of epithelial and/or progenitor cells. Epithelial cell–based repair processes implicated during lung infection include actions of IL-22 and Stat3 (100, 101), β-catenin (102), FOXM1 (103), and p63 (104). Such regenerative pathways in the lung extend beyond epithelial cells to include multipotent stem cells (105), an emerging area with promise for repairing lungs injured by infection.
Mucus is a constitutively expressed epithelial product serving as a platform for eliminating microbes from the airways via mucociliary clearance (44). Enhanced mucus production is triggered by airway infection but is temporally associated with the resolution stages rather than with early or peak microbial activity. The exaggerated mucus is sustained long past the point at which it can productively benefit resistance, suggesting that mucus hyperproduction is more closely related to resilience. In support of this, evidence connects mucus hyperproduction to anti-inflammatory effects. First, the biosynthetic pathways of mucus metaplasia have direct anti-inflammatory signaling activities. For example, the SPDEF transcription factor definitive for mucus metaplasia inhibits MyD88 and TRIF and the activation of NF-κB in those mucus-elaborating epithelial cells, and overexpression of this transcription factor decreases neutrophil recruitment and compromises antibacterial defense in the mouse lung (106). Analogously, the FOXA3 transcription factor enhances mucus production and simultaneously suppresses expression of inflammatory and immune resistance genes (107). Second, mucins themselves are anti-inflammatory. This fact is especially well established for the membrane-bound mucin MUC1. MUC1 suppresses epithelial NF-κB activation in an autocrine fashion by interrupting signaling from Toll-like receptors (108). During P. aeruginosa pneumonia, MUC1 expression peaks after >99.9% of bacteria are already eliminated, remains elevated long past any detection of live microbes, and is essential to the rapid resolution of pulmonary inflammation (108, 109). Mucins can also be paracrine anti-inflammatory signals, as elegantly demonstrated in the gastrointestinal tract, where MUC2 is an immunoregulatory agent stimulating anti-inflammatory pathways in dendritic cells through a novel, multicomponent receptor complex triggering the β-catenin pathway (110). Together, the kinetics plus functional data strongly suggest that mucus hyperproduction triggered by lung infection functions primarily as an anti-inflammatory and proresolution tissue resilience program.
Alveolar Macrophage Roles in Pneumonia Resilience
Alveolar macrophages can be directed away from their sentinel alarm states (Figure 2) to instead actively curb inflammation and promote tissue repair. Environmental cues can push alveolar macrophages toward an M2 alternatively activated macrophage state in which they generate factors like IL-10 and IL-1RA (23, 111). The M2 state has been observed in the resolution stages of acute respiratory infections by P. aeruginosa (112), S. aureus (113), or influenza virus (23). Consensus is yet to emerge on functional significance or if, when, and how alveolar macrophages polarize toward M2-like phenotypes with lung infections (23, 111). Intravital microscopy has recently revealed that, following an intrapulmonary challenge with LPS or P. aeruginosa, a fraction of alveolar macrophages become immobilized on the alveolar surface (114). These sessile macrophages form connexin 43–containing gap junctions with the underlying epithelium, through which they coordinate Ca2+ signaling. Ca2+-dependent Akt activation reduces alveolar neutrophilia and cytokine induction in wild-type mice, in contrast to the case for mice in which intercellular gap junctions are eliminated by mutation of connexin 43 in either macrophages or epithelial cells (114). This presents a novel perspective in which a subset of alveolar macrophages is nonmotile, connected physically and chemically with the epithelium, and primarily anti-inflammatory (Figure 2). Our understanding of these sessile cells is in its infancy, and their development, phenotype, and function merit substantial further exploration.
As implied by their original moniker dust cells, alveolar macrophages are waste-collecting cells in the air spaces. This is also true in the resolution stages of pneumonia, when alveolar macrophages help dispose of the dead and dying inflammatory cells that were recruited for immune resistance. The phagocytosis of apoptotic cells by macrophages, known as efferocytosis, eliminates effete leukocytes, prevents the necrotic release of intracellular danger signals, and actively suppresses inflammation (Figure 2). Efferocytosis reprograms macrophages to diminish expression of proinflammatory cytokines and to promote expression of anti-inflammatory cytokines, and efferocytosis by alveolar macrophages induces TGFβ to enhance resilience during acute pulmonary inflammation (115). Efferocytosis occurs in concert with the induction of proresolving lipid mediators such as lipoxins, resolvins, protectins, and maresins (116). These mediators form a positive feedback loop in which efferocytosis stimulates the generation of lipid mediators that stimulate efferocytosis, with alveolar macrophages being both a source and a site of action for these lipids (116). Difficulties in inhibiting the endogenous generation of or signaling by specific members of this proresolution lipid family has complicated the dissection of their natural roles during pneumonia, but they are made in the injured lung and have proresolving and anti-inflammatory resilience properties (116). Suggesting therapeutic potential, administration of exogenous proresolving lipid mediators improves lung injury in mouse models (116).
As emphasized above, alveolar macrophage sentinel functions are essential to effective defense, so tissue resilience pathways that blunt alveolar macrophage responses may be deleterious by fostering microbial growth. Lipocalin 2 (Lcn2), which enhances immune resistance against siderophore-dependent bacteria like K. pneumoniae (53), is also an alveolar macrophage–deactivating factor via an autocrine IL-10 and STAT3 loop (54). During pneumonia caused by pneumococcus (which does not express siderophores), Lcn2 is abundant in the patients' air spaces, high Lcn2 levels correlate with worse patient outcomes, and Lcn2-deficient mice have far improved defenses (54), altogether suggesting that Lcn2-deactivated alveolar macrophages are counterproductive in such infections. Inactivation of alveolar macrophages by other mechanisms, such as sepsis-induced IRAK-M expression (117), is also deleterious for compromising immune resistance. Just as too much activity in immune resistance pathways fosters lung injury, too much activity in tissue resilience pathways can exacerbate lung infection (Figure 1).
Lymphocyte Roles in Pneumonia Resilience
Lymphocytes are professional immunoregulators and sources of resilience mediators like TGFβ, IL-10, and IL-22. After a bolus of LPS in the lungs, regulatory CD4+ T cells expressing CD25 and FOXP3 (Tregs) accumulate in the lungs during resolution, and these Tregs are sufficient and necessary to limit lung injury through TGFβ expression (118). Similarly, CD8+ T cells have been shown to provide resilience in response to influenza and RSV infections, as sources of IL-10, which curbs lung injury (119); such roles of CD8+ T cells outside of viral infection remain less certain. NK cells are sources of IL-22 and its contributions to resilience during K. pneumoniae or influenza pneumonia (100, 120). A novel set of tissue-protective lymphocytes was identified during influenza infection as a population of lineage-negative CD90+CD25+ innate lymphoid cells that promote resilience through expression of the EGF family member amphiregulin (121). Amphiregulin was also protective when provided exogenously in a model of influenza-induced depressed tissue resilience against Legionella pneumonia (122). Roles of lymphocytes in pneumonia require further study, as emphasized below.
Roles of Other Cells in Resilience During Pneumonia
Tissue resilience is also observed in other tissues and derived from other cells. The pronounced transcriptional responses of hepatocytes described above places physiological stress on the liver. Stat3-mediated gene expression helps mitigate this stress, inducing unfolded protein response genes and secretory protein genes to counter endoplasmic reticulum stress in hepatocytes (78). Although canonical NF-κB signaling is especially important in resistance, it has roles in resilience as well, particularly in the liver. RelA prevents death receptor–induced apoptosis, which is essential for preventing liver injury after a systemic high dose of TNF-α or LPS challenge (123). Liver injury is not observed in mice with RelA mutated in their hepatocytes during pneumococcal pneumonia (79), but whether and when RelA-induced resilience protects the liver during lung infection require more study. In addition to protecting the liver, the acute phase response involves the synthesis of many factors with tissue-protective properties, such as antiprotease and antioxidant proteins (77). These hepatic actions may help protect the lungs from injury during pneumonia, but this is presently speculative. During systemic sepsis, gp130-dependent Stat3 activity in hepatocytes is required for the mobilization and tissue accumulation of a myeloid-derived suppressor cell (MDSC) population generating anti-inflammatory signals to prevent inflammatory injury (124). Although the degree to which MDSCs rely on the lung-liver axis is unknown, a population of MDSCs accumulates in the lungs during K. pneumoniae pneumonia; these cells can efferocytose apoptotic neutrophils and synthesize IL-10, implicating them as local contributors to resilience (125). Inflammatory monocyte macrophages are another myeloid source of recruited resilience. Following intrapulmonary challenges with either LPS or K. pneumoniae, exudate macrophages are sources of IL-1RA, without which lung injury is exacerbated (126). And in a model in which exposures to low doses of LPS protect mice from subsequent and otherwise lethal doses of LPS or P. aeruginosa, IL-10 is the protective factor and derived from recruited monocyte macrophages (127). Finally, the bone marrow–derived mesenchymal stem cell can be a source of cell-mediated tissue resilience and represents a promising therapeutic strategy. In the context of pneumonia, delivery of mesenchymal stem cells markedly diminishes acute lung injury in mouse models of pneumonia (128) and in human models of injury to isolated and perfused lung preparations (129). The potential contribution of mesenchymal stem cells to therapeutic use exemplifies the translational importance of understanding whether and how resilience pathways can be calibrated in pneumonic lungs.
Remodeling: Responses to Lung Infection Differ Throughout a Lifetime
Pneumonia over a Life Course
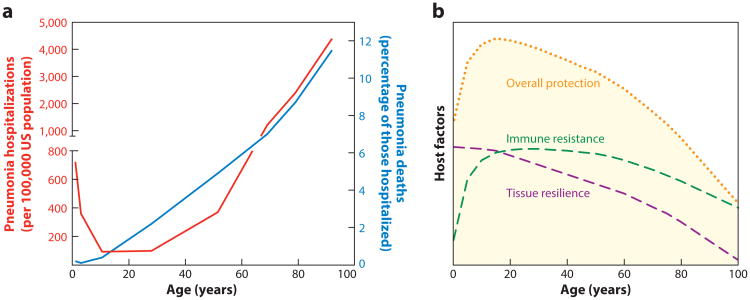
Pneumonia hospitalization rates plummet during early childhood, remain low through young adulthood, and rise again in later years (Figure 4a). Alterations in resistance likely contribute to both the early deceleration and the later acceleration of pneumonia rates (Figure 4b). For those with pneumonia, the risk of death steadily increases over the life course (Figure 4a), suggesting that withstanding the challenges of pneumonia becomes more difficult as we age, perhaps because of declines in tissue resilience (Figure 4b). We propose that the combined decreases in both immune resistance and tissue resilience are responsible for pneumonia being so excessively common and unacceptably severe among the elderly. The ways in which resistance and resilience vary over a lifetime are poorly understood and are an area in which new insights could have especially substantial impacts.
Figure 4.
Remodeling responses to pneumonia throughout the lifetime. (a) Effect of age on pneumonia in the United States, from 2007 to 2009. The red line shows age-stratified hospitalizations for pneumonia of any cause per 100,000 US population, reflecting a U-shaped curve between birth and retirement ages, followed by a steeper incline to far greater hospitalization rates among the elderly (note break in y-axis at left). The blue line shows rates of in-hospital death related to pneumonia from any cause, reflecting a steady increase beginning in early childhood and continuing throughout the ensuing years. The graph was constructed from data communicated by Griffin et al. (155), with ages plotted as the midpoint of each age range reported. (b) Conceptual framework portraying how changes in resistance and resilience may be responsible for the effects of age on pneumonia (as in panel a). We propose that immune resistance increases dramatically in childhood due to the development of heterotypic adaptive immunity and lung-resident memory against respiratory pathogens, which together protect younger adults but (we predict) wane in the elder years. We propose that tissue resilience declines throughout the lifetime, increasing pneumonia death rates even among those with strong resistance and then synergizing with the decreasing resistance in the elder years to render pneumonia incidence and severity especially elevated at those ages. Overall protection against pneumonia, plotted here as a simple sum of resistance plus resilience, plummets when both parameters of lung defense simultaneously decrease. Panel b does not reflect actual data but rather reflects a conceptual framework that we constructed to communicate the paradigms spotlighted in this review.
Heterotypic Immune Protection in the Trough Period of Low Susceptibility
Even in people with deficiencies of MyD88 or IRAK-4, severe infections with respiratory pathogens decrease dramatically by late childhood (21), revealing that protection involves factors other than the maturation of innate immunity. Patients with defects in adaptive immunity, as are seen in asplenia or AIDS, are very susceptible to pneumonia as adults. A considerable component of adaptive immune protection against respiratory pathogens appears to involve heterotypic immunity, pathways that are elicited by conserved epitopes within a species and provide effective but suboptimal protection (e.g., less than type-specific immunity within a serotype or strain). This is particularly well documented for influenza. Both observational and experimental studies demonstrate that humans with preexisting memory T cells responsive to conserved influenza epitopes have less severe respiratory infection by influenza viruses to which the subjects were previously naive (130, 131). Protective correlates include CD4+ and CD8+ memory T cells, IFNγ expressing and cytotoxic in both cases (130, 131). Mouse models reveal similar heterotypic protection and activities from CD4+ and CD8+ cells during influenza pneumonia (119, 132). Thus, naturally acquired heterotypic immune responses substantially limit the severity of respiratory infection by new strains of influenza virus.
Heterotypic immunity is implicated for bacterial pneumonias as well. In mouse experiments, adjuvanted killed bacteria vaccines (for pneumococcus or K. pneumoniae) elicit heterotypic protection against diverse serotypes of those bacteria, and this serotype-independent protection depends on Th17 cells (and neutrophils), but not on antibodies (133, 134). Similarly, nasal colonization of mice with pneumococcus can lead to antibody-independent and Th17-dependent immune resistance (135, 136). Adults have memory T cells in their blood that respond to pneumococcal protein antigens with proliferation and IL-17 production (137), and pneumococcal exposure of the respiratory tract increases Th17 cells in the bronchoalveolar lavage fluid (138), suggesting naturally developed heterotypic immune memory. Although not implicated in the above-described mouse colonization or vaccination experiments, heterotypic antibodies may also be protective in adult humans. A peptide screening approach reveals human serum to contain antibodies against perhaps >140 different protein antigens of pneumococcus, some of which provide protection in mouse models of pneumonia (139). Thus, early-life exposures lead to memory CD4+ T cells and antibodies that provide heterotypic immune resistance against bacterial respiratory pathogens. Although less than optimal (e.g., compared with capsular polysaccharide-specific antibodies), these heterotypic responses are an improvement over naive infections. Heterotypic memory Th17 cells plus antibodies against conserved virulence factors may provide much of the adaptive immune resistance of the so-called trough period, which is characterized by lower susceptibility to pneumonia.
Lung-Resident Immune Cells and Protection from Pneumonia
Adult human lungs contain abundant lymphocytes, with memory CD4+ T cells especially prominent (140, 141). Most studies of adaptive immunity against respiratory pathogens involve lymphocytes from the blood, mucosal surfaces (lavageates), or secondary lymphoid organs, but lung-resident lymphocytes may be distinct. Mouse models of pulmonary influenza infection differentiate between two populations of memory CD4+ T cells: (a) those that circulate and are found in the blood and secondary lymphoid organs and (b) those that reside in the lung tissues instead and do not recirculate (142). The latter, resident memory T cells (TRM) from the lung, provide greater protection against influenza infection (142); the underlying mechanisms are unknown. In humans, more of the T cells from lung tissue are responsive to influenza than are T cells from the blood or skin (140), but little else is known about the antigen specificity of lung TRM. They show polyfunctionality after antigen-independent activation (140, 141), but almost nothing is appreciated about the cytokine profiles, skewing, or other activities of naturally stimulated lung TRM. In mice, lung TRM but not memory cells from secondary lymphoid organs express IFITM3, an antiviral protein permitting cell survival when the lung is infected by influenza virus (143), indicating an adaptation to tissue residence. The specificities and activities of lung TRM are large and likely significant gaps in our understanding of lung defense against respiratory pathogens.
Innate-like lymphocytes, including NKT cells (144), MAIT cells (145), γδ-T cells (146), and NK cells (120), can also provide resistance against bacteria in the lungs. Roles of these cells and how they change over the life course demand further study. Furthermore, it is not unusual for lungs to develop ectopic tertiary lymphoid structures, particularly with chronic lung diseases (147), and the effect of these structures on pneumonia susceptibility deserves further attention. The animal models currently used to study pneumonia biology and pulmonary defenses typically fail to account for the complex relationships between lymphocytes and lungs observed in adult humans. New model systems and thoughtful integration of human and animal experimentation will be needed to generate insights into the specialized immune system of the lung and its roles in protection against respiratory pathogens.
Resistance and Resilience in the Elderly
Pneumonias are exceptionally common and severe in the elderly (Figure 4a), which we attribute to combined decreases in both resistance and resilience (Figure 4b). With immune resistance mechanisms preventing severe pneumonias in young adults only beginning to be understood, it is difficult to identify specific defects that occur with aging and that contribute to pneumonia susceptibility. Greater insights into resistance pathways responsible for decelerating pneumonias in childhood and maintaining the trough period of young adulthood are needed to rationally guide further studies of the effects of aging on resistance. Although much is known about immunosenescence (148), little to date specifically applies to heterotypic immunity against respiratory pathogens and none (to our knowledge) to lung-resident immune cells. Regarding tissue resilience, perhaps all organs of elderly individuals are less capable of withstanding stress, due to cellular senescence, telomere shortening, genome instability, epigenetic alterations, stem cell loss, and more (149–151). The lungs show signs of aging-induced changes that accelerate with comorbidities (152, 153), which may contribute to pneumonia in the elderly. Mechanisms behind the loss of tissue resilience in the lung, and contributions to infection outcomes, are speculative. Supporting the concept that decreases in tissue resilience begin early (as in Figure 4b), children are less likely than adults to develop acute lung injury in a variety of severe infections or other challenges (154). Scant if any data directly demonstrate how declines in tissue resilience impact pneumonia biology, but such data could be important to understanding and preventing the increased pneumonia incidence and severity in older individuals.
Conclusions
The public health crisis attributable to pneumonia is comparable to or worse than many diseases that garner greater attention. Pneumonia warrants more awareness by advocates and stronger effort by researchers. Elucidating the basic biological principles governing resistance, resilience, and their age-dependent remodeling will help to identify novel targets for diagnostic, prognostic, and therapeutic intervention in patients with or at risk for pneumonia. Imbalances among these processes that evolved to counter pressures of acute respiratory infection are paramount in shaping the pathogenesis of and susceptibility to many chronic lung diseases (4), including COPD, pulmonary fibrosis, asthma, and lung cancer. Continued insights into immune resistance, tissue resilience, and their remodeling will advance our fight against lung diseases directly and/or indirectly linked to respiratory infection.
Acknowledgments
This work was funded in part by National Institutes of Health grants R01HL079392 (J.P.M.), R01HL068153 (J.P.M.), R01HL111449 (L.J.Q.), and R00HL092956 (L.J.Q.).
Footnotes
Disclosure Statement: The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Lee J. Quinton, Email: lquinton@bu.edu.
Joseph P. Mizgerd, Email: jmizgerd@bu.edu.
Literature Cited
- 1.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–66. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Johnson HL, Cousens S, Perin J, Scott S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 4.Mizgerd JP. Respiratory infection and the impact of pulmonary immunity on lung health and disease. Am J Respir Crit Care Med. 2012;186:824–29. doi: 10.1164/rccm.201206-1063PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curns AT, Holman RC, Sejvar JJ, Owings MF, Schonberger LB. Infectious disease hospitalizations among older adults in the United States from 1990 through 2002. Arch Intern Med. 2005;165:2514–20. doi: 10.1001/archinte.165.21.2514. [DOI] [PubMed] [Google Scholar]
- 6.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA. 2005;294:2712–19. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 7.Mizgerd JP. Lung infection—a public health priority. PLOS Med. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Eeden S, Leipsic J, Paul Man SF, Sin DD. The relationship between lung inflammation and cardiovascular disease. Am J Respir Crit Care Med. 2012;186:11–16. doi: 10.1164/rccm.201203-0455PP. [DOI] [PubMed] [Google Scholar]
- 9.Edmond K, Scott S, Korczak V, Ward C, Sanderson C, et al. Long term sequelae from childhood pneumonia; systematic review and meta-analysis. PLOS ONE. 2012;7:e31239. doi: 10.1371/journal.pone.0031239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah FA, Pike F, Alvarez K, Angus D, Newman AB, et al. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med. 2013;188:586–92. doi: 10.1164/rccm.201212-2154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol. 2012;30:271–94. doi: 10.1146/annurev-immunol-020711-075030. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–41. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cilloniz C, Ewig S, Polverino E, Marcos MA, Esquinas C, et al. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax. 2011;66:340–46. doi: 10.1136/thx.2010.143982. [DOI] [PubMed] [Google Scholar]
- 14.Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51(Suppl. 1):81–87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 15.Mizgerd JP, Skerrett SJ. Animal models of human pneumonia. Am J Physiol Lung Cell Mol Physiol. 2008;294:L387–98. doi: 10.1152/ajplung.00330.2007. [DOI] [PubMed] [Google Scholar]
- 16.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med. 2008;358:716–27. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinton LJ, Mizgerd JP. NF-κB and STAT3 signaling hubs for lung innate immunity. Cell Tissue Res. 2011;343:153–65. doi: 10.1007/s00441-010-1044-y. [DOI] [PubMed] [Google Scholar]
- 19.Alcamo E, Mizgerd JP, Horwitz BH, Bronson R, Beg AA, et al. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-κB in leukocyte recruitment. J Immunol. 2001;167:1592–600. doi: 10.4049/jimmunol.167.3.1592. [DOI] [PubMed] [Google Scholar]
- 20.Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, et al. Functions and regulation of NF-κB RelA during pneumococcal pneumonia. J Immunol. 2007;178:1896–903. doi: 10.4049/jimmunol.178.3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picard C, von Bernuth H, Ghandil P, Chrabieh M, Levy O, et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine. 2010;89:403–25. doi: 10.1097/MD.0b013e3181fd8ec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pannicke U, Baumann B, Fuchs S, Henneke P, Rensing-Ehl A, et al. Deficiency of innate and acquired immunity caused by an IKBKB mutation. N Engl J Med. 2013;369:2504–14. doi: 10.1056/NEJMoa1309199. [DOI] [PubMed] [Google Scholar]
- 23.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 24.Pittet LA, Quinton LJ, Yamamoto K, Robson BE, Ferrari JD, et al. Earliest innate immune responses require macrophage RelA during pneumococcal pneumonia. Am J Respir Cell Mol Biol. 2011;45:573–81. doi: 10.1165/rcmb.2010-0210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinton LJ, Jones MR, Robson BE, Mizgerd JP. Mechanisms of the hepatic acute-phase response during bacterial pneumonia. Infect Immun. 2009;77:2417–26. doi: 10.1128/IAI.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hess C, Herr C, Beisswenger C, Zakharkina T, Schmid RM, Bals R. Myeloid RelA regulates pulmonary host defense networks. Eur Respir J. 2010;35:343–52. doi: 10.1183/09031936.00196408. [DOI] [PubMed] [Google Scholar]
- 27.Cakarova L, Marsh LM, Wilhelm J, Mayer K, Grimminger F, et al. Macrophage tumor necrosis factor-α induces epithelial expression of granulocyte–macrophage colony-stimulating factor: impact on alveolar epithelial repair. Am J Respir Crit Care Med. 2009;180:521–32. doi: 10.1164/rccm.200812-1837OC. [DOI] [PubMed] [Google Scholar]
- 28.Marriott HM, Gascoyne KA, Gowda R, Geary I, Nicklin MJ, et al. IL-1β regulates CXCL8 release and influences disease outcome in response to Streptococcus pneumoniae, defining intracellular cooperation between pulmonary epithelial cells and macrophages. Infect Immun. 2011;80:1140–49. doi: 10.1128/IAI.05697-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210:1977–92. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy J, Summer R, Wilson AA, Kotton DN, Fine A. The prolonged life-span of alveolar macrophages. Am J Respir Cell Mol Biol. 2008;38:380–85. doi: 10.1165/rcmb.2007-0224RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, et al. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med. 2011;184:547–60. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marriott HM, Bingle CD, Read RC, Braley KE, Kroemer G, et al. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J Clin Investig. 2005;115:359–68. doi: 10.1172/JCI21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aberdein JD, Cole J, Bewley MA, Marriott HM, Dockrell DH. Alveolar macrophages in pulmonary host defence—the unrecognized role of apoptosis as a mechanism of intracellular bacterial killing. Clin Exp Immunol. 2013;174:193–202. doi: 10.1111/cei.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinwede K, Henken S, Bohling J, Maus R, Ueberberg B, et al. TNF-related apoptosis-inducing ligand (TRAIL) exerts therapeutic efficacy for the treatment of pneumococcal pneumonia in mice. J Exp Med. 2012;209:1937–52. doi: 10.1084/jem.20120983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Investig. 2013;123:1630–37. doi: 10.1172/JCI66142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dela Cruz CS, Liu W, He CH, Jacoby A, Gornitzky A, et al. Chitinase 3-like-1 promotes Streptococcus pneumoniae killing and augments host tolerance to lung antibacterial responses. Cell Host Microbe. 2012;12:34–46. doi: 10.1016/j.chom.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber M, Lambeck S, Ding N, Henken S, Kohl M, et al. Hepatic induction of cholesterol biosynthesis reflects a remote adaptive response to pneumococcal pneumonia. FASEB J. 2012;26:2424–36. doi: 10.1096/fj.11-191957. [DOI] [PubMed] [Google Scholar]
- 38.Alymova IV, Samarasinghe A, Vogel P, Green AM, Weinlich R, McCullers JA. A novel cytotoxic sequence contributes to influenza A viral protein PB1-F2 pathogenicity and predisposition to secondary bacterial infection. J Virol. 2014;88:503–15. doi: 10.1128/JVI.01373-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakahira K, Choi AM. Autophagy: a potential therapeutic target in lung diseases. Am J Physiol Lung Cell Mol Physiol. 2013;305:L93–107. doi: 10.1152/ajplung.00072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrne BG, Dubuisson JF, Joshi AD, Persson JJ, Swanson MS. Inflammasome components coordinate autophagy and pyroptosis as macrophage responses to infection. MBio. 2013;4:e00620–12. doi: 10.1128/mBio.00620-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan K, Huang C, Fox J, Laturnus D, Carlson E, et al. Autophagy plays an essential role in the clearance of Pseudomonas aeruginosa by alveolar macrophages. J Cell Sci. 2012;125:507–15. doi: 10.1242/jcs.094573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam JM, Mansour MK, Khan NS, Seward M, Puranam S, et al. Dectin-1-dependent LC3 recruitment to phagosomes enhances fungicidal activity in macrophages. J Infect Dis. 2014;210:1844–54. doi: 10.1093/infdis/jiu290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188:913–22. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, et al. Muc5b is required for airway defence. Nature. 2014;505:412–16. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 46.Mijares LA, Wangdi T, Sokol C, Homer R, Medzhitov R, Kazmierczak BI. Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1-dependent pathway. J Immunol. 2011;186:7080–88. doi: 10.4049/jimmunol.1003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moser C, Weiner DJ, Lysenko E, Bals R, Weiser JN, Wilson JM. β-Defensin 1 contributes to pulmonary innate immunity in mice. Infect Immun. 2002;70:3068–72. doi: 10.1128/IAI.70.6.3068-3072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto K, Ahyi AN, Pepper-Cunningham ZA, Ferrari JD, Wilson AA, et al. Roles of lung epithelium in neutrophil recruitment during pneumococcal pneumonia. Am J Respir Cell Mol Biol. 2014;50:253–62. doi: 10.1165/rcmb.2013-0114OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto K, Ferrari JD, Cao Y, Ramirez MI, Jones MR, et al. Type I alveolar epithelial cells mount innate immune responses during pneumococcal pneumonia. J Immunol. 2012;189:2450–59. doi: 10.4049/jimmunol.1200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Starner TD, Barker CK, Jia HP, Kang Y, McCray PB., Jr CCL20 is an inducible product of human airway epithelia with innate immune properties. Am J Respir Cell Mol Biol. 2003;29:627–33. doi: 10.1165/rcmb.2002-0272OC. [DOI] [PubMed] [Google Scholar]
- 51.Kovach MA, Ballinger MN, Newstead MW, Zeng X, Bhan U, et al. Cathelicidin-related antimicrobial peptide is required for effective lung mucosal immunity in Gram-negative bacterial pneumonia. J Immunol. 2012;189:304–11. doi: 10.4049/jimmunol.1103196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markart P, Korfhagen TR, Weaver TE, Akinbi HT. Mouse lysozyme M is important in pulmonary host defense against Klebsiella pneumoniae infection. Am J Respir Crit Care Med. 2004;169:454–58. doi: 10.1164/rccm.200305-669OC. [DOI] [PubMed] [Google Scholar]
- 53.Chan YR, Liu JS, Pociask DA, Zheng M, Mietzner TA, et al. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol. 2009;182:4947–56. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warszawska JM, Gawish R, Sharif O, Sigel S, Doninger B, et al. Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes. J Clin Investig. 2013;123:3363–72. doi: 10.1172/JCI67911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi SM, McAleer JP, Zheng M, Pociask DA, Kaplan MH, et al. Innate Stat3-mediated induction of the antimicrobial protein Reg3γ is required for host defense against MRSA pneumonia. J Exp Med. 2013;210:551–61. doi: 10.1084/jem.20120260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Bartlett JA, Di ME, Bomberger JM, Chan YR, et al. SPLUNC1/BPIFA1 contributes to pulmonary host defense against Klebsiella pneumoniae respiratory infection. Am J Pathol. 2013;182:1519–31. doi: 10.1016/j.ajpath.2013.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Di ME, Chu HW, Liu X, Wang L, et al. Increased susceptibility to pulmonary Pseudomonas infection in Splunc1 knockout mice. J Immunol. 2013;191:4259–68. doi: 10.4049/jimmunol.1202340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fischer AJ, Lennemann NJ, Krishnamurthy S, Pocza P, Durairaj L, et al. Enhancement of respiratory mucosal antiviral defenses by the oxidation of iodide. Am J Respir Cell Mol Biol. 2011;45:874–81. doi: 10.1165/rcmb.2010-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr, et al. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–83. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Evans SE, Xu Y, Tuvim MJ, Dickey BF. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol. 2010;72:413–35. doi: 10.1146/annurev-physiol-021909-135909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cleaver JO, You D, Michaud DR, Pruneda FA, Juarez MM, et al. Lung epithelial cells are essential effectors of inducible resistance to pneumonia. Mucosal Immunol. 2014;7:78–88. doi: 10.1038/mi.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mei J, Liu Y, Dai N, Favara M, Greene T, et al. CXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity. 2010;33:106–17. doi: 10.1016/j.immuni.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bozinovski S, Jones J, Beavitt SJ, Cook AD, Hamilton JA, Anderson GP. Innate immune responses to LPS in mouse lung are suppressed and reversed by neutralization of GM-CSF via repression of TLR-4. Am J Physiol Lung Cell Mol Physiol. 2004;286:L877–85. doi: 10.1152/ajplung.00275.2003. [DOI] [PubMed] [Google Scholar]
- 64.Unkel B, Hoegner K, Clausen BE, Lewe-Schlosser P, Bodner J, et al. Alveolar epithelial cells orchestrate DC function in murine viral pneumonia. J Clin Investig. 2012;122:3652–64. doi: 10.1172/JCI62139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kallal LE, Schaller MA, Lindell DM, Lira SA, Lukacs NW. CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC. Eur J Immunol. 2010;40:1042–52. doi: 10.1002/eji.200939778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phadke AP, Akangire G, Park SJ, Lira SA, Mehrad B. The role of CC chemokine receptor 6 in host defense in a model of invasive pulmonary aspergillosis. Am J Respir Crit Care Med. 2007;175:1165–72. doi: 10.1164/rccm.200602-256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuzaki Y, Xu Y, Ikegami M, Besnard V, Park KS, et al. Stat3 is required for cytoprotection of the respiratory epithelium during adenoviral infection. J Immunol. 2006;177:527–37. doi: 10.4049/jimmunol.177.1.527. [DOI] [PubMed] [Google Scholar]
- 68.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2008;38:699–706. doi: 10.1165/rcmb.2007-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laan M, Prause O, Miyamoto M, Sjostrand M, Hytonen AM, et al. A role of GM-CSF in the accumulation of neutrophils in the airways caused by IL-17 and TNF-α. Eur Respir J. 2003;21:387–93. doi: 10.1183/09031936.03.00303503. [DOI] [PubMed] [Google Scholar]
- 70.Shui JW, Larange A, Kim G, Vela JL, Zahner S, et al. HVEM signalling at mucosal barriers provides host defence against pathogenic bacteria. Nature. 2012;488:222–25. doi: 10.1038/nature11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jaillon S, Galdiero MR, Del Prete D, Cassatella MA, Garlanda C, Mantovani A. Neutrophils in innate and adaptive immunity. Semin Immunopathol. 2013;35:377–94. doi: 10.1007/s00281-013-0374-8. [DOI] [PubMed] [Google Scholar]
- 72.Brandes M, Klauschen F, Kuchen S, Germain RN. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell. 2013;154:197–212. doi: 10.1016/j.cell.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ichikawa A, Kuba K, Morita M, Chida S, Tezuka H, et al. CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am J Respir Crit Care Med. 2013;187:65–77. doi: 10.1164/rccm.201203-0508OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamada M, Gomez JC, Chugh PE, Lowell CA, Dinauer MC, et al. Interferon-γ production by neutrophils during bacterial pneumonia in mice. Am J Respir Crit Care Med. 2011;183:1391–401. doi: 10.1164/rccm.201004-0592OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Puga I, Cols M, Barra CM, He B, Cassis L, et al. B cell–helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–80. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tillett WS, Francis T. Serological reactions in pneumonia with non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–71. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 78.Ahyi AN, Quinton LJ, Jones MR, Ferrari JD, Pepper-Cunningham ZA, et al. Roles of STAT3 in protein secretion pathways during the acute-phase response. Infect Immun. 2013;81:1644–53. doi: 10.1128/IAI.01332-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quinton LJ, Blahna MT, Jones MR, Allen E, Ferrari JD, et al. Hepatocyte-specific mutation of both NF-κB RelA and STAT3 abrogates the acute phase response in mice. J Clin Investig. 2012;122:1758–63. doi: 10.1172/JCI59408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mizgerd JP, Spieker MR, Doerschuk CM. Early response cytokines and innate immunity: essential roles for TNF receptor 1 and type I IL-1 receptor during Escherichia coli pneumonia in mice. J Immunol. 2001;166:4042–48. doi: 10.4049/jimmunol.166.6.4042. [DOI] [PubMed] [Google Scholar]
- 81.Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcγR by innate pentraxins. Nature. 2008;456:989–92. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuste J, Botto M, Bottoms SE, Brown JS. Serum amyloid P aids complement-mediated immunity to Streptococcus pneumoniae. PLOS Pathog. 2007;3:1208–19. doi: 10.1371/journal.ppat.0030120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–63. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freeman AF, Holland SM. Clinical manifestations, etiology, and pathogenesis of the hyper-IgE syndromes. Pediatr Res. 2009;65:R32–37. doi: 10.1203/PDR.0b013e31819dc8c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freeman AF, Kleiner DE, Nadiminti H, Davis J, Quezado M, et al. Causes of death in hyper-IgE syndrome. J Allergy Clin Immunol. 2007;119:1234–40. doi: 10.1016/j.jaci.2006.12.666. [DOI] [PubMed] [Google Scholar]
- 86.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, et al. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–76. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, et al. Defective IL-10 signaling in hyper-IgE syndrome results in impaired generation of tolerogenic dendritic cells and induced regulatory T cells. J Exp Med. 2011;208:235–49. doi: 10.1084/jem.20100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, et al. Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Investig. 2004;113:28–37. doi: 10.1172/JCI200419491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ikegami M, Falcone A, Whitsett JA. STAT-3 regulates surfactant phospholipid homeostasis in normal lung and during endotoxin-mediated lung injury. J Appl Physiol. 2008;104:1753–60. doi: 10.1152/japplphysiol.00875.2007. [DOI] [PubMed] [Google Scholar]
- 90.Kida H, Mucenski ML, Thitoff AR, Le Cras TD, Park KS, et al. GP130-STAT3 regulates epithelial cell migration and is required for repair of the bronchiolar epithelium. Am J Pathol. 2008;172:1542–54. doi: 10.2353/ajpath.2008.071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lian X, Qin Y, Hossain SA, Yang L, White A, et al. Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J Immunol. 2005;174:7250–56. doi: 10.4049/jimmunol.174.11.7250. [DOI] [PubMed] [Google Scholar]
- 92.Li Y, Du H, Qin Y, Roberts J, Cummings OW, Yan C. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res. 2007;67:8494–503. doi: 10.1158/0008-5472.CAN-07-0647. [DOI] [PubMed] [Google Scholar]
- 93.Jones MR, Quinton LJ, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Roles of interleukin-6 in activation of STAT proteins and recruitment of neutrophils during Escherichia coli pneumonia. J Infect Dis. 2006;193:360–69. doi: 10.1086/499312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heymann D, L'Her E, Nguyen JM, Raher S, Canfrere I, et al. Leukaemia inhibitory factor (LIF) production in pleural effusions: comparison with production of IL-4, IL-8, IL-10 and macrophage-colony stimulating factor (M-CSF) Cytokine. 1996;8:410–16. doi: 10.1006/cyto.1996.0056. [DOI] [PubMed] [Google Scholar]
- 95.Jorens PG, De Jongh R, Bossaert LL, De Backer W, Herman AG, et al. High levels of leukaemia inhibitory factor in ARDS. Cytokine. 1996;8:873–76. doi: 10.1006/cyto.1996.9999. [DOI] [PubMed] [Google Scholar]
- 96.Dagoneau N, Scheffer D, Huber C, Al-Gazali LI, Di Rocco M, et al. Null leukemia inhibitory factor receptor (LIFR) mutations in Stuve-Wiedemann/Schwartz-Jampel type 2 syndrome. Am J Hum Genet. 2004;74:298–305. doi: 10.1086/381715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Quinton LJ, Mizgerd JP, Hilliard KL, Jones MR, Kwon CY, Allen E. Leukemia inhibitory factor signaling is required for lung protection during pneumonia. J Immunol. 2012;188:6300–8. doi: 10.4049/jimmunol.1200256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 99.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–81. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2013;6:69–82. doi: 10.1038/mi.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, et al. IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol. 2013;182:1286–96. doi: 10.1016/j.ajpath.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zemans RL, Briones N, Campbell M, McClendon J, Young SK, et al. Neutrophil transmigration triggers repair of the lung epithelium via β-catenin signaling. Proc Natl Acad Sci USA. 2011;108:15990–95. doi: 10.1073/pnas.1110144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Y, Sadikot RT, Adami GR, Kalinichenko VV, Pendyala S, et al. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J Exp Med. 2011;208:1473–84. doi: 10.1084/jem.20102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–38. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kotton DN. Next-generation regeneration: the hope and hype of lung stem cell research. Am J Respir Crit Care Med. 2012;185:1255–60. doi: 10.1164/rccm.201202-0228PP. [DOI] [PubMed] [Google Scholar]
- 106.Korfhagen TR, Kitzmiller J, Chen G, Sridharan A, Haitchi HM, et al. SAM-pointed domain ETS factor mediates epithelial cell–intrinsic innate immune signaling during airway mucous metaplasia. Proc Natl Acad Sci USA. 2012;109:16630–35. doi: 10.1073/pnas.1208092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, et al. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med. 2014;189:301–13. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim KC. Role of epithelial mucins during airway infection. Pulm Pharmacol Ther. 2012;25:415–19. doi: 10.1016/j.pupt.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Choi S, Park YS, Koga T, Treloar A, Kim KC. TNF-α is a key regulator of MUC1, an anti-inflammatory molecule, during airway Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 2011;44:255–60. doi: 10.1165/rcmb.2009-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–53. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aggarwal NR, King LS, D'Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306:L709–25. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47:417–26. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J, Li F, Sun R, Gao X, Wei H, et al. Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nat Commun. 2013;4:2106. doi: 10.1038/ncomms3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, et al. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–6. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J Clin Investig. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol. 2014;76:467–92. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, et al. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Investig. 2006;116:2532–42. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Investig. 2009;119:2898–913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu X, Weiss ID, Zhang HH, Singh SP, Wynn TA, et al. Conventional NK cells can produce IL-22 and promote host defense in Klebsiella pneumoniae pneumonia. J Immunol. 2014;192:1778–86. doi: 10.4049/jimmunol.1300039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–54. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jamieson AM, Pasman L, Yu S, Gamradt P, Homer RJ, et al. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science. 2013;340:1230–34. doi: 10.1126/science.1233632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Geisler F, Algul H, Paxian S, Schmid RM. Genetic inactivation of RelAp65 sensitizes adult mouse hepatocytes to TNF-induced apoptosis in vivo and in vitro. Gastroenterology. 2007;132:2489–503. doi: 10.1053/j.gastro.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 124.Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 2010;207:1453–64. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Poe SL, Arora M, Oriss TB, Yarlagadda M, Isse K, et al. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol. 2013;6:189–99. doi: 10.1038/mi.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, et al. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med. 2011;183:1380–90. doi: 10.1164/rccm.201009-1431OC. [DOI] [PubMed] [Google Scholar]
- 127.Aggarwal NR, Tsushima K, Eto Y, Tripathi A, Mandke P, et al. Immunological priming requires regulatory T cells and IL-10–producing macrophages to accelerate resolution from severe lung inflammation. J Immunol. 2014;92:4453–64. doi: 10.4049/jimmunol.1400146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533–39. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–62. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. 2013;19:1305–12. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 131.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–80. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 132.Strutt TM, McKinstry KK, Marshall NB, Vong AM, Dutton RW, Swain SL. Multipronged CD4+ T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus. Immunol Rev. 2013;255:149–64. doi: 10.1111/imr.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Malley R, Anderson PW. Serotype-independent pneumococcal experimental vaccines that induce cellular as well as humoral immunity. Proc Natl Acad Sci USA. 2012;109:3623–27. doi: 10.1073/pnas.1121383109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, et al. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity. 2011;35:997–1009. doi: 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci USA. 2005;102:4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cohen JM, Khandavilli S, Camberlein E, Hyams C, Baxendale HE, Brown JS. Protective contributions against invasive Streptococcus pneumoniae pneumonia of antibody and Th17-cell responses to nasopharyngeal colonisation. PLOS ONE. 2011;6:e25558. doi: 10.1371/journal.pone.0025558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lundgren A, Bhuiyan TR, Novak D, Kaim J, Reske A, et al. Characterization of Th17 responses to Streptococcus pneumoniae in humans: comparisons between adults and children in a developed and a developing country. Vaccine. 2012;30:3897–907. doi: 10.1016/j.vaccine.2012.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wright AK, Bangert M, Gritzfeld JF, Ferreira DM, Jambo KC, et al. Experimental human pneumococcal carriage augments IL-17A-dependent T-cell defence of the lung. PLOS Pathog. 2013;9:e1003274. doi: 10.1371/journal.ppat.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Giefing C, Meinke AL, Hanner M, Henics T, Bui MD, et al. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med. 2008;205:117–31. doi: 10.1084/jem.20071168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLOS ONE. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–97. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–14. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wakim LM, Gupta N, Mintern JD, Villadangos JA. Enhanced survival of lung tissue-resident memory CD8+ T cells during infection with influenza virus due to selective expression of IFITM3. Nat Immunol. 2013;14:238–45. doi: 10.1038/ni.2525. [DOI] [PubMed] [Google Scholar]
- 144.Paget C, Trottein F. Role of type 1 natural killer T cells in pulmonary immunity. Mucosal Immunol. 2013;6:1054–67. doi: 10.1038/mi.2013.59. [DOI] [PubMed] [Google Scholar]
- 145.Gold MC, Lewinsohn DM. Co-dependents: MR1-restricted MAIT cells and their antimicrobial function. Nat Rev Microbiol. 2013;11:14–19. doi: 10.1038/nrmicro2918. [DOI] [PubMed] [Google Scholar]
- 146.Nakasone C, Yamamoto N, Nakamatsu M, Kinjo T, Miyagi K, et al. Accumulation of γ/δ T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes Infect. 2007;9:251–58. doi: 10.1016/j.micinf.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 147.Randall TD, Mebius RE. The development and function of mucosal lymphoid tissues: a balancing act with micro-organisms. Mucosal Immunol. 2014;7(3):455–66. doi: 10.1038/mi.2014.11. [DOI] [PubMed] [Google Scholar]
- 148.Krone CL, van de Groep K, Trzcinski K, Sanders EA, Bogaert D. Immunosenescence and pneumococcal disease: an imbalance in host-pathogen interactions. Lancet Respir Med. 2014;2:141–53. doi: 10.1016/S2213-2600(13)70165-6. [DOI] [PubMed] [Google Scholar]
- 149.Vijg J, Suh Y. Genome instability and aging. Annu Rev Physiol. 2013;75:645–68. doi: 10.1146/annurev-physiol-030212-183715. [DOI] [PubMed] [Google Scholar]
- 150.Tevy MF, Giebultowicz J, Pincus Z, Mazzoccoli G, Vinciguerra M. Aging signaling pathways and circadian clock–dependent metabolic derangements. Trends Endocrinol Metab. 2013;24:229–37. doi: 10.1016/j.tem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lalley PM. The aging respiratory system—pulmonary structure, function and neural control. Respir Physiol Neurobiol. 2013;187:199–210. doi: 10.1016/j.resp.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 153.Faner R, Rojas M, Macnee W, Agusti A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:306–13. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 154.Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antivir Res. 2013;99:417–35. doi: 10.1016/j.antiviral.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 155.Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. US hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–63. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]