Abstract
We present two cases of pulmonary aspergillosis in which calcium oxalate crystals in the sputum proved to be a useful diagnostic clue. In case 1, Aspergillus hyphae was not identified; however, calcium oxalate crystals were present, and chronic necrotizing pulmonary aspergillosis was diagnosed. In case 2, calcium oxalate was detected and Aspergillus fumigatus was identified later. Thus, the presence of calcium oxalate in the sputum may be an important indicator for an A. fumigatus infection.
Keywords: Pulmonary aspergillosis, Calcium oxalate, Aspergillus fumigatus
1. Introduction
Invasive pulmonary aspergillosis is extremely difficult to diagnose, and a combination of culture tests, clinical attributes, imaging tests, and serological diagnosis must be used [1]. In approximately 40% of all cases, invasive pulmonary aspergillosis is not clinically diagnosed while the patient is still alive; it is only discovered during post-mortem examination. The diagnostic criteria for Chronic progressive necrotizing pulmonary aspergillosis is defined as follows: (1) it progresses >1 month with lower respiratory tract symptoms; (2) the clinical manifestations are exacerbated on new imaging findings; (3) the exacerbated imaging findings confirm an Aspergillus infection serologically or pathologically; and (4) the condition of the patient on antifungal treatment is inexplicably necessary in terms of general bacterial infection or another disorder; and (5) elevated inflammatory reactions or advanced lesions attributable to Aspergillosis must meet criteria 1–4 even in the absence of an elevated inflammatory response [2]. Invasive pulmonary aspergillosis is normally diagnosed on the basis of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) diagnostic criteria for invasive fungal infections [3].
The presence of calcium oxalate in sputum culture samples is regarded as potentially useful in the diagnosis of Aspergillus niger [4] and Aspergillus fumigatus [5,6]. A study comparing 65 patients who tested positive for Aspergillus and 60 control patients (with or without other types of infection) showed that calcium oxalate crystals are specific markers of an Aspergillus infection [7]. However, the value of calcium oxalate in a sputum culture for the diagnosis of pulmonary aspergillosis has very rarely been examined [7].
We treated two patients in whom calcium oxalate crystals in the sputum proved to be a useful clue in the diagnosis of Aspergillus. Since calcium oxalate in sputum samples aids in diagnosing A. niger and A. fumigatus, which account for most cases of pulmonary aspergillosis [8,9], this suggests that it should be added to the diagnostic criteria for all cases of aspergillosis, including A. niger.
2. Case reports
2.1. Case 1
A 66-year-old man presented to an outlying hospital with complaints of coughing, hemoptysis, and back pain. He had type 2 diabetes managed with kinesitherapy and dietotherapy but without medication. He had no particular contact with any disease agents, no history of owning pets or of traveling North America (Los Angeles), South America (Cancun and Mexico). He smoked 40 cigarettes/day and had no history of alcohol use or allergies. He developed chest and back pain around November 8, 20XX (day –7). On November 12 (day –3), he visited a local ear-nose-throat clinic; leukocytosis and an inflammatory response were detected. On November 15 (day 0), he was referred to our hospital's emergency department for detailed testing and treatment. After imaging tests were performed, he was admitted for further testing and treatment (Table 1).
Table 1.
The blood test results for case 1.
| General blood tests | Blood biochemistry tests | ||||
|---|---|---|---|---|---|
| WBC | 14,900/µL | T-bil | 0.5 mg/dL | CI | 107 mEg/L |
| Seg | 88.6% | TP | 7.8 g/dL | CRP | 30.78 mg/dL |
| Eo | 1.0% | Alb | 3.6 g/dL | β-D glucan | (−) |
| Ly | 5.4% | AST | 29 IU/L | QFT | (−) |
| Mo | 4.2% | ALT | 29 IU/L | Aspergillus antibody | (−) |
| RBC | 367×104/µL | Ch-E | 225 IU/L | Aspergillus antigen | (+) |
| Hb | 12.1 g/dL | γ-GTP | 28 IU/L | Cryptococcus antibody | (+) |
| Ht | 35.5% | ALP | 169 IU/L | PR3-ANCA | (−) |
| MCV | 96.7 fL | LDH | 161 IU/L | MPO-ANCA | (−) |
| MCH | 34.1 Pg | BUN | 24 mg/dL | HIV antibody | (−) |
| MCHC | 35.2% | Cre | 0.66 mg/dL | TP antibody | (−) |
| Plt | 30.4×104/µL | Na | 140 mEq/L | RPR | (−) |
| K | 4.4 mEq/L | ||||
Upon arrival, the patient was hyperthermic (38.7 °C) with a fast pulse (99 beats/min). His respiration and blood pressure were 16/min and 104/76 mmHg, respectively.
His breath sounds were reduced in the left upper lung field only. He had a ground-glass shadow in the left upper lung field on thoracic plain radiograph (Fig. 1), and there was a reverse halo shadow in the left S3 with ground-glass attenuation surrounding the consolidation in S1 and S2 on the thoracic computed tomography (CT) scan (Fig. 2).
Fig. 1.

Case 1: thoracic plain radiograph.
Fig. 2.

Case 1: thoracic plain computed tomography scan.
On the basis of the clinical course and imaging findings, the differential diagnosis in case 1 included community-acquired pneumonia, pulmonary mycobacteriosis (tuberculosis or nontuberculous mycobacteriosis), pulmonary mycosis (chronic pulmonary aspergillosis, cryptococcosis, or histoplasmosis), pulmonary nocardiosis, actinomycosis, alveolar cell carcinoma, pulmonary malignant lymphoma, and cryptogenic organizing pneumonia. The samples were tested for the human immunodeficiency virus antibody, β-d-glucan, Aspergillus and Cryptococcus antigens, and sputum cytology. Sputum Gram stain test was negative; however, considering the frequency of the community-acquired pneumonia, ceftriaxone (2 g/day) was started on day 1.
In addition to the aforementioned tests, that for the Aspergillus galactomannan antigen yielded positive results; however, the sputum smear test (three consecutive expectorations) for Mycobacterium yielded negative results, and the sputum culture was negative. No significant findings were detected in the sputum culture or the cytology acquired by bronchoscopy. Given the presence of calcium oxalate crystals (Fig. 3) as well as the fact that the diagnosis of chronic pulmonary aspergillosis was consistent with the imaging findings, treatment with voriconazole (loading dose: 300 mg intravenously every 12 h on the first day; subsequent doses: 200 mg intravenously every 12 h) was started on day 8. The test for the Aspergillus galactomannan antigen performed on day 12 yielded positive results. Since the patient's symptoms improved, as did the blood test results, the medication was switched to oral voriconazole (600 mg/day) from day 16. The patient was discharged on day 23, and oral voriconazole was continued.
Fig. 3.

The presence of calcium oxalate in the sputum of case 1.
2.2. Case 2
A 66-year-old man presented to an outlying hospital with complaints of fever and a productive cough. The patient had emphysema and stomach cancer (total gastrectomy). He smoked 20 cigarettes/day for 40 years (until 6 years previously) and drank 350 mL of beer/day. He had no history of pets and never traveled to any foreign country.
The patient developed a fever, coughing, and expectoration around September 12, 20XX (day –12). He had been taking some antibiotics that were prescribed by a medical practitioner. The patient visited our hospital on September 24 (day –2). Emphysematous changes in the upper lobe of the right lung and niveau formation in the bulla were evident, and consolidation was also present in the left upper lung. The patient was monitored as an outpatient but was eventually admitted to our hospital's Department of Respiratory Medicine on September 26 (day 0) after hemoptysis was observed (Table 2).
Table 2.
The blood test results for case 2.
| General blood tests | Blood biochemistry tests | ||||
|---|---|---|---|---|---|
| WBC | 14,300/µL | T-bil | 0.3 mg/dL | CI | 107 mEg/L |
| Seg | 89.0% | D-bil | 0.2 mg/dL | CRP | 10.57 mg/dL |
| Eo | 0.0% | TP | 7.8 g/dL | β-d glucan | (−) |
| Ly | 4.0% | Alb | 3.6 g/dL | QFT | (−) |
| Mo | 1.0% | AST | 29 IU/L | Aspergillus antibody | (+) |
| AT-Ly | 2.0% | ALT | 29 IU/L | Aspergillus antigen | (+) |
| RBC | 298×104/µL | Ch-E | 14 IU/L | Cryptococcus antibody | (−) |
| Hb | 9.5 g/dL | γ-GTP | 14 IU/L | HIV antibody | (−) |
| Ht | 29.7% | ALP | 297 IU/L | TP antibody | (−) |
| MCV | 99.7 fL | LDH | 252 IU/L | RPR | (−) |
| MCH | 31.9 Pg | BUN | 6 mg/dL | ||
| MCHC | 32.0% | Cre | 0.36 mg/dL | ||
| Plt | 20.6×104/µL | Na | 2.9 mEq/L | ||
| K | 4.4 mEq/L | ||||
He had ground-glass shadow in the left upper lung field on the thoracic plain radiograph (Fig. 4) and consolidation associated with air bronchogram in the left S3 on thoracic computed tomography scan (Fig. 5).
Fig. 4.

The thoracic plain radiograph of case 2.
Fig. 5.
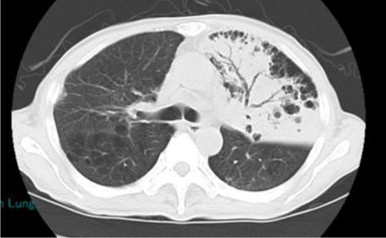
The thoracic computed tomography scan of case 2.
Klebsiella pneumoniae was identified from an initial sputum sample, which was collected at day −12, and treatment with ampicillin/sulbactam (3 g intravenously every 6 h) was started on day 1. However, his symptoms failed to improve, and since the tests for the Aspergillus antigen and antibody were both positive on day 10, our department was consulted. The calcium oxalate crystals were also evident in the sputum culture (Figs. 6 and 7); thus, chronic necrotizing pulmonary aspergillosis was suspected and treatment with voriconazole was started (loading dose: 300 mg intravenously every 12 h on the first day; subsequent doses: 200 mg intravenously every 12 h). A. fumigatus was identified in a sputum culture test on day 12. However, the patient's general condition on admission was poor, and he died on day 14.
Fig. 6.

The calcium oxalate observed in the Gram stain for case 2.
Fig. 7.

The calcium oxalate Gram staining in case 2, as viewed through a simple polarizing filter.
The study was approved by the Ethics committee of Kanto Rosai Hospital, and all the patients provided written informed consent to publish the manuscript.
3. Discussion
The main causative species of pulmonary aspergillosis are A. fumigatus, A. niger, Aspergillus nidulans, and Aspergillus flavus [10], of which A. fumigatus accounts for most cases [8,9,11]. Calcium oxalate is formed when oxalic acid is released into tissue as a metabolite when Aspergillus binds with tissue calcium [8]. Calcium oxalate itself is toxic and destroys tissue [12], causing the progression of lesions. Studies have suggested that calcium oxalate may be useful in the diagnosis of A. niger [4–6], and some have suggested that it may also be useful in the diagnosis of A. fumigatus, as it is present in the pathological samples [1]. However, no previous study has investigated the value of calcium oxalate for diagnosing pulmonary aspergillosis caused by A. fumigatus in living patients. Given this scenario, the cases presented here are of particular interest.
The Aspergillus galactomannan antigen is one of the parameters used for diagnosing chronic necrotizing pulmonary aspergillosis and is among the microbial parameters of the EORTC/MSG diagnostic criteria for invasive fungal infections. However, it has been reported that its sensitivity for A. fumigatus may be lower than that for non-fumigatus infections [13], and it is possible that the presence of calcium oxalate in particular may be a sufficient cause for suspecting A. fumigatus pulmonary aspergillosis in chronic lower respiratory lesions.
In conclusion, calcium oxalate may aid in the diagnosis of pulmonary aspergillosis caused by A. fumigatus. The exact use of calcium oxalate as a diagnostic parameter of chronic necrotizing pulmonary aspergillosis as well as a microbial parameter of the EORTC/MSG diagnostic criteria for invasive fungal infections should be confirmed through the examination of more similar cases.
Conflict of interest
There are no conflicts of interest.
Acknowledgments
There are none.
References
- 1.A. Araoka, T. Ohmagari, A. Ueda, T. Fujita, N. Kishida, Y. Ainoda, Aspergillosis, in: Pulmonary Infections in Immunocompromised Hosts (in Japanese), Nanzando, Japan, 2011, pp. 217–219.
- 2.Kohno S., Masaoka T., Yamaguchi H., Stevens D.A., Edwards J.E., Calandra T. A multicenter, open-label clinical study of micafungin (FK463) in the treatment of deep-seated mycosis in Japan. Scand. J. Infect. Dis. 2004;36:372–379. doi: 10.1080/00365540410020406. [DOI] [PubMed] [Google Scholar]
- 3.De Pauw B., Walsh T.J., Donnelly J.P., Stevens D.A., Edwards J.E., Calandra T. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Disease Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pabuccuoglu U. Aspects of oxalosis associated with aspergillosis in pathology specimens. Pathol. Res. Pract. 2005;201:363–368. doi: 10.1016/j.prp.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Hara K., Yoshioka T., Katsura Y., Satonaka K. A case of pulmonary aspergillosis discovered due to calcium oxalate crystals in bronchial brushing cytological specimens [in Japanese] J. Jpn. Soc. Clin. Cytol. 1999;38:431–435. [Google Scholar]
- 6.Kaneoka A., Kawabe T., Katsuyama E., Takasu K., Hamamoto K. Calcium oxalate crystals in the aspiration cytology specimen of the pulmonary Aspergillosis [in Japanese]. A case report. J. Kyoto City Hosp. 1999;19:58–61. [Google Scholar]
- 7.Farley M.L., Mabry L., Muñoz L.A., Diserens H.W. Crystals occurring in pulmonary cytology specimens. Association with Aspergillus infection. Acta Cytol. 1985;29:737–744. [PubMed] [Google Scholar]
- 8.Yamaguchi H., Uchida K. A pictorial guide to laboratory diagnosis of mycosis [in Japanese] Eiken Chem. (first edition) 1994:95–119. [Google Scholar]
- 9.Ando T., Shibuya K., Kume H. Clinical presentation and pathology of pulmonary aspergillosis. Pathological diagnosis of fungal infections [in Japanese] Pathol. Diagn. Update Rev. (first edition) 2012:23–24. [Google Scholar]
- 10.Yasue T. Histochemical identification of calcium oxalate. Acta Histochem. Cytochem. 1969;2:83–95. [Google Scholar]
- 11.Takayoshi T. Pulmonary aspergillosis and clinical significance of Aspergillus isolation from respiratory samples [in Japanese] J. Jpn. Soc. Clin. Microbiol. 2009;19:5. [Google Scholar]
- 12.Metzger J.B., Garagusi V.F., Kerwin D.M. Pulmonary oxalosis caused by Aspergillus niger. Am. Rev. Respir. Dis. 1984;129:501–502. doi: 10.1164/arrd.1984.129.3.501. [DOI] [PubMed] [Google Scholar]
- 13.R.Y. Hachem, D.P. Kontoyiannis, R.F. Chemaly, Y. Jiang, R. Reitzel, I. Raad, Utility of galactomannan enzyme immunoassay and (1,3)β-D-glucan in diagnosis of invasive fungal infection: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients, J. Clin. Microbiol. 47 (2009) 129–133. [DOI] [PMC free article] [PubMed]


