Abstract
Objective To examine the role of temperament (i.e., surgency/positive affect, negative affect, and effortful control) in the social behavior of pediatric brain tumor survivors and comparison classmates. Methods Parent-, peer-, and self-report data were collected for 75 children after treatment for a brain tumor, and 67 comparison classmates. Tests of mediation and moderated mediation were run to examine whether effortful control accounted for group differences in social behavior and whether this indirect effect was moderated by surgency/positive or negative affectivity. Results Peers described survivors as lower in Leadership-popularity and higher in Sensitivity-isolation and victimization than comparison classmates. Parent and self-report of surgency/positive affect revealed survivors were lower on this dimension. Survivors were rated by parents as demonstrating less effortful control. Effortful control did not consistently account for group differences in social behavior. There was limited evidence of moderated mediation. Conclusions Research on the implications of potential changes in temperament following treatment is warranted.
Keywords: brain tumor, pediatric, social, temperament
Brain tumors, the second most common form of pediatric cancer, affect 2,200 children in the United States each year (Ries et al., 1999). Advances in treatment have led to 5-year survival rates that now approach 74% as opposed to 51% in the 1970s (Jemal et al., 2009). However, this progress may have been achieved at the cost of neurocognitive and psychosocial problems for affected children. Increasing evidence indicates that brain tumor survivors are at risk for disruption in social competence (Schulte & Barrera, 2010). This article examines how temperament may inform our understanding of pediatric brain tumor survivors’ social behavior. To obtain unique perspectives, we used multiple reporters to gather information regarding temperament (parent and self-report) and social behavior (peer report) for survivors and healthy comparison classmates.
Social competence can include social performance (e.g., behavior in social interactions), social adjustment (e.g., quality of relationships and self-perceptions of loneliness, social support, or social self-esteem), and social skills (e.g., abilities needed to behave competently in social settings) (Cavell, 1990; Yeates et al., 2007). Across studies, brain tumor survivors are often found to have less social competence, including clinically significant social problems and social skills deficits compared with normative samples (Carey et al., 2001), healthy controls (Bhat et al., 2005), and children with other chronic conditions (Bonner et al., 2008). Parents describe brain tumor survivors as less involved in social activities and friendships than survivors of cancers outside the central nervous system (CNS; Carpentieri, Mulhern, Douglas, Hanna, & Fairclough, 1993).
Our research group has examined social functioning by assessing peer report of children’s behaviors in the school setting. Peers have been recognized as a strong source of information regarding children’s social behaviors (Parker & Asher, 1987). Using the Revised Class Play (RCP) (see Methods section below for more information regarding the RCP), we have found that classmates describe brain tumor survivors as socially withdrawn and excluded (e.g., RCP items such as “Someone who likes spending time alone, feelings are easily hurt, often left out”) and victimized at school (e.g., RCP items such as “Someone picked on by other children”) with lower levels of both leadership (e.g., RCP items such as “Someone everyone listens to, is a good leader”) and aggressive disruptive behavior (e.g., RCP items such as “Someone who gets into fights a lot, is too bossy”) than control classmates (Vannatta et al., 2011). This is in contrast to a general consensus that survivors of non-CNS cancers generally do not have poorer social outcomes than their peers (Fuemmeler, Mullins, & Carpentier, 2006), unless they are treated with more intensive CNS-directed treatment (Vannatta, Gerhardt, Wells, & Noll, 2007). These patterns of findings suggest that the intensive treatments targeting the CNS that are required to successfully treat pediatric brain tumors may lead to detrimental effects in children’s social competence after treatment.
The current project was developed to gain greater understanding of survivors’ social behavior. It is likely that a number of individual, environmental/systemic, and treatment-related factors contribute to pediatric brain tumor survivors’ social behaviors (Yeates et al., 2007). This study examined a specific individual characteristic, temperament, in an effort to better understand survivors’ social behaviors. Temperament has been defined by Rothbart and colleagues as constitutionally based, individual differences in emotional, motor, and attentional reactivity to stimulus events and self-regulation (Rothbart & Bates, 2006; Rothbart, Sheese, & Posner, 2007). “Constitutional” refers to the enduring biological makeup of the individual, which may be influenced across time by heredity, maturation, and experience. Temperament includes three components: surgency/positive affect, negative affect, and effortful control. These systems involve a number of CNS structures, and therefore it is possible that survivors’ temperament may differ from peers who have not been treated with CNS-directed agents (Derryberry & Tucker, 2006; Kagan & Fox, 2006). To our knowledge, however, temperament has not been investigated previously as an explanatory variable with respect to behavioral outcomes of pediatric brain tumor survivors.
Temperament models address how individual differences in emotional reactivity and self-regulation may affect behavior in social settings (Eisenberg et al., 1995; Rothbart, 1989). Contemporary psychobiological theory suggests that temperament encompasses two reactive or motivational systems, surgency/positive affect and negative affect, as well as a more voluntary control system, effortful control. Effortful control includes a child’s ability to “choose a course of action under conditions of conflict, plan for the future, and detect errors” (Rothbart, 2007), and it is positively related to adaptive social outcomes, such as socially appropriate behavior, popularity, and friendships (Buckner, Mezzacappa, & Beardslee, 2009; Spinrad et al., 2006). This regulatory system interacts with surgency/positive affect and negative affect by activating attention or behavior and conversely inhibiting behavior when necessary (Rothbart & Bates, 1998). For example, effortful control can allow an individual to approach an event or stimulus even when they are frightened (e.g., high negative affect) (Rothbart, 1989). Particularly high (or low) levels of surgency/positive affect or negative affect may place greater demands on the regulatory system. Surgency/positive affect, or the appetitive system, is characterized by approach behavior, sensation seeking, activity level, impulsivity, sociability, positive anticipation, and low levels of shyness (Rothbart, 2007). Negative affect is the defensive system and is described as observable behaviors that include frustration, fear, discomfort, sadness, and soothability.
Surgency/positive affect and negative affect appear to influence social outcomes differently. Children high in surgency/positive affect are generally positive and energetic, directing much of their energy toward social affiliation because of the expectation and experience of social relationships as rewarding (Derryberry & Tucker, 2006). Those low on surgency/positive affect tend to be quieter and more inhibited (Shiner & Caspi, 2003). In some samples, high levels of surgency/positive affect have been associated with social problems and inappropriate social skills (Sallquist et al., 2009). Negative affect has been associated with negative social outcomes (Coplan, Wilson, Frohlick, & Zelenski, 2006), lower levels of prosocial/sociable behavior (Eisenberg et al., 1995), and declining social skills over time (Sallquist et al., 2009). There is also evidence that negative affect moderates the association between effortful control and social outcomes, suggesting that behavioral regulation and socially inappropriate behavior may be correlated with social competence only for children with high negative emotionality (Eisenberg et al., 1997). Thus, while both surgency/positive affect and negative affect have been found to have direct effects on social behavior, it also appears that they may have a moderating effect on the association between effortful control and behavioral outcomes.
There is an increasing recognition that biological dispositions, or temperament, may play a role in outcomes for children with cancer; yet, to our knowledge, only three studies have examined temperament in children with cancer (Barrera et al., 2003; Harper et al., 2014; Miller etal., 2009). Those studies were based on varying models of temperament, did not restrict inclusion to children with a brain tumor specifically, nor included child self-report of temperament. Barrera et al. (2003) found that an easy temperament is positively associated with better psychological adjustment in children with cancer. Harper et al. (2014) reported that higher effortful control was positively associated with ego resilience, which predicted better quality of life. In the study conducted by Miller et al. (2009), data indicated that negative affect was positively associated with anxiety and depression. There was no evidence of an interaction between effortful control and surgency/positive affect or negative affect in predicting anxiety or depression. While these studies were developed to look at temperament differently or related to different outcomes than the current study, they point to the importance of considering these constructs in children who have been treated for cancer. It is particularly important to understand a number of factors, particularly those that are biologically based, in examining outcomes for children treated for a brain tumor.
We expected that pediatric brain tumor survivors in our sample would have lower effortful control than comparison peers and that effortful control would predict social behavior. That is, it was hypothesized that effortful control would mediate the association between group (brain tumor vs. comparison) and social behavior. This hypothesis stems from findings in two areas. First, prior research on temperament suggests that effortful control lies within the executive attention network (one of the three neural attention networks) and attention control capacity is a key component of effortful control (Rothbart et al., 2007; Rueda, Posner, & Rothbart, 2005). Deficits in attention have been well documented for survivors (Mulhern & Butler, 2004), and therefore it seems likely that survivors would be at risk for deficits in effortful control as well. Second, attention control capacity has been linked to social outcomes in samples of healthy children and those with attention-deficit/hyperactivity disorder (ADHD) where poorer attention is associated with less social competence. Given the association between attention and effortful control and social outcomes, it is plausible to expect that if brain tumors and their treatment have deleterious effects on attention in survivors, there may be adverse effects in effortful control, which may then affect social behavior. The potential impact of brain tumors and their treatment on surgency/positive affect and negative affect is less clear. However, given similar and distinct neurological underpinnings of these components of temperament, group differences were explored.
The aim of this study was to investigate temperament (i.e., effortful control, surgency/positive affect, and negative affect) for children treated for brain tumors and comparison peers. In addition to group differences in these constructs, we examined the association between temperament and social behavior for children treated for brain tumors and comparison peers. We hypothesized that survivors would demonstrate less effortful control than peers and that effortful control would mediate group differences in social behavior. In exploratory analyses, we also examined whether the effect of effortful control on each dimension of social behavior was moderated by the level ofa child’s surgency/positive affect and negative affect (moderated mediation).
Method
Procedure
This project was part of a larger multisite study (Vannatta et al., 2011) investigating psychosocial outcomes for pediatric brain tumor survivors and their parents. Data were obtained during school- and home-based assessments with pediatric brain tumor survivors and healthy comparison classmates. This project reports data obtained from two participating pediatric oncology centers. Following institutional review board approval, tumor registries were used to identify children who were (a) aged 8–15 years1 and (b) 1–5 years posttreatment for an intracranial tumor without current or progressive disease. Children were excluded if they (a) had a preexisting neurobehavioral disorder (e.g., neurofibromatosis), (b) resided >100–125 miles from the medical center, (c) were not fluent in English, or (d) were home-schooled or received full-time special education.2
After obtaining parent and principal approval, data collection was coordinated with the primary classroom teacher for elementary school students or the teacher of a required academic class for students in middle or high school. Consent forms were distributed to all classmates, and only children with parental consent participated in a group data collection session. To reduce stigma and bias, the study was described as a project about children’s friendships with no mention of brain tumors, cancer, the medical center, or the ill child.
Following data collection in each class, one classmate matched on race, gender, and age to the brain tumor survivor was recruited for inclusion in the comparison sample. Parents of brain tumor survivors and potential comparison classmates were contacted about participation in a home-based assessment. Potential comparison children were not eligible if they or any child in their home had been treated for a chronic medical condition. When potential comparison families declined or were ineligible, the next closely matched classmate was invited to participate. Families in both groups were invited to participate in a second home-based assessment that included the measures reported in this article. The participating child and a primary caregiver completed the measures of children’s temperament. Families were compensated for their time.
Participants
Eighty-six percent (N = 113 of 131) of parents with eligible children treated for a brain tumor gave permission to contact the child’s school. Data collection was successfully completed at 87% of these schools (N = 98) with parental consent for 89% of classmates. Survivors were an average of 3.69 years from diagnosis (SD = 2.21 years) at study enrollment. The home visit that involved completion of temperament measures was completed with 75 families of the 93 brain tumor survivors (81%) who remained eligible (e.g., had not moved or relapsed after school data were collected). The final sample of brain tumor survivors had an average age of 12.25 years (SD = 3.39 years), 57% (n = 43) were male, and 85% (n = 64) were White. Diagnoses included astrocytoma (n = 35; 47%), medulloblastoma (n = 17; 23%), and other brain tumors (n = 23; 30%). Nearly all children had undergone surgical tumor resection (n = 62; 83%), 32 (43%) had received treatment with radiation, and 32 (43%) had received chemotherapy. Sixty-seven comparison families also completed temperament measures during a home-based assessment. Comparison classmates had an average age of 12.25 years (SD = 2.30), 55% (n = 43) male, and 87% (n = 58) White. A majority of those participating were the first (n = 30, 45%), second (n = 16, 24%), or third (n = 13, 19%) choice, or best-matched eligible classmate, while the remainder (n = 8, 12%) were recruited from remaining same-gender classmates. No significant differences were found between children enrolled in the study who did and did not consent to participate in this follow-up home visit on the five RCP subscales, age at recruitment, or gender. The majority of participating parents (93%) were female. A reported diagnosis of ADHD did not exclude children in either the survivor or comparison group. Common reasons for withdrawal from the prior home visit to the current home visit included ineligibility due to medical factors (n = 7) within the brain tumor group, family declined (n = 11), or unable to be contacted (n = 14). The comparison and survivor groups vary in sample size owing to variable withdrawal rates between time points. Pediatric brain tumor survivors and comparison peers did not differ significantly with regard to age, ethnicity, family income, or number of parents in the home (see Tables I and II). Families of pediatric brain tumor survivors were characterized by lower socioeconomic status (SES), t(140) = − 1.99, p = .05, as indicated by lower parental occupational prestige, than comparison families.
Table I.
Demographic Differences Between Pediatric Brain Tumor Survivors and Comparison Classmates
| Variable | Brain tumor | Comparison | ta | p | db |
|---|---|---|---|---|---|
| M ± SD | M ± SD | ||||
| Child age | 12.28 ± 2.39 | 12.25 ± 2.30 | 0.07 | .946 | .01 |
| Family income (in dollars) | 59,500 ± 41,700 | 73,200 ± 47,800 | −1.82 | .069 | −.31 |
| SES | 52.21 ± 22.21 | 59.43 ± 20.75 | −1.99* | .048 | −.34 |
| Variable | Brain tumor | Comparison | χ2 | p | |
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| Child’s race | |||||
| White | 64 (85%) | 58 (87%) | 1.72 | .424 | |
| Black | 7 (10%) | 8 (12%) | |||
| Other | 4 (5%) | 1 (1%) | |||
| Child’s ethnicity | |||||
| Hispanic | 2 (3%) | 1 (1%) | 0.24 | .627 | |
| Non-Hispanic | 73 (97%) | 64 (99%) | |||
| Number of parents in household | |||||
| One-parent home | 24 (32%) | 13 (19%) | 2.92 | .088 | |
| Two-parent home | 51 (68%) | 54 (81%) | |||
| Child gender | |||||
| Male | 43 (57%) | 37 (55%) | 0.12 | .730 | |
| Female | 32 (43%) | 30 (45%) | |||
Note. Demographic characteristics reported by parents. Child age = child age at second home data collection; Family income = mother report of family income; SES = occupational prestige based on Revised Duncan (TSEI) (Nakao & Treas, 1992).
adf = 140. bEffect sizes for t tests represented with Cohen’s d.
*p < .05.
Table II.
Differences in Peer-Reported Social Behavior for Pediatric Brain Tumor Survivors and Comparison Classmates: Univariate Main Effects
| RCP subscale | Brain tumor | Comparison | Fa | p | db |
|---|---|---|---|---|---|
| M ± SD | M ± SD | ||||
| Leadership-popularity | −0.41 ± 0.75 | 0.29 ± 0.98 | 20.20*** | .000 | −.79 |
| Prosocial | 0.11 ± 0.90 | 0.18 ± 0.96 | 0.016 | .900 | −.07 |
| Aggressive-disruptive | −0.24 ± 0.80 | 0.00 ± 0.99 | 3.169 | .077 | −.27 |
| Sensitive-isolated | 0.67 ± 1.10 | −0.13 ± 0.89 | 20.174*** | .000 | .79 |
| Victimization | 0.49 ± 1.20 | −0.04 ± 0.91 | 7.591** | .007 | .49 |
Note. MANCOVA analyses run with SES entered as a covariate. SES based on parental occupational prestige. RCP = Revised Class Play completed by classroom peers.
adf = 138. bEffect sizes represented with Cohen’s d.
**p < .01; ***p < .001.
Measures
Classroom Data Collection
Revised Class Play
This measure assesses social behavior by asking students to “cast” their classmates into 42 behavioral “roles” (Masten, Morison, & Pellegrini, 1985). Nominations were limited to students who were of the same gender as the target child to avoid sex-role stereotyping, and self-nominations were not allowed. Classmates nominated only the girls or boys in the classroom for roles on the RCP. The number of nominations received by each child for each role was tallied and standardized (M = 0, SD = 1) within each gender in each class to adjust for uneven class sizes, composition, and participation rates. Four subscales, (a) Leadership-popularity, (b) Prosocial, (c) Aggressive-disruptive, and (d) Sensitive-isolated, were created based on previous factor analytic work (Zeller, Vannatta, Schafer, & Noll, 2003). Three additional items were included to construct a Victimization subscale based on previous work (Crick & Nelson, 2002). Scale scores were created by summing the z scores for items loading on each behavioral dimension and standardized to adjust for unequal numbers of items on each scale. Cronbach’s α ranged from .81 to .90 in the current study.
Home Data Collection
Demographic Questionnaire
Parents reported background information about the family (e.g., parental marital status, education). SES was coded using revised Duncan (TSEI) scores assigned to occupations of parents residing in that child’s home (Nakao & Treas, 1992), with the higher value representing family SES in families with two working caregivers.
Early Adolescent Temperament Questionnaire-Revised
This 62-item parent-report questionnaire assesses dimensions of temperament relevant to emotion regulation in children on a 5-point Likert scale ranging from 1 = almost never true to 5 = almost always true (Capaldi & Rothbart, 1992; Ellis & Rothbart, 2001). Exploratory factor analyses of the Early Adolescent Temperament Questionnaire-Revised (EATQ-R) have identified scales reflecting four factors, including the three used in this project: (a) negative affectivity, which includes scales of irritability and frustration and items such as “Worries about our family when s/he is not with us,” (b) surgency, which includes scales of high-intensity pleasure, low levels of shyness, and low levels of fear and items such as “Thinks it would be exciting to move to a new city,” and (c) effortful control, which includes scales of attentional control, activation control, and inhibitory control and items such as “When asked to do something, does it right away, even if s/he doesn’t want to.” The nine scores are created by calculating the mean of included items, and the three temperament factors are created by calculating the mean of the included scales. Cronbach’s α values were .83, .69, and .85 for effortful control, surgency, and negative affect, respectively, in the current sample.
Effortful Control Scale
This 24-item measure assesses child perceptions of self-regulatory abilities. The Persistence/Low Distractibility subscale consists of 12 items ranging from 1 = not at all to 5 = very much (e.g., “When an activity or task is difficult, I give up”) (Lonigan & Phillips, 2001). The mean of these items is the resulting score and reflects the attention and activation control aspects of effortful control. This subscale has good internal consistency (α = .85) and correlates with measures of anxiety and depression (e.g., Muris, 2006). Cronbach’s α for the current sample was .80.
Positive and Negative Affectivity Schedule
This 27-item self-report measure asks children to rate the extent to which different positive and negative feeling/emotion adjectives describe them on a 5-point scale (Watson, Clark, & Tellegen, 1988). Negative Affect (e.g., sad, nervous, miserable) and Positive Affect (e.g., cheerful, happy, delighted) subscales are created by calculating the mean of items. Cronbach’s α for the current sample were .91 and .88, respectively.
Analysis Plan
Separate one-way multivariate analyses of covariance (MANCOVA) were run to examine group differences in (a) social behavior for the five RCP3 subscales, (b) parent report of temperament, and (c) self-report of temperament. SES was entered as a covariate for each MANCOVA. Two-tailed Pearson correlation coefficients were calculated to investigate agreement among parent and child report for the three temperament dimensions.
Mediation analyses examined whether group differences in social behavior were at least partially accounted for by effortful control. These models were run separately for parent report of effortful control and for each of the five RCP subscales (five models) and for child report of effortful control and for each of the five RCP subscales (five models), resulting in 10 mediational models. SES was entered as a covariate for each model. The significance of the indirect effects was tested using bootstrapping, a nonparametric procedure that constructs a confidence interval for the indirect effect by computing the effect in 5,000 subsamples constructed with replacement (Preacher & Hayes, 2004).
Subsequent exploratory analyses investigated whether the effect of effortful control on each dimension of social behavior was moderated by surgency/positive affect or negative affect. These models were run for parent report of effortful control from the EATQ-R with surgency from the EATQ-R (five models) and then with negative affect from the EATQ-R (five models) for each of the five RCP subscales. They were then run for child report of effortful control from the Effortful Control Scale (ECS) with positive affect from the Positive and Negative Affectivity Schedule (PANAS; five models) and then with negative affect from the PANAS (five models) for each of the five RCP subscales. SES was entered as a covariate for each model. Twenty moderated mediation analyses were run using the PROCESS macro for SPSS provided by Hayes (2013). This macro estimates the mediation effect at “low,” “moderate,” and “high” levels of the moderator (reflecting the mean and ±1 standard deviation). In all mediation and moderated mediation analyses, comparison children were entered as “0” and brain tumor survivors were entered as “1” to assist with interpretation of the results.
Results
Group Differences in Social Behavior
A MANCOVA revealed a significant multivariate effect for group for domains of social behavior, Wilks’ λ = .802, F(5, 133) = 6.580, p < .001, partial η2 = .198. Univariate main effects revealed survivors were perceived by classmates as lower on Leadership-popularity, but higher on Sensitive-isolated and Victimization, than comparison peers. See Table II for means and standard deviations of RCP subscales.
Group Differences in Temperament and Correlation Between Parent and Child Report
A MANCOVA revealed a significant multivariate effect for group for parent report of temperament, Wilks’ λ = .937, F(3, 137) = 3.063, p < .05, partial η2 = .063. Univariate main effects revealed pediatric brain tumor survivors were rated by parents as demonstrating less effortful control and less surgency/positive affect than comparison peers. A MANCOVA revealed a significant multivariate effect for group for child self-report of temperament, Wilks’ λ = .930, F(3, 136) = 3.425, p < .05, partial η2 = .070. Univariate main effects revealed survivors reported lower surgency/positive affect than comparison peers. See Table III for means and standard deviations for temperament constructs. Parent report and self-report of effortful control (r(140) = .28, p = .00) were significantly correlated, but reports of surgency/positive affect (r(140) = .11, p = .19) and negative affect (r(140) = .10, p = .24) were not.
Table III.
Differences in Temperament for Pediatric Brain Tumor Survivors and Comparison Classmates: Univariate Main Effects
| Variable | Brain tumor | Comparison | Fa | p | db |
|---|---|---|---|---|---|
| M ± SD | M ± SD | ||||
| Parent report | |||||
| Surgency | 3.05 ± .45 | 3.22 ± .45 | 5.401* | .022 | −.38 |
| NA | 2.67 ± .63 | 2.54 ± .61 | 0.487 | .487 | .21 |
| EC | 3.17 ± .60 | 3.41 ± .56 | 4.071* | .046 | −.41 |
| Child report | |||||
| PA | 3.61 ± .79 | 3.99 ± .64 | 1.501 | .003 | −.60 |
| NA | 1.90 ± .74 | 1.71 ± .71 | 2.425 | .122 | .26 |
| EC | 3.89 ± .70 | 4.04 ± .55 | 9.155** | .223 | −.21 |
Note. MANCOVA analyses run with SES entered as a covariate. SES based on parental occupational prestige. Parent report Surgency = Surgency subscale of the EATQ-R; Parent report NA = Negative Affectivity subscale of the EATQ-R; Parent report EC = EATQ-R; Child report PA = Positive Affect on the PANAS; Child report NA = Negative Affect on the PANAS; Child report EC = child-report of Distractibility on the ECS.
adf = 140. bEffect sizes represented with Cohen’s d.
*p < .05, **p < .01.
Mediation of Group Differences in Social Behavior by Effortful Control
We expected that group differences in social behavior would be mediated by effortful control (Figure 1). A significant indirect effect was found in the model evaluating parent report of effortful control as a mediator of the association between group and both prosocial behavior and aggressive-disruptive behavior, as indicated by the bootstrapped 95% confidence interval for the coefficient for indirect effect that did not include zero (Table IV). The coefficient for the indirect effect involving prosocial behavior was negative, suggesting that reductions in effortful control for brain tumor survivors may account for decreases in reported prosocial behavior for survivors. In contrast, the indirect effect of group on aggressive-disruptive behavior via effortful control was positive because lower levels of effortful control demonstrated by brain tumor survivors were associated with higher levels of aggressive and disruptive behavior. Parent report of effortful control did not mediate or account for group differences in the other dimensions of social behavior, as confidence intervals for these indirect effects did include zero (Table IV). Finally, child self-report of effortful control did not account for group differences for any dimension of social behavior.
Figure 1.
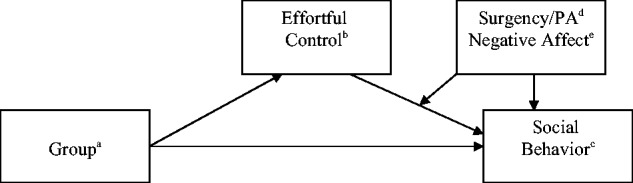
Model of analyses run. Initial analyses examined if effortful control mediated the association between group and indicators of social behavior. Secondary analyses examined if surgency/positive affect and negative affect moderated the pathway from effortful control to social behavior.
aGroup = brain tumor survivors versus comparison peers; bEffortful Control = EATQ-R effortful control construct, ECS child report of persistence/low distractibility; cSocial Behavior = RCP Leadership-popularity scale, RCP Prosocial scale, RCP Aggressive-disruptive scale, RCP Sensitive-isolated scale, RCP Victimization scale; dSurgency/PA = EATQ-R Surgency, PANAS Surgency/Positive Affect; eNegative Affect = EATQ-R Negative Affect, PANAS Negative Affect.
Table IV.
Indirect Effects of Group on Social Behavior via Effortful Control Using Parent Report Data
| Predictor | Coefficient | Standard error | P | Coefficient for indirect effect via EC a | 95% bootstrap CI for the indirect effect |
|---|---|---|---|---|---|
| Outcome: Leadership-popularity R2 = .155; F(3, 136) = 8.326, p < .001 | |||||
| Group | −0.649 | 0.151 | <.001 | −.019 | −0.112 to 0.027 |
| EC | 0.093 | 0.134 | .491 | ||
| SES | 0.004 | 0.004 | .278 | ||
| Outcome: Prosocial R2 = .107; F(3, 136) = 5.453, p < .001 | |||||
| Group | 0.074 | 0.154 | .630 | −.094 | −0.224 to −0.013 |
| EC | 0.464 | 0.137 | <.001 | ||
| SES | 0.004 | 0.004 | .274 | ||
| Outcome: Aggressive-disruptive R2 = .124; F(3, 136) = 6.387, p < .001 | |||||
| Group | −0.373 | 0.149 | .013 | .100 | 0.021 to 0.249 |
| EC | −0.494 | 0.132 | <.001 | ||
| SES | −0.002 | 0.004 | .641 | ||
| Outcome: Sensitive-isolated R2 = .147; F(3, 136) = 7.798, p < .001 | |||||
| Group | 0.754 | 0.175 | <.001 | .020 | −0.027 to 0.116 |
| EC | −0.096 | 0.156 | .537 | ||
| SES | −0.003 | 0.004 | .456 | ||
| Outcome: Victimization R2 = .078; F(3, 136) = 3.816, p = .012 | |||||
| Group | 0.458 | 0.187 | .016 | .051 | −0.006 to 0.177 |
| EC | −0.250 | 0.166 | .134 | ||
| SES | −0.001 | 0.004 | .760 | ||
Note. Group = Brain tumor survivors (1) versus Comparison classmates (0); EC = Effortful Control subscale of the EATQ-R; SES based on parental occupational prestige.
aCoefficient for group =−0.203, p = .034 in model predicting ECS with SES as a covariate.
Exploratory Analyses: Examination of Affectivity as Moderating the Mediating Role of Effortful Control on the Group Effect on Social Behavior
We expected that the association of effortful control with social behavior would vary in the context of high versus low negative affect or high versus low surgency/positive affect. Moderated mediation analyses using parent report indicated that effortful control accounted for the association between group and prosocial behavior at low to moderate, but not high, levels of negative affect. The confidence intervals for the indirect effect derived from bootstrapping included zero when negative affectivity was high but not when low or moderate. This suggests that at high levels of negative affectivity, prosocial behavior is reduced, regardless of whether effortful control is low or high. However, at low or moderate levels, prosocial behavior is reduced in brain tumor survivors as a function of their lower level of effortful control. However, this was the only significant finding in the 20 exploratory analyses and is not more than expected by chance. Models examining the RCP subscales reflecting social Sensitivity-isolation, Leadership-popularity, Aggressive-disruptive, and Victimization did not find evidence of moderated mediation involving negative affectivity. Similarly, parent report of surgency/positive affect did not moderate any indirect effects. Finally, none of the models using child self-report of surgency/positive affect or negative affect found evidence of moderated mediation.
Discussion
Although research has documented that children treated for a brain tumor demonstrate social deficits, the specific nature of these deficits is not well understood. The current study investigated temperament and its role in survivors’ social behavior. Previous findings regarding group differences in social behavior were replicated (Vannatta et al., 2011), and survivors were found to have less effortful control and surgency/positive affect than comparison peers. Temperament showed utility in explaining some aspects of social behavior in this sample.
Traditionally, temperament is conceptualized as biologically based individual differences that are present from birth and relatively stable across time (Rothbart, 2007). However, by using a group of matched comparison peers, we investigated the possibility that temperament, or dispositional levels of affectivity and behavioral regulation, may be altered, or at least systematically different, after children have been treated for a CNS tumor. As expected, we documented that a behavioral correlate of executive functioning, effortful control, may be adversely affected. This decrease in self-regulatory capacities could lead to behavioral deficits in both restraint and initiation (Brière, Scott, McNall-Knapp, & Adams, 2008). This difference was noted only by parents, suggesting they may be better able to judge children’s effortful control than children themselves, although, interestingly, parent and child reports of effortful control were significantly correlated, suggesting some agreement between reporters. Verstraeten, Vasey, Claes, and Bijttebier (2010) found that parent report of effortful control was more stable across time and more consistently correlated with indicators of psychopathology than child report. Interestingly, in their sample, parent report of effortful control on the EATQ-R and self-report on the Persistence/Low Distractibility subscale of the ECS had a correlation coefficient of .50, whereas in our combined sample of brain tumor survivors and comparison peers the correlation coefficient was .28. The correlation was somewhat, but not significantly, stronger in the comparison group (r(65) = .34, p = .01) relative to the survivor group (r(73) = .22, p = .06), indicating that children treated for a brain tumor may be slightly less accurate at providing self-report of effortful control than children not treated for CNS disease. This is consistent with other data suggesting that survivors’ self-reports may be discrepant from others’ reports (Salley et al., 2014).
While it seemed likely that survivors would be described as demonstrating less effortful control, it was unclear how they would be perceived in terms of surgency/positive affect and negative affect. Results revealed these children were described as lower in surgency/positive affect than comparison peers by both parent report and self-report, and developmental literature has revealed that low surgency could be a risk factor for social difficulties (Shiner & Caspi, 2003). These group differences indicate that affectivity, like effortful control, may be altered after treatment for a brain tumor during childhood, suggesting the relative stability of these constructs may be disrupted by neurological insult during childhood. Interestingly, positive emotionality involves the prefrontal cortex, which is the same neurological substrate of effortful control. Unfortunately, we were unable to test longitudinal changes in temperament due to CNS-directed treatment in this study. We considered our findings relative to those reported for a sample of children newly diagnosed with cancer by Miller et al. (2009). Our brain tumor sample appeared to demonstrate greater positive affect, less effortful control, and similar negative affect in comparison with children recently diagnosed and undergoing active treatment for cancer as reported by parents on the same measure of temperament.
We hypothesized that effortful control would mediate group differences in social behavior, and we explored whether mediation would depend on (i.e., be moderated by) a child’s level of surgency/positive affect and negative affect. Significant mediation was found in models of parent-reported temperament in predicting prosocial and aggressive-disruptive behavior. Analyses indicated that children treated for a brain tumor have lower effortful control and that effortful control is positively associated with prosocial behavior. Therefore, survivors may demonstrate less prosocial behavior because of less effortful control. Results also indicated that effortful control is negatively associated with aggressive and disruptive behavior. This finding suggests that deficits in effortful control could increase the extent to which brain tumor survivors demonstrate aggressive and disruptive behavior with peers. Interestingly, the brain tumor survivors in this study were described overall as less, not more, aggressive and disruptive than comparison peers. There are other factors that may mitigate or protect brain tumor survivors from developing this type of maladaptive behavior despite difficulties in effortful control (Gartstein, Vannatta, & Noll, 2000). For example, some of the physical effects of treatment for a brain tumor, such as chronic fatigue or small stature (Turner, Rey-Casserly, Liptak, & Chordas, 2009), may decrease risk for externalizing behavior. Only 1 of 20 moderated mediation models was significant, which is not more than we would expect by chance.
Interestingly, the models in which mediation or moderated mediation were statistically significant showed that low effortful control contributes to less prosocial and more aggressive and disruptive behavior for brain tumor survivors. However, the group difference main effects of these subscales did not reveal statistically significant differences in these behaviors. These findings further support the importance of considering moderators and mediators in understanding outcomes for children with chronic illness.
The results of this study should be considered within the context of several limitations. Due to the novelty of these analyses and the constructs studied in this population, as well as difficulty in defining what would be logical families of tests, we did not correct for multiple comparisons. Although our sample size was larger than most pediatric brain tumor outcome studies, a larger sample size would have increased power and potential to detect significant effects where we found only trends (e.g., group differences in aggressive-disruptive behavior). Further, tests of interactions are typically low in power unless sample sizes are large, and therefore our exploratory analyses of moderated mediation were underpowered (McClelland & Judd, 1993). Our limited sample size also precluded examination of the influence of treatment (i.e., radiation, chemotherapy, surgery, or combined treatment), illness (e.g., tumor type and location, time since treatment), or demographic (e.g., age, gender) factors as moderators of temperament. Examination of such factors may be warranted, as evidence suggests that children treated at a younger age (Reimers et al., 2003), children receiving higher doses of radiation (Mulhern et al., 1998), and females (Brown et al., 1998) are at greatest risk for neurocognitive deficits following CNS-directed treatment. As such, these subgroups may exhibit more dramatic changes in temperament, in particular effortful control. Owing to a lack of prospective data, we were unable to track changes in temperament and social behavior over time. Survivors also had to be in at least one main-streamed classroom, thereby excluding the most impaired survivors. Thus, the results of this study may not generalize to all those treated for a CNS tumor.
Despite these limitations, this project has several strengths. The sample size was larger than most studies investigating psychosocial outcomes in pediatric brain tumor survivors (Schulte & Barrera, 2010). We were also able to examine the temperament and social behavior of survivors relative to a well-matched control group. Another strength was the use of multiple informants, though notably analyses were run only with the same reporter (parent report or self-report) of the mediator and moderator. Ratings of social behavior were provided by all peers within a child’s classroom, creating a more reliable measure of children’s behavior than using a single informant alone. Furthermore, the collection of both parent report and self-report of temperament allowed us to consider their unique perspectives.
This study was developed to provide a possible explanation for differences in social behavior among pediatric brain tumor survivors. We found that two components of temperament (effortful control and surgency/positive affect) were different for brain tumor survivors. Analyses revealed that temperament did not explain where there were statistically significant differences in social behavior (Leadership-popularity, Sensitivity-isolation, Victimization). This may indicate that changes in temperament do not help explain some of the problematic changes in social interaction patterns observed for brain tumor survivors (i.e., higher levels of exclusion and victimization by peers, social withdrawal, and decreases in leadership or dominance in the peer group) or that other factors not examined in this article may compete or interact with temperament to affect behavior. Future work should continue to consider other aspects of social information processing that may influence social outcomes for pediatric brain tumor survivors. For example, other constructs may be affected by neurological insult, including affect recognition, social problem solving, or pragmatic language skills (Yeates et al., 2007). Identification of such variables may aid in the development of interventions to promote positive social relationships and improve overall quality of life for survivors.
Clinicians might consider how changes in temperament may influence a child’s self-regulation and motivation for interaction in their environment, as well as the reaction of key figures in the child’s life. Both anecdotally and qualitatively, parents have expressed how their child seems “different” following treatment (Vance, Eiser, & Horne, 2004). Assisting parents in processing these changes and developing an understanding or expectation of these changes may be helpful, particularly because family processes may moderate the extent to which neurocognitive impact may influence psychosocial outcomes (Patel & Carlson-Green, 2005). Similarly, it may be helpful to provide information to teachers about why a child who had a brain tumor may struggle with self-regulation and may appear less interested in social interaction.
Funding
This work was supported by awards from the American Cancer Society (RSGPB-03-098-01-PBP) and the National Cancer Institute (R03 CA138122-02).
Portions of these data and analyses were presented at the 12th World Congress of Psycho-Oncology, Quebec City, Canada (2010).
Conflicts of interest: None declared.
Footnotes
1 This age range reflects the inclusion criteria for the larger study from which participants were recruited for the current study. This age range was chosen to address the age requirements and limitations in measures chosen for both the current study and the larger study.
2 Many self-contained special education classrooms have a small class size that may compromise the reliability and validity of the sociometric measures. However, children who received part-time special education services were included if they did not exclusively receive instruction in required academic subjects (e.g., English, math, social studies, science) in small special education classes.
3 The current RCP data overlap with the data reported by Vannatta et al. (2011). The current article includes approximately 34% of the pediatric brain tumor survivors from Vannatta et al. (2011). The RCP is the only measure that overlaps in these two papers.
References
- Barrera M, Wayland L A, D’Agostino N, Gibson J, Weksberg R, Malkin D. Developmental differences in psychological adjustment and health related quality of life in pediatric cancer patients. Children’s Health Care. 2003;12:215–232. [Google Scholar]
- Bhat S R, Goodwin T L, Burwinkle T M, Lansdale M F, Dahl G V, Huhn S L, Gibbs I C, Donaldson S S, Rosenblum R K, Varni J W, Fisher P G. Profile of daily life in children with brain tumors: An assessment of health-related quality of life. Journal of Clinical Oncology. 2005;23:5493–5500. doi: 10.1200/JCO.2005.10.190. [DOI] [PubMed] [Google Scholar]
- Bonner M J, Hardy K K, Willard V W, Anthony K K, Hood M, Gururangen S. Social functioning and facial expression recognition in survivors of pediatric brain tumors. Journal of Pediatric Psychology. 2008;33:1142–1152. doi: 10.1093/jpepsy/jsn035. [DOI] [PubMed] [Google Scholar]
- Brière M, Scott J G, McNall-Knapp R Y, Adams R L. Cognitive outcome in pediatric brain tumor survivors: Delayed attention deficit at long-term follow-up. Pediatric Blood & Cancer. 2008;50:337–340. doi: 10.1002/pbc.21223. [DOI] [PubMed] [Google Scholar]
- Brown R T, Madan-Swain A, Walco G A, Cherrick I, Ievers C E, Conte P M, Vega R, Bell B, Lauer S J. Cognitive and academic late effects among children previously treated for acute lymphocytic leukemia receiving chemotherapy as CNS prophylaxis. Journal of Pediatric Psychology. 1998;23:333–340. doi: 10.1093/jpepsy/23.5.333. [DOI] [PubMed] [Google Scholar]
- Buckner J C, Mezzacappa E, Beardslee W R. Self-regulation and its relations to adaptive functioning in low income youths. American Journal of Orthopsychiatry. 2009;79:19–30. doi: 10.1037/a0014796. [DOI] [PubMed] [Google Scholar]
- Capaldi D M, Rothbart M K. Development and validation of an early adolescent temperament measure. Journal of Early Adolescence. 1992;12:153–173. [Google Scholar]
- Carey M E, Barakat L P, Foley B, Gyato K, Phillips P C. Neuropsychological functioning and social functioning of survivors of pediatric brain tumors: Evidence of nonverbal learning disability. Child Neuropsychology. 2001;7:265–272. doi: 10.1076/chin.7.4.265.8730. [DOI] [PubMed] [Google Scholar]
- Carpentieri S C, Mulhern R K, Douglas S, Hanna S, Fairclough D L. Behavioral resiliency among children surviving brain tumors: A longitudinal study. Journal of Clinical Child Psychology. 1993;22:236–246. [Google Scholar]
- Cavell T A. Social adjustment, social performance, and social skills: A tri-component model of social competence. Journal of Clinical Child Psychology. 1990;19:111–122. [Google Scholar]
- Coplan R J, Wilson J, Frohlick S L, Zelenski J. A person-oriented analysis of behavioral inhibition and behavioral activation in children. Personality and Individual Differences. 2006;41:917–927. [Google Scholar]
- Crick N R, Nelson D A. Relational and physical victimization within friendships: Nobody told me there’d be friends like these. Journal of Abnormal Child Psychology. 2002;30:599–607. doi: 10.1023/a:1020811714064. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Tucker D M. Motivation, self-regulation, and self-organization. In: Cicchetti D, Cohen D J, editors. Developmental psychopathology: Vol. 2: Developmental Neuroscience. 2006. (2nd ed., pp. 502–532). New York: Wiley. [Google Scholar]
- Eisenberg N, Fabes R A, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children’s social functioning: A longitudinal study. Child Development. 1995;66:1360–1384. [PubMed] [Google Scholar]
- Eisenberg N, Guthrie I K, Fabes R A, Reiser M, Murphy B C, Holgren R, Maszk P, Losoya S. The relations of regulation and emotionality to resiliency and competent social functioning in elementary school children. Child Development. 1997;68:295–311. [PubMed] [Google Scholar]
- Ellis L K, Rothbart M K. Revision of the early adolescent temperament scale. Minneapolis, MN: Society for Research in Child Development; 2001. [Google Scholar]
- Fuemmeler B F, Mullins L L, Carpentier M. Peer friendship issues and emotional well-being. In: Brown R T, editor. Pediatric hematology/oncology: A biopsychosocial approach. New York: Oxford University Press; 2006. [Google Scholar]
- Gartstein M A, Vannatta K, Noll R B. Childhood aggression and chronic illness: Possible protective mechanisms. Journal of Applied Developmental Psychology. 2000;21:315–333. [Google Scholar]
- Harper F W, Goodlett B D, Trentacosta C J, Albrecht T L, Taub J W, Phipps S, Penner L A. Temperament, personality, and quality of life in pediatric cancer patients. Journal of Pediatric Psychology. 2014;39:459–468. doi: 10.1093/jpepsy/jst141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A F. Introduction to mediation, moderation, and conditional process analysis. New York, NY: Guilford Press; 2013. [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun M J. 2009. Cancer statistics. CA: A Cancer Journal for Clinicians, 59, 225–249. doi: 10.3322/caac.20006. [Google Scholar]
- Kagan J, Fox N A. 2006. Biology, culture, and temperamental biases. In W. Damon & R. Lerner (Book Eds.) & N. Eisenberg (Vol. Ed), Handbook of child psychology: Vol. 3. Social, emotional, and personality development (6th ed., pp.167–225). New York: Wiley. [Google Scholar]
- Lonigan C J, Phillips B M. Temperamental influences on the development of anxiety disorders. In: Vasey M W, Dadds M R, editors. The developmental psychopathology of anxiety. New York: Oxford University Press; 2001. pp. 60–91. [Google Scholar]
- Masten A S, Morison P, Pellegrini D S. A revised class play method of peer assessment. Developmental Psychology. 1985;21:523–533. [Google Scholar]
- McClelland G H, Judd C M. Statistical difficulties of detecting interactions and moderator effects. Psychological Bulletin. 1993;114:376–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- Miller K S, Vannatta K, Compas B E, Vasey M, McGoron K D, Salley C G, Gerhardt C A. The role of coping and temperament in the adjustment of children with cancer. Journal of Pediatric Psychology. 2009;34:1135–1143. doi: 10.1093/jpepsy/jsp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhern R K, Butler R W. Neurocognitive sequelae of childhood cancers and their treatment. Pediatric Rehabilitation. 2004;7:1–14. doi: 10.1080/13638490310001655528. [DOI] [PubMed] [Google Scholar]
- Mulhern R K, Kepner J L, Thomas P R, Armstrong F D, Friedman H S, Kun L E. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: A pediatric oncology group study. Journal of Clinical Oncology. 1998;16:1723–1728. doi: 10.1200/JCO.1998.16.5.1723. [DOI] [PubMed] [Google Scholar]
- Muris P. Unique and interactive effects of neuroticism and effortful control on psychopathological symptoms in non-clinical adolescents. Personality and Individual Differences. 2006;40:1409–1419. [Google Scholar]
- Nakao K, Treas J. The 1989 socioeconomic index of occupations: Construction from the 1989 occupational prestige scores (general social survey methodological report no. 74) Chicago, IL: National Opinion Research Center, University of Chicago; 1992. [Google Scholar]
- Parker J G, Asher S R. Peer relations and later personal adjustment: Are low-accepted children at risk? Psychological Bulletin. 1987;107:357–389. doi: 10.1037//0033-2909.102.3.357. [DOI] [PubMed] [Google Scholar]
- Patel S K, Carlson-Green B. Commentary: Toward greater integration and specificity in conceptual models of neurocognitive functioning in childhood cancer survivors. Journal of Pediatric Psychology. 2005;30:85–88. doi: 10.1093/jpepsy/jsi019. [DOI] [PubMed] [Google Scholar]
- Preacher K J, Hayes A F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, and Computers. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Reimers T S, Ehrenfels S, Mortensen E L, Schmiegelow M, Sonderkaer S, Carstensen H, Schmiegelow K, Muller J. Cognitive deficits in long-term survivors of childhood brain tumors: Identification of predictive factors. Medical Pediatric Oncology. 2003;40:26–34. doi: 10.1002/mpo.10211. [DOI] [PubMed] [Google Scholar]
- Ries L G, Smith M A, Gurney J G, Linet M, Tamra T, Young J L, Bunin G R, editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. National Cancer Institute; SEER Program. NIH Pub. No. 99-4649. Bethesda, MD, 1999. [Google Scholar]
- Rothbart M K. Temperament in childhood: A framework. In: Kohnstamm G A, Bates J E, Rothbart M K, editors. Temperament in childhood. Chichester, UK: Wiley; 1989. pp. 59–73. [Google Scholar]
- Rothbart M K. Temperament, development, and personality. Current Directions in Psychological Science. 2007;16:207–212. [Google Scholar]
- Rothbart M K, Bates J E. 1998. Temperament. In W. Damon (Series Ed.) and N. Eisenberg (Vol. Ed). Handbook of child psychology: Vol. 3. Social, emotional, and personality development (pp. 105–176). New York: Wiley. [Google Scholar]
- Rothbart M K, Bates J E. 2006. Temperament in children’s development. In W. Damon & R. Lerner (Book Eds.) & N. Eisenberg (Vol. Ed), Handbook of child psychology: Vol. 3. Social, emotional, and personality development (6th ed., pp. 99–166). New York: Wiley. [Google Scholar]
- Rothbart M K, Sheese B E, Posner M I. Executive attention and effortful control: Linking temperament, brain networks, and genes. Child Development Perspectives. 2007;1:2–7. [Google Scholar]
- Rueda M R, Posner M I, Rothbart M K. The development of executive attention: Contributions to the emergence of self-regulation. Developmental Neuropsychology. 2005;28:573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- Salley C G, Gerhardt C A, Fairclough D L, Patenaude A F, Kupst M J, Barrera M, Vannatta K. Social self-perception among pediatric brain tumor survivors compared with peers. Journal of Developmental and Behavioral Pediatrics. 2014;35:427–434. doi: 10.1097/DBP.0000000000000077. doi:10.1097/dbp.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallquist J V, Eisenberg N, Spinrad T L, Reiser M, Hofer C, Liew J, Zhou Q, Eggum N. Positive and negative emotionality: Trajectories across six years and relations with social competence. Emotion. 2009;9:15–28. doi: 10.1037/a0013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte F, Barrera M. Social competence in childhood brain tumor survivors: A comprehensive review. Supportive Care in Cancer. 2010;18:1499–1513. doi: 10.1007/s00520-010-0963-1. [DOI] [PubMed] [Google Scholar]
- Shiner R, Caspi A. Personality differences in childhood and adolescence: Measurement, development, and consequences. Journal of Child Psychology and Psychiatry. 2003;44:2–32. doi: 10.1111/1469-7610.00101. [DOI] [PubMed] [Google Scholar]
- Spinrad T L, Eisenberg N, Cumberland A, Fabes R A, Valiente C, Shepard S A, Reiser M, Losoya S, Guthrie I K. Relation of emotion-related regulation to children’s social competence: A longitudinal study. Emotion. 2006;6:498–510. doi: 10.1037/1528-3542.6.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C D, Rey-Casserly C, Liptak C C, Chordas C. Late effects of therapy for pediatric brain tumor survivors. Journal of Child Neurology. 2009;11:1455–1463. doi: 10.1177/0883073809341709. [DOI] [PubMed] [Google Scholar]
- Vance Y H, Eiser C, Horne B. Parents’ views of the impact of childhood brain tumours and treatment on young people’s social and family functioning. Clinical Child Psychology and Psychiatry. 2004;9:271–288. [Google Scholar]
- Vannatta K, Barrera M, Gerhardt C A, Fairclough D, Kupst M J, Meyer E A, Patenaude A F, Turner C. Social and behavioral adjustment of pediatric brain tumor survivors: Examination of factors that may modify risk. Pediatric Blood and Cancer. 2011;56:1162. [Google Scholar]
- Vannatta K, Gerhardt C A, Wells R J, Noll R B. Intensity of CNS treatment for pediatric cancer: Prediction of social outcomes in survivors. Pediatric Blood and Cancer. 2007;49:716–722. doi: 10.1002/pbc.21062. [DOI] [PubMed] [Google Scholar]
- Verstraeten K, Vasey M W, Claes L, Bijttebier P. The assessment of effortful control in childhood: Questionnaires at the test of everyday attention for children compared. Personality and Individual Differences. 2010;48:59–65. [Google Scholar]
- Watson D, Clark L A, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yeates K O, Bigler E D, Dennis M, Gerhardt C A, Rubin K H, Stancin T, Taylor H G, Vannatta K. Social outcomes in childhood brain disorder: A heuristic integration of social neuroscience and developmental psychology. Psychological Bulletin. 2007;133:535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller M, Vannatta K, Schafer J, Noll R B. Behavioral reputation: A cross-age perspective. Developmental Psychology. 2003;39:129–139. doi: 10.1037//0012-1649.39.1.129. [DOI] [PubMed] [Google Scholar]


