Abstract
Aims
This study aimed to evaluate the incidence and prevalence of blindness, sight impairment, and other visual acuity (VA) states in patients receiving ranibizumab for neovascular age-related macular degeneration (nAMD) in Gloucestershire.
Methods
Serial VA and injection data for all treatment-naive patients receiving their first intravitreal injections of ranibizumab for nAMD in the Gloucestershire National Health Service Ophthalmology department between 2008 and 2010 were extracted from an electronic medical record system.
Results
The prevalence of blindness (VA in the better-seeing eye ≤25 Early Treatment Diabetic Retinopathy Study (ETDRS) letters) at the time of first intravitreal injection was 0.8%, increasing to 3.5% after 3 years. The prevalence of sight impairment (VA in the better-seeing eye 26–39 ETDRS letters) increased from 4.1% at baseline to 5.5% after 3 years. The incidence of initiating ranibizumab treatment for nAMD in people aged ≥50 years in Gloucestershire was 111 people per 100 000 population in 2009, and 97 people in 2010. The incidence of patients meeting the visual criteria for blindness and sight impairment registration from treated nAMD in people aged ≥50 years in Gloucestershire was 3.5 and 9.7 people, respectively per 100 000 population in 2010.
Conclusion
This is the first real-world study on the incidence and prevalence of eligibility for blindness and sight impairment registration in treated nAMD in the UK based on VA data. The incidence and prevalence of eligibility for certification of blindness or sight impairment in patients treated with ranibizumab for nAMD is low in Gloucestershire, with only 3.6% of the incident population progressing to blindness in 2010.
Introduction
Before the introduction of antivascular endothelial growth factor (anti-VEGF) treatments, neovascular age-related macular degeneration (nAMD) was the leading cause of blindness in the USA, UK, and other developed countries, accounting for two-thirds of new cases of blindness in the elderly population and between 50 and 60% of new cases of blindness overall.1, 2, 3, 4, 5 The prevalence of nAMD was predicted to continue increasing over the next decade, from 415 000 cases in 2010 to 516 000 in 2020 in the UK,6 and from 1 million in 2010 to over 2 million in 2050 in the USA.7
A temporal and causative relationship has been noted between the widespread introduction of anti-VEGF drugs and a decrease in blindness registration caused by nAMD.5, 8, 9, 10, 11 To date, the annual incidence of blindness in England and Wales has been estimated from the number of patients newly registered as severely sight impaired. The limitations of certification data to estimate the incidence of blindness and sight impairment have been demonstrated repeatedly, with some studies showing that fewer than 50% of eligible patients are registered.4, 12, 13, 14
The aim of this study was to use visual acuity (VA) data routinely collected within an electronic medical record (EMR) in the context of a paperless nAMD service to analyse the incidence of blindness and other degrees of sight impairment in patients receiving anti-VEGF for nAMD in a well-defined region of the UK.
Method
Before the NICE Technology Appraisal 155, issued in August 2008, there were restrictions on patient access to ranibizumab treatment within the National Health Service (NHS). Therefore, 2008 was chosen as the start of the study period, as universal NHS availability of ranibizumab was achieved during this year, initially for second affected eyes and after August 2008 for all affected eyes.
Data were collected prospectively for all patients receiving intravitreal injections of ranibizumab (Lucentis; Novartis Pharmaceuticals, Camberley, UK; Genentech, Inc., San Francisco, CA, USA) for nAMD in the NHS ophthalmology department serving Gloucestershire, a geographically well-defined county of the UK where very few, if any, patients seek care from neighbouring centres. To date the department has exclusively used ranibizumab to treat NHS patients with nAMD.
Prospective collection of a standardised data set was achieved as by-product of routine clinical care using an EMR system (Medisoft Ophthalmology, Medisoft Limited, Leeds, UK), in the context of paperless clinics, guaranteeing high levels of data completeness. VA with habitual correction is measured at baseline (before treatment starts) and at all follow-up appointments using Early Treatment Diabetic Retinopathy Study (ETDRS) logMAR charts. The department uses a pro re nata treatment regimen after an initial loading phase of three injections at monthly intervals. Patients are followed up at monthly intervals with spectral domain optical coherence tomography and fundal examination until no injections have been required to either eye for 6 months after which follow-up intervals are gradually extended. If no injections have been required for 1 year patients are discharged and advised to return if they notice any new symptoms of blurring or distortion of vision in either eye.
Analysis in this study was restricted to treatment-naive exclusively NHS-treated patients receiving ranibizumab for nAMD for the first time in one or both eyes between 3 January 2008 and 31 December 2010, for whom a minimum of 12 months follow-up data were recorded. Data were extracted from the EMR on 21 October 2013 and analysed in Excel (Microsoft, Redmond, WA, USA), Ruby (https://www.ruby-lang.org/en), and R (http://www.r-project.org). Population denominators for the Gloucestershire region were obtained from the Office of National Statistics as the mid-year population estimates.
Results
Participants
Pseudonymised data were extracted from the EMR for 927 eyes of 818 patients receiving their first intravitreal injection of an anti-VEGF drug between 2008 and 2010 using standardised auditing queries built into the EMR. See Supplementary Figure 1 for the consolidated standards of reporting trials-style diagram showing patients and eyes analysed in this study. Of these 208 patients were excluded from analysis: 90 had eyes treated with anti-VEGF for non-AMD indications or bevacizumab for any indication; 79 had <12 months follow-up acuity data available; 38 were treated privately; and 1 patient aged 48 years at first injection. Six hundred and ten patients (653 eyes) met the inclusion criteria: 143 patients received their first intravitreal treatment in 2008; 248 in 2009; and 219 in 2010. Table 1 summarises the characteristics of the study patients.
Table 1. Patient characteristics.
| Total | First injection 2008 | First injection 2009 | First injection 2010 | |
|---|---|---|---|---|
| Number of patients | 610 | 143 | 248 | 219 |
| Mean age at first injection, years (range) | 80.3 (50–98) | 80.9 (50–98) | 80.4 (54–97) | 80.0 (50–95) |
| Female : male | 1.95 : 1 | 2 : 1 | 1.76 : 1 | 2.1 : 1 |
| Mean VA in the first treated eye (ETDRS letters) | 55 | 54 | 53 | 58 |
| Mean VA in the better-seeing eye at first injection (ETDRS letters) | 68 | 66 | 68 | 70 |
Follow-up/attrition
Of those meeting the inclusion criteria, 491 (81%) completed 3 years or more of follow-up. Among them, 31 (5%) patients died, 54 (9%) patients were discharged, 21 (3%) patients were lost to follow-up, 6 (1%) patients moved away, 6 (1%) patients declined further treatment, and 1 (0.2%) patient developed a suspected allergy to ranibizumab.
Visual acuity
The first treated eye was the better-seeing eye in 41% cases overall. Excluding the 7% (n=41) patients who received treatment to both eyes at baseline, the better-seeing eye was the first treated in 52, 38, and 37% in the 2008, 2009, and 2010 cohort, respectively. The fellow eye was treated in 14.9% of patients overall. The fellow eye was treated at any time in 29% (n=42) of patients receiving first intravitreal injection in 2008, in 20% (n=50) of the 2009 cohort, and in 23% (n=50) of the 2010 cohort.
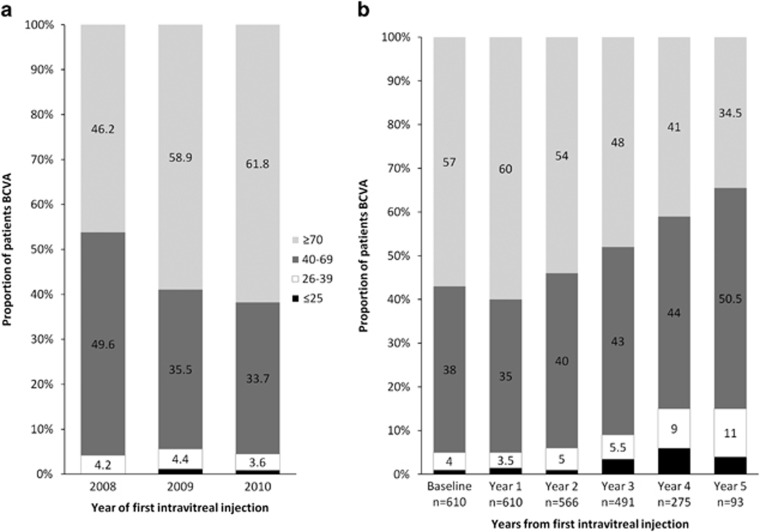
The baseline VA with habitual correction in the better-seeing eye for all patients at baseline by year of first injection is presented in Figure 1a. The VA with habitual correction in the better-seeing eye for all patients at annual time points from first injection is presented in Figure 1b.
Figure 1.
(a) Baseline VA with habitual correction in the better-seeing eye for all patients at baseline by year of first injection (b) VA with habitual correction in the better-seeing eye for all patients at annual time points from first injection. ETDRS letters are shown.
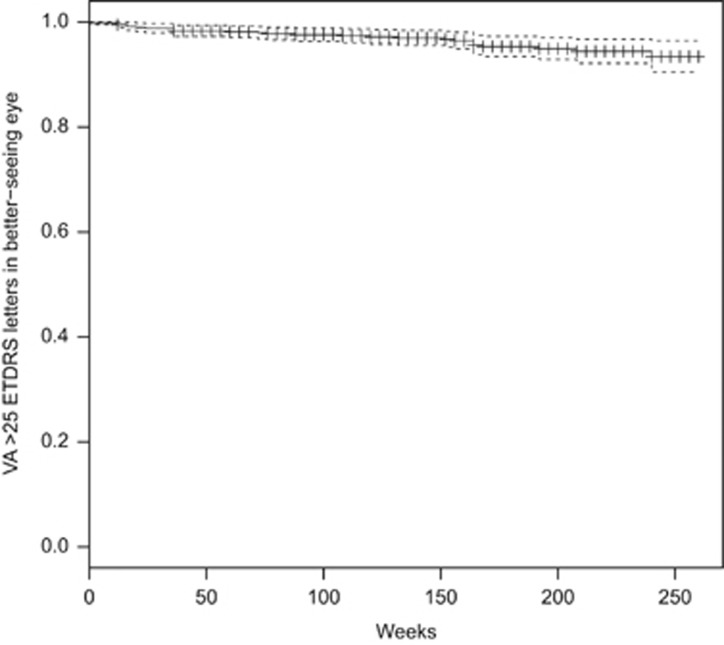
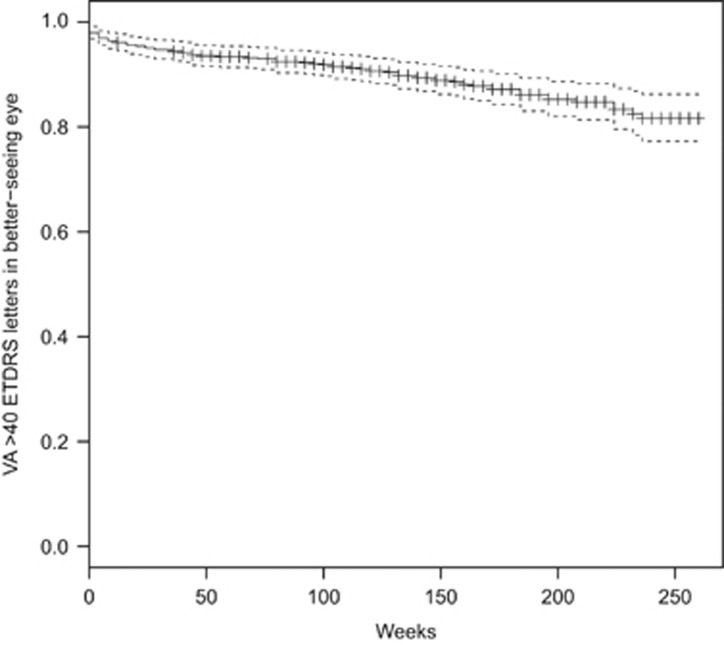
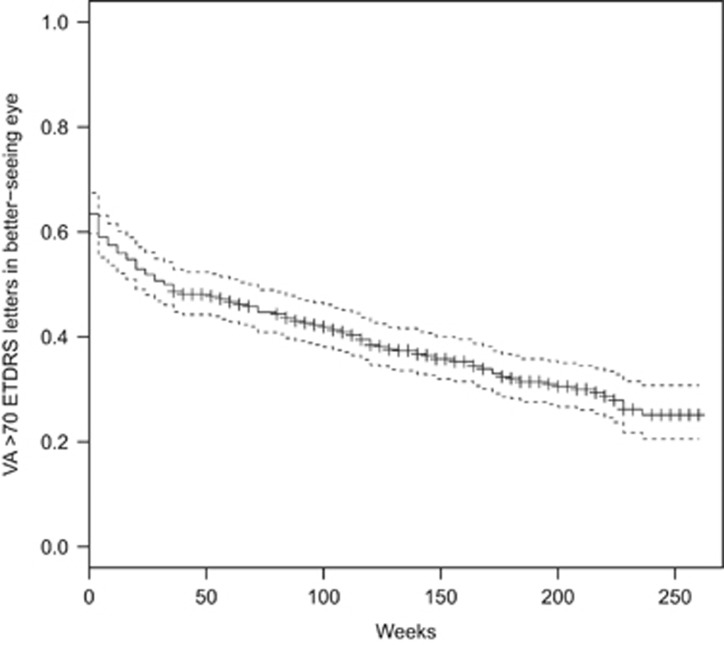
The prevalence of blindness (VA in the better-seeing eye 25 ETDRS letters or fewer at two consecutive visits or one visit with no further follow-up) in all patients at the time of first intravitreal injection was 5 patients (0.8%), increasing to 17 (3.5%) after 3 years. The prevalence of sight impairment (VA in the better-seeing eye 26–39 ETDRS letters at two consecutive visits or one visit with no further follow-up) increased from 25 patients (4.1%) at baseline to 27 (5.5%) after 3 years. The prevalence of vision approximately sufficient to meet the criteria for holding a UK drivers' licence (VA in the better-seeing eye 70 or more ETDRS letters at two consecutive visits or one visit with no further follow-up) reduced from 347 patients (57%) at baseline to 237 (48%) at 3 years. In patients who received their first intravitreal injection of ranibizumab in 2008, at 5 years the prevalence of blindness was 4 patients (4.3%), the prevalence of sight impairment was 10 patients (10.8%), and the prevalence of driving standard vision was 32 patients (34.4%). The event rates for blindness, sight impairment, and failure to meet driving standard vision are illustrated in Kaplan–Meier plots, Figures 2, 3, 4.
Figure 2.
Kaplan–Meier chart of new blindness.
Figure 3.
Kaplan–Meier chart of new sight impairment.
Figure 4.
Kaplan–Meier chart of loss of the driving standard VA.
Confining the patients to those with a Gloucestershire postcode, the incidence of initiating NHS ranibizumab treatment for nAMD in people aged 50 years and older was 65 people per 100 000 population in 2008, 111 people per 100 000 population in 2009, and 97 people per 100 000 population in 2010. The incidence of blindness from treated nAMD in people aged 50 years and older was 1.3 people in 2009 (n=3) and 3.5 people in 2010 (n=8). The figures for sight impairment were 8.5 people in 2009 (n=19) and 9.7 people in 2010 (n=22).
Discussion
To our knowledge this is the first paper to assess the impact of anti-VEGF treatment in the UK based on changes in VA in the better-seeing eye, which is known to be the most significant factor influencing patient-reported vision-related function.15, 16
As blindness is a definition pertaining to the VA in the better-seeing eye, we must consider the better-seeing eye of the patient to understand the natural history of blindness from untreated nAMD, and the impact of anti-VEGF treatment on blindness, and other levels of visual disability from nAMD. The natural history of nAMD in individual eyes (placebo or sham arm) and the impact of nAMD treatment in individual eyes have been described in the study arm of clinical trials.17, 18, 19, 20 When analysis is restricted to one eye per patient, study results cannot be used to comment on rates of blindness or other levels of visual disability from untreated nAMD, or reduction in disability in treated nAMD.
Analysis of the trends in our data is complicated by the changing nature of the baseline characteristics over time. In 2008, the first treated eye was significantly more frequently the better-seeing eye (P=0.002) because it was often the second affected eye; the first eye having been affected before the availability of anti-VEGF within the NHS. In subsequent cohorts, the first treated eye was more commonly the first eye affected by nAMD, and was less frequently the better-seeing eye.
In 2012, Bloch et al9 reported that between 2000 and 2010 the incidence of legal blindness from all types of AMD fell to half the baseline incidence (from 52.2 cases/year per 100 000 in 2000 to 25.7 cases/year per 100 000 population in 2010 of the population aged ≥50 years). The bulk of the reduction occurred after the introduction of intravitreally injected inhibitors of vascular endothelial growth factor in 2006. Similarly in 2013, Skaat et al5 reported that between 1999 and 2008, the incidence of blindness registration from all types of AMD more than halved (from 208 cases/year per 100 000 population in 1999 to 90 cases/year per 100 000 in 2008 of the population aged 66–80 years). In our study, the incidence of blindness from nAMD was 3.5 people per 100 000 population aged ≥50 years in 2010. We note our population includes only those with nAMD treated with anti-VEGF rather than all AMD subtypes; we report the incidence in the third year of availability of anti-VEGF drugs; and we report a different age range that may contribute to the lower incidence rate observed in our population.
Rostron et al10 reported an incidence rate of blindness registration in patients with nAMD (all ages) of 13.8/year per 100 000 population in 2010. In this study, we restricted the analysis to patients aged ≥50 years, but the nature of the condition is such that this age restriction is unlikely to have excluded sufficient cases of nAMD to account for the lower incidence rate in our region. It may be that registration of blindness is a ‘lagging' indicator, reflecting both treated and untreated cases, whereas our data are contemporary and only analyse patients treated with anti-VEGF from 2008 to 2010.
In 2011, Bressler et al11 used Markov modelling to estimate the impact of anti-VEGF treatment on blindness from nAMD in the USA. Legal blindness was defined as a letter score of 38 or lower in the better-seeing eye. If no treatment was given, of the 103 582 individuals in the USA predicted to develop nAMD for which ranibizumab would be indicated and available, 16 268 were predicted to become legally blind in 2 years (15.7%). Monthly ranibizumab was predicted to reduce the incidence of legal blindness in 2 years by 72% to 4484 individuals (4%).
In our data, 6.9% (n=42) had become legally blind after 2 years, according to the Bressler study definition. Our real-world data is higher than the modelled figure of 4% of treated nAMD patients progressing to blindness after 2 years, but compares favourably with the predicted figure of 15.7% progression to blindness in untreated nAMD after 2 years.
In 2012, Campbell et al8 compared the prevalence of blindness and visual impairment in patients with treated and untreated nAMD. Retrospective VA data were collected for both eyes of patients with incident nAMD for 24 months from 2002 (84 patients, 91 eyes), before the introduction of anti-VEGF, and from 2008 (41 patients, 43 eyes). Mean age was 77 years in 2002 and 76 years in 2008. Twenty-nine percent of patients were legally blind (best corrected VA in the better-seeing eye ≤20/200 (38 ETDRS letters or fewer) after 24 months follow-up in 2002, compared with 2% in 2008.
In our data, 6.9% (n=42) of all patients had progressed to blindness according to the Campbell study definition by 2 years. Mean age was significantly older (80.3 years), which is associated with a poorer visual prognosis.21 This study also has a much larger sample size, which should provide a more reliable estimate of rates of progression. These differences notwithstanding, our data compares favourably with the 29% progression to blindness at 2 years in the untreated nAMD group from 2002, and is comparable to the 2% progression to blindness in their 2008 group.
In 2013, Rofagha et al22 assessed 65 patients with nAMD who had been treated in the Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration (MARINA), Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularisation in Age-Related Macular Degeneration (ANCHOR), and Open-Label Extension Trial of Ranibizumab for Choroidal Neovascularization Secondary to Age-Related Macular Degeneration (HORIZON) trials. At a mean of 7.3 years (range, 6.3–8.5 years) after entry into ANCHOR or MARINA, four patients (6%) were legally blind, defined as BCVA in better-seeing eye ≤20/200 (38 ETDRS letters or fewer). In our data, 6.9% (n=42) were blind after 2 years, according to the study definition. This result is worse than the RCTs, but real-world studies have no upper age limits and include patients with structural foveal changes and systemic co-pathologies that would be excluded from trials.
The incidence of new cases of nAMD in Gloucestershire accessing NHS care was 111 people per 100 000 population aged ≥50 years in 2009. Having established the incidence of eligibility for blindness and sight impairment registration in patients receiving ranibizumab for nAMD in Gloucestershire in 2009, we note that only 1.2% of this population became eligible to be registered blind, and 7.7% sight impaired. In 2010 only 3.6% became eligible to be registered blind and 10% sight impaired.
Conclusion
This is the first study on the incidence and prevalence of eligibility for blindness and sight impairment registration in treated nAMD in the UK based on VA data. Availability of anti-VEGF treatment has markedly improved the visual prognosis for patients with nAMD. The incidence of eligibility for certification of blindness or sight impairment in patients treated with ranibizumab for nAMD in Gloucestershire in 2010 is low, at 3.5 and 9.7 people aged ≥50 years per 100 000 population, respectively. Only 3.6% of the incident treated population became eligible to be registered blind in 2010. Such real-world evidence is important to measure the cost effectiveness of these new therapies.
RJ is a director of Medisoft Limited, the electronic medical record used in this study. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
Author contributions
MB collected and analysed the data, and drafted the paper; AL analysed the data; RH collected the data; AT and RJ devised the concept, and redrafted and edited the paper; QM, EF, and AS edited the paper.
Supplementary Material
References
- Buch H, Vinding T, La Cour M, Appleyard M, Jensen GB, Nielsen NV. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults. Ophthalmology. 2004;111 (1:53–61. doi: 10.1016/j.ophtha.2003.05.010. [DOI] [PubMed] [Google Scholar]
- Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82 (11:844–851. [PMC free article] [PubMed] [Google Scholar]
- Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291 (15:1900–1901. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- Bunce C, Wormald R. Causes of blind certification in England and Wales: April 1999 - March 2000. Eye. 2008;22:905–911. doi: 10.1038/sj.eye.6702767. [DOI] [PubMed] [Google Scholar]
- Skaat A, Chetrit A, Belkin M, Kalter-Leibovici O. Time trends in the incidence and causes of blindness in Israel. Am J Ophthalmol. 2012;153 (2:214–221. doi: 10.1016/j.ajo.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Minassian DC, Reidy A, Lightstone A, Desai P. Modelling the prevalence of age-related macular degeneration (2010-2020) in the UK: expected impact of anti-vascular endothelial growth factor (VEGF) therapy. Br J Ophthalmol. 2011;95 (10:1433–1436. doi: 10.1136/bjo.2010.195370. [DOI] [PubMed] [Google Scholar]
- Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127 (4:533–540. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- Campbell JP, Bressler SB, Bressler NM. Impact of availability of anti-vascular endothelial growth factor therapy on visual impairment and blindness due to neovascular age-related macular degeneration. Arch Ophthalmol. 2012;130 (6:794–795. doi: 10.1001/archophthalmol.2011.2480. [DOI] [PubMed] [Google Scholar]
- Bloch S, Larsen M, Munch I. Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol. 2012;153 (2:209–213. doi: 10.1016/j.ajo.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Rostron E, McKibbin M. Visual impairment certification secondary to ARMD in Leeds 2005-2010: is the incidence falling. Eye. 2012;26 (7:933–936. doi: 10.1038/eye.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler NM, Doan QV, Varma R, Lee PP, Suner IJ, Dolan C, et al. Estimated cases of legal blindness and visual impairment avoided using ranibizumab for choroidal neovascularisation: non-Hispanic white population in the United States with age-related macular degeneration. Arch Ophthalmol. 2011;129 (6:709–717. doi: 10.1001/archophthalmol.2011.140. [DOI] [PubMed] [Google Scholar]
- Robinson R, Deutsch J, Jones H, Youngson-Reilly S, Hamlin D, Dhurjon L. Unrecognised and unregistered visual impairment. Br J Ophthalmol. 1994;78 (10:736–740. doi: 10.1136/bjo.78.10.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R, Murray P. Unregistered visual impairment: is registration a failing system. Br J Ophthalmol. 2005;89 (8:995–998. doi: 10.1136/bjo.2004.059915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AN, Bunce C, Wormald R, Suleman M, Stratton I, Muir Gray JA. Geographical variation in certification rates of blindness and sight impairment in England, 2008-2009. BMJ Open. 2012;2 (6 doi: 10.1136/bmjopen-2012-001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder M, Chang TS, Scott IU, Hay D, Chambers K, Sibley LM, et al. Validity of the visual function index (VF-14) in patients with retinal disease. Arch Ophthalmol. 1999;117 (12:1611–1616. doi: 10.1001/archopht.117.12.1611. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Chang TS, Suner IJ, Fine JT, Dolan CM, Ward J, et al. Vision-related function after ranibizumab treatment by better- or worse-seeing eye: clinical trial results from MARINA AND ANCHOR. Ophthalmology. 2010;117 (4:747–756. doi: 10.1016/j.ophtha.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Verteporfin photodynamic therapy cohort study: report 1: effectiveness and factors influencing outcomes Ophthalmology. 2009. pp. e1–e8. [DOI] [PubMed]
- TAP Study Group Photodynamic therapy of subfoveal choroidal neovascularisation in age-related macular degeneration with verteporfin. Arch Ophthalmol. 1999;117:1329–1345. [PubMed] [Google Scholar]
- TAP and VIP Study Groups Effect of lesion size, visual acuity, and lesion composition on visual acuity change with and without verteporfin therapy for choroidal neovascularisation secondary to age-related macular degeneration: TAP and VIP Report No. 1. Am J Ophthalmol. 2003;136:407–418. doi: 10.1016/s0002-9394(03)00223-x. [DOI] [PubMed] [Google Scholar]
- Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, et al. Randomised, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145 (2:239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Writing Committee for the UK Age-Related Macular Degeneration EMR Users' Group The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014;121 (5:1092–1101. doi: 10.1016/j.ophtha.2013.11.031. [DOI] [PubMed] [Google Scholar]
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA and HORIZON. Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.