Abstract
Aim
To compare the choroidal thickness and ocular pulse amplitude (OPA) measurements obtained during the attack period in migraine patients and age and gender matched control group participants using high definition optical coherence tomography (OCT).
Methods
Thirty eyes at the side of the headache of 30 subjects with a diagnosis of migraine with or without aura and unilateral migraine and 29 age and gender matched healthy participants were enrolled in this observational, cross-sectional study. OCT scans were performed to all participants. Choroidal thicknesses were measured at the fovea, 1500 μm nasal and 1500 μm temporal to the fovea. Intraocular pressure (IOP) and OPA were also measured.
Results
The choroidal thickness measurements obtained during the attack period in migraine patients were (mean±SD) 279.82±35.87, 250.05±29.49, and 239.58±27.92 and in control group were 308.20±44.97, 276.95±41.39, and 281.60±41.38 at foveal, nasal, and temporal measurement points, respectively. Choroidal thickness significantly decreased according to the control group (P<0.05) at all measured points in migraine patients during attack. IOP (mean±SD) values were 16.71±3.26 and17.40±3.19 and OPA (mean±SD) values were 2.26±0.81 and 2.64±1.03 in migraine and control groups, respectively, and did not seem to be changed (P>0.05).
Conclusions
Choroidal thickness was found to be significantly decreased in unilateral migraine patients during the attack period when compared with the control group, whereas OPA did not change. The possible implications of these findings on the association between migraine and glaucoma are discussed.
Introduction
Migraine is a chronic neurovascular disorder with recurrent attacks of pulsating headache and autonomic nervous system dysfunction and its pathogenesis is still unclear.1 The prevalence of migraine is 24.4% among the women aged between 30 and 40 years.2 Visual symptoms are common in migraine patients and patients with migraine have an increased prevalence of visual field defects and a relation with glaucoma.3 Vasospasm is important to explain visual aura and headache in migraine patients and vasospasm- and ischemia-related effects on the retina and optic nerve head were reported before.4
The electrophysiological activation of trigeminovascular neurons during a migraine attack was reported.5 Sensorial innervations of the eye and dura mater are commonly originated from the trigeminal ganglion is also supplied by the trigeminal nerve. Long and short ciliary nerve branches of the ophthalmic division of the trigeminal nerve innervate different parts of the eye and autonomic nerve fibers that innervate the choroidal vasculature are also carried by the short ciliary nerves.6
The choroid is a highly vascularized tissue. The choroid provides oxygen and nourishment to the outer retinal layers and regulates heat at the foveal region. Nearly 90% of the ophthalmic artery blood flow is received by the choroid.7 It is shown that the anatomy and physiologic functions of choroid is quite important in ocular diseases like glaucoma.8 Several studies have reported noninvasive in vivo measurements of the choroidal thickness with the optical coherence tomography (OCT) in normal subjects and choroidal or retinal diseases.9, 10
The difference between the systolic and diastolic intraocular pressure (IOP) is called the ocular pulse amplitude (OPA) and it is caused by cardiovascular pulsations and ocular blood flow. The OPA is an index of choroidal perfusion.11 There are many different methods for measuring the OPA and choroidal perfusion.12 The dynamic contour tonometer (DCT; Swiss Micro technology AG, Port, Switzerland) is a relatively new technology that allows the measurement of both IOP and OPA.
To the best of our knowledge, there are no previous studies in the literature evaluating choroidal thickness and OPA together in migraine. In our study, we aimed to show the relation between migraines, choroidal thickness, and OPA as this may help to understand the relation between migraine and glaucoma. The relationship between glaucoma and migraine was explored later in two large population-based studies, the Blue Mountain Eye Study and the Beaver Dam Eye Study, and their results were conflicting as the former supported the relationship, whereas the latter did not.13, 14
Materials and methods
This cross-sectional prospective observational study was carried out with the patients, patients' relatives, and hospital stuff of the Turgut Özal University Hospital from 1 May to 1 November 2013. The study was performed in adherence with the tenets of the Declaration of Helsinki and was approved by the local ethics committee. Informed consent was obtained from all of the study participants. Subjects diagnosed with migraines with or without aura according to the criteria established by the International Headache Society were recruited from the neurology clinic at the Turgut Özal University hospital15, and only the patients with unilateral headache and the eyes at the side of the headache in the phase of pain were enrolled. Non migraine subjects of the control group were recruited from the ophthalmology clinic and stuff of the same hospital. The control group consisted of age and gender matched healthy participants and the right eyes were evaluated. The migraine group consisted of 30 eyes of 30 participants (25 female and 5 male). In the control group, 29 eyes of 29 participants (25 female and 4 male) were investigated. History was taken to ensure that all patients were free of systemic diseases and not taking any systemic medications known to affect the visual functions. None of the migraine patients were receiving a specific therapy for migraine except for simple analgesics. The age of onset, duration, frequency, predominant side of headache, presence or absence of aura, and family history of migraine if present were recorded. A detailed questionnaire was given to the control subjects to rule out any history of migraine. Migraine subjects with other central nervous system diseases or abnormal MRI findings were excluded. Visual acuity, refraction, and IOP were measured in all subjects followed by an anterior segment slit lamp exam and a dilated fundoscopic exam. The IOP and the OPA were measured with the DCT by an experienced operator (MSD) after administering topical anesthesia. Subjects with >3 diopter myopia and history of glaucoma were excluded from both the groups. Participants also underwent a complete neurologic examination and subjects with neurological diseases other than migraines or with other types of migraine (that is, hemiplegic migraine and retinal migraine) were excluded. Subjects with a history of systemic diseases, such as diabetes, hypertension, collagen vascular disease, pregnancy, and smoking were also excluded from the study.
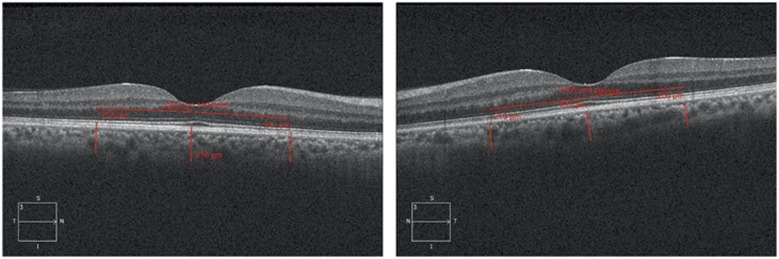
All OCT scans were performed by the same experienced technician who was blind to the study protocol. Before the scan, it was verified that none of the patients had consumed drinks with caffeine or had taken analgesic medications, triptans, or ergot alkaloids for at least 24 h previously. The choroidal thickness was measured by the Cirrus high definition OCT (HD-OCT, Cirrus Version 6.0; Carl Zeiss Meditec, Dublin, CA, USA). Each participant's head was fixed on the sustainer with the eye focusing on the international fixation target without blinking and moving the eye while six radial retinal scans were performed. The scans were only accepted if they were completed, well centered, had signal strength of at least 6 and had no motion or blinking artifacts. After each examination, two independent masked graders (MSD and YT) evaluated the best image that is projected on a computer screen. The image was accepted and used for analysis when two graders determined that both inner and outer borders of the choroid were clearly distinguishable. The choroidal image was obtained according to the previous method.16 The protocol of HD 5 Line Raster centered foveally and spaced at 0.25 mm was performed. This protocol consisted of 6 mm parallel lines with 1024 A-scans/B-scans and averaging four B-scans per image. As the inversion of the image by using the Cirrus software results in a pixelated and low resolution image, the images were not inverted to bring the choroid into closer proximity to the zero delay line. The image of the thinnest point of the macula was chosen to prevent the affect of positioning on the measured thickness of the fovea. The image size was doubled, foveally centered and the subfoveal choroidal thickness was measured manually from the outer portion of the hyper reflective line corresponding to the retina pigment epithelium to the inner surface of sclera by the linear measuring tool of Cirrus software. The thickness of the choroid at the areas 1500 μm nasal and 1500 μm temporal to the fovea was also examined (Figure 1). Two independent raters (MSD and YT) measured images without the information of the eye or other observer, as described previously17 and the average of the two measurements was taken; the differences between readings of the masked physicians were found to be within 10% of the mean.
Figure 1.
Choroidal thickness in a participant using enhanced depth imaging OCT.
Statistical analyses were performed using SPSS V.20.0 (SPSS Inc., IBM Corp,Chicago, IL, USA). The normal distribution of the data was checked using the Kolmogorov–Smirnov test. The mean age and distribution of gender among the patients in migraine and the control groups were compared with the Mann–Whitney U-test and χ2-test, respectively. The Mann–Whitney U-test was used to test the differences in choroidal thickness and OPA values between the migraine and the control groups. Values of P<0.05 were considered as statistically significant. Partial Pearson correlation was used to evaluate the influence of various measured parameters on the choroidal thickness and OPA. Multiple regression models were used to identify the associations between central choroidal thickness and age, IOP and OPA.
Results
Thirty eyes of 30 participants (25 female and 5 male) in the migraine group and 29 right eyes of 29 participants (25 female and 4 male) in the control group were investigated. Demographic data of migraine and control groups are shown in Table 1. All migraine patients had unilateral headaches. There were no differences between participants in the migraine and control groups with respect to age or gender (P>0.05).
Table 1. Demographic data of migraine and control groups.
| Migraine group (n=30) | Control group (n=29) | P-value | |
|---|---|---|---|
| Age (mean±SD) (years) | 39.52±1.97 | 39.45±1.95 | 0.41 |
| Male/female (%) | 5/25 (20/80%) | 4/25 (16/84%) | 0.40 |
The choroidal thickness measurements were significantly decreased in the migraine group in all measured points (P<0.05) but the IOP and OPA measurements did not differ between the groups (P>0.05) (Table 2).
Table 2. Choroidal thickness, IOP, and OPA measurements in groups.
| Measurement parameters | Migraine (mean±SD in μm) N=30 | Control (mean±SD in μm) N=29 | P-value |
|---|---|---|---|
| F | 279.82±35.87 | 308.20±44.97 | 0.032 |
| N | 250.05±29.49 | 276.95±41.39 | 0.025 |
| T | 239.58±27.92 | 281.60±41.38 | 0.002 |
| IOP | 16.71±3.26 | 17.40±3.19 | 0.483 |
| OPA | 2.26±0.81 | 2.64±1.03 | 0.233 |
Abbreviations: F, choroidal thickness at fovea; N, choroidal thickness at 1500 μm nasal to the fovea; T, choroidal thickness at 1500 μm temporal to the fovea; IOP, intraocular pressure; OPA; ocular pulse amplitude.
Pearson's correlation coefficient test did not reveal any significant correlation between age, gender, IOP, OPA, and choroidal thicknesses in the migraine group (r-values ranged between -0.09and -0.61 and P-values ranged between 0.11 and 0.97). In the control group, Pearson's correlation coefficient test revealed a fair and statistically significant correlation between the OPA and the central (r=−0.58, P=0.09), nasal (r=−0.53, P=0.19), and temporal (r=−0.63, P=0.03) choroidal thicknesses.
In the migraine group, multiple linear regression models did not identify significant association between choroidal thickness and age, IOP, and OPA (F (6, 33)=1.403, P=0.21).
In the control group, multiple linear regression models did not identify significant association between choroidal thickness and age, IOP, and OPA (F (5, 24)=1.264, P=0.36).
Discussion
Migraine is accepted as a neurovascular disorder of unknown etiology. To the best of our knowledge, this is the first investigation of choroidal thickness and OPA together in migraine patients during attack. In our study, the mean choroidal thickness of migraine patients was significantly thinner than that of controls in all points during migraine attack but OPA did not seem to be changed (Table 2). These findings may be important in understanding the pathophysiology of migraine and its association with glaucoma.
In different studies, ocular disorders and their association with choroidal thickness were reported. Choroidal thickness was reported to decrease in high myopia, retinal dystrophy, and age-related choroidalatrophy while increased in central serous chorioretinopathy and Vogt–Koyanagi–Harada disease.18 Peripapillary choroidal thickness was found to be lower in patients with normal-tension glaucoma compared with normal controls.18 There are also OCT studies regarding the haemodynamic effects of chemicals like sildenafil and cigarette smoke, on choroid.18
The choroid is a highly vascularized tissue and it is directly influenced by intraocular and perfusion pressures. It is shown that OCT is superior to histology in term of accuracy of measuring the choroidal thickness.19 Measurements of the OPA and the choroidal thickness together may be important as the relationship between choroidal thickness and choroidal perfusion is still debate. OPA is caused by cardiovascular pulsations and ocular blood flow and it is an index of choroidal perfusion.11 There are many different methods for measuring the OPA and choroidal perfusion including DCT and it is suggested that choroidal blood flow may not be correlated with choroidal thickness in an experimental study that measurements with the laser Doppler flowmetry showed increased choroidal blood flow precede the increase in choroidal thickness during recovery from deprivation myopia.20 In contrast, it has been reported that sildenafil citrate increases choroidal thickness owing to a vasodilator effect on choroidal circulation.21 In another recent study, subfoveal choroidal thickness, measured by enhanced depth imaging OCT (EDI-OCT), was not found to be significantly correlated with choroidal blood flow in young healthy eyes.22 The authors speculated and suggested that further prospective studies are required to determine the changes in choroidal thickness during the stimulation that can cause choroidal circulation changes. In our study, we could not find a statistically significant difference in OPA between the migraine patients during attack and the control group (P=), and this may indicate that the auto regulatory mechanisms of the choroidal perfusion may overcome the decrease of the choroidal thickness in migraine patients.
An increase in the choroidal thickness in migraine patients during attack was reported.23 In contrast, a different study, similar to our results, reported a decrease in the choroidal thickness in migraine patients, especially five of the patients during attack, according to the control group, which supports our suggestion of decreased blood flow according to the vasogenic theory of migraine.18
It is also reported that the patients with migraine often suffer from vascular dysregulation involving the choroid24 and this may be in relation with the choroidal thickness changes in migraine patients. Our study had several limitations. First of all, the number of cases was limited but it is very difficult to obtain OCT images during a painful migraine attack. Another limitation was related to the effect of diurnal rhythm of choroidal thickness (a change of ∼20–30 lm), which was demonstrated in previous studies.19 However, in our study, we performed the OCT examinations at the same time of day (10 : 00–12 : 00) during the migraine attack and excluded the attack patients out of this time limit so it was unlikely to be a potential bias in our study. The last major limitation of this study was that the choroidal thickness measurements had to be performed manually, which remains a potential cause of interobserver bias. To overcome this limitation, two different observers performed measurements at three different points as described in previous reports25 but the time needed to measure the choroidal thickness manually may lead to observer fatigue and may increase the possibility of false measurements. Although this is still the most commonly used method for determining choroidal thickness, there is still a need to minimize the bias associated with this measurement method. To eliminate factors that may have an effect on choroidal circulation, patients on systemic and topical medications patients with a known systemic or ocular disease, smokers, and pregnant subjects were excluded.
In conclusion, despite these limitations we found that the choroidal thickness was significantly decreased in migraine patients during the attack period when compared with the control group. These findings may be useful in future studies investigating the neurovascular structures of the eye in migraine patients and may be a step to understand the pathogenesis of migraine and the association between migraine and glaucoma.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors declare no conflict of interest.
References
- Eadie MJ. Pathogenesis of migraine-17th to early 20th century understandings. J Clin Neurosci. 2005;12:383–388. doi: 10.1016/j.jocn.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, et al. Migraine prevalence, disease burden and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- Klein BEK, Klein R, Meuer SM, Goetz LA. Migraine headache and its association with open angle glaucoma: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1993;34:3024–3028. [PubMed] [Google Scholar]
- Solomon S, Grosberg BM, Friedman DI, Lipton RB. Retinal migraine. J Neuroophthalmol. 2007;27:243–244. doi: 10.1097/WNO.0b013e31814a6243. [DOI] [PubMed] [Google Scholar]
- Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011;69:855–865. doi: 10.1002/ana.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhuber W, Schrödl F. Autonomic control of the eye and the iris. Auton Neurosci. 2011;165:67–79. doi: 10.1016/j.autneu.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Segmental nature of the choroidal vasculature. Br J Ophthalmol. 1975;59:631–648. doi: 10.1136/bjo.59.11.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Jonas JB, Naumann GO. Decreased choroidal thickness in eyes with secondary angle-closure glaucoma: an aetiological factor for deep retinal changes in glaucoma. Br J Ophthalmol. 1993;77:430–432. doi: 10.1136/bjo.77.7.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A, Binns A, Margrain T, Drexler W, Považay B, Esmaeelpour M, et al. Retinal and choroidal thickness in early age-related macular degeneration. Am J Ophthalmol. 2011;152:1030–1038. doi: 10.1016/j.ajo.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Usui S, Ikuno Y, Miki A, Matsushita K, Yasuno Y, Nishida K. Evaluation of the choroidal thickness using high-penetration optical coherence tomography with long wavelength in highly myopic normal-tension glaucoma. Am J Ophthalmol. 2012;153:10–16. doi: 10.1016/j.ajo.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Walker RE, Litovitz TL. An experimental and theoretical study of pneumatictonometer. Exp Eye Res. 1972;13:14–23. doi: 10.1016/0014-4835(72)90120-0. [DOI] [PubMed] [Google Scholar]
- Schmetterer L, Dallinger S, Findl O, et al. Noninvasive investigations of the normal ocular circulation in humans. Invest Ophthalmol Vis Sci. 1998;39:1210–1220. [PubMed] [Google Scholar]
- Wang JJ, Mitchell P, Smith W. Is there an association between migraine headache and open-angle glaucoma? Findings from the Blue Mountains Eye Study. Ophthalmology. 1997;104:1714–1719. doi: 10.1016/s0161-6420(97)30075-x. [DOI] [PubMed] [Google Scholar]
- Klein BEK, Klein R, Meuer SM, Goetz LA. Migraine headache and its association with open-angle glaucoma: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1993;34:3024–3027. [PubMed] [Google Scholar]
- Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia. 1988;8 (Suppl 7:1–96. [PubMed] [Google Scholar]
- Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150:325–329. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchini L, Regatieri CV, Flores-Moreno I, Baumann B, Fujimoto JG, Duker JS. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems. Ophthalmology. 2012;119:119–123. doi: 10.1016/j.ophtha.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin MO, Elmas Z, Cinar E, Kucukerdonmez C.Choroidal thickness changes in patients with migraine Acta Neurol Belge-pub ahead of print 8 May 2014. [DOI] [PubMed]
- Maul EA, Friedman DS, Chang DS, Boland MV, Ramulu PY, Jampel HD, et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011;118 (8:1571–1579. doi: 10.1016/j.ophtha.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ME, Wildsoet CF, Reiner A. Temporal relationship of choroidal blood flow and thickness changes during recovery from form deprivation myopia in chicks. Exp Eye Res. 2002;2002;74 (5:561–570. doi: 10.1006/exer.2002.1142. [DOI] [PubMed] [Google Scholar]
- Kim DY, Silverman RH, Chan RV, Khanifar AA, Rondeau M, Lloyd H, et al. Measurement of choroidal perfusion and thickness following systemic sildenafil (Viagra) Acta Ophthalmol. 2013;91 (2:183–188. doi: 10.1111/j.1755-3768.2011.02305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa K, Nagaoka T, Takahashi A, Tanano I, Tani T, Ishibazawa A, et al. Relationship between choroidal thickness and choroidal circulation in healthy young subjects. Am J Ophthalmol. 2012;153 (6:1129–1132. doi: 10.1016/j.ajo.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Dadaci Z, Doganay F, Acir NO, Aydin HD, Borazan M, et al. Enhanced depth imaging optical coherence tomography of the choroid in migraine patients: implications for the association of migraine and glaucoma. Br J Ophthalmol. 2014;98:972–975. doi: 10.1136/bjophthalmol-2013-304711. [DOI] [PubMed] [Google Scholar]
- Hasler PW, Orgül S, Gugleta K, Vogten H, Zhao X, Gherghel D, et al. Vascular dysregulation in the choroid of subjects with acral vasospasm. Arch Ophthalmol. 2003;120 (3:302–307. doi: 10.1001/archopht.120.3.302. [DOI] [PubMed] [Google Scholar]
- Ulaş F, Çelik F, Doğan Ü, Çelebi S. Effect of smoking on choroidal thickness in healthy smokers. Curr Eye Res. 2014;39 (5:504–511. doi: 10.3109/02713683.2013.850099. [DOI] [PubMed] [Google Scholar]