Abstract
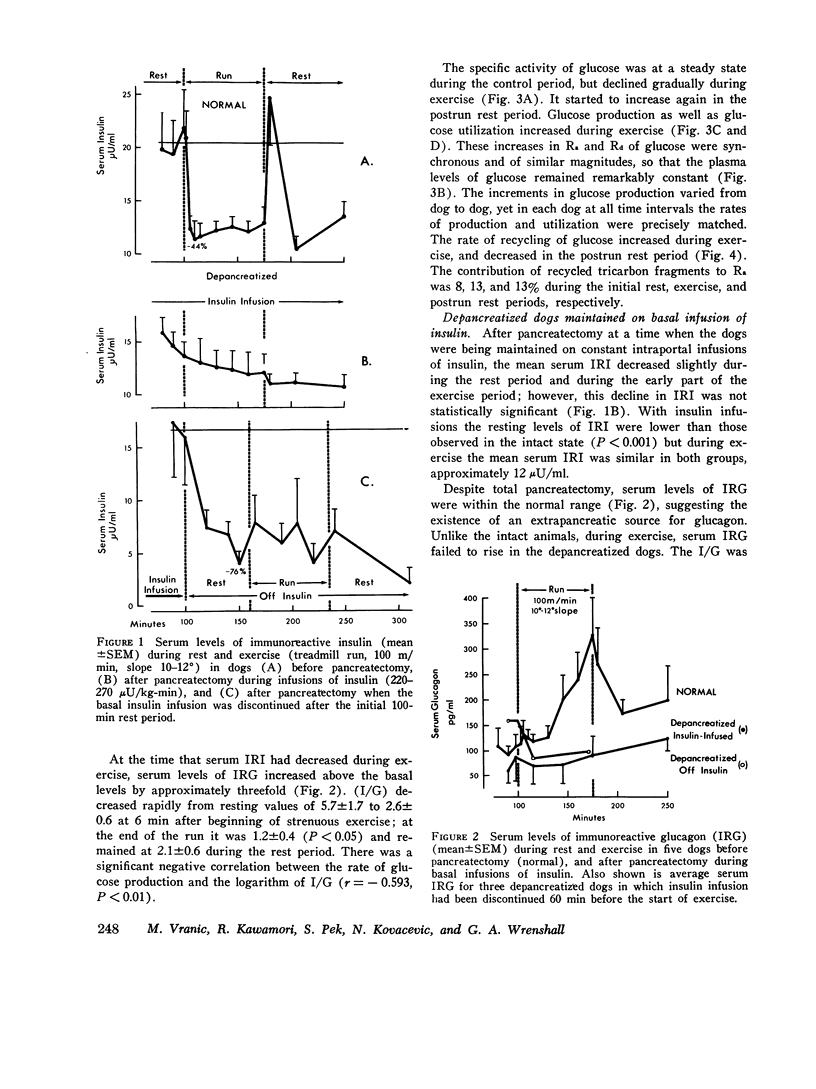
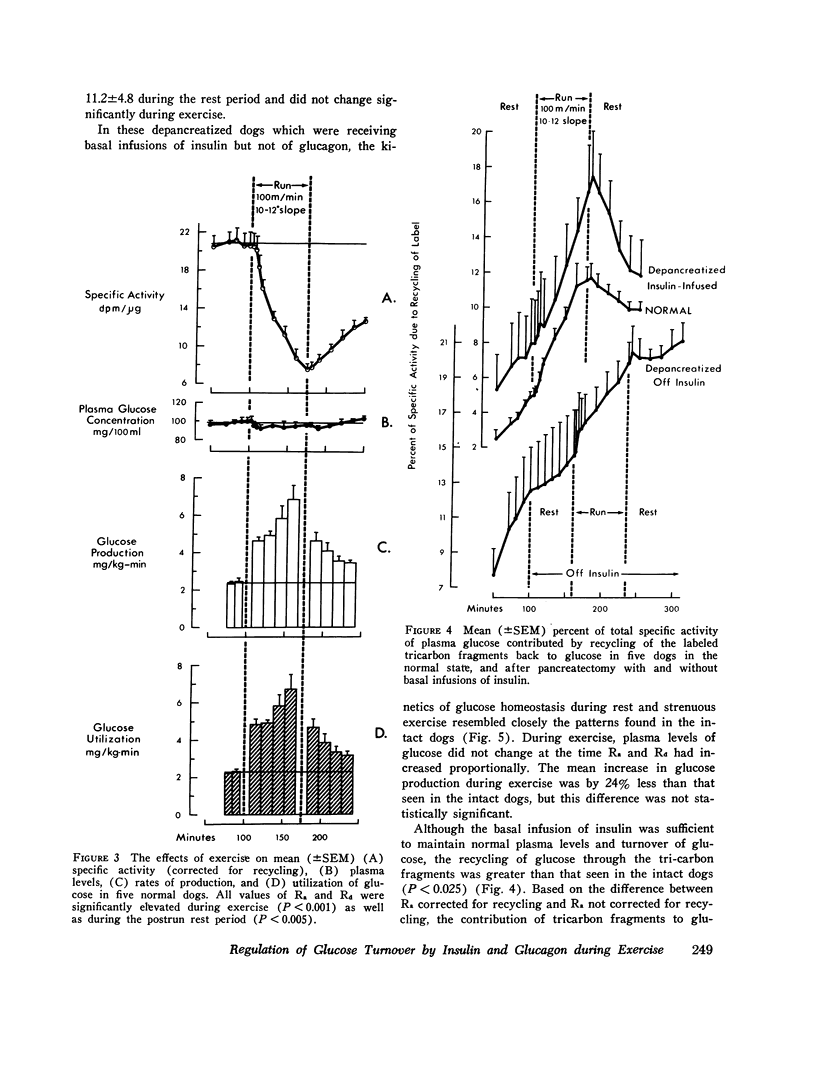
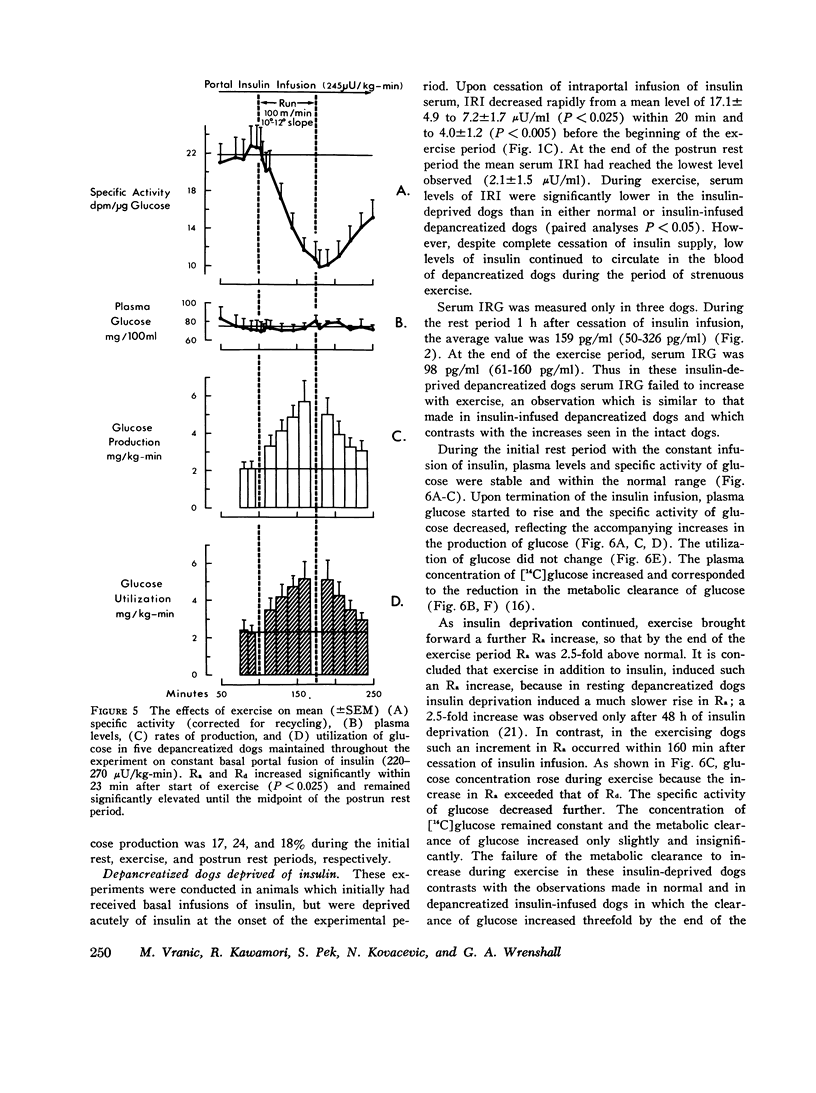
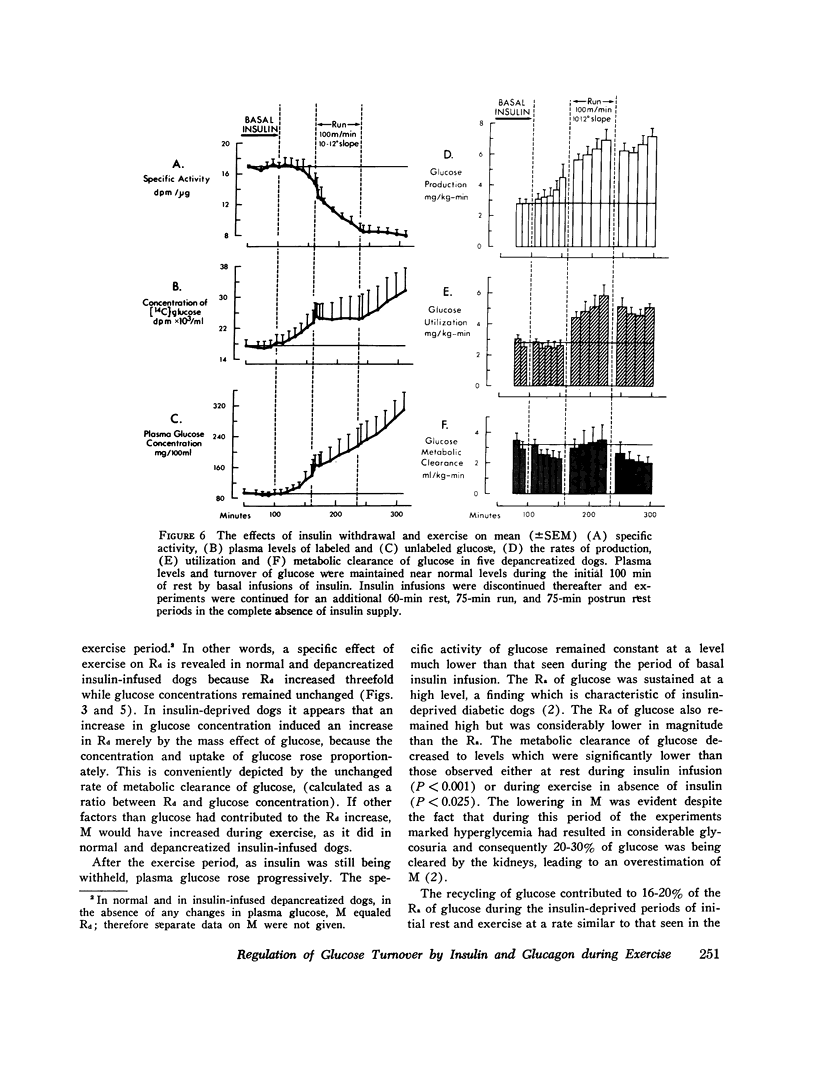
In order to elucidate the role of insulin and glucagon during strenuous exercise (100 m/min, slope 10-12 degrees), we have determined the rates of production (Ra), utilization (Rd), and metabolic clearance (M) of glucose in normal dogs before pancreatectomy and 2 wk after total pancreatectomy (a) when they were being maintained on constant intraportal basal insulin infusion, (245 muU/kg-min) and (b) when insulin supply had been withheld before and during exercise. Such an intense exercise induced in normal dogs a prompt decrease in mean immunoreactive serum insulin (IRI) from 20 +/- 3 to 11 +/- 2 muU/ml. In depancreatized insulin-infused dogs serum IRI during rest and exercise was between 14 +/- 1 and 12 +/- 2 muU/ml. In the third group, after cessation of insulin infusion, IRI decreased by 76% (from 17 +/- 5 to 4 +/- 1) and did not decrease futher during exercise. During exercise, serum immunoreactive glucagon (IRG) increased threefold in normal dogs. In depancreatized dogs serum IRG was the same as in normal resting dogs (indicating a nonpancreatic source of the hormone) but it did not increase during exercise. In normal dogs exercise induced proportional increases in Ra, Rd, and M (threefold) and normoglycemia was maintained. Changes in glucose turnover in depancreatized insulin-infused dogs were similar to those seen in normal dogs suggesting that a decrease in insulin secretion and a rise in IRG are not essential to prevent hypoglycemia in diabetic dogs. With the cessation of insulin infusion in resting depancreatized dogs, Ra increased, M decreased, and hyperglycemia ensued. During exercise, Ra continued to rise, but M did not increase significantly. Conclusions: (a) Regulation of glucose production by liver during exercise is multifactorial. A decrease in IRI and an increase in IRG are not the only factors which can promote delivery of glucose to the peripheral tissues. The insulin glucagon molar ratio was found not to be an essential metabolic functional unit in regulating glucose metabolism during exercise. (b) It is hypothesized that increases in blood flow and capillary surface area can lead to an increase in the amount of insulin delivered to the muscle even when serum levels of IRI are reduced during exercies. It is suggested that small, but adequate amounts of insulin (as found in normal and depancreatized insulin-infused dogs) are essential in regulating glucose uptake in the working muscle. (c) Since totally depancreatized dogs had normal serum levels of IRG (originating presumably from the gastrointestinal tract), the question of essentiality of basal glucagon activity in glucose homeostasis during exercise could not be resolved by these experiments. It appears, however, that regulation of secretion of nonpancreatic glucagon differs from that of pancreatic glucagon.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bihler I., Hollands M., Dresel P. E. Stimulation of sugar transport by a factor released from gas-perfused hearts. Can J Physiol Pharmacol. 1970 Jun;48(6):327–332. doi: 10.1139/y70-054. [DOI] [PubMed] [Google Scholar]
- Böttger I., Schlein E. M., Faloona G. R., Knochel J. P., Unger R. H. The effect of exercise on glucagon secretion. J Clin Endocrinol Metab. 1972 Jul;35(1):117–125. doi: 10.1210/jcem-35-1-117. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Kawamori R., Pek S., Vranic M. Arginine infusion in dogs. Model for the roles of insulin and glucagon in regulating glucose turnover and free fatty acid levels. Diabetes. 1974 Oct;23(10):805–815. doi: 10.2337/diab.23.10.805. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Vranic M. Effect of arginine on glucose turnover and plasma free fatty acids in normal dogs. Diabetes. 1973 Jul;22(7):537–543. doi: 10.2337/diab.22.7.537. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Vranic M. Effect of interaction between insulin and glucagon on glucose turnover and FFA concentration in normal and depancreatized dogs. Metabolism. 1974 Aug;23(8):729–744. doi: 10.1016/0026-0495(74)90005-5. [DOI] [PubMed] [Google Scholar]
- Cherrington A., Vranic M., Fono P., Kovacevic N. Effect of glucagon on glucose turnover and plasma free fatty acids in depancreatized dogs maintained on matched insulin infusions. Can J Physiol Pharmacol. 1972 Oct;50(10):946–954. doi: 10.1139/y72-137. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Vranic M., Wrenshall G. A. Effects of preceding diet and fasting on glucose turnover in normal dogs. Metabolism. 1969 Apr;18(4):319–330. doi: 10.1016/0026-0495(69)90053-5. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R., Ahlborg G. Plasma glucagon levels in exercising man. N Engl J Med. 1972 Jul 27;287(4):184–185. doi: 10.1056/NEJM197207272870412. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Soule R. G., Taylor A. W., Williams C., Ianuzzo C. D. Exercise-induced glycogenolysis and lipolysis in the rat: hormonal influence. Am J Physiol. 1970 Sep;219(3):729–733. doi: 10.1152/ajplegacy.1970.219.3.729. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVIVI E., WERTHEIMER H. E. A MUSCLE ACTIVITY FACTOR INCREASING SUGAR UPTAKE BY RAT DIAPHRAGMS IN VITRO. J Physiol. 1964 Aug;172:342–352. doi: 10.1113/jphysiol.1964.sp007422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley L. H., Mason J. W., Hogan R. P., Jones L. G., Kotchen T. A., Mougey E. H., Wherry F. E., Pennington L. L., Ricketts P. T. Multiple hormonal responses to graded exercise in relation to physical training. J Appl Physiol. 1972 Nov;33(5):602–606. doi: 10.1152/jappl.1972.33.5.602. [DOI] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Norwich K. H. Validity of the rates of production and utilization of metabolites as determined by tracer methods in intact animals. Fed Proc. 1974 Jul;33(7):1841–1848. [PubMed] [Google Scholar]
- Issekutz B., Jr, Issekutz A. C., Nash D. Mobilization of energy sources in exercising dogs. J Appl Physiol. 1970 Nov;29(5):691–697. doi: 10.1152/jappl.1970.29.5.691. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Paul P., Miller H. I. Metabolism in normal and pancreatectomized dogs during steady-state exercise. Am J Physiol. 1967 Oct;213(4):857–862. doi: 10.1152/ajplegacy.1967.213.4.857. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyckx A. S., Lefebvre P. J. Mechanisms involved in the exercise-induced increase in glucagon secretion in rats. Diabetes. 1974 Feb;23(2):81–93. doi: 10.2337/diab.23.2.81. [DOI] [PubMed] [Google Scholar]
- Mashiter K., Harding P. E., Chou M., Mashiter G. D., Stout J., Diamond D., Field J. B. Persistent pancreatic glucagon but not insulin response to arginine in pancreatectomized dogs. Endocrinology. 1975 Mar;96(3):678–693. doi: 10.1210/endo-96-3-678. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Foà P. P. Plasma glucose, insulin, pancreatic, and enteroglucagon levels in normal and depancreatized dogs. Proc Soc Exp Biol Med. 1974 Oct;147(1):97–102. doi: 10.3181/00379727-147-38288. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Brennan M. F., Tan M. H., Aoki T. T. Studies of glucagon secretion in pancreatectomized patients. Diabetes. 1974 Jun;23(6):512–516. doi: 10.2337/diab.23.6.512. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Measurement and validation of nonsteady turnover rates with applications to the inulin and glucose systems. Fed Proc. 1974 Jul;33(7):1855–1864. [PubMed] [Google Scholar]
- Sasaki H., Rubalcava B., Baetens D., Blazquez E., Srikant C. B., Orci L., Unger R. H. Identification of glucagon in the gastrointestinal tract. J Clin Invest. 1975 Jul;56(1):135–145. doi: 10.1172/JCI108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranic M., Pek S., Kawamori R. Increased "glucagon immunoreactivity" in plasma of totally depancreatized dogs. Diabetes. 1974 Nov;23(11):905–912. doi: 10.2337/diab.23.11.905. [DOI] [PubMed] [Google Scholar]
- Vranic M., Wrenshall G. A. Exercise, insulin and glucose turnover in dogs. Endocrinology. 1969 Jul;85(1):165–171. doi: 10.1210/endo-85-1-165. [DOI] [PubMed] [Google Scholar]
- WRENSHALL G. A., RAPPAPORT A. M., BEST C. H., COWAN J. S., HETENYI G., Jr ABSOLUTE RATES OF GLUCOSE PRODUCTION, ACCUMULATION, AND UTILIZATION IN THE DOG AT PANCREATECTOMY AND THEREAFTER. Diabetes. 1964 Sep-Oct;13:500–508. doi: 10.2337/diab.13.5.500. [DOI] [PubMed] [Google Scholar]
- Wahren J., Hagenfeldt L., Felig P. Splanchnic and leg exchange of glucose, amino acids, and free fatty acids during exercise in diabetes mellitus. J Clin Invest. 1975 Jun;55(6):1303–1314. doi: 10.1172/JCI108050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]