Abstract
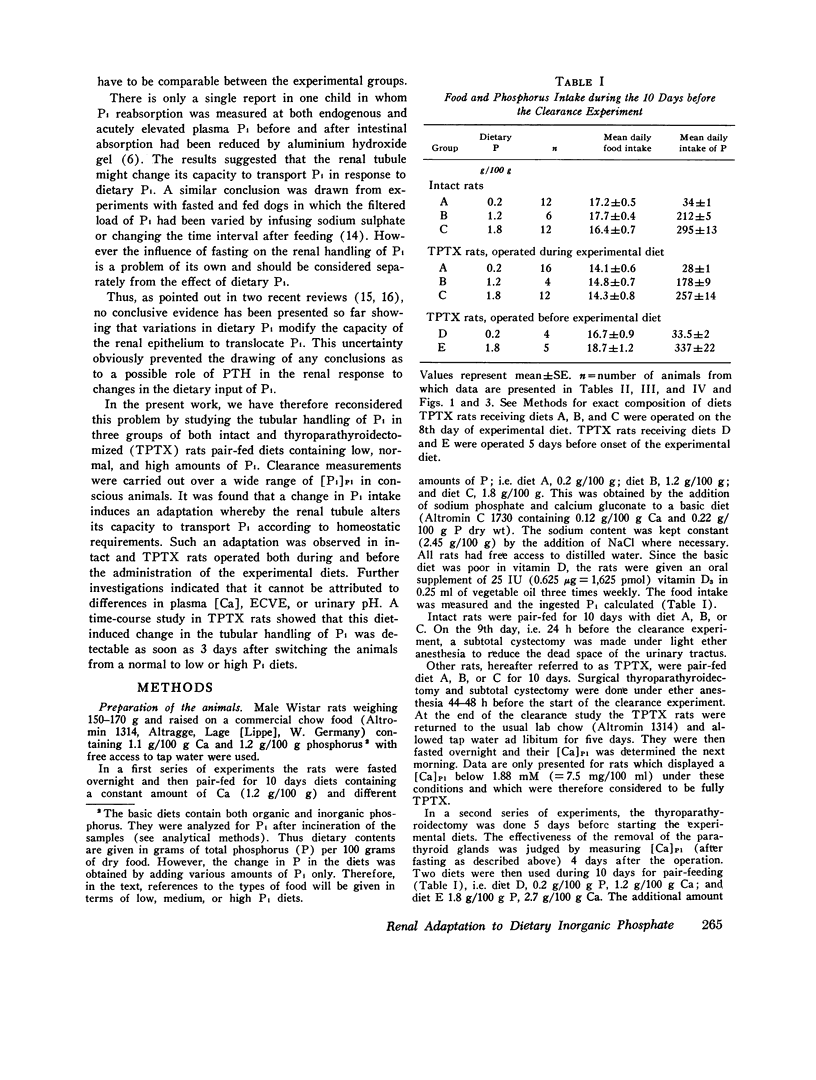
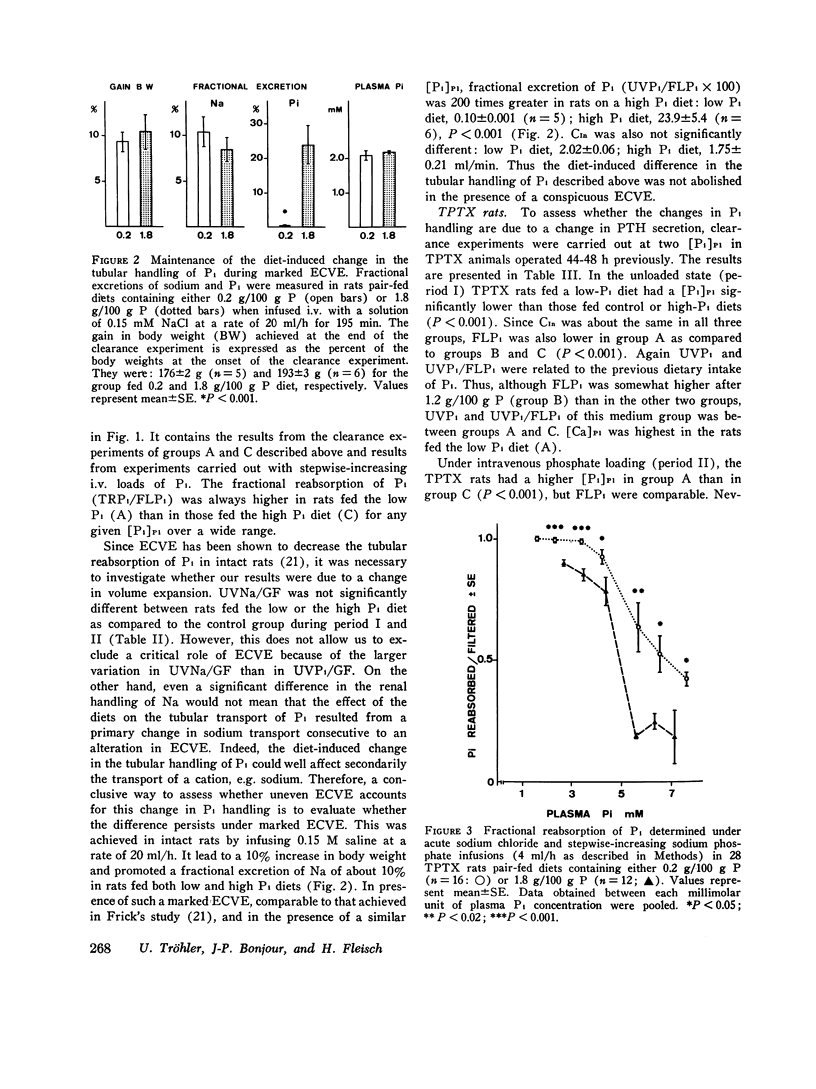
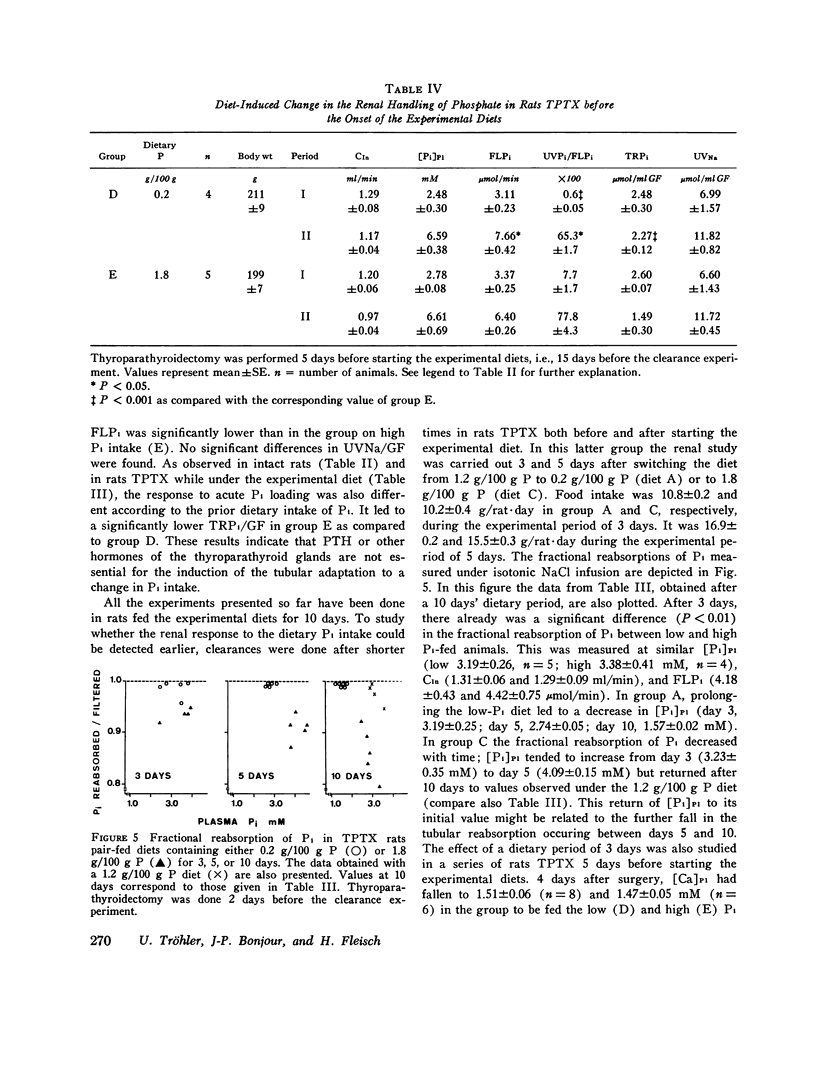
The possibility of renal tubular adaptation to variations in dietary inorganic phosphate (Pi) was investigated in intact and thyroparathyroidectomized (TPTX) rats pair-fed diets containing low, normal, and high amounts of Pi for periods up to 10 days. Clearances were measured before and during active i.v. infusions with Pi in conscious animals. Thus tubular reabsorption of phosphate (TRPi) could be assessed over a wide range of plasma phosphate concentrations ([Pi]P1). It was found that the renal tubule could adapt its capacity to transport Pi according to the dietary Pi: TRPi was always higher, for a given [Pi]P1, in the animals fed low than in those fed higher Pi diets. This diet-induced modification also occurred in the absence of thyroparathyroid glands, in the presence of the same calcemia and urinary pH, and during marked extracellular volume expansion. A time-course study in rats TPTX both before and during the administration of the experimental diets showed that a difference in the tubular handling of Pi was detectable as early as 3 days after switching the animals from a normal to low- or high-Pi diets. These results indicate that factors other than parathyroid hormone are implicated in the tubular response to variations in the dietary intake of inorganic phosphate.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. H., Draper H. H. Effect of dietary phosphorus on calcium metabolism in intact and parathyroidectomized adult rats. J Nutr. 1972 Sep;102(9):1123–1132. doi: 10.1093/jn/102.9.1123. [DOI] [PubMed] [Google Scholar]
- CHAMBERS E. L., Jr, GOLDMAN L., GORDAN G. S., REIFENSTEIN E. C., Jr Tests for hyperparathyroidism: tubular reabsorption of phosphate, phosphate deprivation, and calcium infusion. J Clin Endocrinol Metab. 1956 Nov;16(11):1507–1521. doi: 10.1210/jcem-16-11-1507. [DOI] [PubMed] [Google Scholar]
- CRAWFORD J. D., OSBORNE M. M., Jr, TALBOT N. B., TERRY M. L., MORRILL M. F. The parathyroid glands and phosphorus homeostasis. J Clin Invest. 1950 Nov;29(11):1448–1461. doi: 10.1172/JCI102385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo L. S., Sheehe P. R., Weiner I. M. Renal actions of vitamin D in D-deficient rats. Am J Physiol. 1974 Jun;226(6):1490–1495. doi: 10.1152/ajplegacy.1974.226.6.1490. [DOI] [PubMed] [Google Scholar]
- Eisenberg E. Effects of varying phosphate intake in primary hyperparathyroidism. J Clin Endocrinol Metab. 1968 May;28(5):651–660. doi: 10.1210/jcem-28-5-651. [DOI] [PubMed] [Google Scholar]
- FOULKS J. G. Homeostatic adjustment in the renal tubular transport of inorganic phosphate in the dog. Can J Biochem Physiol. 1955 Jul;33(4):638–650. [PubMed] [Google Scholar]
- Frick A. Mechanism of inorganic phosphate diuresis secondary to saline infusions in the rat. Excretion of sodium, inorganic phosphate, and calcium in normal and in parathyroidectomized rats. Pflugers Arch. 1969;313(2):106–122. doi: 10.1007/BF00586239. [DOI] [PubMed] [Google Scholar]
- Frick A. Reabsorption of inorganic phosphate in the rat kidney. I. Saturation of transport mechanism. II. Suppression of fractional phosphate reabsorption due to expansion of extracellular fluid volume. Pflugers Arch. 1968;304(4):351–364. doi: 10.1007/BF00587710. [DOI] [PubMed] [Google Scholar]
- Fulop M., Brazeau P. The phosphaturic effect of sodium bicarbonate and acetazolamide in dogs. J Clin Invest. 1968 May;47(5):983–991. doi: 10.1172/JCI105813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN R., BASSETT S. H. Renal regulation of phosphorus excretion. J Clin Endocrinol Metab. 1958 Sep;18(9):981–990. doi: 10.1210/jcem-18-9-981. [DOI] [PubMed] [Google Scholar]
- Gold L. W., Massry S. G., Arieff A. I., Coburn J. W. Renal bicarbonate wasting during phosphate depletion. A possible cause of altered acid-base homeostasis in hyperparathyroidism. J Clin Invest. 1973 Oct;52(10):2556–2561. doi: 10.1172/JCI107447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALDEN A., EISENBERG E., GORDAN G. S. PARATHYROID HORMONE AND PHOSPHATE HOMEOSTASIS IN MAN. Acta Endocrinol (Copenh) 1964 Jun;46:285–291. doi: 10.1530/acta.0.0460285. [DOI] [PubMed] [Google Scholar]
- HELLMAN D., BAIRD H. R., BARTTER F. C. RELATIONSHIP OF MAXIMAL TUBULAR PHOSPHATE REABSORPTION TO DIETARY PHOSPHATE IN THE DOG. Am J Physiol. 1964 Jul;207:97–103. doi: 10.1152/ajplegacy.1964.207.1.97. [DOI] [PubMed] [Google Scholar]
- Knox F. G., Schneider E. G., Willis L. R., Strandhoy J. W., Ott C. E. Editorial: Site and control of phosphate reabsorption by the kidney. Kidney Int. 1973 Jun;3(6):347–353. doi: 10.1038/ki.1973.56. [DOI] [PubMed] [Google Scholar]
- Kuntziger H., Amiel C., Gaudebout C. Phosphate handling by the rat nephron during saline diuresis. Kidney Int. 1972 Dec;2(6):318–323. doi: 10.1038/ki.1972.115. [DOI] [PubMed] [Google Scholar]
- LOTSPEICH W. D., MALVIN R. L. Relation between tubular transport of inorganic phosphate and bicarbonate in the dog. Am J Physiol. 1956 Sep;187(1):51–56. doi: 10.1152/ajplegacy.1956.187.1.51. [DOI] [PubMed] [Google Scholar]
- Lotz M., Zisman E., Bartter F. C. Evidence for a phosphorus-depletion syndrome in man. N Engl J Med. 1968 Feb 22;278(8):409–415. doi: 10.1056/NEJM196802222780802. [DOI] [PubMed] [Google Scholar]
- Maesaka J. K., Levitt M. F., Abramson R. G. Effect of saline infusion on phosphate transport in intact and thyroparathyroidectomized rats. Am J Physiol. 1973 Dec;225(6):1421–1429. doi: 10.1152/ajplegacy.1973.225.6.1421. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Friedler R. M., Coburn J. W. Excretion of phosphate and calcium. Physiology of their renal handling and relation to clinical medicine. Arch Intern Med. 1973 Jun;131(6):828–859. doi: 10.1001/archinte.131.6.828. [DOI] [PubMed] [Google Scholar]
- McCRORY W. W., FORMAN C. W., McNAMARA H., BARNETT H. L. Renal excretion of inorganic phosphate in newborn infants. J Clin Invest. 1952 Apr;31(4):357–366. doi: 10.1172/JCI102616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman W. F., Neuman M. W., Sammon P. J., Simon W., Lane K. The metabolism of labeled parathyroid hormone. III. Studies in rats. Calcif Tissue Res. 1975 Sep 17;18(4):251–261. doi: 10.1007/BF02546244. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Fernandez P. C., Boyle I. T., Gray R. W., Omdahl J. L., DeLuca H. F. The acute renal tubular effects of 1,25-dihydroxycholecalciferol. Proc Soc Exp Biol Med. 1972 Oct;141(1):379–384. doi: 10.3181/00379727-141-36781. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Goldberg M. The relationship between the renal handling of phosphate and bicarbonate in man. J Lab Clin Med. 1969 Jun;73(6):956–969. [PubMed] [Google Scholar]
- Puschett J. B., Moranz J., Kurnick W. S. Evidence for a direct action of cholecalciferol and 25-hydroxycholecalciferol on the renal transport of phosphate, sodium, and calcium. J Clin Invest. 1972 Feb;51(2):373–385. doi: 10.1172/JCI106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH D. A., NORDIN B. E. THE EFFECT OF A HIGH PHOSPHORUS INTAKE ON TOTAL AND ULTRAFILTRABLE PLASMA CALCIUM AND ON PHOSPHATE CLEARANCE. Clin Sci. 1964 Jun;26:479–486. [PubMed] [Google Scholar]
- STEENBOCK H., HERTING D. C. Vitamin D and growth. J Nutr. 1955 Dec 10;57(4):449–468. doi: 10.1093/jn/57.4.449. [DOI] [PubMed] [Google Scholar]
- STRICKLER J. C., THOMPSON D. D., KLOSE R. M., GIEBISCH G. MICROPUNCTURE STUDY OF INORGANIC PHOSPHATE EXCRETION IN THE RAT. J Clin Invest. 1964 Aug;43:1596–1607. doi: 10.1172/JCI105035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON D. D., HIATT H. H. Effects of phosphate loading and depletion on the renal excretion and reabsorption of inorganic phosphate. J Clin Invest. 1957 Apr;36(4):566–572. doi: 10.1172/JCI103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973 Feb;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Troehler U., Bonjour J. P., Fleisch H. Renal secretion of diphosphonates in rats. Kidney Int. 1975 Jul;8(1):6–13. doi: 10.1038/ki.1975.70. [DOI] [PubMed] [Google Scholar]



