Abstract
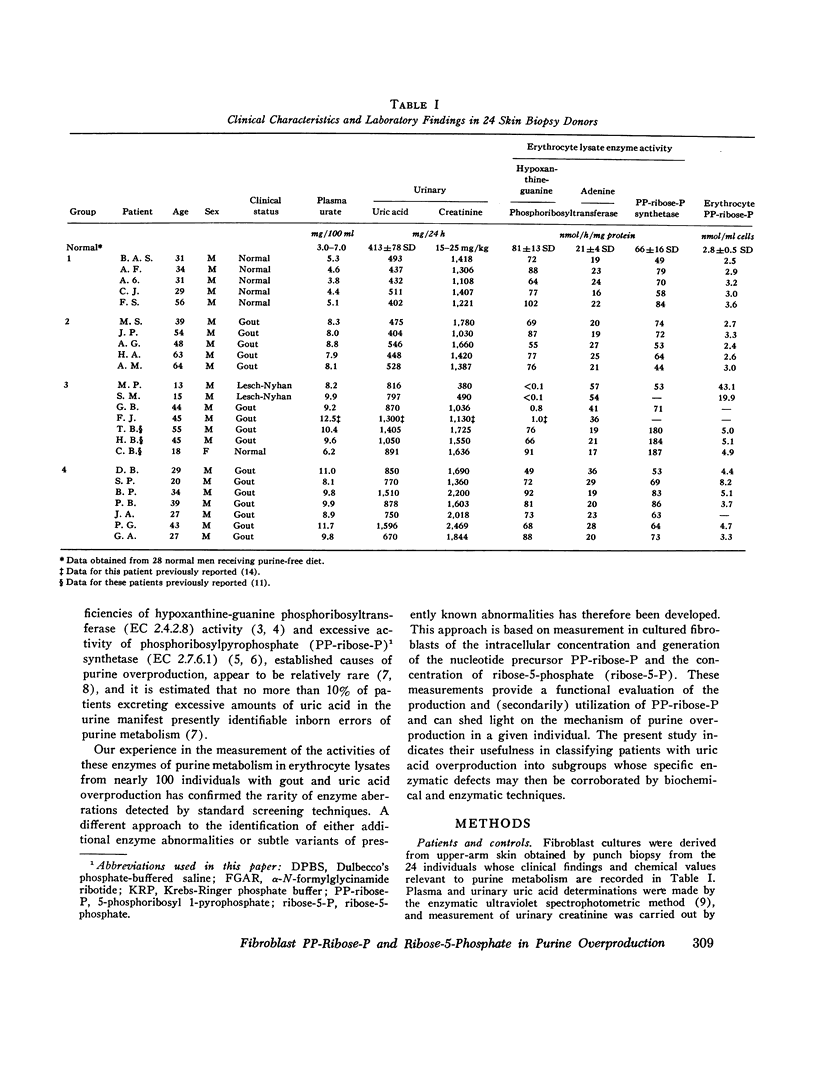
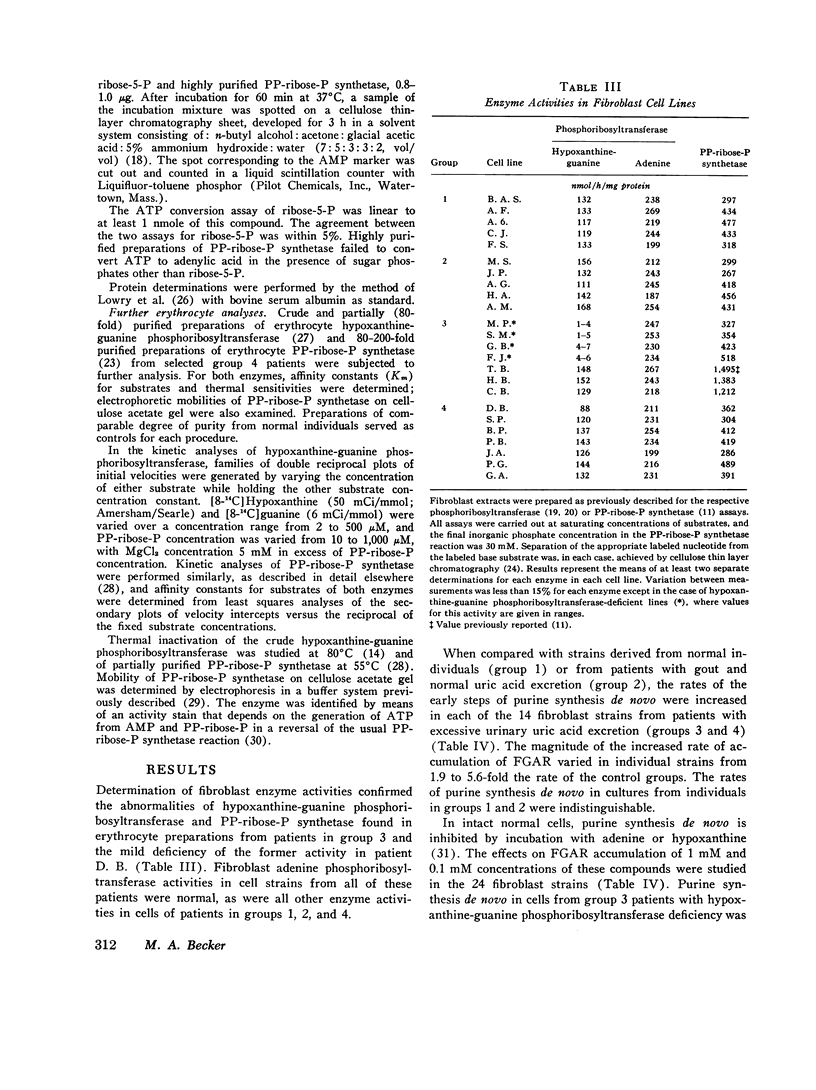
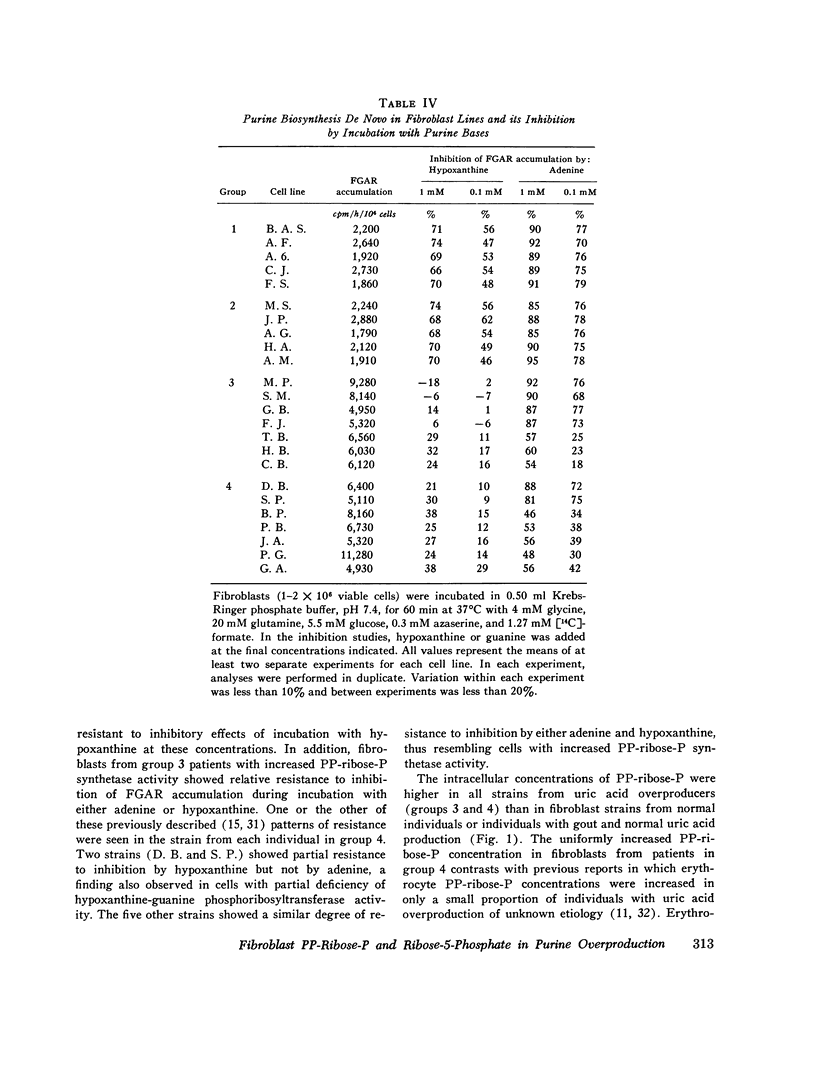
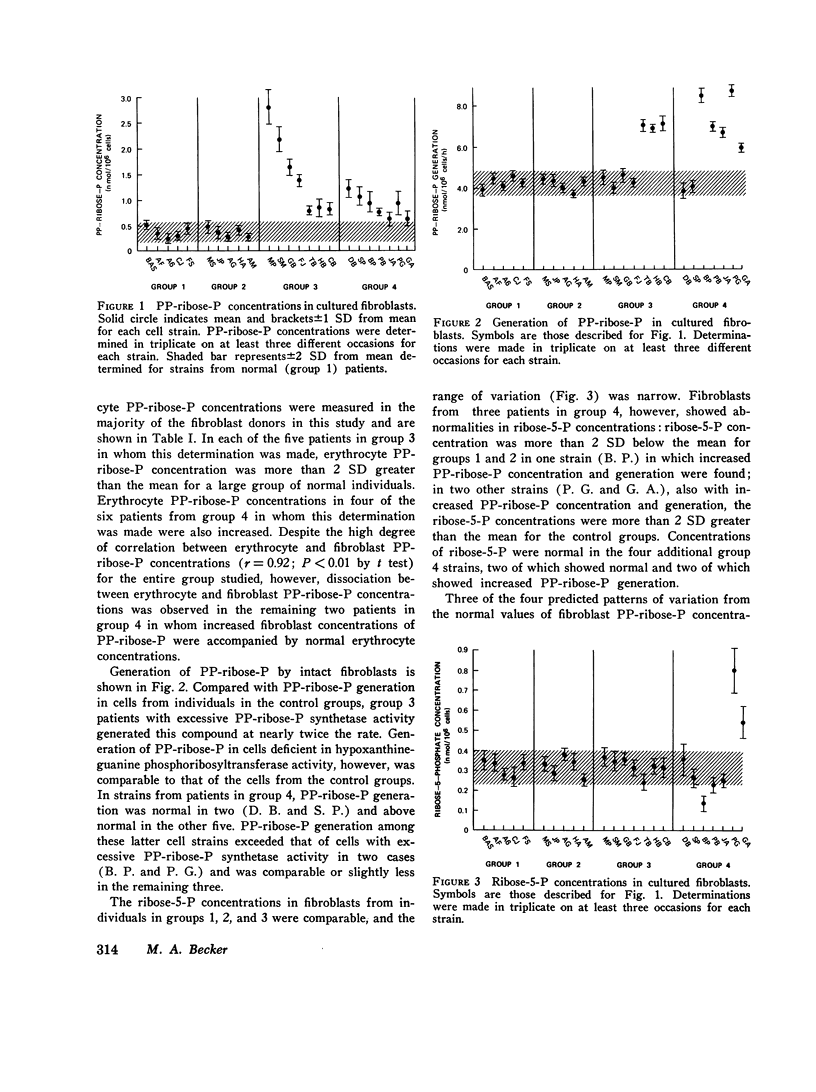
In the majority of patients with gout and excessive uric acid production, underlying enzyme abnormalities have not been identified. In the present study, measurement of both the rate of generation and concentration of phosphoribosylpyrophosphate (PP-ribose-P) and the concentration of ribose-5-phosphate in cultured cells were undertaken to establish a classification of purine overproducers to direct study of additional enzyme defects. Fibroblasts were cultured from 24 individuals assigned to 4 groups: group 1, 5 normal controls; group 2, 5 patients with gout and normal dialy urinary uric acid excretion (gouty controls); group 3, 7 patients with well-defined enzyme abnormalities and excessive urinary acid excretion (4 with hypoxanthine-guanine phosphoribosyltransferase deficiency and 3 with excessive PP-ribose-P synthetase activity); and group 4, 7 patients with gout and excessive uric acid excretion but without grossly abnormal activities of the above enzymes in erythrocyte lysates. In all 14 fibroblast strains from patients showing excessive production of uric acid (groups 3 and 4), rates of purine synthesis de novo and PP-ribose-P concentrations exceeded values for cells from control groups. Cells from group 3 patients with hypoxanthine-guanine phosphoribosyltransferase deficiency showed normal PP-ribose-P generation, while those with excessive PP-ribose-P synthetase activity demonstrated increased generation of this regulatory substrate. All strains from group 3 patients had normal ribose-5-phosphate concentrations. Five cell strains from group 4 patients showed one of the two patterns of abnormalities in these measurements seen in strains from group 3 patients: two resembled hypoxanthine-guanine phosphoribosyltransferase-deficient cells, and three resembled cells with excessive PP-ribose-P synthetase activity. Analyses of erythrocyte enzyme preparations from two of these patients in group 4 have led to identification of a kinetic variant of each enzyme as predicted from the foregoing patterns. Two additional group 4 cell lines that showed increased ribose-5-phosphate concentrations in addition to increased PP-ribose-P concentrations and generation were classified in a separate subgroup, since in the individuals excessive purine synthesis appeared to result from increases ribose-5-phosphate concentration, leading to increased availability of PP-ribose-P. No abnormality in either hypoxanthine-guanine phosphoribosyltransferase or PP-ribose-P synthetase has been found in erythrocyte preparations from one patient so classified.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alepa F. P., Howell R. R., Klinenberg J. R., Seegmiller J. E. Relationships between glycogen storage disease and tophaceous gout. Am J Med. 1967 Jan;42(1):58–66. doi: 10.1016/0002-9343(67)90006-x. [DOI] [PubMed] [Google Scholar]
- Arnold W. J., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Purification and subunit structure. J Biol Chem. 1971 Dec 10;246(23):7398–7404. [PubMed] [Google Scholar]
- BRIN M., YONEMOTO R. H. Stimulation of the glucose oxidative pathway in human erythrocytes by methylene blue. J Biol Chem. 1958 Jan;230(1):307–317. [PubMed] [Google Scholar]
- Bagnara A. S., Letter A. A., Henderson J. F. Multiple mechanisms of regulation of purine biosynthesis de novo in intact tumor cells. Biochim Biophys Acta. 1974 Dec 20;374(3):259–270. doi: 10.1016/0005-2787(74)90247-0. [DOI] [PubMed] [Google Scholar]
- Becker M. A., Kostel P. J., Meyer L. J. Human phosphoribosylpyrophosphate synthetase. Comparison of purified normal and mutant enzymes. J Biol Chem. 1975 Sep 10;250(17):6822–6830. [PubMed] [Google Scholar]
- Becker M. A., Kostel P. J., Meyer L. J., Seegmiller J. E. Human phosphoribosylpyrophosphate synthetase: increased enzyme specific activity in a family with gout and excessive purine synthesis. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2749–2752. doi: 10.1073/pnas.70.10.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M. A., Meyer L. J., Seegmiller J. E. Gout with purine overproduction due to increased phosphoribosylpyrophosphate synthetase activity. Am J Med. 1973 Aug;55(2):232–242. doi: 10.1016/0002-9343(73)90174-5. [DOI] [PubMed] [Google Scholar]
- Becker M. A., Meyer L. J., Wood A. W., Seegmiller J. E. Purine overproduction in man associated with increased phosphoribosylpyrophosphate synthetase activity. Science. 1973 Mar 16;179(4078):1123–1126. doi: 10.1126/science.179.4078.1123. [DOI] [PubMed] [Google Scholar]
- Becker M. A., Seegmiller J. E. Genetic aspects of gout. Annu Rev Med. 1974;25(0):15–28. doi: 10.1146/annurev.me.25.020174.000311. [DOI] [PubMed] [Google Scholar]
- Boyle J. A., Raivio K. O., Becker M. A., Seegmiller J. E. Effects of nicotinic acid on human fibroblast purine biosynthesis. Biochim Biophys Acta. 1972 May 10;269(2):179–183. doi: 10.1016/0005-2787(72)90424-8. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Human phosphoribosylpyrophosphate synthetase. Distribution, purification, and properties. J Biol Chem. 1971 Sep 25;246(18):5739–5748. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Human phosphoribosylpyrophosphate synthetase. Kinetic mechanism and end product inhibition. J Biol Chem. 1972 Apr 10;247(7):2126–2131. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Phosphoribosylpyrophosphate in man: biochemical and clinical significance. Ann Intern Med. 1971 Mar;74(3):424–433. doi: 10.7326/0003-4819-74-3-424. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Wyngaarden J. B., Kelley W. N. Depletion of erythrocyte phosphoribosylpyrophosphate in man. N Engl J Med. 1970 Nov 26;283(22):1177–1182. doi: 10.1056/NEJM197011262832201. [DOI] [PubMed] [Google Scholar]
- HENDERSON J. F. Feedback inhibition of purine biosynthesis in ascites tumor cells. J Biol Chem. 1962 Aug;237:2631–2635. [PubMed] [Google Scholar]
- Henderson J. F., Rosenbloom F. M., Kelley W. N., Seegmiller J. E. Variations in purine metabolism of cultured skin fibroblasts from patients with gout. J Clin Invest. 1968 Jul;47(7):1511–1516. doi: 10.1172/JCI105844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Hershko C., Mager J. Increased formation of 5-phosphoriboxyl-1-pyrophosphate in red blood cells of some gouty patients. Isr J Med Sci. 1968 Sep-Oct;4(5):939–944. [PubMed] [Google Scholar]
- Hershko A., Razin A., Mager J. Regulation of the synthesis of 5-phosphoribosyl-I-pyrophosphate in intact red blood cells and in cell-free preparations. Biochim Biophys Acta. 1969 Jun 17;184(1):64–76. doi: 10.1016/0304-4165(69)90099-3. [DOI] [PubMed] [Google Scholar]
- Holmes E. W., McDonald J. A., McCord J. M., Wyngaarden J. B., Kelley W. N. Human glutamine phosphoribosylpyrophosphate amidotransferase. Kinetic and regulatory properties. J Biol Chem. 1973 Jan 10;248(1):144–150. [PubMed] [Google Scholar]
- Holmes E. W., Wyngaarden J. B., Kelley W. N. Human glutamine phosphoribosylpyrophosphate amidotransferase. Two molecular forms interconvertible by purine ribonucleotides and phosphoribosylpyrophosphate. J Biol Chem. 1973 Sep 10;248(17):6035–6040. [PubMed] [Google Scholar]
- JONES O. W., Jr, ASHTON D. M., WYNGAARDEN J. B. Accelerated turnover of phosphoribosylpyrophosphate, a purine nucleotide precursor, in certain gouty subjects. J Clin Invest. 1962 Sep;41:1805–1815. doi: 10.1172/JCI104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. G., Rosenweig S., Switzer R. L., Becker M. A., Seegmiller J. E. Evaluation of the role of 5-phosphoribosyl-alpha-1-pyrophosphate synthetase in congenital hyperuricemia and gout: a simple isotopic assay and an activity stain for the enzyme. Biochem Med. 1974 Jul;10(3):266–275. doi: 10.1016/0006-2944(74)90030-1. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Fox I. H., Wyngaarden J. B. Regulation of purine biosynthesis in cultured human cells. I. Effects of orotic acid. Biochim Biophys Acta. 1970 Sep 22;215(3):512–516. doi: 10.1016/0304-4165(70)90101-7. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Greene M. L., Fox I. H., Rosenbloom F. M., Levy R. I., Seegmiller J. E. Effects of orotic acid on purine and lipoprotein metabolism in man. Metabolism. 1970 Dec;19(12):1025–1035. doi: 10.1016/0026-0495(70)90026-0. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Greene M. L., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency in gout. Ann Intern Med. 1969 Jan;70(1):155–206. doi: 10.7326/0003-4819-70-1-155. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Meade J. C. Studies on hypoxanthine-guanine phosphoribosyltransferase in fibroblasts from patients with the Lesch-Nyhan syndrome. Evidence for genetic heterogeneity. J Biol Chem. 1971 May 10;246(9):2953–2958. [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. A specific enzyme defect in gout associated with overproduction of uric acid. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1735–1739. doi: 10.1073/pnas.57.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. N. Studies on the adenine phosphoribosyltransferase enzyme in human fibroblasts lacking hypoxanthine-guanine phosphoribosyltransferase. J Lab Clin Med. 1971 Jan;77(1):33–38. [PubMed] [Google Scholar]
- Kelley W. N., Wyngaarden J. B. Effects of allopurinol and oxipurinol on purine synthesis in cultured human cells. J Clin Invest. 1970 Mar;49(3):602–609. doi: 10.1172/JCI106271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESCH M., NYHAN W. L. A FAMILIAL DISORDER OF URIC ACID METABOLISM AND CENTRAL NERVOUS SYSTEM FUNCTION. Am J Med. 1964 Apr;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- LIDDLE L., SEEGMILLER J. E., LASTER L. The enzymatic spectrophotometric method for determination of uric acid. J Lab Clin Med. 1959 Dec;54:903–913. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Raivio K. O., Seegmiller E. Adenine, hypoxanthine and guanine metabolism in fibroblasts from normal individuals and from patients with hypoxanthine phosphoribosyltransferase deficiency. Biochim Biophys Acta. 1973 Mar 19;299(2):273–282. doi: 10.1016/0005-2787(73)90350-x. [DOI] [PubMed] [Google Scholar]
- Rosenbloom F. M., Henderson J. F., Caldwell I. C., Kelley W. N., Seegmiller J. E. Biochemical bases of accelerated purine biosynthesis de novo in human fibroblasts lacking hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968 Mar 25;243(6):1166–1173. [PubMed] [Google Scholar]
- Rubin C. S., Balis M. E., Piomelli S., Berman P. H., Dancis J. Elevated AMP pyrophosphorylase activity in congenital IMP pyrophosphorylase deficiencey (Lesch-Nyhan disease). J Lab Clin Med. 1969 Nov;74(5):732–741. [PubMed] [Google Scholar]
- SEEGMILLER J. E., GRAYZEL A. I., LASTER L., LIDDLE L. Uric acid production in gout. J Clin Invest. 1961 Jul;40:1304–1314. doi: 10.1172/JCI104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Klinenberg J. R., Miller J., Watts R. W. Suppression of glycine-15N incorporation into urinary uric acid by adenine-8-13C in normal and gouty subjects. J Clin Invest. 1968 May;47(5):1193–1203. doi: 10.1172/JCI105808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Sperling O., Boer P., Persky-Brosh S., Kanarek E., De Vries A. Altered kinetic property of erythrocyte phosphoribosylpsyrophosphate synthetase in excessive purine production. Rev Eur Etud Clin Biol. 1972 Aug-Sep;17(7):703–706. [PubMed] [Google Scholar]
- Sperling O., Eilam G., Persky-Brosh S., De Vries A. Simpler method for the determination of 5-phosphoribosyl-1-pyrophosphate in red blood cells. J Lab Clin Med. 1972 Jun;79(6):1021–1026. [PubMed] [Google Scholar]
- Sperling O., Ophir R., de Vries A. Purine base incorporation into erythrocyte nucleotides and erythrocyte phosphoribosyltransferase activity in primary gout. Rev Eur Etud Clin Biol. 1971 Feb;16(2):147–151. [PubMed] [Google Scholar]
- TAUSSKY H. H. A microcolorimetric determination of creatine in urine by the Jaffe reaction. J Biol Chem. 1954 Jun;208(2):853–861. [PubMed] [Google Scholar]
- Thomas C. B., Arnold W. J., Kelley W. N. Human adenine phosphoribosyltransferase. Purification, subunit structure, and substrate specificity. J Biol Chem. 1973 Apr 10;248(7):2529–2535. [PubMed] [Google Scholar]
- Wood A. W., Becker M. A., Seegmiller J. E. Purine nucleotide synthesis in lymphoblasts cultured from normal subjects and a patient with Lesch-Nyhan syndrome. Biochem Genet. 1973 Jul;9(3):261–274. doi: 10.1007/BF00485739. [DOI] [PubMed] [Google Scholar]
- Yü T. F., Balis M. E., Krenitsky T. A., Dancis J., Silvers D. N., Elion G. B., Gutman A. B. Rarity of X-linked partial hypoxanthine-guanine phosphoribosyltransferase deficiency in a large gouty population. Ann Intern Med. 1972 Feb;76(2):255–264. doi: 10.7326/0003-4819-76-2-255. [DOI] [PubMed] [Google Scholar]


