Abstract
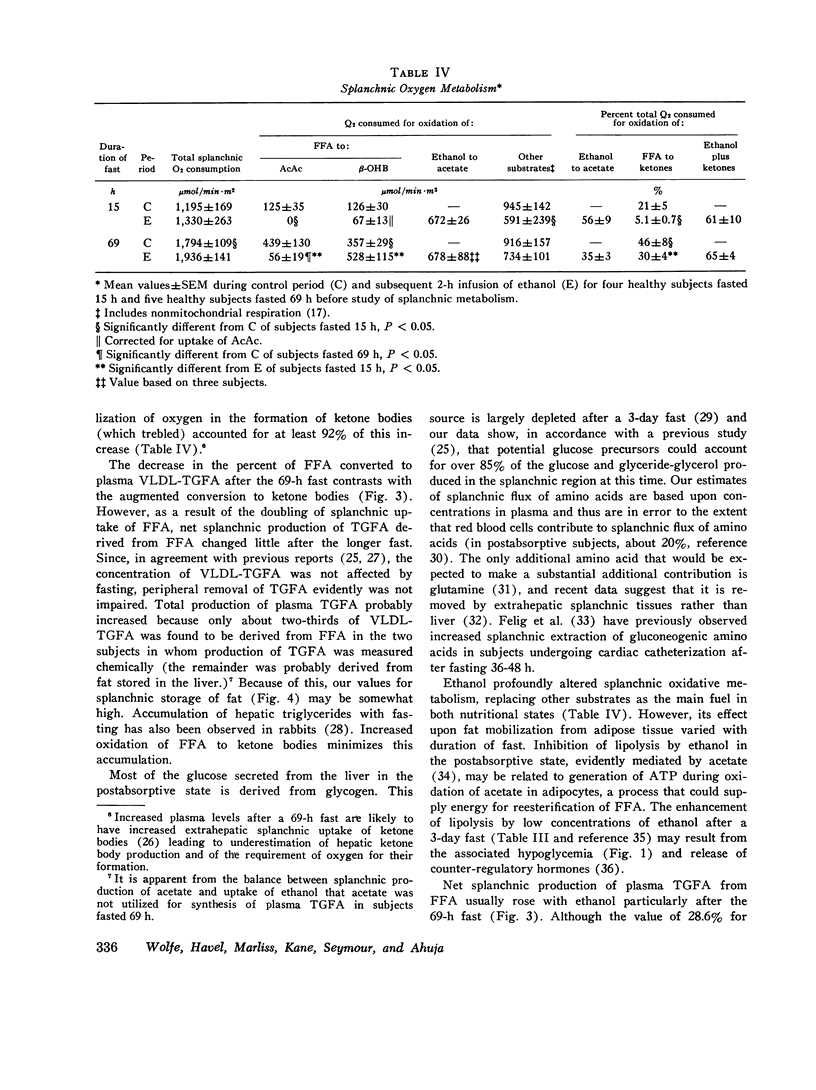
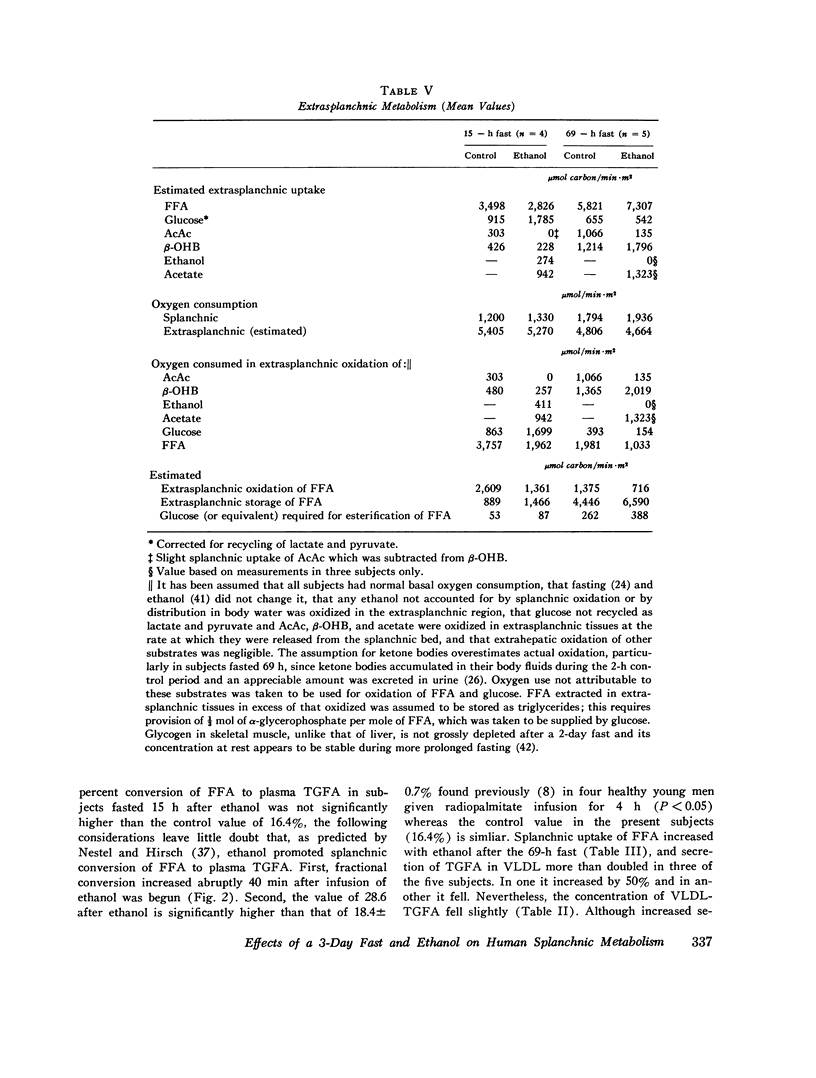
Splanchnic metabolism was studied to quantify changes underlying the fatty liver, hyperlipemia, and hypoglycemia produced by ethanol. Four subjects fasted for 15 h were compared with five subjects fasted for 69 h under basal conditions and during continuous intravenous infusion of sufficient ethanol to give a concentration of 3-5 mM in arterial blood plasma. Splanchnic storage of fatty acids was estimated from the difference between uptake of FFA and secretion of derived products. Basal values for splanchnic uptake of FFA were twofold higher after the 69-h fast while splanchnic storage of fatty acids and production of ketone bodies increased threefold. Values for basal secreation into the blood of triglycerides derived from FFA were similar in the two groups. In both nutritional states, the fraction of FFA taken up in the splanchnic region oxidized to ketone bodies and to CO2 fell when ethanol was given because of preferential oxidation of ethanol to acetate, and the fraction esterified rose. However, systemic transport and splanchnic uptake of FFA fell with ethanol in subjects fasted 15 h, so that neither storage of triglycerides in splanchnic tissues nor secretion into the blood increased. In subjects fasted 69 h, ethanol increased transport of FFA and splanchnic storage of fat. In all but one subject it also increased secretion of triglycerides into the blood. The concentration of glucose in blood fell during ethanol infusion in all five subjects undergoing the 69-h fast. Mean splanchnic glucose production was maintained at about one-half of the pre-ethanol value, despite virtual cessation of splanchnic uptake of lactate and of those amino acids that are metabolized via malate. Quantitative estimates of extrasplanchnic metabolism suggest that enhanced formation of alpha-glycerophosphate from glucose, in addition to impaired hepatic gluconeogenesis, may contribute to ethanol-induced hypoglycemia in man.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F., LINDSAY D. B. Acetate utilization in sheep. Biochem J. 1961 Apr;78:777–785. doi: 10.1042/bj0780777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasse E. O., Havel R. J. Evidence for an effect of inulin on the peripheral utilization of ketone bodies in dogs. J Clin Invest. 1971 Apr;50(4):801–813. doi: 10.1172/JCI106551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W., Kronfeld D. S. Gluconeogenesis and lipogenesis in tissue from ruminant and nonruminant animals. Fed Proc. 1969 Jan-Feb;28(1):218–231. [PubMed] [Google Scholar]
- Basso L. V., Havel R. J. Hepatic metabolism of free fatty acids in normal and diabetic dogs. J Clin Invest. 1970 Mar;49(3):537–547. doi: 10.1172/JCI106264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer H. U., Moellering H. Enzymatische Bestimmung von Acetat. Biochem Z. 1966 Mar 28;344(2):167–189. [PubMed] [Google Scholar]
- Block W. D., Markovs M. E., Steele B. F. Comparison between free amino acid levels in plasma deproteinated with picric acid and with sulfosalicylic acid. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1089–1091. doi: 10.3181/00379727-122-31333. [DOI] [PubMed] [Google Scholar]
- Crouse J. R., Gerson C. D., DeCarli L. M., Lieber C. S. Role of acetate in the reduction of plasma free fatty acids produced by ethanol in man. J Lipid Res. 1968 Jul;9(4):509–512. [PubMed] [Google Scholar]
- EDELMAN I. S., HALEY H. B., SCHLOERB P. R., SHELDON D. B., FRIIS-HANSEN B. J., STOLL G., MOORE F. D. Further observations on total body water. I. Normal values throughout the life span. Surg Gynecol Obstet. 1952 Jul;95(1):1–12. [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J., Karl I., Cerasi E., Luft R., Kipnis D. M. Glutamine and glutamate metabolism in normal and diabetic subjects. Diabetes. 1973 Aug;22(8):573–576. doi: 10.2337/diab.22.8.573. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Räf L. Evidence of inter-organ amino-acid transport by blood cells in humans. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1775–1779. doi: 10.1073/pnas.70.6.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freinkel N., Singer D. L., Arky R. A., Bleicher S. J., Anderson J. B., Silbert C. K. ALCOHOL HYPOGLYCEMIA. I. CARBOHYDRATE METABOLISM OF PATIENTS WITH CLINICAL ALCOHOL HYPOGLYCEMIA AND THE EXPERIMENTAL REPRODUCTION OF THE SYNDROME WITH PURE ETHANOL. J Clin Invest. 1963 Jul;42(7):1112–1133. doi: 10.1172/JCI104797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. J., Menzel P. H., Boden G., Owen O. E. Hepatic ketogenesis and gluconeogenesis in humans. J Clin Invest. 1974 Oct;54(4):981–989. doi: 10.1172/JCI107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P., Balasse E. O., Segel N., Basso L. V. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest. 1970 Nov;49(11):2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISSELBACHER K. J., GREENBERGER N. J. METABOLIC EFFECTS OF ALCOHOL ON THE LIVER. N Engl J Med. 1964 Feb 13;270:351–CONTD. doi: 10.1056/NEJM196402132700707. [DOI] [PubMed] [Google Scholar]
- Kedenburg C. P. A lithium buffer system for accelerated single-column amino acid analysis in physiological fluids. Anal Biochem. 1971 Mar;40(1):35–42. doi: 10.1016/0003-2697(71)90081-9. [DOI] [PubMed] [Google Scholar]
- Kikuchi T., Kako K. J. Metabolic effects of ethanol on the rabbit heart. Circ Res. 1970 May;26(5):625–634. doi: 10.1161/01.res.26.5.625. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Freedland R. A., Hems R., Stubbs M. Inhibition of hepatic gluconeogenesis by ethanol. Biochem J. 1969 Mar;112(1):117–124. doi: 10.1042/bj1120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg R. A., Owen W. C., Siegal A. M. Ethanol-induced hyperlacticacidemia: inhibition of lactate utilization. J Clin Invest. 1971 Jan;50(1):166–174. doi: 10.1172/JCI106470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDQUIST F., TYGSTRUP N., WINKLER K., MELLEMGAARD K., MUNCK-PETERSEN S. Ethanol metabolism and production of free acetate in the human liver. J Clin Invest. 1962 May;41:955–961. doi: 10.1172/JCI104574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner A., Wulff J., Madison L. L. Ethanol-induced hypoglycemia. I. The acute effects of glucose output and peripheral glucose utilization in fasted dogs. Metabolism. 1967 Jan;16(1):1–18. doi: 10.1016/0026-0495(67)90154-0. [DOI] [PubMed] [Google Scholar]
- Marliss E. B., Aoki T. T., Pozefsky T., Most A. S., Cahill G. F., Jr Muscle and splanchnic glutmine and glutamate metabolism in postabsorptive andstarved man. J Clin Invest. 1971 Apr;50(4):814–817. doi: 10.1172/JCI106552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson J. H., Mello N. K. Alcohol-induced hyperlipidemia and beta lipoproteins. Science. 1973 Jun 29;180(4093):1372–1374. doi: 10.1126/science.180.4093.1372. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Hirsch E. Z. Mechanism of alcohol-induced hypertriglyceridemia. J Lab Clin Med. 1965 Sep;66(3):357–365. [PubMed] [Google Scholar]
- Nilsson S., Scherstén T. Synthesis of phospholipids and triglycerides in human liver slices. I. Experimental conditions and the synthesis rate in normal liver tissue. Scand J Clin Lab Invest. 1969 Oct;24(3):237–249. doi: 10.3109/00365516909080159. [DOI] [PubMed] [Google Scholar]
- PERMAN E. S. The effect of ethyl alcohol on the secretion from the adrenal medulla in man. Acta Physiol Scand. 1958 Dec 15;44(3-4):241–247. doi: 10.1111/j.1748-1716.1958.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Ensinck J. W. Stimulation of glucagon secretion by ethanol-induced hypoglycemia in man. Diabetes. 1975 Mar;24(3):295–300. doi: 10.2337/diab.24.3.295. [DOI] [PubMed] [Google Scholar]
- RUBIN L., ALADJEM F. Serum lipoprotein changes during fasting in man. Am J Physiol. 1954 Aug;178(2):263–266. doi: 10.1152/ajplegacy.1954.178.2.263. [DOI] [PubMed] [Google Scholar]
- Searle G. L., Shames D., Cavalieri R. R., Bagdade J. D., Porte D., Jr Evaluation of ethanol hypoglycemia in man: turnover studies with C-6 14C glucose. Metabolism. 1974 Nov;23(11):1023–1035. doi: 10.1016/0026-0495(74)90069-9. [DOI] [PubMed] [Google Scholar]
- Wolfe B. M., Kane J. P., Havel R. J., Brewster H. P. Mechanism of the hypolipemic effect of clofibrate in postabsorptive man. J Clin Invest. 1973 Sep;52(9):2146–2159. doi: 10.1172/JCI107399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. In vivo sampling of cardiac triglyceride from dogs during ethanol infusion. J Lipid Res. 1974 Jan;15(1):50–55. [PubMed] [Google Scholar]
- ZILVERSMIT D. B. The design and analysis of isotope experiments. Am J Med. 1960 Nov;29:832–848. doi: 10.1016/0002-9343(60)90117-0. [DOI] [PubMed] [Google Scholar]



