Abstract
BACKGROUND
Progesterone is a key hormonal regulator of the female reproductive system. It plays a major role to prepare the uterus for implantation and in the establishment and maintenance of pregnancy. Actions of progesterone on the uterine tissues (endometrium, myometrium and cervix) are mediated by the combined effects of two progesterone receptor (PR) isoforms, designated PR-A and PR-B. Both receptors function primarily as ligand-activated transcription factors. Progesterone action on the uterine tissues is qualitatively and quantitatively determined by the relative levels and transcriptional activities of PR-A and PR-B. The transcriptional activity of the PR isoforms is affected by specific transcriptional coregulators and by PR post-translational modifications that affect gene promoter targeting. In this context, appropriate temporal and cell-specific expression and function of PR-A and PR-B are critical for normal uterine function.
METHODS
Relevant studies describing the role of PRs in uterine physiology and pathology (endometriosis, uterine leiomyoma, endometrial cancer, cervical cancer and recurrent pregnancy loss) were comprehensively searched using PubMed, Cochrane Library, Web of Science, and Google Scholar and critically reviewed.
RESULTS
Progesterone, acting through PR-A and PR-B, regulates the development and function of the endometrium and induces changes in cells essential for implantation and the establishment and maintenance of pregnancy. During pregnancy, progesterone via the PRs promotes myometrial relaxation and cervical closure. Withdrawal of PR-mediated progesterone signaling triggers menstruation and parturition. PR-mediated progesterone signaling is anti-mitogenic in endometrial epithelial cells, and as such, mitigates the tropic effects of estrogen on eutopic normal endometrium, and on ectopic implants in endometriosis. Similarly, ligand-activated PRs function as tumor suppressors in endometrial cancer cells through inhibition of key cellular signaling pathways required for growth. In contrast, progesterone via PR activation appears to increase leiomyoma growth. The exact role of PRs in cervical cancer is unclear. PRs regulate implantation and therefore aberrant PR function may be implicated in recurrent pregnancy loss (RPL). PRs likely regulate key immunogenic factors involved in RPL. However, the exact role of PRs in the pathophysiology of RPL and the use of progesterone for therapeutic benefit remains uncertain.
CONCLUSIONS
PRs are key mediators of progesterone action in uterine tissues and are essential for normal uterine function. Aberrant PR function (due to abnormal expression and/or function) is a major cause of uterine pathophysiology. Further investigation of the underlying mechanisms of PR isoform action in the uterus is required, as this knowledge will afford the opportunity to create progestin/PR-based therapeutics to treat various uterine pathologies.
Keywords: progesterone, progesterone receptors, uterine pathophysiology
Introduction
Progesterone is an essential hormone in the female reproductive system. In conjunction with estrogen (mainly in the form of estradiol), it controls uterine function to facilitate reproduction. The central role of these hormones is reflected in the fact that progesterone/estrogen therapy alone is sufficient to produce a receptive uterus capable of supporting a viable pregnancy in post-menopausal women receiving donor embryo transfer. The principal uterine targets for progesterone are stromal and epithelial cells in the endometrium, smooth muscle cells in myometrium, and stromal fibroblasts and glandular epithelial cells in the cervix. Effects of progesterone in these cells are mediated by its interaction with specific progesterone receptors (PRs), and its pleiotropic actions are due to cell type-specific variations in PR signaling. The etiology of uterine pathologies, including endometriosis, leiomyoma, endometrial cancer, cervical cancer and recurrent pregnancy loss has been associated with aberrant PR signaling. Consequently, therapies targeted to correct problems with PR-mediated progesterone signaling have promise for the treatment of multiple uterine disorders. The goal of this review is to synthesize the current understanding of how PRs mediate progesterone actions in the endometrium, myometrium and cervix to facilitate normal uterine function, and how PR dysregulation may contribute to uterine pathophysiology.
The human progesterone receptor
Effects of progesterone on target cells are mediated by cellular PRs whose function is altered by progesterone binding. To date two groups of PRs have been identified: (i) the nuclear PRs that function as ligand-activated transcription factors and mediate genomic actions (i.e. affect gene expression) (Evans, 1988) and (ii) a family of PRs that reside at the cell surface and are structurally related to G-protein coupled receptors and single transmembrane receptors and appear to mediate direct non-genomic actions of progesterone (Gerdes et al., 1998; O'Brien et al., 1998; Saner et al., 2003; Welter et al., 2003; Zhu et al., 2003a,b; Price et al., 2005; Younglai et al., 2006; Behera et al., 2009; Lee et al., 2010). Actions of progesterone in the female reproductive system are thought to be primarily mediated by the nuclear PRs. The physiologic relevance of the membrane PR is unclear since their capacity to bind progesterone is relatively low, compared with the nuclear PRs, and some studies suggest that they are not activated by progesterone (Krietsch et al., 2006). In light of this controversy, the following discussion focuses on the role of nuclear PRs in uterine pathophysiology.
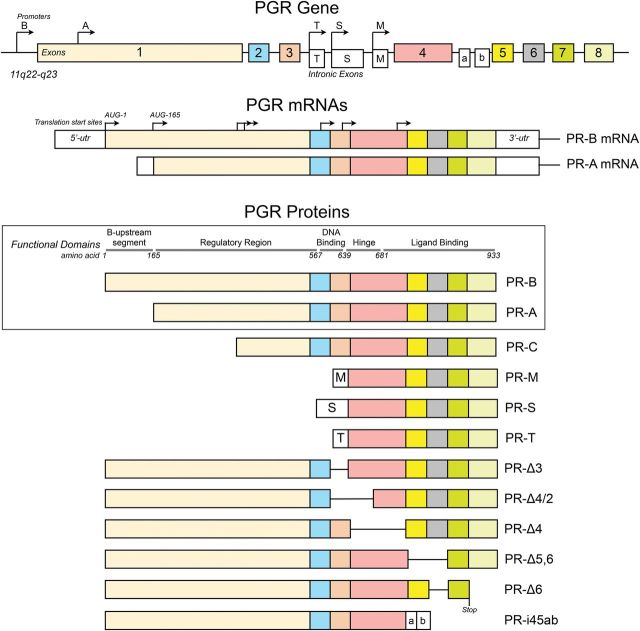
The human nuclear PRs are encoded by a single gene (PGR) located on chromosome 11 (11q22-q23). Expression of PGR is controlled by two promoters to produce two major mRNA transcripts that encode two proteins: the full-length PR-B (116 kDa) controlled by the distal PR-B promoter region and initiated from the first AUG translational start codon, and PR-A (94 kDa) controlled by the proximal PR-A promoter region and initiated from the second AUG (492 bases upstream) translational start codon (Kastner et al., 1990; Sartorius et al., 1994; Wen et al., 1994; Leonhardt et al., 2003) (Fig. 1). Other PR isoforms are thought to be generated by the initiation of translation from further downstream AUG start sites (e.g. PR-C), exon splicing and exon insertions (Fig. 1) (reviewed by Hirata et al., 2003; Cork et al., 2008); but their physiologic relevance is uncertain, and for some of these variants, especially the putative PR-C, their production in vivo is unclear since the natural AUG start sites lacks an upstream Kosak sequence needed for translation initiation (Samalecos and Gellersen, 2008). The following discussion will therefore be limited to PR-A and PR-B.
Figure 1.
Structure of the human PR isoforms produced from the PGR gene. The major mRNA transcripts are derived from translational start sites controlled by the PR-B (distal) and PR-A (proximal) promoters. The major proteins products (boxed) are the full-length PR-B produced from PR-B mRNA and initiated from the first AUG, and the PR-A which is produced from PR-A mRNA and initiated from the second AUG. The receptors contain functional domains that are typical of the nuclear receptors family. The structures of other putative splice variants are shown below the boxed area.
PR-A and PR-B belong to a family of ligand-activated transcription factors and share common structural and functional elements (i.e. regulatory region, DNA binding domain, hinge region and ligand binding domain) with other steroid hormone receptors (Fig. 1) (Evans, 1988; Mangelsdorf et al., 1995; Escriva et al., 2004; McEwan, 2009). The DNA binding domain, hinge region and ligand binding domain are identical in PR-A and PR-B with the difference between the two PRs being in the N-terminal regulatory domain that is truncated by 164 amino acids in PR-A. Differences in the activities of PR-A and PR-B are thought to be conferred by the N-terminal segment (B-upstream segment) unique to PR-B.
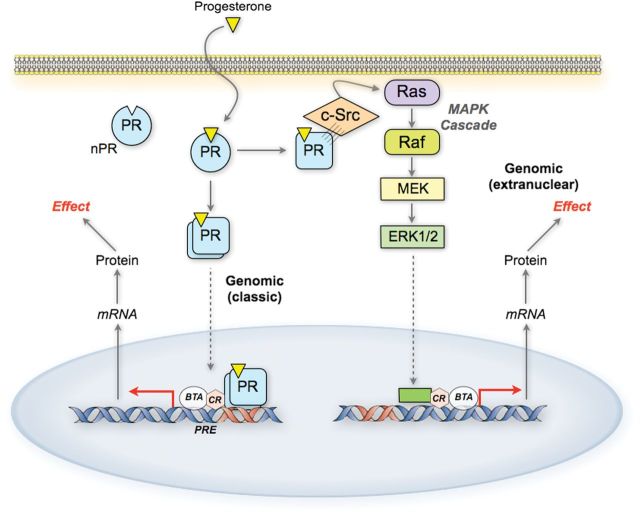
Effects of progesterone are generally considered to represent the combined activities of PR-A and PR-B. Upon ligand binding, PR-A and PR-B affect cellular function by altering gene expression via two modes of action: (i) the direct genomic mode, whereby the PRs function as ligand-activated transcription factors to directly interact with specific DNA promoter/enhancer elements and transcriptional co-regulators to modulate the expression of downstream genes; and (ii) the indirect extranuclear mode whereby the PRs interact with Src tyrosine kinases in the cytoplasm to activate mitogen-activated protein kinases (MAPKs) which then affect gene expression (Boonyaratanakornkit et al., 2001; Leonhardt et al., 2003; Boonyaratanakornkit and Edwards, 2007) (Fig. 2).
Figure 2.
Genomic signaling pathways for PR action. In response to ligand, PRs undergo a conformational change and dimerize. The receptors then translocate to the nucleus where they function as ligand-activated transcription factors. The receptors also activate cytoplasmic signaling cascades such as the ERK/MAPK pathway by interacting directly with extranuclear signaling molecules. BTA, basal transcriptional apparatus; CR, co-regulators.
PR-A and PR-B are co-expressed in varying relative amounts depending on the cell type and pathophysiologic condition. Studies to assess the abundance and cellular localization of PR-A and PR-B are complicated by technical limitations due to the common sequence of PR-A and PR-B. Consequently, antibodies specific for PR-A are not available, and PR-A mRNA abundance cannot be directly measured by quantitative RT–PCR-based techniques. The abundance of PR-A and its level relative to PR-B can only be determined by immunoblotting, which is semi-quantitative at best. In vitro approaches, however, using various cell types genetically modified to express PR-A and/or PR-B in conjunction with PR-reporter systems, have revealed key functions of PR-A and PR-B, and how they interact to affect transcription in specific cell types. In addition, significant progress in understanding PR function has been gained from studies of mice genetically modified to abolish the PR-A and PR-B isoforms together or individually (see below).
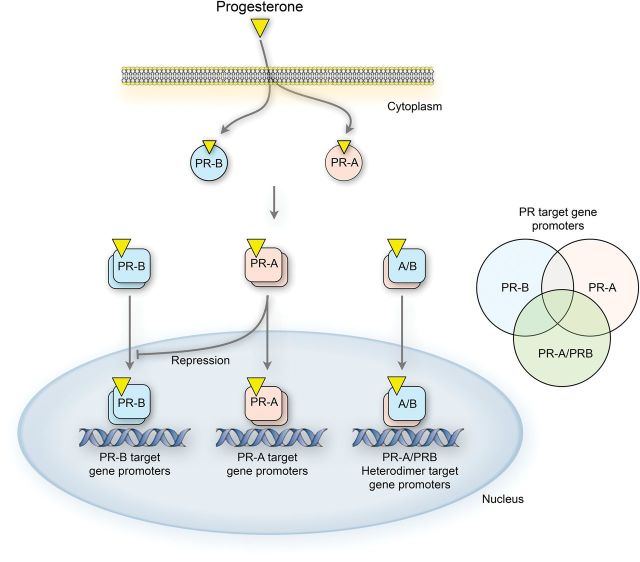
Initial studies of PR transcriptional activity were performed using artificial reporter genes controlled by canonical progesterone responsive elements (PREs). In that assay, PR-B is a strong transactivator in response to progesterone, whereas PR-A is less active and in most cases inhibits the transcriptionally active PR-B, especially when its level exceeds that of PR-B (i.e. PR-A:PR-B ratio >1) (Tung et al., 1993; Giangrande et al., 1997, 2000; Pieber et al., 2001; Richer et al., 2002; Condon et al., 2006; Merlino et al., 2007). Those observations led to the concept that PR-A and PR-B have opposing transcriptional activity and that as such net progesterone responsiveness is inversely related to the PR-A:PR-B ratio. Such a mechanism permits control of progesterone responsiveness by the target cell via modulation of isoform levels. Recent studies, however, using global gene expression analyses, show that both isoforms are transcriptionally active at diverse sets of endogenous promoters, most of which lack a canonical PRE. Importantly, the repressive activity of PR-A was found to be minimal at endogenous genes promoters (Richer et al., 2002; Graham et al., 2005; Jacobsen et al., 2005; Leo et al., 2005; Yudt et al., 2006; Khan et al., 2012). It is now generally accepted that response to progesterone is determined by the combined actions of PR-A and PR-B, which upon ligand binding form homodimers or heterodimers that have distinct transcriptional activities at specific sets of gene promoters (Fig. 3).
Figure 3.
Functional interaction between PR-A and PR-B. Upon ligand binding, the receptors form transcriptionally active homo- and hetero-dimers that affect the expression of specific and common gene sets. PR-A also acts as a trans-repressor of PR-B at some promoters.
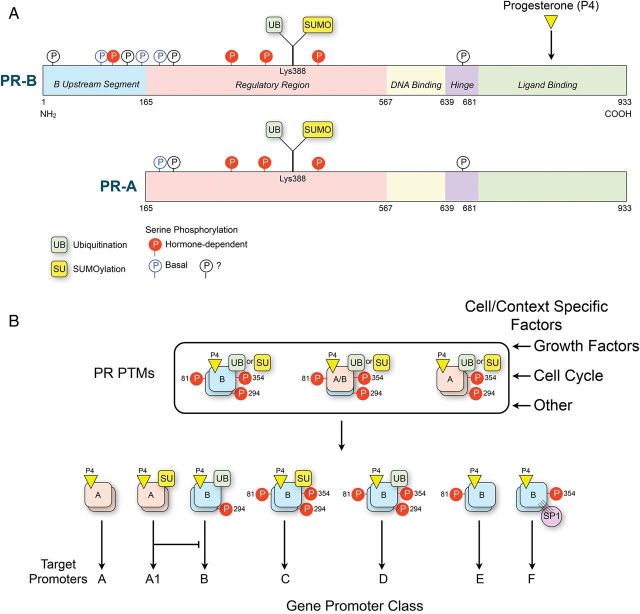
The capacity for the PR isoforms to target distinct promoters and affect the expression of diverse downstream genes is qualitatively and quantitatively affected in a cell- and context-specific manner by: (i) transcriptional co-regulators that form a functional bridge connecting the PRs with the basal transcription machinery (McKenna et al., 1999); (ii) the functional interaction of PRs with other transcription factors such as NFκB (Kalkhoven et al., 1996), AP-1 (Bamberger et al., 1996) and SP1 (Faivre et al., 2008); and (iii) post-translational modifications (PTMs) including serine phosphorylation, ubiquitination and sumoylation of the PRs that affects their stability, trafficking, transcriptional activity and target gene selectivity (Abdel-Hafiz and Horwitz, 2012, 2014; Knutson et al., 2012) (Fig. 4). Regulation of numerous coregulators (coactivators and corepressors) is pivotal for modulation of PR-mediated gene transcription leading to activation or repression of specific target genes (Xu and Li, 2003). For example, the p160 steroid receptor coactivator-1 (SRC-1) is a transcriptional coactivator for PR, which interacts with liganded-PR and serves to recruit histone acetyl transferases and methyltransferases to specific gene promoter regions to facilitate transcription by altering chromatin structure (McKenna et al., 1999). These factors account for most of the cell- and context-specific pleitropic actions of progesterone.
Figure 4.
(A) Sites for known serine phosphorylation and ubiquitination/sumoylation in PR-A and PR-B that are induced in response to ligand binding and/or in response to growth factors and other kinases. (B) It is proposed that post-translational modifications (PTMs) affect the targeting of ligand-activated PRs to specific gene promoter classes. This mechanism explains how the PRs mediate pleiotropic actions of progesterone in different cell types and different physiologic conditions.
PGR expression by uterine cells is stimulated by estrogens via estrogen receptor-α (ERα) and consequently progesterone responsiveness is dependent on the presence of an estrogenic drive (Tsai et al., 1998). The affect is especially relevant in the endometrium where estrogen exposure during the follicular phase promotes PR expression in endometrial cells to augment progesterone responsiveness during the luteal phase. In fact, low levels of estrogen are required for progesterone responsiveness throughout the luteal phase (Janne et al., 1975; Kreitmann et al., 1979; Katzenellenbogen et al., 1980; Bergeron et al., 1988; Lessey et al., 1988; Press et al., 1988; Okulicz et al., 1989; Savouret et al., 1990; Fung et al., 1994; Ingamells et al., 1996; Moutsatsou and Sekeris, 1997). Conversely, ERα expression in uterine cells is inhibited by progesterone via PRs (Haluska et al., 1990). This functional feedback interaction between the progesterone and estrogen hormonal systems is crucial for normal uterine function and for balancing the often-opposing actions of the progesterone/PR and estrogen/ER systems. Steady state levels of PR are decreased by progesterone through ligand-induced phosphorylation of the PR, which increases transcriptional activity but also induced PR ubiquitination that targets the protein for degradation through the proteosome (Lange et al., 2000; Shen et al., 2001; Abdel-Hafiz and Horwitz, 2014). Similar to ligand-dependent PR down-regulation through the ubiquitin-proteosome pathway, the presence of ligand also contributes to down-regulation of specific PR coregulators, including SRC-1 (Amazit et al., 2011). Thus, in the presence of ligand, levels of PR (especially PR-B) are inversely correlated with transcriptional activity.
Other PR-independent mechanisms could also operate to facilitate target cell control of progesterone responsiveness. One important mechanism is via the intracrine mode of hormonal control whereby a hormone is metabolized to a more or less active form within the target cell prior to its interaction with a cognate receptor. In the case of progesterone, target cells may express enzymes such as 5α-reductase and 20α-hydroxysteroid dehydrogenase that metabolize progesterone to less active forms prior to its interaction with intracellular receptors. This PR-independent regulatory mode may be critical for controlling progesterone actions in the cervix during pregnancy and especially at parturition (Mahendroo et al., 1999). Intracrine mechanisms also may control progesterone actions on the brain (Mellon, 2007).
Progesterone receptors in normal uterine function
Effects of progesterone on uterine physiology occur during the post-ovulatory luteal/secretory phase of the menstrual cycle and during pregnancy; i.e. when circulating levels of progesterone are sufficient to activate PRs in uterine cells. Progesterone affects cells in each of the functional tissue types of the uterus, and PR-A and PR-B are detectable in epithelial and stromal/decidual cells in the endometrium (Mote et al., 1999), smooth muscle cells in the myometrium (Fig. 5) (Mesiano, 2007), and stromal fibroblasts in the cervix (Cowan et al., 2004). The overall scope of progesterone action in the uterus has been delineated through studies utilizing PR antagonists such as mifepristone (RU486) for emergency contraception. While administration of high doses of RU486 in the mid- or late follicular phase delays the luteinizing hormone (LH) surge and inhibits ovulation, low doses can cause infertility by delaying endometrial maturation (Spitz et al., 1994; Gemzell-Danielsson and Marions, 2004; Lakha et al., 2007). Similarly, if administered during pregnancy, RU486 induces abortion, fetal loss or parturition depending on the gestational age (Chwalisz, 1994; Wildschut et al., 2011; Shaw et al., 2013). Thus, PR-mediated progesterone actions that are disrupted by RU486 are essential for normal uterine function.
Figure 5.
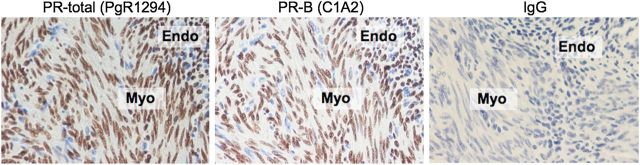
Immunohistochemical localization of PR-A and PR-B at the human myometrial/endometrial interface during the secretory phase of the menstrual cycle. Total PR (PR-A and PR-B) was detected with antibody PgR1294 (Dako Corp) that reacts with an epitope in the N-terminal region common to both PR-A and PR-B. PR-B was detected with antibody C1A2 (Cell Signaling, Inc.) that reacts with an epitope in the N-terminus unique to PR-B. IgG represents the negative control using the non-immune immunoglobulin of the same isotype as the PgR1294 (mouse) and C1A2 (rabbit) primary antibodies. Positive staining is indicated by brown coloration. Sections were counterstained with hematoxylin (stains nuclei blue). Magnification = ×200.
The endometrium is a remarkably dynamic uterine tissue that exhibits dramatic steroid hormone dependent changes in morphology and function during the menstrual cycle and during pregnancy. It is composed of stromal and epithelial cells arranged into two morphologically and functionally distinct zones: the inner basalis, made up mainly of stromal cells, and the outer functionalis, which contains stromal and epithelial cells. Progesterone exerts specific effects on endometrial epithelial and stromal cells. During the pre-ovulatory follicular phase, the functionalis thickens in response to estrogens (mainly estradiol) by epithelial and stromal cell proliferation. Cell proliferation is then inhibited by progesterone during the post-ovulatory luteal phase and a morphologic and functional change occurs to establish a glandular secretory endometrial epithelium and a vascular stroma conducive for blastocyst implantation and the establishment of pregnancy. In endometrial stromal cells, progesterone increases proliferation during the follicular phase and during the peri-implantation phase via activation of the ERK/AKT pathway (Vallejo et al., 2014). This effect highlights the complex functional interaction between the ER and PR systems especially via their extranuclear modes of action (see Fig. 2). Importantly, progesterone induces stromal cell decidualization in the late luteal phase and is essential for maintenance of the decidual phenotype (Lydon et al., 1995; Das et al., 2009). In the decidualization process, which initiates prior to implantation and is independent of the presence of a blastocyst, endometrial stromal cells proliferate, become rounded, and accumulate cytoplasmic glycogen (Bergeron et al., 1988; Lessey et al., 1988). In non-conception cycles, the functional lifespan of the corpus luteum is limited, and within 14–18 days after its formation, it undergoes apoptosis leading to a rapid fall in circulating progesterone levels. If pregnancy is not established, falling progesterone levels due to luteolysis reverse decidualization and induce the expression of chemokines, pro-inflammatory cytokines and matrix metalloproteinases that cause endometrial inflammation, cell death and extracellular matrix degradation leading to shedding of the functionalis, i.e. menstruation (Gellersen and Brosens, 2003; Jabbour et al., 2006; Gellersen et al., 2007). The functionalis then regenerates from the remaining basalis layer in response to estrogen during the proliferative phase of the following cycle (Ferenczy et al., 1979; Graham et al., 2005). If conception occurs, the steroidogenic life span of the corpus luteum is prolonged for up to 10 weeks by chorionic gonadotrophin (CG) secreted by trophoblast cells of the successfully implanted blastocyst. The extended time of progesterone exposure causes the endometrium to undergo complete decidualization within the first week of pregnancy. Complex paracrine signaling between the decidua and trophoblast cells in the chorionic membrane is critical for establishing and maintaining pregnancy. The appropriate role of decidual cells in that dialogue is dependent on progesterone. PR is expressed in endometrial epithelium immediately prior to blastocyst implantation but decreases markedly during the implantation process. At the same time PR expression in stromal cells increases and remains high during the decidualization process (Tan et al., 1999; Spencer et al., 2004). Specific ablation of PR expression in endometrial epithelial cells showed that expression of the receptors in these cells is critical for embryo attachment, stromal decidualization and the inhibition of estrogen-induced epithelial hyperplasia (Fernandez-Valdivia et al., 2010). Loss of PR expression in decidual cells at term is thought to cause functional progesterone withdrawal that triggers inflammation at the maternal–fetal interface leading to parturition (Lockwood et al., 2010).
Differential effects of progesterone on endometrial epithelial and stromal cells are thought to be due to cell type-specific differences in PR-A and PR-B expression and function. PR-A and PR-B are present in endometrial epithelium during the proliferative phase and increase concordantly with estrogen levels, consistent with the known induction of PR expression by estrogen. Late in the secretory phase, PR-A levels decline, whereas PR-B levels remain constant in the epithelial cells, suggesting that it is involved in the control of glandular secretion. Stromal cells, in contrast, exhibit a predominance of PR-A throughout the menstrual cycle, which likely reflects the need for prolonged progesterone-PR-A signaling in this compartment to support the establishment of pregnancy (Mote et al., 1999, 2000; Attia et al., 2000).
Studies of mice genetically modified to alter PR expression demonstrated fundamental roles of the PRs in mediating progesterone actions on the uterus. Animals lacking PR expression develop normally to adulthood regardless of sex (Lydon et al., 1995; Fernandez-Valdivia et al., 2010). Female PR-null homozygous mice, however, have multiple defects in uterine growth and function, the most notable being hypertrophy and inflammation of the glandular epithelium and failure to exhibit decidualization in response to a traumatic stimulus. PR-null mice fail to reproduce due to defects in ovulation and implantation. Studies targeting PR-A or PR-B demonstrated specific roles of each PR isoform in mediating progesterone actions on the murine uterus. Progesterone actions mediated by PR-A (i.e. phenotype of PR-B-knockout mice) were sufficient to restore normal uterine function. Ovarian function, implantation, pregnancy and parturition are normal in mice expressing only PR-A (Mulac-Jericevic et al., 2003). Progesterone actions mediated by PR-B (i.e. reproductive phenotype of PR-A-knockout mice) lead to increased hyperplasia of the endometrial epithelium and inflammation, and no decidualization in the endometrial stroma (Mulac-Jericevic et al., 2000). Taken together the data from PR knockout mice show that PR-A is critical for normal function of the endometrial epithelium and stroma, and that PR-B functions to promote hyperplasia of the epithelium, an effect which is repressed by PR-A. Both receptors appear to mediate anti-inflammatory actions of progesterone on the endometrium. Studies in mice over-expressing PR-A show that the relative levels of PR-A and PR-B are critical for normal response to progesterone (Fleisch et al., 2009). Over-expression of PR-A was associated with enlargement of the uterus, and hyperplasia of the endometrium that included atypical lesions, endometritis and pelvic inflammatory disease.
The myometrium and cervix also undergo changes in response to estrogen and progesterone during the menstrual cycle, albeit less dynamic compared with those in the endometrium. The myometrium, which forms the bulk of the uterus, is composed of myometrial smooth muscle cells arranged into randomly orientated interlacing bundles. Progesterone and estrogen promote myometrial growth mainly by stimulating hyperplasia and hypertrophy of myometrial cells. After menopause, the myometrium becomes atrophic and the size of the uterus decreases to about half its size. Myometrial cells contain PR-A and PR-B throughout the menstrual cycle and during pregnancy. Progesterone affects the contractile activity of myometrial cells. During the estrogen-dominated proliferative phase, peristaltic waves of myometrial contractions gradually increase in frequency and intensity and at the time of ovulation the direction of the waves is predominantly from cervix to fundus (Chalubinski et al., 1993; Kunz et al., 1996). The waves decrease during the progesterone-dominated post-ovulatory secretory phase presumably due to the relaxatory actions of progesterone, and contractions increase in association with the decrease in progesterone levels late in the secretory phase and during menstruation. These effects are mediated by the nuclear PRs since administration of the nuclear PR antagonist, RU486, increases uterine contractions during the secretory phase (Bygdeman et al., 1993; Gemzell-Danielsson et al., 1993). Likewise, during pregnancy, nuclear PRs expressed by myometrial cells mediate relaxatory actions of progesterone and inhibition of this activity by RU486 treatment increases myometrial contractility and excitability and, in most cases, induces labor and delivery (Avrech et al., 1991). Withdrawal of progesterone in human pregnancy is thought to be mediated by increased expression of PR-A, possibly due to altered methylation of the PR-A promoter region (Chai et al., 2014), leading to a switch in the PR-A:PR-B ratio to PR-A-dominance, which is thought to inhibit PR-B-mediated pro-gestational actions (Mesiano et al., 2002). The withdrawal or disruption of PR-mediated progesterone actions generally leads to the uterine emptying, i.e. mensuration or parturition, that involves increased myometrial contractity and tissue level inflammation within the endometrium, myometrium and cervix (Thomson et al., 1999; Jabbour et al., 2006).
The cervix is composed of stromal fibroblasts and squamous epithelial cells and can be divided into three anatomically distinct compartments: (i) the ectocervix that projects into the vagina and is lined by a thick stratified squamous epithelium, (ii) the endocervix that forms the lining of the cervical canal and comprises single layer of columnar mucus-secreting epithelial cells that form deep furrows and tunnels; and (iii) the stroma which comprises the bulk of the cervix and is composed of cervical fibroblasts that produce a tough collagenous extracellular matrix (ECM) (Bathgate et al., 2006). PRs localize to the nucleus in stromal fibroblasts and basal squamous epithelial cells, but are absent in intermediate and superficial squamous epithelial cells (www.nordiqc.org/Run-18-B2/assessment/assessment-PR.htm). During the proliferative phase of the menstrual cycle, the endocervical epithelium, in response to estrogen, produces a thin watery mucus that is conducive to the passage of sperm into the uterus. In contrast, during the secretory phase, progesterone promotes the production of a highly viscous cervical mucus, which forms a plug that restricts the passage of sperm and micro-organisms from the vagina. Estrogen softens the cervical stroma by affecting collagen synthesis and breakdown and promoting water imbibition. In contrast, progesterone, especially during pregnancy, promotes cervical closure by increasing collagen production and rigidity. These effects are mediated by PRs that, in response to progesterone, modulate the expression of genes whose products promote collagen synthesis and inhibit its breakdown (Di Nezza et al., 2003; Jaffe et al., 2007; Ward et al., 2008; Neubauer et al., 2011). Progesterone also antagonizes estrogen-induced collagenase expression (Shiozawa et al., 1998; Kyo et al., 2011) and inhibits hyaluronate synthesis in human endocervical fibroblasts (Uchida et al., 2005, 2007). A decrease in hyaluronate prevents water imbibition and collagen dissolution and, therefore, maintains ECM rigidity. In all species studied so far, treatment with PR antagonists at any stage of pregnancy promotes cervical ripening (Dai et al., 2001; Smid-Koopman et al., 2003; Gielen et al., 2006; Paulssen et al., 2008; Moe et al., 2009), which demonstrates that PR signaling is essential for progesterone-induced cervical competence. Sensitivity of the cervix to PR antagonist-induced ripening increases as pregnancy nears term, suggesting that the capacity for progesterone to maintain cervical competence wanes and pro-softening influences increase as pregnancy progresses (Orbo et al., 2009).
In the gravid uterus, progesterone promotes relaxation of the myometrium, closure and rigidity of the cervix, and inflammatory quiescence in the chorion/decidua. The pro-gestational actions of progesterone are mediated by PRs expressed in myometrial, cervical and decidual cells (Merlino et al., 2007). Progesterone promotes pregnancy, in part, by decreasing responsiveness of myometrial, cervical and decidual cells to pro-inflammatory/pro-labor stimuli (Hardy et al., 2006; Tan et al., 2012). In myometrial cells, the anti-inflammatory effects of progesterone are mediated by PR-B and inhibited by PR-A (Tan et al., 2012).
In all viviparous species studies to date, the process of parturition is triggered by withdrawal of the progesterone block to labor, and depending on the species, occurs by a either a decrease in circulating progesterone levels (referred to as systemic progesterone withdrawal) or desensitization to PR-mediated progesterone action (referred to as functional progesterone withdrawal). Human parturition is triggered by a functional progesterone withdrawal mediated, in part, by changes in the transcriptional activities of PR-A and PR-B such that the capacity for progesterone to exert anti-inflammatory actions via PR-B is inhibited by transrepressive actions of PR-A (Mesiano et al., 2011; Tan et al., 2012). Transcriptional activity of PR-B is further impaired at parturition by decreased expression of progesterone-PR coregulators such as cAMP-response element-binding protein (CREB-binding protein) and steroid receptor coactivators 2 and 3 (Condon et al., 2003). Secondary to their inherent histone acetyltransferase activity, decreased expression of these coactivators may result in the decline of histone acetylation, and therefore, chromatin inaccessibility and transcriptional repression during parturition (Chen et al., 1997; Condon et al., 2003).
Thus, a common theme of PR-mediated progesterone actions in the human uterus (gravid and non-gravid) is that PRs mediate pro-gestational and anti-inflammatory actions and withdrawal of this activity, either by functional inhibition of PR-B signaling or a systemic decrease in progesterone levels, leads to tissue level inflammation, which causes menstruation in the non-gravid uterus and parturition and involution in the gravid uterus. In both contexts, the key physiologic consequence of progesterone/PR withdrawal is the resumption of the menstrual cycle.
Progesterone receptors in uterine pathophysiology
The following discussion focuses on the role of PR-A and PR-B in the development and progression of endometriosis, uterine leiomyoma, endometrial cancer, cervical cancer, and recurrent pregnancy loss. As discussed above, progesterone affects normal uterine function via a finely tuned and tissue/cell type specific balance between PR-A- and PR-B-mediated transcriptional activities. Most pathophysiologies of myometrium, endometrium and cervix are responsive to progesterone, albeit in an abnormal manner. PR-mediated progesterone actions vary according to the cell type. In the breast, PR (mainly PR-B) mediates proliferative actions of progesterone, whereas in the uterus progesterone stimulates growth of leiomyomas but inhibits growth of the endometrium. In general, studies examining the role of PRs in the etiology of uterine pathophysiologies have assessed only the expression level and cellular location of PR-A and PR-B, without considering net transcriptional activity. Clearly, it is now evident that functional pleiotropy in PR activity can arise not only through cell type-specific differences in PR-A:PR-B levels, but also by changes in PR PTMs, and differences in transcriptional co-regulators and target gene promoter structure. Nonetheless, temporo-spatial information of PR isoform expression has provided a better understanding of PR function in uterine pathophysiology.
Endometriosis
Endometriosis is the presence of glandular and stromal endometrial implants at an extrauterine (ectopic) site. The disease affects 5–10% of women of reproductive age and is markedly influenced by estrogen and progesterone. Inflamed endometriotic lesions are usually found in the peritoneal cavity and on the ovaries, and it is generally considered that they derive from abnormal endometrial cells that access the peritoneum by retrograde menstruation (Sampson, 1927; Giudice and Kao, 2004; Giudice, 2010). Lymphatic/hematogenous dissemination of abnormal endometrial cells and metaplastic transformation of native peritoneal tissue may also be responsible for implants at distant sites (Giudice, 2010).
Endometriotic implants undergo estrogen- and progesterone-induced changes in growth and morphology during the menstrual cycle in parallel with the eutopic endometrium (Jiang et al., 2002). Consequently, the clinical severity of endometriosis is affected by estrogens and progesterone, with estrogen exposure being a major endocrine risk factor for disease development and progression (Halme et al., 1995), whereas progesterone has inhibitory effects on endometriotic implants and is associated with disease regression (Kaunitz, 1998; Olive et al., 2004). This pattern of response is consistent with estrogen-induced proliferation of endometrial epithelial cells during the proliferative phase and the inhibition of proliferative activity by progesterone during the secretory phase.
Whether aberrant PR signaling in endometrial cells plays a role in the etiology of endometriosis is unclear. Endometriosis appears to be associated with decreased progesterone responsiveness in endometrial stromal cells (Bulun et al., 2006; Yin et al., 2007, 2012) that may be due to decrease in PR expression. Studies of PR-A and PR-B levels and cell localization in endometriosis however are equivocal. Most studies using assays that have not discriminated between PR-A and PR-B have reported lower PR levels in ectopic compared with eutopic endometrium (Lyndrup et al., 1987; Prentice et al., 1992; Bergqvist and Ferno, 1993a,b; Bergqvist et al., 1993), with one study (Nisolle et al., 1994) reporting no difference. Immunoblot analysis of PR-A and PR-B shows that peritoneal endometriotic tissue does not express PR-B and has reduced levels of PR-A compared with eutopic endometrium (Attia et al., 2000). In contrast, mRNA analysis of ovarian endometriotic tissue shows dominant expression of PR-B (Misao et al., 1999), whereas studies of PR gene structure in endometriosis show hypermethylation of the PR-B promoter consistent with decreased PR-B expression (Wu et al., 2006).
A key unanswered question regarding the etiology of endometriosis is why it affects 5–10% of women even though retrograde menstruation occurs in most women (Eskenazi and Warner, 1997). One explanation is that endometriosis arises due to changes in eutopic endometrial cell progesterone responsiveness, especially decreased responsiveness, that confers a predisposition to form ectopic implants. To test this hypothesis, several studies analyzed PR expression in eutopic endometrium from women with and without endometriosis. Outcomes, however, conflicted with some studies reporting dysregulation of PR isoform expression associated with the presence of disease (Kao et al., 2003; Burney et al., 2007) and others reporting no difference (Prentice et al., 1992; Attia et al., 2000; Igarashi et al., 2005; Aghajanova et al., 2009). Taken together, published data regarding the role of PRs in the etiology of endometriosis produce no clear consensus. Nonetheless, aberrant PR signaling, especially reduced capacity for progesterone to oppose estrogen-induced endometrial cell proliferation and/or responsiveness to pro-inflammatory stimuli in eutopic endometrium may contribute to the development of endometriosis once the cells relocate to the peritoneum (Osteen et al., 2005). Progesterone is clearly a central player in the disease, and it is likely that further studies using more specific and sensitive assays for PR-A and PR-B expression, PTM forms, and transcriptional activity will elucidate the role of the PRs in the pathophysiology of endometriosis.
Leiomyoma
Uterine leiomyoma, also known as uterine fibroids, are benign monoclonal smooth muscle cell tumors thought to arise from a genetically modified myometrial cell. The tumors comprise modified myometrial smooth muscle cells, known as leiomyoma cells that overproduce ECM (Williams et al., 1997; Sumitani et al., 2000; Barbarisi et al., 2001; Park et al., 2008). Leiomyoma is the leading indication for hysterectomy occurring in ∼70–80% of reproductive age females and causes multiple gynecologic symptoms including pelvic pain and dysfunctional uterine bleeding (Flynn et al., 2006; Parker, 2007).
Clinical observations provide strong circumstantial evidence that leiomyoma is estrogen and progesterone responsive. The disease has not been reported in pre-pubescent girls but the incidence and burden of tumors increase in association with high estrogen and progesterone levels, especially during the reproductive years, and decrease after menopause when ovarian steroids are low. Moreover, treatment with gonadotrophin releasing hormone (GnRH) agonist, which causes hypogonadism, suppresses leiomyoma growth, whereas estrogen and progesterone replacement therapy increases the tumor size in menopausal women (Sener et al., 1996; Palomba et al., 2001; Yang et al., 2002). The mitogenic potential of estrogen and progesterone on leiomyoma cells is curbed by concurrent treatment with GnRH agonists (Chegini et al., 2002). As side effects related to hypoestrogenism preclude long-term treatment with GnRH analogs, low doses of estrogen and progesterone can be utilized as add-back therapy without stimulation of leiomyoma growth (Thomas, 1996; Takeuchi et al., 2000).
Clinical evidence shows that progesterone stimulates leiomyoma growth. An elevated proliferative index (assessed by Ki67 staining) and epidermal growth factor receptor expression were detected in leiomyoma cells compared with adjacent myometrium, and are highest during the progesterone-dominant secretory phase (Tiltman 1985; Kawaguchi et al., 1989; Brandon et al., 1993; Harrison-Woolrych et al., 1994). In post-menopausal women, the leiomyoma proliferative index is higher in women receiving combined estrogen and progesterone replacement therapy compared with those receiving estrogen alone (Lamminen et al., 1992). Progestin (synthetic progestogen) therapy decreases the efficacy of GnRH agonist treatment to reduce leiomyoma size (Friedman et al., 1993) and increases leiomyoma size in post-menopausal women (Lamminen et al., 1992; Palomba et al., 2002). Importantly, RU486 and other selective progesterone receptor modulators (SPRMs) significantly reduce the leiomyoma tumor burden, suggesting that targeting progesterone signaling in leiomyoma is an effective strategy for reducing the burden of this disease (Murphy and Mahesh, 1985; Murphy et al., 1993, 1995; Cermik et al., 2002; Eisinger et al., 2003, 2005; Steinauer et al., 2004; Fiscella et al., 2006; Xu et al., 2006, 2008; Chwalisz et al., 2007; Williams et al., 2007; Wilkens et al., 2008; Donnez et al., 2012a, b).
Actions of progesterone in leiomyoma are thought to be mediated by PR-A and PR-B. Both receptors have been detected in leiomyoma tissue and in immortalized leiomyoma cell lines (Kawaguchi et al., 1991; Nisolle et al., 1999; Carney et al., 2002), and some studies show increased expression of PR-A and PR-B in leiomyoma compared with normal myometrium from the same subjects (Brandon et al., 1993; Viville et al., 1997; Fujimoto et al., 1998). Interestingly, PR-A and PR-B expression in leiomyoma is decreased by GnRH agonist therapy (Nisolle et al., 1999), which is consistent with estrogen-induced PR expression. Extranuclear actions of PRs, especially via the PI3K/AKT signaling cascade, which augments cell viability and prevents apoptosis, may contribute to progesterone/PR-induced leiomyoma growth (Hoekstra et al., 2009). In addition, progesterone via the PRs may inhibit apoptosis by directly augmenting expression of Bcl-2 (Yin et al., 2007) and promote proliferation by increasing expression of EGF (Shimomura et al., 1998; Matsuo et al., 1999).
Leiomyoma are characterized as benign fibrous tumors with excessive deposition of disorganized ECM. The composition of the leiomyoma ECM, and in particular the relative levels of specific proteoglycans such as decorin and versican, are thought to affect tumor growth by modulating the activity of growth factors, especially transforming growth factor-β (TGF-β), that bind to proteoglycans (Harper et al., 1994). Compared with unaffected tissues, leiomyoma contain higher relative amounts of versican and lower relative amounts of decorin (Carrino et al., 2012). Higher TGF-β levels have been found in leiomyoma, and decorin has been shown to antagonize TGF-β signaling. A decrease in decorin would be expected to increase TGF-β activity in the leiomyoma microenvironment. Importantly, progesterone and estrogen increase ECM production and TGF-β expression in leiomyoma cells (Arici and Sozen, 2000; Joseph et al., 2010) and potentially initiate a positive feedback interaction whereby excessive deposition of the decorin-deficient ECM leads to excessive TGF-β signaling which increases the proliferation of cells that produce the aberrant decorin-deficient ECM and over-express TGF-β in response to progesterone. Although studies assessing the link between proteoglycan synthesis and PR transcriptional activity in leiomyoma are lacking, the use of SPRMs targeting this interaction to treat leiomyoma disease has been proposed (Kim et al., 2013).
To date, numerous SPRMs have been investigated as potential treatments for symptomatic leiomyomas and two, asoprisnil (DeManno et al., 2003) and ulipristal acetate (Yoshida et al., 2010), have been examined in detail. Asoprisnil reduces leiomyoma volume and associated symptoms (Chwalisz et al., 2007) possibly by inhibiting proliferation and inducing apoptosis of leiomyoma cells without effecting normal myometrial cells (Chen et al., 2006; Wang et al., 2006; Sasaki et al., 2007). Asoprisnil also decreases leiomyoma collagen synthesis and enhances the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs), thereby reducing collagen deposition into the leiomyoma ECM (Stewart et al., 1994; Morikawa et al., 2008). Ulipristal acetate also has demonstrated anti-proliferative, anti-fibrotic and pro-apoptotic effects on cultured leiomyoma cells, but not on normal myometrial cells (Yoshida et al., 2010) and has recently received approval by both the US Food and Drug Administration and the European Medicine Agency for preoperative treatment of leiomyoma (Talaulikar and Manyonda, 2012; Biglia et al., 2014). Ulipristal has been shown to reduce both leiomyoma and uterine size in women with symptomatic leiomyoma (Levens et al., 2008; Nieman et al., 2011). Repeated doses of ulipristal acetate at 3-month intervals are effective in treating symptomatic leiomyoma, with induction of amenorrhea and reduced leiomyoma volume (Donnez et al., 2014). Ulipristal may inhibit the proliferation of leiomyoma cells and induce apoptosis through increased expression of pro-apoptotic caspase 3 and decreased expression of anti-apoptotic Bcl-2 (Maruo et al., 2000; Maruo, 2007; Biglia et al., 2014). Ulipristal also down-regulates expression of key angiogenic growth factors including vascular endothelial growth factor (VEGF), and similar to asoprisnil, may reduce deposition of collagen in the extracellular matrix (Spitz, 2009; Talaulikar and Manyonda, 2012; Biglia et al., 2014). Overall, SPRMs are associated with fewer adverse effects compared with pure progesterone receptor antagonists. In some studies, however, SPRM use has been associated with adverse endometrial effects, including endometrial hyperplasia. Endometrial hyperplasia is classified by the World Health Organization (WHO) based on cystic glandular dilation/crowding of the epithelium and a gland to stromal ratio of >50% in the absence of secretory endometrial changes (Owings and Quick, 2014). Simple hyperplasia is associated with few mitoses and glands with minimal outpouching. In contrast, complex hyperplasia is associated with numerous mitoses, disorganized glands with luminal outpouching. Additionally, nuclear atypia can accompany either of these classifications. As such, endometrial hyperplasia is a precursor to endometrial adenocarcinoma. These endometrial effects associated with SPRMs appear to be related to the dose of PR ligand utilized, hormonal status at the time of treatment, and the animal model used in previous studies. For instance, mifepristone (RU486), a PR antagonist, has both anti-proliferative and hyperplastic effects depending on the dose and subject. In premenopausal subjects, low dose mifepristone has an anti-proliferative effect, while higher doses result in various degrees of hyperplasia (Chwalisz et al., 1998, 2000; Spitz and Chwalisz, 2000; Chabbert-Buffet et al., 2005). The exact mechanism of endometrial hyperplasia with SPRM use remains unclear. SPRMs bind minimally to the estrogen receptor (Chabbert-Buffet et al., 2005). Like mifepristone, the hyperplastic effect of SPRMs on the endometrium may be related to their antiglucocorticoid effects. The ensuing increase in adrenocorticotropic hormone (ACTH) may increase production of androstenedione and testosterone from the adrenal cortex, which are then aromatized to estrone and estradiol respectively, thus resulting in an overall increase in estrogen-drive in the endometrium (Lamberts et al., 1991; Heikinheimo 1997; Heikinheimo et al., 2000; Chabbert-Buffet et al., 2005). However, histological endometrial changes noted with SPRMs are distinct from estrogen-associated endometrial hyperplasia or disordered proliferative endometrium (Biglia et al., 2014). The term ‘progesterone receptor modulator-associated endometrial changes’ (PAECs) has been used to describe various histological patterns noted in the endometrium of women receiving SPRMs that do not include malignant or premalignant changes (Mutter et al., 2008; Biglia et al., 2014). Patients receiving ulipristal have demonstrated PAECs, with noted cystic changes in the endometrium and increased endometrial thickness. Despite the clinical finding of increased endometrial thickness in these patients, PAECs are distinct from endometrial hyperplasia as there is pronounced dyssynchrony of endometrial and stromal proliferation resulting in cystic dilation admixed with both estrogen-induced (mitotic) and progesterone-induced (secretory) epithelial changes (Mutter et al., 2008). Overall, PAECs differ from classic unopposed estrogen effects in demonstrating lower or absent mitotic activity, exhibiting varying levels of epithelial secretory change, and having occasional stromal pseudodecidualization. Additionally, these changes are reversible after cessation of ulipristal therapy, with restoration of endometrial thickness to baseline levels. It is important to note, however, that these endometrial changes are of unknown pathologic significance as biopsies from both control subjects and patients prior to treatment have demonstrated these endometrial changes at times (Ioffe et al., 2009). Thus, taken together, the current data regarding SPRM use for the treatment of leiomyoma are promising and further studies are needed to determine their exact mechanism of action and especially their effects on PR-A and PR-B activity in the leiomyoma cell context.
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, accounting for 6% of all cancers in women (Siegel et al., 2011; American Cancer Society 2013). The majority of endometrial cancers occur in postmenopausal women, and 80% of patients are diagnosed when the tumor is confined to the uterus (stage 1 disease). As described above, endometrial hyperplasia is a precursor to endometrial adenocarcinoma and is characterized by disordered proliferation of endometrial glands, resulting in a greater gland-to-stroma ratio than observed in normal endometrium (Kurman et al., 1994).
In normal endometrium, expression of PR is induced during the estrogen-dominated proliferative phase. During the secretory phase, when circulating concentrations of progesterone are maximal, activation of PR results in reduced proliferation of the endometrial epithelium, and its differentiation into a secretory phenotype. If progesterone effects are disrupted, as might occur during anovulatory cycles when its production by the ovary is reduced, the epithelium can become hyperplastic in response to unopposed estrogen. Indeed, many of the established risk factors for developing endometrial cancer (most notably type I) are associated with excess exposure to estrogen unopposed by progesterone. For instance, risk of developing endometrial cancer is increased by estrogen-only hormone replacement therapy (Beral et al., 2005) and a high body mass index (BMI), which is associated with higher estrogen levels and anovulation (Rieck and Fiander, 2006). Polycystic ovary syndrome (PCOS), a condition characterized by hyperandrogenism and chronic anovulation, is associated with approximately a 2.7 fold increase in endometrial cancer (Fauser et al., 2012; Barry et al., 2014). In addition, endometrial adenocarcinomas express enzymes involved in estrogen biosynthesis (Bulun et al., 1994; Utsunomiya et al., 2001), which may further increase the local estrogenic drive. Thus, progesterone plays a critical role in restricting the tropic actions of estrogen on the endometrium, and as such PR expression status in endometrial carcinoma is considered to be an independent prognostic factor (Ballester et al., 2013; Zhang et al., 2013). While positive immunohistochemical staining of ER in endometrial adenocarcinoma is one of the most important prognostic factors for survival, PR expression may also predict survival (Ballester et al., 2013; Zhang et al., 2013; Carlson et al., 2014). However, controversy around this issue exists. While some small studies have not shown specific advantages with respect to PR expression status and survival (Thigpen et al., 1999; Singh et al., 2007; Carlson et al., 2014; Gunderson et al., 2014), a recent prospective multicenter trial including specimens from 832 women with endometrial carcinoma found decreased survival in patients with ER and PR receptor loss (Trovik et al., 2013).
The understanding that progesterone exerts PR-mediated anti-mitotic effects on the endometrium has led to the use of various progestins as therapeutics for low-grade endometrial adenocarcinoma (Lentz et al., 1996; Thigpen et al., 1999; Fiorica et al., 2004; Whitney et al., 2004; Decruze and Green, 2007). Progestin therapy markedly affects the histopathologic characteristics of endometrial cancers (Wheeler et al., 2007). Gland-to-stroma ratio, glandular cellularity, mitotic activity and cytologic atypia are each decreased by progestin therapy, and these changes are associated with complete resolution of disease after 12 months of treatment in some patients. Additionally, the anti-gonadotropic activity of progesterone suppresses endogenous estrogen production by the ovaries (Banno et al., 2012). Progesterone also decreases ER expression in endometrial cells and activates enzymatic pathways that inactivate estradiol by its conversion to estrone by the 17-β hydroxysteroid dehydrogenase type 2 and sulfotransferase within endometrial cells (Banno et al., 2012).
Although increased PR-A expression has been found in ER positive endometrial cancer, the specific roles of PR-A and PR-B in the etiology of endometrial cancer are unclear (Singh et al., 2007). Endometrial cancer is associated with mutations in progesterone/PR responsive genes whose products are thought to mediate anti-proliferative and immunosuppressive actions of progesterone (Shiozawa et al., 1998; Di Nezza et al., 2003; Jaffe et al., 2007; Ward et al., 2008; Kyo et al., 2011). One such PR-responsive gene, forkhead box protein O1 (FOXO1), is emerging as a key mediator for cellular senescence in endometrial carcinomas (Kyo et al., 2011). The transcription factor FOXO1 is a downstream target of the phosphatidylinositol-3-kinase/Akt signaling pathway (Goto et al., 2008) and, as such, FOXO1 is a regulator of the cell-cycle and plays a role in cellular apoptosis. FOXO1 expression is decreased in the majority of endometrial cancers, and treatment with progesterone increases its expression, an effect which is mediated by PR-B (Ward et al., 2008). Progesterone, through PR-B but not PR-A, also induces expression of the anti-mitogen, insulin-like growth factor binding protein 1 (IGFBP-1), in endometrial cancer cells (Nakamura et al., 2013). IGFBP-1 requires upstream binding of FOXO1 to its promoter in order to exert progesterone-induced anti-proliferative effects in endometrial cells, thereby lending credence to the concept that progesterone-induced FOXO1 is key to tumor suppression in endometrial cells.
Progesterone and PRs also may mediate anti-proliferative effects through regulation of cell-cycle dependent kinases (CDKs) (Banno et al., 2012). CDKs advance the cell cycle through functional interactions with other transcription factors including PR (Hagan et al., 2011). This has been proposed as a key mechanism for progesterone/PR induced proliferation of breast epithelial cells and the etiology of breast cancer (Hagan et al., 2011). Progesterone induces expression of p27, a cyclin E/CDK2 inhibitor, thereby suppressing the cell cycle (Banno et al., 2012).
Some endometrial cancers exhibit a complete loss of PR expression. In a recent study, Yang et al. examined the possibility of restoring PR expression in endometrial cancer cells by epigenetic modulation treating cells with histone deacetylase inhibitors (Yang et al., 2014). Importantly, they found that treatment of endometrial cancer cells with a histone deacetylase inhibitor increased PR expression and conferred progesterone responsiveness at various target genes relevant to endometrial cancer biology, including FOXO1, p21 and p27. This innovative therapeutic approach may be used to sensitize endometrial tumors to progestin therapy. Further understanding of how PGR is controlled in endometrial cancer and how PR gene targets are affected by PR-A and PR-B in response to various progestins, and of their roles in disease etiology and progression, will increase the therapeutic arsenal to treat this disease specifically by exploiting the protective action of progesterone.
Cervical cancer
Cervical cancer is a consequence of infection with human papilloma virus (HPV) (Van Ranst et al., 1996). Specifically, viral gene E2, an important regulator of protooncogenes E6 and E7, is deleted in malignantly transformed cells. Oncogenes E6 and E7 interact with cellular proteins that regulate the cell cycle and apoptosis (zur Hausen, 2002). Notably, E6 promotes proteosome-mediated degradation of tumor suppressor protein p53 and E7 inactivates the tumor suppressor retinoblastoma protein.
The link between sex steroids and cervical cancer surfaced when a higher incidence of cervical carcinoma was noted among long-term oral contraceptive users (Franceschi, 2005). This relationship appears to have temporal effects as cervical cancer risk increases 4-fold in women who have used oral contraceptives for >10 years (Moreno et al., 2002). Estrogen and progesterone appear to facilitate HPV DNA integration into the host cell genome (Webster et al., 2001), and progesterone facilitates the oncogenic transformation of HPV DNA with the ras oncogene (Pater et al., 1990) and increases expression of the E6 and E7 oncogenes (Chen et al., 1996; Michelin et al., 1997; Yuan et al., 1999). These effects are likely mediated by PRs whose expression is increased in stromal cells of both squamous cell and adenocarcinoma cervical lesions compared with normal controls (Bodner et al., 2010; Kwasniewska et al., 2011). Intensity of PR staining also directly correlates with grade of precancerous cervical lesions, with high-grade precancerous lesions staining intensely for PR (Monsonego et al., 1991).
Despite much of this evidence, it is important to note that the exact role of progesterone and the PRs in cervical cancer still remains unclear. Although most studies have shown a positive correlation between progesterone and the development of cervical cancer, epidemiological data regarding the use of medroxyprogesterone acetate (MPA), a potent progestational agent, and cervical carcinoma have yielded conflicting results. While many studies have suggested that MPA use increases the risk of neoplastic disease in the cervix (Thomas et al., 1985, 1995; McFarlane-Anderson et al., 2008), several case–control studies show that MPA use is not a risk factor for the disease (Thomas and Ray 1995; Kaunitz 1996), and may, in fact, inversely correlate with disease (Harris et al., 2009). Furthermore, from studies in a transgenic mouse model expressing E6 and/or E7, high doses of MPA may actually cause regression of cervical dysplasia (Yoo et al., 2013). Interpretation of these effects is complicated by the fact the MPA also activates the glucocorticoid receptor. Prospective studies are required to provide clear insight into the clinical outcomes of cervical cancer with progesterone use and the possible utility of various SPRMs as therapeutic measures for cervical carcinoma.
Recurrent pregnancy loss
Recurrent pregnancy loss (RPL) is defined as two or more clinically recognized failed pregnancies and affects ∼0.5–1% of couples (Baek et al., 2007). Embryonic and parental cytogenetic abnormalities, anatomic malformations, thrombophilic disorders, and hormonal aberrations have been implicated in this condition. Progesterone deficiency and aberrant PR-mediated signaling may play a role in RPL. As discussed above, progesterone induces the endometrial phenotype that is conducive to embryo implantation and to the establishment and maintenance of pregnancy (Norwitz et al., 2001). Progesterone deficiency and a shortened luteal phase may result in retarded endometrial development, which has been associated with RPL. While progesterone acting through PRs undoubtedly plays a pivotal role in implantation and maintenance of pregnancy, progesterone supplementation for patients with sporadic miscarriage does not improve pregnancy outcomes (Haas and Ramsey, 2013). However, a small number of studies involving patients with RPL have shown that administration of progestin may be of some benefit in preventing miscarriage in this population (Oates-Whitehead et al., 2003). Larger prospective clinical trials are needed to definitively elucidate the benefits of progestin therapy in patients with RPL. Clearly, in most cases the problem lies with progesterone responsiveness rather than hormone availability. In these cases the abnormal expression and/or function of the PRs is implicated. RPL is associated with decreased PR expression by the embryo (Hickman et al., 2002) and in the endometrium (Carranza-Lira et al., 2000). Specific PGR polymorphisms have been reported in patients with idiopathic RPL (Su et al., 2011). Of particular interest is a 306 base pair insertion polymorphism in intron G of the PGR gene that correlates with RPL and is linked to implantation failure in in vitro fertilization cycles (Cramer et al., 2003). Interestingly, the polymorphism also segregates with progesterone-dependent neoplasms (Romano et al., 2006).
The establishment of pregnancy involves a complex hormonal dialogue between the mother and the fetus that modulates the maternal immune system such that it does not reject the fetal allograft. Progesterone is thought to play a key role in this process through multiple effects on the maternal immune system. Progesterone has been reported to reduce natural killer (NK)-cell activity (Hansen et al., 1992), increase HLA-G production in trophoblast cells (Yie et al., 2006b), increase suppressor-cell levels (Brierley and Clark, 1987), inhibit cytotoxic T-cell activity (Mannel et al., 1990), induce the production of lymphocyte-blocking proteins (Barakonyi et al., 1999), and modify the cytokine response from the Th-1 to the pro-pregnancy Th-2 pattern (Piccinni et al., 1995; Choi et al., 2000). The mechanism for this effect is unclear and the involvement of the nuclear PRs is controversial (Van Voorhis et al., 1989; Szekeres-Bartho et al., 1990, 2001; Mansour et al., 1994; Schust et al., 1996). Indeed, the effects may be indirect via increased trophoblast HLA-G production (Yie et al., 2006a) or via production of a factor in lymphocytes, referred to as progesterone-induced blocking factor (PIBF) (Szekeres-Bartho et al., 1985, 2001). Interestingly, progesterone-treated lymphocytes of pregnant women showing clinical symptoms of threatened preterm delivery fail to release PIBF (Szekeres-Bartho et al., 1990; Yie et al., 2006a). The uncertainty of the role of progesterone and PRs in RPL underscores the need for further research to delineate the exact role of endometrial PR modulation in RPL.
Conclusions
As its name implies, progesterone is a pro-gestation hormone and has long been considered the master hormone of pregnancy. Its effects on the uterus are essential for the establishment and maintenance of pregnancy, and as such it plays a central and critical role in the viviparous reproductive cycle. The discovery of PR antagonists such as mifepristone (RU486) and onapristone demonstrated that the nuclear PRs mediate most, if not all, actions of progesterone on the uterus. Understanding of PR molecular biology has advanced significantly in the last 20–30 years, and it is now clear that progesterone actions in the human uterus are mediated by the combined, and sometimes opposing, effects of the two PR isoforms, PR-A and PR-B. The dual PR isoform systems allow for qualitative and quantitative control of progesterone responsiveness through modulation of relative PR-A and PR-B levels and transcriptional activities and by the presence and activity of transcriptional co-regulators in a cell-specific manner. This paradigm together with cell- and context-specific PR post-translational modifications, explains the pleiotropic actions of progesterone in the various uterine cell types, and allows for control of progesterone action secondary to the presence of hormone. Given the essential role of progesterone in human reproduction and the fact that its levels can vary markedly between menstrual cycles, such a system may have evolved to favor the establishment of pregnancy despite a wide range of circulating progesterone levels. It is not surprising therefore that the etiology and clinical trajectory of multiple uterine pathophysiologies involves defects in PR-A/PR-B signaling and its relationship with the estrogen/ER system. Thus, PR expression and activity in conjunction with the associated signal transduction systems are a key element in progesterone actions in the human uterus. A clear understanding of the molecular biology of progesterone/PR signaling in each of the uterine target cells will be essential for the development of SPRM-based therapies to treat uterine pathophysiologies.
Authors' roles
B.P., S.E., S.T., S.M. contributed to the research, verification and writing of this review. W.D. and M.B. contributed to Fig. 5 in this review. All authors had full access to the manuscript.
Funding
No external funding was sought for this review.
Conflict of interest
None declared.
Acknowledgements
We thank the librarians at Case Western Reserve University for their assistance in our literature search and full access to all articles.
References
- Abdel-Hafiz HA, Horwitz KB. Control of progesterone receptor transcriptional synergy by SUMOylation and deSUMOylation. BMC Mol Biol. 2012;13:10. doi: 10.1186/1471-2199-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Hafiz HA, Horwitz KB. Post-translational modifications of the progesterone receptors. J Steroid Biochem Mol Biol. 2014;140:80–89. doi: 10.1016/j.jsbmb.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Velarde MC, Giudice LC. The progesterone receptor coactivator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology. 2009;150:3863–3870. doi: 10.1210/en.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amazit L, Roseau A, Khan JA, Chauchereau A, Tyagi RK, Loosfelt H, Leclerc P, Lombes M, Guiochon-Mantel A. Ligand-dependent degradation of SRC-1 is pivotal for progesterone receptor transcriptional activity. Mol Endocrinol. 2011;25:394–408. doi: 10.1210/me.2010-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- Arici A, Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73:1006–1011. doi: 10.1016/s0015-0282(00)00418-0. [DOI] [PubMed] [Google Scholar]
- Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85:2897–2902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- Avrech OM, Golan A, Weinraub Z, Bukovsky I, Caspi E. Mifepristone (RU486) alone or in combination with a prostaglandin analogue for termination of early pregnancy: a review. Fertil Steril. 1991;56:385–393. doi: 10.1016/s0015-0282(16)54527-0. [DOI] [PubMed] [Google Scholar]
- Baek KH, Lee EJ, Kim YS. Recurrent pregnancy loss: the key potential mechanisms. Trends Mol Med. 2007;13:310–317. doi: 10.1016/j.molmed.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Ballester M, Canlorbe G, Cortez A, Gonin J, Laas E, Bendifallah S, Graesslin O, Darai E. Histological and immunohistochemical profiles predict lymph node status in women with low-intermediate risk endometrial cancer. Gynecol Oncol. 2013;130:457–462. doi: 10.1016/j.ygyno.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Bamberger AM, Bamberger CM, Gellersen B, Schulte HM. Modulation of AP-1 activity by the human progesterone receptor in endometrial adenocarcinoma cells. Proc Natl Acad Sci USA. 1996;93:6169–6174. doi: 10.1073/pnas.93.12.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno K, Kisu I, Yanokura M, Tsuji K, Masuda K, Ueki A, Kobayashi Y, Yamagami W, Nomura H, Susumu N, et al. Progestin therapy for endometrial cancer: the potential of fourth-generation progestin (review) Int J Oncol. 2012;40:1755–1762. doi: 10.3892/ijo.2012.1384. [DOI] [PubMed] [Google Scholar]
- Barakonyi A, Polgar B, Szekeres-Bartho J. The role of gamma/delta T-cell receptor-positive cells in pregnancy: part II. Am J Reprod Immunol. 1999;42:83–87. [PubMed] [Google Scholar]
- Barbarisi A, Petillo O, Di Lieto A, Melone MA, Margarucci S, Cannas M, Peluso G. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. J Cell Physiol. 2001;186:414–424. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1040>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748–758. doi: 10.1093/humupd/dmu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathgate R, Hsueh A, Sherwood D. Knobil and Neill's Physiology of Reproduction. 3rd edn. Academic Press; 2006. St. Louis. [Google Scholar]
- Behera MA, Dai Q, Garde R, Saner C, Jungheim E, Price TM. Progesterone stimulates mitochondrial activity with subsequent inhibition of apoptosis in MCF-10A benign breast epithelial cells. Am J Physiol Endocrinol Metab. 2009;297:E1089–E1096. doi: 10.1152/ajpendo.00209.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beral V, Bull D, Reeves G Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365:1543–1551. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- Bergeron C, Ferenczy A, Toft DO, Schneider W, Shyamala G. Immunocytochemical study of progesterone receptors in the human endometrium during the menstrual cycle. Lab Invest. 1988;59:862–869. [PubMed] [Google Scholar]
- Bergqvist A, Ferno M. Estrogen and progesterone receptors in endometriotic tissue and endometrium: comparison according to localization and recurrence. Fertil Steril. 1993a;60:63–68. [PubMed] [Google Scholar]
- Bergqvist A, Ferno M. Oestrogen and progesterone receptors in endometriotic tissue and endometrium: comparison of different cycle phases and ages. Hum Reprod. 1993b;8:2211–2217. doi: 10.1093/oxfordjournals.humrep.a138005. [DOI] [PubMed] [Google Scholar]
- Bergqvist A, Ljungberg O, Skoog L. Immunohistochemical analysis of oestrogen and progesterone receptors in endometriotic tissue and endometrium. Hum Reprod. 1993;8:1915–1922. doi: 10.1093/oxfordjournals.humrep.a137960. [DOI] [PubMed] [Google Scholar]
- Biglia N, Carinelli S, Maiorana A, D'Alonzo M, Lo Monte G, Marci R. Ulipristal acetate: a novel pharmacological approach for the treatment of uterine fibroids. Drug Des Devel Ther. 2014;8:285–292. doi: 10.2147/DDDT.S54565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodner K, Laubichler P, Kimberger O, Czerwenka K, Zeillinger R, Bodner-Adler B. Oestrogen and progesterone receptor expression in patients with adenocarcinoma of the uterine cervix and correlation with various clinicopathological parameters. Anticancer Res. 2010;30:1341–1345. [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med. 2007;25:139–153. doi: 10.1055/s-2007-973427. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- Brandon DD, Bethea CL, Strawn EY, Novy MJ, Burry KA, Harrington MS, Erickson TE, Warner C, Keenan EJ, Clinton GM. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993;169:78–85. doi: 10.1016/0002-9378(93)90135-6. [DOI] [PubMed] [Google Scholar]
- Brierley J, Clark DA. Characterization of hormone-dependent suppressor cells in the uterus of mated and pseudopregnant mice. J Reprod Immunol. 1987;10:201–217. doi: 10.1016/0165-0378(87)90087-8. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Economos K, Miller D, Simpson ER. CYP19 (aromatase cytochrome P450) gene expression in human malignant endometrial tumors. J Clin Endocrinol Metab. 1994;79:1831–1834. doi: 10.1210/jcem.79.6.7989490. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- Bygdeman M, Swahn ML, Gemzell-Danielsson K, Svalander P. Mode of action of RU 486. Ann Med. 1993;25:61–64. doi: 10.3109/07853899309147859. [DOI] [PubMed] [Google Scholar]
- Carlson MJ, Thiel KW, Leslie KK. Past, present, and future of hormonal therapy in recurrent endometrial cancer. Int J Womens Health. 2014;6:429–435. doi: 10.2147/IJWH.S40942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney SA, Tahara H, Swartz CD, Risinger JI, He H, Moore AB, Haseman JK, Barrett JC, Dixon D. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest. 2002;82:719–728. doi: 10.1097/01.lab.0000017499.51216.3e. [DOI] [PubMed] [Google Scholar]
- Carranza-Lira S, Blanquet J, Tserotas K, Calzada L. Endometrial progesterone and estradiol receptors in patients with recurrent early pregnancy loss of unknown etiology—preliminary report. Med Sci Monit. 2000;6:759–762. [PubMed] [Google Scholar]
- Carrino DA, Mesiano S, Barker NM, Hurd WW, Caplan AI. Proteoglycans of uterine fibroids and keloid scars: similarity in their proteoglycan composition. Biochem J. 2012;443:361–368. doi: 10.1042/BJ20111996. [DOI] [PubMed] [Google Scholar]
- Cermik D, Arici A, Taylor HS. Coordinated regulation of HOX gene expression in myometrium and uterine leiomyoma. Fertil Steril. 2002;78:979–984. doi: 10.1016/s0015-0282(02)03366-6. [DOI] [PubMed] [Google Scholar]
- Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM. Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update. 2005;11:293–307. doi: 10.1093/humupd/dmi002. [DOI] [PubMed] [Google Scholar]
- Chai SY, Smith R, Fitter JT, Mitchell C, Pan X, Ilicic M, Maiti K, Zakar T, Madsen G. Increased progesterone receptor A expression in labouring human myometrium is associated with decreased promoter occupancy by the histone demethylase JARID1A. Mol Hum Reprod. 2014;20:442–453. doi: 10.1093/molehr/gau005. [DOI] [PubMed] [Google Scholar]
- Chalubinski K, Deutinger J, Bernaschek G. Vaginosonography for recording of cycle-related myometrial contractions. Fertil Steril. 1993;59:225–228. doi: 10.1016/s0015-0282(16)55644-1. [DOI] [PubMed] [Google Scholar]
- Chegini N, Ma C, Tang XM, Williams RS. Effects of GnRH analogues, ‘add-back’ steroid therapy, antiestrogen and antiprogestins on leiomyoma and myometrial smooth muscle cell growth and transforming growth factor-beta expression. Mol Hum Reprod. 2002;8:1071–1078. doi: 10.1093/molehr/8.12.1071. [DOI] [PubMed] [Google Scholar]
- Chen YH, Huang LH, Chen TM. Differential effects of progestins and estrogens on long control regions of human papillomavirus types 16 and 18. Biochem Biophys Res Commun. 1996;224:651–659. doi: 10.1006/bbrc.1996.1080. [DOI] [PubMed] [Google Scholar]
- Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- Chen W, Ohara N, Wang J, Xu Q, Liu J, Morikawa A, Sasaki H, Yoshida S, Demanno DA, Chwalisz K, et al. A novel selective progesterone receptor modulator asoprisnil (J867) inhibits proliferation and induces apoptosis in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab. 2006;91:1296–1304. doi: 10.1210/jc.2005-2379. [DOI] [PubMed] [Google Scholar]
- Choi BC, Polgar K, Xiao L, Hill JA. Progesterone inhibits in-vitro embryotoxic Th1 cytokine production to trophoblast in women with recurrent pregnancy loss. Hum Reprod. 2000;15(Suppl 1):46–59. doi: 10.1093/humrep/15.suppl_1.46. [DOI] [PubMed] [Google Scholar]
- Chwalisz K. The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery. Hum Reprod. 1994;9(Suppl 1):131–161. doi: 10.1093/humrep/9.suppl_1.131. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Stockemann K, Fritzemeier KH, Fuhrmann U. Modulation of oestrogenic effects by progesterone antagonists in the rat uterus. Hum Reprod Update. 1998;4:570–583. doi: 10.1093/humupd/4.5.570. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Brenner RM, Fuhrmann UU, Hess-Stumpp H, Elger W. Antiproliferative effects of progesterone antagonists and progesterone receptor modulators on the endometrium. Steroids. 2000;65:741–751. doi: 10.1016/s0039-128x(00)00190-2. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil Steril. 2007;87:1399–1412. doi: 10.1016/j.fertnstert.2006.11.094. [DOI] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA. 2003;100:9518–9523. doi: 10.1073/pnas.1633616100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- Cork DMW, Lennard TWJ, Tyson-Capper AL. Alternative splicing and the progesterone receptor in breast cancer. Breast Cancer Res. 2008;10:207–215. doi: 10.1186/bcr2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan S, Calder AA, Kelly RW. Decidualisation of cervical stromal cells. Eur J Obstet Gynecol Reprod Biol. 2004;114:189–196. doi: 10.1016/j.ejogrb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Hornstein MD, McShane P, Powers RD, Lescault PJ, Vitonis AF, De Vivo I. Human progesterone receptor polymorphisms and implantation failure during in vitro fertilization. Am J Obstet Gynecol. 2003;189:1085–1092. doi: 10.1067/s0002-9378(03)00517-9. [DOI] [PubMed] [Google Scholar]
- Dai D, Kumar NS, Wolf DM, Leslie KK. Molecular tools to reestablish progestin control of endometrial cancer cell proliferation. Am J Obstet Gynecol. 2001;184:790–797. doi: 10.1067/mob.2001.113844. [DOI] [PubMed] [Google Scholar]
- Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, Bagchi IC. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci USA. 2009;106:12542–12547. doi: 10.1073/pnas.0901647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decruze SB, Green JA. Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer. 2007;17:964–978. doi: 10.1111/j.1525-1438.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- DeManno D, Elger W, Garg R, Lee R, Schneider B, Hess-Stumpp H, Schubert G, Chwalisz K. Asoprisnil (J867): a selective progesterone receptor modulator for gynecological therapy. Steroids. 2003;68:1019–1032. doi: 10.1016/j.steroids.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Di Nezza LA, Jobling T, Salamonsen LA. Progestin suppresses matrix metalloproteinase production in endometrial cancer. Gynecol Oncol. 2003;89:325–333. doi: 10.1016/s0090-8258(03)00089-1. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, Mara M, Jilla MP, Bestel E, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012a;366:409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tomaszewski J, Vazquez F, Bouchard P, Lemieszczuk B, Baro F, Nouri K, Selvaggi L, Sodowski K, Bestel E, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012b;366:421–432. doi: 10.1056/NEJMoa1103180. [DOI] [PubMed] [Google Scholar]
- Donnez J, Vazquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BC, Barlow DH, Palacios S, Donnez O, Bestel E, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril. 2014;101:1565–1573. doi: 10.1016/j.fertnstert.2014.02.008. e1561–1518. [DOI] [PubMed] [Google Scholar]
- Eisinger SH, Meldrum S, Fiscella K, le Roux HD, Guzick DS. Low-dose mifepristone for uterine leiomyomata. Obstet Gynecol. 2003;101:243–250. doi: 10.1016/s0029-7844(02)02511-5. [DOI] [PubMed] [Google Scholar]
- Eisinger SH, Bonfiglio T, Fiscella K, Meldrum S, Guzick DS. Twelve-month safety and efficacy of low-dose mifepristone for uterine myomas. J Minim Invasive Gynecol. 2005;12:227–233. doi: 10.1016/j.jmig.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Escriva H, Bertrand S, Laudet V. The evolution of the nuclear receptor superfamily. Essays Biochem. 2004;40:11–26. doi: 10.1042/bse0400011. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol. 2008;22:823–837. doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. e25. [DOI] [PubMed] [Google Scholar]
- Ferenczy A, Bertrand G, Gelfand MM. Proliferation kinetics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol. 1979;133:859–867. doi: 10.1016/0002-9378(79)90302-8. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Jeong J, Mukherjee A, Soyal SM, Li J, Ying Y, Demayo FJ, Lydon JP. A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland. Genesis. 2010;48:106–113. doi: 10.1002/dvg.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorica JV, Brunetto VL, Hanjani P, Lentz SS, Mannel R, Andersen W Gynecologic Oncology Groups. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:10–14. doi: 10.1016/j.ygyno.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS. Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol. 2006;108:1381–1387. doi: 10.1097/01.AOG.0000243776.23391.7b. [DOI] [PubMed] [Google Scholar]
- Fleisch MC, Chou YC, Cardiff RD, Asaithambi A, Shyamala G. Overexpression of progesterone receptor A isoform in mice leads to endometrial hyperproliferation, hyperplasia and atypia. Mol Hum Reprod. 2009;15:241–249. doi: 10.1093/molehr/gap013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn M, Jamison M, Datta S, Myers E. Health care resource use for uterine fibroid tumors in the United States. Am J Obstet Gynecol. 2006;195:955–964. doi: 10.1016/j.ajog.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Franceschi S. The IARC commitment to cancer prevention: the example of papillomavirus and cervical cancer. Recent Results Cancer Res. 2005;166:277–297. doi: 10.1007/3-540-26980-0_18. [DOI] [PubMed] [Google Scholar]
- Friedman AJ, Daly M, Juneau-Norcross M, Rein MS, Fine C, Gleason R, Leboff M. A prospective, randomized trial of gonadotropin-releasing hormone agonist plus estrogen-progestin or progestin ‘add-back’ regimens for women with leiomyomata uteri. J Clin Endocrinol Metab. 1993;76:1439–1445. doi: 10.1210/jcem.76.6.8501148. [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Hirose R, Ichigo S, Sakaguchi H, Li Y, Tamaya T. Expression of progesterone receptor form A and B mRNAs in uterine leiomyoma. Tumour Biol. 1998;19:126–131. doi: 10.1159/000029983. [DOI] [PubMed] [Google Scholar]
- Fung HY, Wong YL, Wong FW, Rogers MS. Study of oestrogen and progesterone receptors in normal human endometrium during the menstrual cycle by immunocytochemical analysis. Gynecol Obstet Invest. 1994;38:186–190. doi: 10.1159/000292476. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178:357–372. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Briese J, Oberndörfer M, Redlin K, Samalecos A, Richter DU, Löning T, Schulte HM, Bamberger AM. Expression of the metastasis suppressor KAI1 in decidual cells at the human maternal-fetal interface: regulation and functional implications. Am J Pathol. 2007;170:126–139. doi: 10.2353/ajpath.2007.060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemzell-Danielsson K, Marions L. Mechanisms of action of mifepristone and levonorgestrel when used for emergency contraception. Hum Reprod Update. 2004;10:341–348. doi: 10.1093/humupd/dmh027. [DOI] [PubMed] [Google Scholar]
- Gemzell-Danielsson K, Swahn ML, Svalander P, Bygdeman M. Early luteal phase treatment with mifepristone (RU 486) for fertility regulation. Hum Reprod. 1993;8:870–873. doi: 10.1093/oxfordjournals.humrep.a138157. [DOI] [PubMed] [Google Scholar]
- Gerdes D, Wehling M, Leube B, Falkenstein E. Cloning and tissue expression of two putative steroid membrane receptors. Biol Chem. 1998;379:907–911. doi: 10.1515/bchm.1998.379.7.907. [DOI] [PubMed] [Google Scholar]
- Giangrande PH, Pollio G, McDonnell DP. Mapping and characterization of the functional domains responsible for the differential activity of the A and B isoforms of the human progesterone receptor. J Biol Chem. 1997;272:32889–32900. doi: 10.1074/jbc.272.52.32889. [DOI] [PubMed] [Google Scholar]
- Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20:3102–3115. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen SC, Hanekamp EE, Hanifi-Moghaddam P, Sijbers AM, van Gool AJ, Burger CW, Blok LJ, Huikeshoven FJ. Growth regulation and transcriptional activities of estrogen and progesterone in human endometrial cancer cells. Int J Gynecol Cancer. 2006;16:110–120. doi: 10.1111/j.1525-1438.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Goto T, Takano M, Albergaria A, Briese J, Pomeranz KM, Cloke B, Fusi L, Feroze-Zaidi F, Maywald N, Sajin M, et al. Mechanism and functional consequences of loss of FOXO1 expression in endometrioid endometrial cancer cells. Oncogene. 2008;27:9–19. doi: 10.1038/sj.onc.1210626. [DOI] [PubMed] [Google Scholar]
- Graham JD, Yager ML, Hill HD, Byth K, O'Neill GM, Clarke CL. Altered progesterone receptor isoform expression remodels progestin responsiveness of breast cancer cells. Mol Endocrinol. 2005;19:2713–2735. doi: 10.1210/me.2005-0126. [DOI] [PubMed] [Google Scholar]
- Gunderson CC, Dutta S, Fader AN, Maniar KP, Nasseri-Nik N, Bristow RE, Diaz-Montes TP, Palermo R, Kurman RJ. Pathologic features associated with resolution of complex atypical hyperplasia and grade 1 endometrial adenocarcinoma after progestin therapy. Gynecol Oncol. 2014;132:33–37. doi: 10.1016/j.ygyno.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Haas DM, Ramsey PS. Progestogen for preventing miscarriage. Cochrane Database Syst Rev. 2013;10:CD003511. doi: 10.1002/14651858.CD003511.pub3. [DOI] [PubMed] [Google Scholar]
- Hagan CR, Regan TM, Dressing GE, Lange CA. ck2-dependent phosphorylation of progesterone receptors (PR) on Ser81 regulates PR-B isoform-specific target gene expression in breast cancer cells. Mol Cell Biol. 2011;31:2439–2452. doi: 10.1128/MCB.01246-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme J, Toma SK, Talbert LM. A case of severe ovarian hyperstimulation in a healthy oocyte donor. Fertil Steril. 1995;64:857–859. doi: 10.1016/s0015-0282(16)57866-2. [DOI] [PubMed] [Google Scholar]
- Haluska GJ, West NB, Novy MJ, Brenner RM. Uterine estrogen receptors are increased by RU486 in late pregnant rhesus macaques but not after spontaneous labor. J Clin Endocrinol Metab. 1990;70:181–186. doi: 10.1210/jcem-70-1-181. [DOI] [PubMed] [Google Scholar]
- Hansen KA, Opsahl MS, Nieman LK, Baker JR, Jr, Klein TA. Natural killer cell activity from pregnant subjects is modulated by RU 486. Am J Obstet Gynecol. 1992;166:87–90. doi: 10.1016/0002-9378(92)91835-x. [DOI] [PubMed] [Google Scholar]
- Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20:2724–2733. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- Harper JR, Spiro RC, Gaarde WA, Tamura RN, Pierschbacher MD, Noble NA, Stecker KK, Border WA. Role of transforming growth factor beta and decorin in controlling fibrosis. Methods Enzymol. 1994;245:241–254. doi: 10.1016/0076-6879(94)45014-5. [DOI] [PubMed] [Google Scholar]
- Harris TG, Miller L, Kulasingam SL, Feng Q, Kiviat NB, Schwartz SM, Koutsky LA. Depot-medroxyprogesterone acetate and combined oral contraceptive use and cervical neoplasia among women with oncogenic human papillomavirus infection. Am J Obstet Gynecol. 2009;200:489. doi: 10.1016/j.ajog.2009.01.030. e481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison-Woolrych ML, Charnock-Jones DS, Smith SK. Quantification of messenger ribonucleic acid for epidermal growth factor in human myometrium and leiomyomata using reverse transcriptase polymerase chain reaction. J Clin Endocrinol Metab. 1994;78:1179–1184. doi: 10.1210/jcem.78.5.8175976. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O. Clinical pharmacokinetics of mifepristone. Clin Pharmacokinet. 1997;33:7–17. doi: 10.2165/00003088-199733010-00002. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O, Ranta S, Grunberg S, Spitz IM. Alterations in sex steroids and gonadotropins in post-menopausal women subsequent to long-term mifepristone administration. Steroids. 2000;65:831–836. doi: 10.1016/s0039-128x(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Hickman TN, Shih LM, Zacur HA, Kurman RJ, Diener-West M, Gearhart JD. Decreased progesterone receptor expression in the intermediate trophoblastic cells of spontaneous abortions. Fertil Steril. 2002;77:1001–1005. doi: 10.1016/s0015-0282(02)02953-9. [DOI] [PubMed] [Google Scholar]
- Hirata S, Shoda T, Kato J, Hoshi K. Isoform/variant mRNAs for sex steroid hormone receptors in humans. Trends Endocrinol Metab. 2003;14:124–129. doi: 10.1016/s1043-2760(03)00028-6. [DOI] [PubMed] [Google Scholar]
- Hoekstra AV, Sefton EC, Berry E, Lu Z, Hardt J, Marsh E, Yin P, Clardy J, Chakravarti D, Bulun S, et al. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J Clin Endocrinol Metab. 2009;94:1768–1774. doi: 10.1210/jc.2008-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, Osteen KG. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril. 2005;84:67–74. doi: 10.1016/j.fertnstert.2005.01.113. [DOI] [PubMed] [Google Scholar]
- Ingamells S, Campbell IG, Anthony FW, Thomas EJ. Endometrial progesterone receptor expression during the human menstrual cycle. J Reprod Fertil. 1996;106:33–38. doi: 10.1530/jrf.0.1060033. [DOI] [PubMed] [Google Scholar]
- Ioffe OB, Zaino RJ, Mutter GL. Endometrial changes from short-term therapy with CDB-4124, a selective progesterone receptor modulator. Mod Pathol. 2009;22:450–459. doi: 10.1038/modpathol.2008.204. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- Jacobsen BM, Schittone SA, Richer JK, Horwitz KB. Progesterone-independent effects of human progesterone receptors (PRs) in estrogen receptor-positive breast cancer: PR isoform-specific gene regulation and tumor biology. J Mol Endocrinol. 2005;19:574–587. doi: 10.1210/me.2004-0287. [DOI] [PubMed] [Google Scholar]
- Jaffe RC, Ferguson-Gottschall SD, Gao W, Beam C, Fazleabas AT. Histone deacetylase inhibition and progesterone act synergistically to stimulate baboon glycodelin gene expression. J Mol Endocrinol. 2007;38:401–407. doi: 10.1677/JME-06-0030. [DOI] [PubMed] [Google Scholar]
- Janne O, Kontula K, Luukkainen T, Vihko R. Oestrogen-induced progesterone receptor in human uterus. J Steroid Biochem. 1975;6:501–509. doi: 10.1016/0022-4731(75)90179-x. [DOI] [PubMed] [Google Scholar]
- Jiang J, Wu RF, Wang ZH, Sun HC, Xu Z, Xiu HM. Effect of mifepristone on estrogen and progesterone receptors in human endometrial and endometriotic cells in vitro. Fertil Steril. 2002;77:995–1000. doi: 10.1016/s0015-0282(02)03081-9. [DOI] [PubMed] [Google Scholar]
- Joseph DS, Malik M, Nurudeen S, Catherino WH. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril. 2010;93:1500–1508. doi: 10.1016/j.fertnstert.2009.01.081. [DOI] [PubMed] [Google Scholar]
- Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem. 1996;271:6217–6224. doi: 10.1074/jbc.271.11.6217. [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Lan NC, Rutledge SK. Estrogen receptors of human endometrium: characterization of nuclear and cytoplasmic forms and comparisons with rat uterine receptors. J Steroid Biochem. 1980;13:113–122. doi: 10.1016/0022-4731(80)90181-8. [DOI] [PubMed] [Google Scholar]
- Kaunitz AM. Depot medroxyprogesterone acetate contraception and the risk of breast and gynecologic cancer. J Reprod Med. 1996;41:419–427. [PubMed] [Google Scholar]
- Kaunitz AM. Injectable depot medroxyprogesterone acetate contraception: an update for U.S. clinicians. Int J Fertil Womens Med. 1998;43:73–83. [PubMed] [Google Scholar]
- Kawaguchi K, Fujii S, Konishi I, Nanbu Y, Nonogaki H, Mori T. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol. 1989;160:637–641. doi: 10.1016/s0002-9378(89)80046-8. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Fujii S, Konishi I, Iwai T, Nanbu Y, Nonogaki H, Ishikawa Y, Mori T. Immunohistochemical analysis of oestrogen receptors, progesterone receptors and Ki-67 in leiomyoma and myometrium during the menstrual cycle and pregnancy. Virchows Arch A Pathol Anat Histopathol. 1991;419:309–315. doi: 10.1007/BF01606522. [DOI] [PubMed] [Google Scholar]
- Khan JA, Bellance C, Guiochon-Mantel A, Lombès M, Loosfelt H. Differential regulation of breast cancer-associated genes by progesterone receptor isoforms PRA and PRB in a new bi-inducible breast cancer cell line. PLoS One. 2012;7:e45993. doi: 10.1371/journal.pone.0045993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson TP, Daniel AR, Fan D, Silverstein KA, Covington KR, Fuqua SA, Lange CA. Phosphorylated and sumoylation-deficient progesterone receptors drive proliferative gene signatures during breast cancer progression. Breast Cancer Res. 2012;14:R95. doi: 10.1186/bcr3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitmann B, Bugat R, Bayard F. Estrogen and progestin regulation of the progesterone receptor concentration in human endometrium. J Clin Endocrinol Metab. 1979;49:926–929. doi: 10.1210/jcem-49-6-926. [DOI] [PubMed] [Google Scholar]
- Krietsch T, Fernandes MS, Kero J, Losel R, Heyens M, Huhtaniemi I, Brosens JJ, Gellersen B. Human homologs of the putative G protein-coupled membrane progestin receptors (mPRα, ß, and γ) localize to the endoplasmic reticulum and are not activated by progesterone. Mol Endocrinol. 2006;20:3146–3164. doi: 10.1210/me.2006-0129. [DOI] [PubMed] [Google Scholar]
- Kunz G, Beil D, Deininger H, Wildt L, Leyendecker G. The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy. Hum Reprod. 1996;11:627–632. doi: 10.1093/humrep/11.3.627. [DOI] [PubMed] [Google Scholar]
- Kurman RJ, Henson DE, Herbst AL, Noller KL, Schiffman MH. Interim guidelines for management of abnormal cervical cytology. The 1992 National Cancer Institute Workshop. JAMA. 1994;271:1866–1869. [PubMed] [Google Scholar]
- Kwasniewska A, Postawski K, Gozdzicka-Jozefiak A, Kwasniewski W, Grywalska E, Zdunek M, Korobowicz E. Estrogen and progesterone receptor expression in HPV-positive and HPV-negative cervical carcinomas. Oncol Rep. 2011;26:153–160. doi: 10.3892/or.2011.1256. [DOI] [PubMed] [Google Scholar]
- Kyo S, Sakaguchi J, Kiyono T, Shimizu Y, Maida Y, Mizumoto Y, Mori N, Nakamura M, Takakura M, Miyake K, et al. Forkhead transcription factor FOXO1 is a direct target of progestin to inhibit endometrial epithelial cell growth. Clin Cancer Res. 2011;17:525–537. doi: 10.1158/1078-0432.CCR-10-1287. [DOI] [PubMed] [Google Scholar]
- Lakha F, Ho PC, Van der Spuy ZM, Dada K, Elton R, Glasier AF, Critchley HO, Williams AR, Baird DT. A novel estrogen-free oral contraceptive pill for women: multicentre, double-blind, randomized controlled trial of mifepristone and progestogen-only pill (levonorgestrel) Hum Reprod. 2007;22:2428–2436. doi: 10.1093/humrep/dem177. [DOI] [PubMed] [Google Scholar]
- Lamberts SW, Koper JW, de Jong FH. The endocrine effects of long-term treatment with mifepristone (RU 486) J Clin Endocrinol Metab. 1991;73:187–191. doi: 10.1210/jcem-73-1-187. [DOI] [PubMed] [Google Scholar]
- Lamminen S, Rantala I, Helin H, Rorarius M, Tuimala R. Proliferative activity of human uterine leiomyoma cells as measured by automatic image analysis. Gynecol Obstet Invest. 1992;34:111–114. doi: 10.1159/000292738. [DOI] [PubMed] [Google Scholar]
- Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA. 2000;97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Dai Q, Hansen EL, Saner CN, Price TM. Modulation of ATP-induced calcium signaling by progesterone in T47D-Y breast cancer cells. Mol Cell Endocrinol. 2010;319:109–115. doi: 10.1016/j.mce.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz SS, Brady MF, Major FJ, Reid GC, Soper JT. High-dose megestrol acetate in advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 1996;14:357–361. doi: 10.1200/JCO.1996.14.2.357. [DOI] [PubMed] [Google Scholar]
- Leo JC, Wang SM, Guo CH, Aw SE, Zhao Y, Li JM, Hui KM, Lin VC. Gene regulation profile reveals consistent anticancer properties of progesterone in hormone-independent breast cancer cells transfected with progesterone receptor. Int J Cancer. 2005;117:561–568. doi: 10.1002/ijc.21186. [DOI] [PubMed] [Google Scholar]
- Leonhardt SA, Boonyaratanakornkit V, Edwards DP. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids. 2003;68:761–770. doi: 10.1016/s0039-128x(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67:334–340. doi: 10.1210/jcem-67-2-334. [DOI] [PubMed] [Google Scholar]
- Levens ED, Potlog-Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL, Blocker W, Nieman LK. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol. 2008;111:1129–1136. doi: 10.1097/AOG.0b013e3181705d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Stocco C, Murk W, Kayisli UA, Funai EF, Schatz F. Human labor is associated with reduced decidual cell expression of progesterone, but not glucocorticoid, receptors. J Clin Endocrinol Metab. 2010;95:2271–2275. doi: 10.1210/jc.2009-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Lyndrup J, Thorpe S, Glenthoj A, Obel E, Sele V. Altered progesterone/estrogen receptor ratios in endometriosis. A comparative study of steroid receptors and morphology in endometriosis and endometrium. Acta Obstet Gynecol Scand. 1987;66:625–629. doi: 10.3109/00016348709022068. [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13:981–992. doi: 10.1210/mend.13.6.0307. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannel DN, Falk W, Yron I. Inhibition of murine cytotoxic T cell responses by progesterone. Immunol Lett. 1990;26:89–94. doi: 10.1016/0165-2478(90)90181-o. [DOI] [PubMed] [Google Scholar]
- Mansour I, Reznikoff-Etievant MF, Netter A. No evidence for the expression of the progesterone receptor on peripheral blood lymphocytes during pregnancy. Hum Reprod. 1994;9:1546–1549. doi: 10.1093/oxfordjournals.humrep.a138746. [DOI] [PubMed] [Google Scholar]
- Maruo T. Progesterone and progesterone receptor modulator in uterine leiomyoma growth. Gynecol Endocrinol. 2007;23:186–187. doi: 10.1080/09513590701350416. [DOI] [PubMed] [Google Scholar]
- Maruo T, Matsuo H, Samoto T, Shimomura Y, Kurachi O, Gao Z, Wang Y, Spitz IM, Johansson E. Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids. 2000;65:585–592. doi: 10.1016/s0039-128x(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Kurachi O, Shimomura Y, Samoto T, Maruo T. Molecular bases for the actions of ovarian sex steroids in the regulation of proliferation and apoptosis of human uterine leiomyoma. Oncology (Williston Park) 1999;57(Suppl 2):49–58. doi: 10.1159/000055275. [DOI] [PubMed] [Google Scholar]
- McEwan IJ. Nuclear receptors: one big family. Methods Mol Biol. 2009;505:3–18. doi: 10.1007/978-1-60327-575-0_1. [DOI] [PubMed] [Google Scholar]
- McFarlane-Anderson N, Bazuaye PE, Jackson MD, Smikle M, Fletcher HM. Cervical dysplasia and cancer and the use of hormonal contraceptives in Jamaican women. BMC Womens Health. 2008;8:9. doi: 10.1186/1472-6874-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- Mellon S. Neurosteroid regulation of CNS development. Pharmacol Ther. 2007;116:107–124. doi: 10.1016/j.pharmthera.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92:1927–1933. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- Mesiano S. Myometrial progesterone responsiveness. Semin Reprod Med. 2007;25:5–13. doi: 10.1055/s-2006-956771. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87:2924–2930. doi: 10.1210/jcem.87.6.8609. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci. 2011;18:6–19. doi: 10.1177/1933719110382922. [DOI] [PubMed] [Google Scholar]
- Michelin D, Gissmann L, Street D, Potkul RK, Fisher S, Kaufmann AM, Qiao L, Schreckenberger C. Regulation of human papillomavirus type 18 in vivo: effects of estrogen and progesterone in transgenic mice. Gynecol Oncol. 1997;66:202–208. doi: 10.1006/gyno.1997.4745. [DOI] [PubMed] [Google Scholar]
- Misao R, Iwagaki S, Fujimoto J, Sun W, Tamaya T. Dominant expression of progesterone receptor form B mRNA in ovarian endometriosis. Horm Res. 1999;52:30–34. doi: 10.1159/000023429. [DOI] [PubMed] [Google Scholar]
- Moe BG, Vereide AB, Orbo A, Sager G. High concentrations of progesterone and mifepristone mutually reinforce cell cycle retardation and induction of apoptosis. Anticancer Res. 2009;29:1053–1058. [PubMed] [Google Scholar]
- Monsonego J, Magdelenat H, Catalan F, Coscas Y, Zerat L, Sastre X. Estrogen and progesterone receptors in cervical human papillomavirus related lesions. Int J Cancer. 1991;48:533–539. doi: 10.1002/ijc.2910480410. [DOI] [PubMed] [Google Scholar]
- Moreno V, Bosch FX, Munoz N, Meijer CJ, Shah KV, Walboomers JM, Herrero R, Franceschi S International Agency for Research on Cancer. Multicentric Cervical Cancer Study Group. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085–1092. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- Morikawa A, Ohara N, Xu Q, Nakabayashi K, DeManno DA, Chwalisz K, Yoshida S, Maruo T. Selective progesterone receptor modulator asoprisnil down-regulates collagen synthesis in cultured human uterine leiomyoma cells through up-regulating extracellular matrix metalloproteinase inducer. Hum Reprod. 2008;23:944–951. doi: 10.1093/humrep/den025. [DOI] [PubMed] [Google Scholar]
- Mote PA, Balleine RL, McGowan EM, Clarke CL. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1999;84:2963–2971. doi: 10.1210/jcem.84.8.5928. [DOI] [PubMed] [Google Scholar]
- Mote PA, Balleine RL, McGowan EM, Clarke CL. Heterogeneity of progesterone receptors A and B expression in human endometrial glands and stroma. Hum Reprod. 2000;15(Suppl 3):48–56. doi: 10.1093/humrep/15.suppl_3.48. [DOI] [PubMed] [Google Scholar]
- Moutsatsou P, Sekeris CE. Estrogen and progesterone receptors in the endometrium. Ann N Y Acad Sci. 1997;816:99–115. doi: 10.1111/j.1749-6632.1997.tb52134.x. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LL, Mahesh VB. Selective release of follicle-stimulating hormone and luteinizing hormone by 5 alpha-dihydroprogesterone and 3 alpha, 5 alpha-tetrahydroprogesterone in pregnant mare's serum gonadotropin-primed immature rats exposed to constant light. Biol Reprod. 1985;32:795–803. doi: 10.1095/biolreprod32.4.795. [DOI] [PubMed] [Google Scholar]
- Murphy AA, Kettel LM, Morales AJ, Roberts VJ, Yen SS. Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J Clin Endocrinol Metab. 1993;76:513–517. doi: 10.1210/jcem.76.2.8432797. [DOI] [PubMed] [Google Scholar]
- Murphy AA, Morales AJ, Kettel LM, Yen SS. Regression of uterine leiomyomata to the antiprogesterone RU486: dose-response effect. Fertil Steril. 1995;64:187–190. [PubMed] [Google Scholar]
- Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merino M, Williams AR, Blithe DL. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol. 2008;21:591–598. doi: 10.1038/modpathol.2008.19. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Takakura M, Fujii R, Maida Y, Bono Y, Mizumoto Y, Zhang X, Kiyono T, Kyo S. The PRB-dependent FOXO1/IGFBP-1 axis is essential for progestin to inhibit endometrial epithelial growth. Cancer Lett. 2013;336:68–75. doi: 10.1016/j.canlet.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Neubauer NL, Ward EC, Patel P, Lu Z, Lee I, Blok LJ, Hanifi-Moghaddam P, Schink J, Kim JJ. Progesterone receptor-B induction of BIRC3 protects endometrial cancer cells from AP1-59-mediated apoptosis. Horm Cancer. 2011;2:170–181. doi: 10.1007/s12672-011-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman LK, Blocker W, Nansel T, Mahoney S, Reynolds J, Blithe D, Wesley R, Armstrong A. Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study. Fertil Steril. 2011;95:767–772. doi: 10.1016/j.fertnstert.2010.09.059. e761–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisolle M, Casanas-Roux F, Wyns C, de Menten Y, Mathieu PE, Donnez J. Immunohistochemical analysis of estrogen and progesterone receptors in endometrium and peritoneal endometriosis: a new quantitative method. Fertil Steril. 1994;62:751–759. doi: 10.1016/s0015-0282(16)57000-9. [DOI] [PubMed] [Google Scholar]
- Nisolle M, Gillerot S, Casanas-Roux F, Squifflet J, Berliere M, Donnez J. Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Hum Reprod. 1999;14:2844–2850. doi: 10.1093/humrep/14.11.2844. [DOI] [PubMed] [Google Scholar]
- Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- Oates-Whitehead RM, Haas DM, Carrier JA. Progestogen for preventing miscarriage. Cochrane Database Syst Rev. 2003:CD003511. doi: 10.1002/14651858.CD003511. [DOI] [PubMed] [Google Scholar]
- O'Brien SN, Welter BH, Mantzke KA, Price TM. Identification of progesterone receptor in human subcutaneous adipose tissue. J Clin Endocrinol Metab. 1998;83:509–513. doi: 10.1210/jcem.83.2.4561. [DOI] [PubMed] [Google Scholar]
- Okulicz WC, Savasta AM, Hoberg LM, Longcope C. Immunofluorescent analysis of estrogen induction of progesterone receptor in the rhesus uterus. Endocrinology. 1989;125:930–934. doi: 10.1210/endo-125-2-930. [DOI] [PubMed] [Google Scholar]
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18:319–328. doi: 10.1016/j.bpobgyn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Orbo A, Moe BT, Gronaas H, Paulssen RH. Early effects of high concentrations of progesterone and mifepristone A gene expression study of endometrial cancer cells (Ishikawa) J Steroid Biochem Mol Biol. 2009;113:139–149. doi: 10.1016/j.jsbmb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Osteen KG, Bruner-Tran KL, Eisenberg E. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril. 2005;83:529–537. doi: 10.1016/j.fertnstert.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Owings RA, Quick CM. Endometrial intraepithelial neoplasia. Arch Pathol Lab Med. 2014;138:484–491. doi: 10.5858/arpa.2012-0709-RA. [DOI] [PubMed] [Google Scholar]
- Palomba S, Sena T, Noia R, Di Carlo C, Zullo F, Mastrantonio P. Transdermal hormone replacement therapy in post-menopausal women with uterine leiomyomas. Obstet Gynecol. 2001;98:1053–1058. doi: 10.1016/s0029-7844(01)01598-8. [DOI] [PubMed] [Google Scholar]
- Palomba S, Sena T, Morelli M, Noia R, Zullo F, Mastrantonio P. Effect of different doses of progestin on uterine leiomyomas in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2002;102:199–201. doi: 10.1016/s0301-2115(01)00588-7. [DOI] [PubMed] [Google Scholar]
- Park SH, Ramachandran S, Kwon SH, Cha SD, Seo EW, Bae I, Cho C, Song DK. Upregulation of ATP-sensitive potassium channels for estrogen-mediated cell proliferation in human uterine leiomyoma cells. Gynecol Endocrinol. 2008;24:250–256. doi: 10.1080/09513590801893315. [DOI] [PubMed] [Google Scholar]
- Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725–736. doi: 10.1016/j.fertnstert.2007.01.093. [DOI] [PubMed] [Google Scholar]
- Pater A, Bayatpour M, Pater MM. Oncogenic transformation by human papillomavirus type 16 deoxyribonucleic acid in the presence of progesterone or progestins from oral contraceptives. Am J Obstet Gynecol. 1990;162:1099–1103. doi: 10.1016/0002-9378(90)91323-5. [DOI] [PubMed] [Google Scholar]
- Paulssen RH, Moe B, Gronaas H, Orbo A. Gene expression in endometrial cancer cells (Ishikawa) after short time high dose exposure to progesterone. Steroids. 2008;73:116–128. doi: 10.1016/j.steroids.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, Parronchi P, Manetti R, Annunziato F, Livi C, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–133. [PubMed] [Google Scholar]
- Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod. 2001;7:875–879. doi: 10.1093/molehr/7.9.875. [DOI] [PubMed] [Google Scholar]
- Prentice A, Randall BJ, Weddell A, McGill A, Henry L, Horne CH, Thomas EJ. Ovarian steroid receptor expression in endometriosis and in two potential parent epithelia: endometrium and peritoneal mesothelium. Hum Reprod. 1992;7:1318–1325. doi: 10.1093/oxfordjournals.humrep.a137848. [DOI] [PubMed] [Google Scholar]
- Press MF, Udove JA, Greene GL. Progesterone receptor distribution in the human endometrium. Analysis using monoclonal antibodies to the human progesterone receptor. Am J Pathol. 1988;131:112–124. [PMC free article] [PubMed] [Google Scholar]
- Price TM, Hansen EL, Oliver TN. Immunofluorescent localization of a novel progesterone receptor(s) in a T47D-Y breast cancer cell line lacking genomic progesterone receptor expression. J Soc Gynecol Investig. 2005;12:610–616. doi: 10.1016/j.jsgi.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- Rieck G, Fiander A. The effect of lifestyle factors on gynaecological cancer. Best Pract Res Clin Obstet Gynaecol. 2006;20:227–251. doi: 10.1016/j.bpobgyn.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Romano A, Lindsey PJ, Fischer DC, Delvoux B, Paulussen AD, Janssen RG, Kieback DG. Two functionally relevant polymorphisms in the human progesterone receptor gene (+331 G/A and progins) and the predisposition for breast and/or ovarian cancer. Gynecol Oncol. 2006;101:287–295. doi: 10.1016/j.ygyno.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Samalecos A, Gellersen B. Systematic expression analysis and antibody screening do not support the existence of naturally occurring PR-C, PR-M or other truncated progesterone receptor isoforms. Endocrinology. 2008;149:5872–5887. doi: 10.1210/en.2008-0602. [DOI] [PubMed] [Google Scholar]
- Sampson JA. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am J Pathol. 1927;3:93–110. 143. [PMC free article] [PubMed] [Google Scholar]
- Saner KJ, Welter BH, Zhang F, Hansen E, Dupont B, Wei Y, Price TM. Cloning and expression of a novel, truncated, progesterone receptor. Mol Cell Endocrinol. 2003;200:155–163. doi: 10.1016/s0303-7207(02)00380-5. [DOI] [PubMed] [Google Scholar]
- Sartorius CA, Melville MY, Hovland AR, Tung L, Takimoto GS, Horwitz KB. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol Endocrinol. 1994;8:1347–1360. doi: 10.1210/mend.8.10.7854352. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Ohara N, Xu Q, Wang JY, DeManno DA, Chwalisz K, Yoshida S, Maruo T. A novel selective progesterone receptor modulator asoprisnil activates tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated signaling pathway in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab. 2007;92:616–623. doi: 10.1210/jc.2006-0898. [DOI] [PubMed] [Google Scholar]
- Savouret JF, Misrahi M, Milgrom E. Molecular action of progesterone. Int J Biochem. 1990;22:579–594. doi: 10.1016/0020-711x(90)90033-y. [DOI] [PubMed] [Google Scholar]
- Schust DJ, Anderson DJ, Hill JA. Progesterone-induced immunosuppression is not mediated through the progesterone receptor. Hum Reprod. 1996;11:980–985. doi: 10.1093/oxfordjournals.humrep.a019335. [DOI] [PubMed] [Google Scholar]
- Sener AB, Seckin NC, Ozmen S, Gokmen O, Dogu N, Ekici E. The effects of hormone replacement therapy on uterine fibroids in postmenopausal women. Fertil Steril. 1996;65:354–357. doi: 10.1016/s0015-0282(16)58098-4. [DOI] [PubMed] [Google Scholar]
- Shaw KA, Topp NJ, Shaw JG, Blumenthal PD. Mifepristone-misoprostol dosing interval and effect on induction abortion times: a systematic review. Obstet Gynecol. 2013;121:1335–1347. doi: 10.1097/AOG.0b013e3182932f37. [DOI] [PubMed] [Google Scholar]
- Shen T, Horwitz KB, Lange CA. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol Cell Biol. 2001;21:6122–6131. doi: 10.1128/MCB.21.18.6122-6131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Matsuo H, Samoto T, Maruo T. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab. 1998;83:2192–2198. doi: 10.1210/jcem.83.6.4879. [DOI] [PubMed] [Google Scholar]
- Shiozawa T, Nikaido T, Nakayama K, Lu X, Fujii S. Involvement of cyclin-dependent kinase inhibitor p27Kip1 in growth inhibition of endometrium in the secretory phase and of hyperplastic endometrium treated with progesterone. Mol Hum Reprod. 1998;4:899–905. doi: 10.1093/molehr/4.9.899. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Singh M, Zaino RJ, Filiaci VJ, Leslie KK. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2007;106:325–333. doi: 10.1016/j.ygyno.2007.03.042. [DOI] [PubMed] [Google Scholar]
- Smid-Koopman E, Blok LJ, Kuhne LC, Burger CW, Helmerhorst TJ, Brinkmann AO, Huikeshoven FJ. Distinct functional differences of human progesterone receptors A and B on gene expression and growth regulation in two endometrial carcinoma cell lines. J Soc Gynecol Investig. 2003;10:49–57. [PubMed] [Google Scholar]
- Spencer TE, Johnson GA, Burghardt RC, Bazer FW. Progesterone and placental hormone actions on the uterus: insights from domestic animals. Biol Reprod. 2004;71:2–10. doi: 10.1095/biolreprod.103.024133. [DOI] [PubMed] [Google Scholar]
- Spitz IM. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol. 2009;21:318–324. doi: 10.1097/GCO.0b013e32832e07e8. [DOI] [PubMed] [Google Scholar]
- Spitz IM, Chwalisz K. Progesterone receptor modulators and progesterone antagonists in women's health. Steroids. 2000;65:807–815. doi: 10.1016/s0039-128x(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Spitz IM, Croxatto HB, Lahteenmaki P, Heikinheimo O, Bardin CW. Effect of mifepristone on inhibition of ovulation and induction of luteolysis. Hum Reprod. 1994;9(Suppl 1):69–76. doi: 10.1093/humrep/9.suppl_1.69. [DOI] [PubMed] [Google Scholar]
- Steinauer J, Pritts EA, Jackson R, Jacoby AF. Systematic review of mifepristone for the treatment of uterine leiomyomata. Obstet Gynecol. 2004;103:1331–1336. doi: 10.1097/01.AOG.0000127622.63269.8b. [DOI] [PubMed] [Google Scholar]
- Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900–906. doi: 10.1210/jcem.79.3.8077380. [DOI] [PubMed] [Google Scholar]
- Su MT, Lee IW, Chen YC, Kuo PL. Association of progesterone receptor polymorphism with idiopathic recurrent pregnancy loss in Taiwanese Han population. J Assist Reprod Genet. 2011;28:239–243. doi: 10.1007/s10815-010-9510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitani H, Shozu M, Segawa T, Murakami K, Yang HJ, Shimada K, Inoue M. In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology. 2000;141:3852–3861. doi: 10.1210/endo.141.10.7719. [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Hadnagy J, Pacsa AS. The suppressive effect of progesterone on lymphocyte cytotoxicity: unique progesterone sensitivity of pregnancy lymphocytes. J Reprod Immunol. 1985;7:121–128. doi: 10.1016/0165-0378(85)90066-x. [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Szekeres G, Debre P, Autran B, Chaouat G. Reactivity of lymphocytes to a progesterone receptor-specific monoclonal antibody. Cell Immunol. 1990;125:273–283. doi: 10.1016/0008-8749(90)90083-4. [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Barakonyi A, Par G, Polgar B, Palkovics T, Szereday L. Progesterone as an immunomodulatory molecule. Int Immunopharmacol. 2001;1:1037–1048. doi: 10.1016/s1567-5769(01)00035-2. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Kobori H, Kikuchi I, Sato Y, Mitsuhashi N. A prospective randomized study comparing endocrinological and clinical effects of two types of GnRH agonists in cases of uterine leiomyomas or endometriosis. J Obstet Gynaecol Res. 2000;26:325–331. doi: 10.1111/j.1447-0756.2000.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Talaulikar VS, Manyonda IT. Ulipristal acetate: a novel option for the medical management of symptomatic uterine fibroids. Adv Ther. 2012;29:655–663. doi: 10.1007/s12325-012-0042-8. [DOI] [PubMed] [Google Scholar]
- Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97:E719–E730. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JT, Brady MF, Alvarez RD, Adelson MD, Homesley HD, Manetta A, Soper JT, Given FT. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736–1744. doi: 10.1200/JCO.1999.17.6.1736. [DOI] [PubMed] [Google Scholar]
- Thomas EJ. Add-back therapy for long-term use in dysfunctional uterine bleeding and uterine fibroids. Br J Obstet Gynaecol. 1996;103(Suppl 14):18–21. [PubMed] [Google Scholar]
- Thomas DB, Ray RM. Depot-medroxyprogesterone acetate (DMPA) and risk of invasive adenocarcinomas and adenosquamous carcinomas of the uterine cervix. WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Contraception. 1995;52:307–312. doi: 10.1016/0010-7824(95)00215-v. [DOI] [PubMed] [Google Scholar]
- Thomas DB, Noonan L, Whitehead A, Roseman D. Invasive cervical cancer and depot-medroxyprogesterone acetate. WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Bull World Health Organ. 1985;63:505–511. [PMC free article] [PubMed] [Google Scholar]
- Thomas DB, Ye Z, Ray RM. Cervical carcinoma in situ and use of depot-medroxyprogesterone acetate (DMPA). WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Contraception. 1995;51:25–31. doi: 10.1016/0010-7824(94)00007-j. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- Tiltman AJ. The effect of progestins on the mitotic activity of uterine fibromyomas. Int J Gynecol Pathol. 1985;4:89–96. doi: 10.1097/00004347-198506000-00001. [DOI] [PubMed] [Google Scholar]
- Trovik J, Wik E, Werner HM, Krakstad C, Helland H, Vandenput I, Njolstad TS, Stefansson IM, Marcickiewicz J, Tingulstad S, et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur J Cancer. 2013;49:3431–3441. doi: 10.1016/j.ejca.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Tsai M-J, Clark JH, Schrader WT, O'Malley BW. Mechanisms of action of hormones that act as transcription-regulatory factors. In: Wilson J, Foster D, Kronenberg H, Larsen P, editors. Williams Textbook of Endocrinology. Philadelphia: W.B. Saunders Company; 1998. pp. 55–94. [Google Scholar]
- Tung L, Mohamed MK, Hoeffler JP, Takimoto GS, Horwitz KB. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol. 1993;7:1256–1265. doi: 10.1210/mend.7.10.8123133. [DOI] [PubMed] [Google Scholar]
- Uchida H, Maruyama T, Nagashima T, Asada H, Yoshimura Y. Histone deacetylase inhibitors induce differentiation of human endometrial adenocarcinoma cells through up-regulation of glycodelin. Endocrinology. 2005;146:5365–5373. doi: 10.1210/en.2005-0359. [DOI] [PubMed] [Google Scholar]
- Uchida H, Maruyama T, Ono M, Ohta K, Kajitani T, Masuda H, Nagashima T, Arase T, Asada H, Yoshimura Y. Histone deacetylase inhibitors stimulate cell migration in human endometrial adenocarcinoma cells through up-regulation of glycodelin. Endocrinology. 2007;148:896–902. doi: 10.1210/en.2006-0896. [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Suzuki T, Kaneko C, Takeyama J, Nakamura J, Kimura K, Yoshihama M, Harada N, Ito K, Konno R, et al. The analyses of 17beta-hydroxysteroid dehydrogenase isozymes in human endometrial hyperplasia and carcinoma. J Clin Endocrinol Metab. 2001;86:3436–3443. doi: 10.1210/jcem.86.7.7661. [DOI] [PubMed] [Google Scholar]
- Vallejo G, La Greca AD, Tarifa-Reischle IC, Mestre-Citrinovitz AC, Ballare C, Beato M, Saragueta P. CDC2 mediates progestin initiated endometrial stromal cell proliferation: a PR signaling to gene expression independently of its binding to chromatin. PLoS One. 2014;9:e97311. doi: 10.1371/journal.pone.0097311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ranst M, Tachezy R, Burk RD. Human papillomaviruses: a neverending story? In: Lacey C, editor. Papillomavirus Reviews: Current Research on Papillomaviruses. Leeds: Leeds University Press; 1996. pp. 1–19. [Google Scholar]
- Van Voorhis BJ, Anderson DJ, Hill JA. The effects of RU 486 on immune function and steroid-induced immunosuppression in vitro. J Clin Endocrinol Metab. 1989;69:1195–1199. doi: 10.1210/jcem-69-6-1195. [DOI] [PubMed] [Google Scholar]
- Viville B, Charnock-Jones DS, Sharkey AM, Wetzka B, Smith SK. Distribution of the A and B forms of the progesterone receptor messenger ribonucleic acid and protein in uterine leiomyomata and adjacent myometrium. Hum Reprod. 1997;12:815–822. doi: 10.1093/humrep/12.4.815. [DOI] [PubMed] [Google Scholar]
- Wang J, Ohara N, Wang Z, Chen W, Morikawa A, Sasaki H, DeManno DA, Chwalisz K, Maruo T. A novel selective progesterone receptor modulator asoprisnil (J867) down-regulates the expression of EGF, IGF-I, TGFbeta3 and their receptors in cultured uterine leiomyoma cells. Hum Reprod. 2006;21:1869–1877. doi: 10.1093/humrep/del035. [DOI] [PubMed] [Google Scholar]
- Ward EC, Hoekstra AV, Blok LJ, Hanifi-Moghaddam P, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ. The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma. Endocrinology. 2008;149:1942–1950. doi: 10.1210/en.2007-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster K, Taylor A, Gaston K. Oestrogen and progesterone increase the levels of apoptosis induced by the human papillomavirus type 16 E2 and E7 proteins. J Gen Virol. 2001;82:201–213. doi: 10.1099/0022-1317-82-1-201. [DOI] [PubMed] [Google Scholar]
- Welter BH, Hansen EL, Saner KJ, Wei Y, Price TM. Membrane-bound progesterone receptor expression in human aortic endothelial cells. J Histochem Cytochem. 2003;51:1049–1055. doi: 10.1177/002215540305100808. [DOI] [PubMed] [Google Scholar]
- Wen DX, Xu YF, Mais DE, Goldman ME, McDonnell DP. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol. 1994;14:8356–8364. doi: 10.1128/mcb.14.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DT, Bristow RE, Kurman RJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007;31:988–998. doi: 10.1097/PAS.0b013e31802d68ce. [DOI] [PubMed] [Google Scholar]
- Whitney CW, Brunetto VL, Zaino RJ, Lentz SS, Sorosky J, Armstrong DK, Lee RB Gynecologic Oncology Group Study. Phase II study of medroxyprogesterone acetate plus tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:4–9. doi: 10.1016/j.ygyno.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Wildschut H, Both MI, Medema S, Thomee E, Wildhagen MF, Kapp N. Medical methods for mid-trimester termination of pregnancy. Cochrane Database Syst Rev. 2011:CD005216. doi: 10.1002/14651858.CD005216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens J, Chwalisz K, Han C, Walker J, Cameron IT, Ingamells S, Lawrence AC, Lumsden MA, Hapangama D, Williams AR, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. J Clin Endocrinol Metab. 2008;93:4664–4671. doi: 10.1210/jc.2008-1104. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Powell WL, Collins T, Morton CC. HMGI(Y) expression in human uterine leiomyomata. Involvement of another high-mobility group architectural factor in a benign neoplasm. Am J Pathol. 1997;150:911–918. [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Critchley HO, Osei J, Ingamells S, Cameron IT, Han C, Chwalisz K. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum Reprod. 2007;22:1696–1704. doi: 10.1093/humrep/dem026. [DOI] [PubMed] [Google Scholar]
- Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1:106–111. doi: 10.4161/epi.1.2.2766. [DOI] [PubMed] [Google Scholar]
- Xu J, Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol. 2003;17:1681–1692. doi: 10.1210/me.2003-0116. [DOI] [PubMed] [Google Scholar]
- Xu Q, Ohara N, Chen W, Liu J, Sasaki H, Morikawa A, Sitruk-Ware R, Johansson ED, Maruo T. Progesterone receptor modulator CDB-2914 down-regulates vascular endothelial growth factor, adrenomedullin and their receptors and modulates progesterone receptor content in cultured human uterine leiomyoma cells. Hum Reprod. 2006;21:2408–2416. doi: 10.1093/humrep/del159. [DOI] [PubMed] [Google Scholar]
- Xu Q, Ohara N, Liu J, Amano M, Sitruk-Ware R, Yoshida S, Maruo T. Progesterone receptor modulator CDB-2914 induces extracellular matrix metalloproteinase inducer in cultured human uterine leiomyoma cells. Mol Hum Reprod. 2008;14:181–191. doi: 10.1093/molehr/gan004. [DOI] [PubMed] [Google Scholar]
- Yang CH, Lee JN, Hsu SC, Kuo CH, Tsai EM. Effect of hormone replacement therapy on uterine fibroids in postmenopausal women—a 3-year study. Maturitas. 2002;43:35–39. doi: 10.1016/s0378-5122(02)00159-7. [DOI] [PubMed] [Google Scholar]
- Yang S, Xiao X, Jia Y, Liu X, Zhang Y, Wang X, Winters CJ, Devor EJ, Meng X, Thiel KW, et al. Epigenetic modification restores functional PR expression in endometrial cancer cells. Curr Pharm Des. 2014;20:1874–1880. doi: 10.2174/13816128113199990532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yie SM, Li LH, Li GM, Xiao R, Librach CL. Progesterone enhances HLA-G gene expression in JEG-3 choriocarcinoma cells and human cytotrophoblasts in vitro. Hum Reprod. 2006a;21:46–51. doi: 10.1093/humrep/dei305. [DOI] [PubMed] [Google Scholar]
- Yie SM, Xiao R, Librach CL. Progesterone regulates HLA-G gene expression through a novel progesterone response element. Hum Reprod. 2006b;21:2538–2544. doi: 10.1093/humrep/del126. [DOI] [PubMed] [Google Scholar]
- Yin P, Lin Z, Cheng YH, Marsh EE, Utsunomiya H, Ishikawa H, Xue Q, Reierstad S, Innes J, Thung S, et al. Progesterone receptor regulates Bcl-2 gene expression through direct binding to its promoter region in uterine leiomyoma cells. J Clin Endocrinol Metab. 2007;92:4459–4466. doi: 10.1210/jc.2007-0725. [DOI] [PubMed] [Google Scholar]
- Yin X, Pavone ME, Lu Z, Wei J, Kim JJ. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J Clin Endocrinol Metab. 2012;97:E35–E43. doi: 10.1210/jc.2011-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo YA, Son J, Mehta FF, DeMayo FJ, Lydon JP, Chung SH. Progesterone signaling inhibits cervical carcinogenesis in mice. Am J Pathol. 2013;183:1679–1687. doi: 10.1016/j.ajpath.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Ohara N, Xu Q, Chen W, Wang J, Nakabayashi K, Sasaki H, Morikawa A, Maruo T. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Semin Reprod Med. 2010;28:260–273. doi: 10.1055/s-0030-1251483. [DOI] [PubMed] [Google Scholar]
- Younglai EV, Wu Y, Foster WG, Lobb DK, Price TM. Binding of progesterone to cell surfaces of human granulosa-lutein cells. J Steroid Biochem Mol Biol. 2006;101:61–67. doi: 10.1016/j.jsbmb.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Yuan F, Auborn K, James C. Altered growth and viral gene expression in human papillomavirus type 16-containing cancer cell lines treated with progesterone. Cancer Invest. 1999;17:19–29. [PubMed] [Google Scholar]
- Yudt MR, Berrodin TJ, Jelinsky SA, Hanna LA, Brown EL, Chippari S, Bhat RA, Winneker RC, Zhang Z. Selective and opposing actions of progesterone receptor isoforms in human endometrial stromal cells. Mol Cell Endocrinol. 2006;247:116–126. doi: 10.1016/j.mce.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Zhang GY, Wu LY, Li B, Huang MN, Zhang R, Li XG. Retrospective analysis of prognostic variables and clinical outcomes in surgically staged intermediate risk endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol. 2013;169:309–316. doi: 10.1016/j.ejogrb.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA. 2003a;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003b;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]