Abstract
OBJECTIVES
Treatment patterns and outcomes in a population-based database were examined to identify patients likely to benefit from surgical resection of locally advanced (T3N0–2) non-small-cell lung cancer (NSCLC).
METHODS
Factors predicting the use of surgery for patients with T3N0–2M0 NSCLC in the Surveillance, Epidemiology and End Results (SEER) database from 1988 to 2010 were assessed using a multivariable logistic regression model. Survival was analysed using the Kaplan–Meier approach and Cox proportional hazard models. Propensity matching was used to compare outcomes after surgery and outcomes in patients who refused surgery and underwent radiation therapy (RT).
RESULTS
Of 17 378 patients identified for study inclusion [8597 (50%) T3N0, 2304 (13%) T3N1 and 6477 (37%) T3N2], surgery was used in 7120 (41%). Only female sex and being married predicted the use of surgery, while older age, black race and N2 nodal disease predicted non-surgical management. Surgical patients overall had better long-term survival than non-surgical patients [odds ratio (OR) 0.42, 95% confidence interval (CI): 0.41–0.45, P < 0.001]. After propensity adjustment, patients who refused surgery and instead were treated with RT had significantly worse long-term survival than matched surgery patients (OR 0.65, 95% CI: 0.48–0.89, P = 0.0074). Sublobar resection and pneumonectomy predicted worse survival in patients who had surgery. Nodal disease also predicted worse survival after surgery, but surgery maintained an association with better overall survival compared with non-operative therapy among patients with both N1 (OR 0.53, P < 0.001) and N2 disease (OR 0.50, P < 0.001) in separate analyses stratified by nodal status. Older age also predicted worse survival after surgery, but patients older than 75 who were treated with surgery had significantly better long-term survival than non-operative patients (OR 0.49, 95% CI: 0.45–0.53, P < 0.001).
CONCLUSIONS
Surgery is used in a minority of patients with locally advanced NSCLC, but is associated with better survival than non-surgical treatment, even for patients older than 75 and patients with nodal disease. Given the very poor outcomes observed with non-operative management, surgical resection should be carefully considered in all patients with locally advanced NSCLC and should not necessarily be denied because of patient age or nodal disease.
Keywords: Lung cancer surgery, Outcomes, Chest wall, Lobectomy, Pneumonectomy
INTRODUCTION
Lung cancer is the leading cause of cancer mortality in the USA, accounting for more than 160 000 deaths annually [1]. Patients with locally advanced disease due to T3 status or node-positive disease comprise 22% of lung cancer patients [2]. The treatment recommendations for patients with T3 non-small- cell lung cancer (NSCLC) that invades local structures such as the chest wall, proximal airway or mediastinal pleura are dependent on the N status [3, 4]. Current National Comprehensive Center Network (NCCN) guidelines recommend that medically inoperable patients be treated with radiation and possibly chemotherapy, and that patients with T3 disease resulting from local invasion and N2 disease be treated with definitive chemoradiation [3]. The recommended treatment for medically operable patients with T3N0–1 disease is surgical resection followed by adjuvant chemotherapy [3, 5].
Patients with T3N0 disease have a 5-year survival rate of ∼40%, although rates as high as 50–60% have been reported after complete surgical resection [6–9]. Factors associated with worse 5-year survival include incomplete resection and the presence of regional lymph node metastasis [7, 10–12]. As optimal management has not been clearly established by any randomized controlled trials, the purpose of this study was to examine treatment patterns and outcomes for locally advanced (T3) NSCLC in a population-based database. Specifically, we sought to examine both factors that predicted the use of surgery and factors that impacted survival following surgical resection to better inform the treatment selection process for patients with locally advanced disease.
PATIENTS AND METHODS
This secondary analysis of the Surveillance, Epidemiology, and End Results (SEER) database was approved by the Institutional Review Board at Duke University. Included for the study were patients 18 years or older diagnosed with NSCLC between 1988 and 2010. Patients were identified using ICD-O-3 location codes for lung cancer (C34.0–C34.9) and appropriate SEER histology codes ranging from 8014 to 8576 for all possible non-small-cell lung tumour histologies. Tumour–node–metastasis (TNM) stage was directly extracted from SEER for patients diagnosed in 2004 or later, and was manually recoded from available SEER variables using the sixth edition of the AJCC Cancer Staging Manual for patients diagnosed between 1988 and 2003. Only patients with T3 N0-2 tumours were kept for analysis, as treatment guidelines include surgical resection as a possible intervention for this subset of patients with locally advanced tumours. TNM staging in SEER is based on clinical data when no surgery is performed or when any cancer-directed neoadjuvant treatment is administered prior to surgery. In contrast, if surgery was the primary cancer-directed therapy, pathological stage data are reported in SEER. Patient age, sex, race, ethnicity, marital status and time to last available reported survival time point were also extracted. Patients living longer than 10 years were right-censored.
The SEER registry collects incidence and survival data from 18 population-based cancer registries across the USA. The entirety of SEER is estimated to comprise roughly one-quarter of the US population, and the registry populations are comparable to the overall population. A more complete overview of the SEER database is available at http://seer.cancer.gov/about/. SEER does not contain information relative to chemotherapy administration, but data regarding surgical interventions and radiation therapy (RT) are readily available. As such, patients were categorized as either having undergone surgery, defined as an operative intervention with therapeutic intent (sublobar resection, lobectomy or pneumonectomy), or no surgery (diagnostic biopsy or no invasive intervention). Trends in the use of surgery over time were evaluated using the Cochran–Armitage trend test. Patients were then stratified into subgroups based on TNM stage (T3N0, T3N1 and T3N2). Our primary analysis was to compare patients undergoing surgery with those without operative interventions. We then performed an a priori defined subgroup analysis, examining patients undergoing surgery stratified by nodal status.
Comparisons of patient and treatment characteristics were performed using Pearson's chi-square test for categorical variables and Student's t-test or one-way ANOVA for continuous variables. Owing to the likelihood of significant selection bias present regarding the patients selected for surgery, multivariable logistic regression was used to estimate predictors of surgical intervention. The following covariates were included in the model: age (18–40, 41–64, 65–74 and 75+ years), race, sex, marital status and N-stage.
Survival analysis
Unadjusted survival analyses were performed using the Kaplan–Meier method. To estimate the impact of surgical intervention on overall survival, a multivariable Cox proportional hazards model was created, using surgical intervention as the primary predictor, and age, race, sex, N-stage, use of RT and marital status as covariates. In a second survival model examining the cohort of patients treated with surgical resection, a multivariable Cox model was created with the extent of surgical resection as the primary predictor variable, and included use and timing of RT, N-stage, age, sex, race and marital status. To examine potential differences in the impact of surgical intervention based on nodal status, patients were then stratified by N-stage (N0, N1 or N2), with surgical intervention as the primary predictor and age, race, sex, N-stage, use of RT and marital status as covariates in each model.
To further assess potential overestimation of the benefit of surgery due to inclusion of patients who were not medically fit for an operation, we then conducted a propensity-matched analysis. We developed propensity scores defined as the probability of a patient being treated with surgery, conditional on known covariates and matched patients using a 2:1 nearest neighbour matching algorithm. To further strengthen this approach, surgery patients were compared only with those non-surgery patients who refused surgery but did receive at least some RT. This strategy was utilized to create a non-operative control group of patients who were at least considered as potentially acceptable surgical candidates and who also received local therapy via RT.
No major model assumptions were violated, and missing data were handled with complete case analysis. Type I error was controlled for at the level of the comparison. A P-value of ≤0.05 indicated statistical significance for all comparisons, and all analyses were performed using R version 3.0.1 (Vienna, Austria).
RESULTS
Utilization of surgery
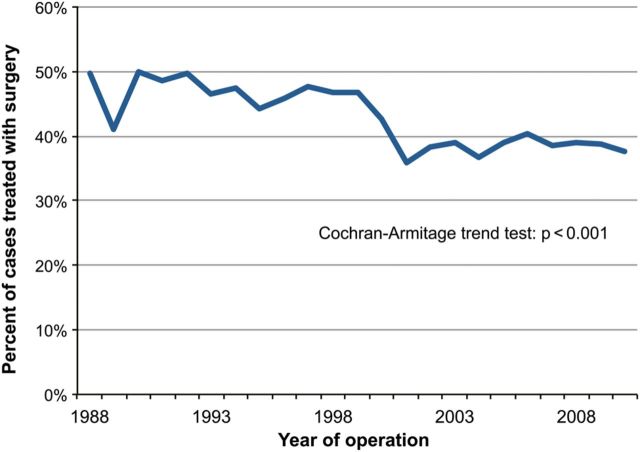
A total of 17 490 patients with T3N0–2 NSCLC from the period 1988–2010 in the SEER database were identified. Data regarding surgery were not available for 112 (0.6%) patients, who were excluded from the analysis. Of the remaining 17 378 patients, 7120 (41%) had surgical resection and 10 258 (59%) had no surgical intervention. Trends in the utilization of surgery for T3N0–2 NSCLC over the course of the study period are shown in Fig. 1. The use of surgery significantly decreased over time (P < 0.001); 50% of patients underwent surgery in 1988, while 38% of patients had surgery in 2010. With respect to N-status for all subjects, 8647 (49%) were N0, 2313 (13%) were N1 and 6530 (37%) were N2 at the time of diagnosis. Among the patients who underwent surgery and were diagnosed prior to 2000, 64% had negative nodes, while 66% of patients treated with surgery and diagnosed after 2000 were node-negative (P = 0.053). Patient, treatment and tumour characteristics stratified by surgery or no surgery are given in Table 1. Compared with patients who did not have surgery, patients undergoing surgical resection were younger, had lower N stage, were less frequently treated with radiotherapy and were more often white and married. Among the 10 258 patients who were not treated with surgery, 8615 (84%) were treated non-operatively due to ‘surgery not recommended, or contraindicated due to other conditions’. Only 7 patients died before recommended surgery, and 167 patients (2%) refused surgery that was recommended. In the remaining 1469 (14%) patients, the reason for no surgery was not documented.
Figure 1:
Trends in the utilization of surgery for locally advanced (T3N0–2M0) NSCLC, 1988–2010.
Table 1:
Baseline demographic and disease-specific characteristics, stratified by treatment strategy
| Characteristics | Surgical approach |
||
|---|---|---|---|
| No surgery (n = 10 258) | Surgery (n = 7120) | P-value | |
| Age (years) | 70 (62, 77) | 67 (58, 73) | <0.001 |
| Sex | |||
| Female | 3820 (37.2%) | 2734 (38.4%) | 0.13 |
| Male | 6438 (62.8%) | 4386 (61.6%) | |
| Race | |||
| White | 8315 (81.1%) | 6049 (85%) | <0.001 |
| Black | 1415 (13.8%) | 643 (9%) | |
| Other | 528 (5.1%) | 428 (6%) | |
| Marital status | |||
| Married | 5242 (51.1%) | 4414 (62%) | <0.001 |
| Not married | 4657 (45.4%) | 2485 (34.9%) | |
| Unknown | 359 (3.5%) | 221 (3.1%) | |
| N-stage | |||
| 0 | 3963 (38.6%) | 4634 (65.1%) | <0.001 |
| 1 | 953 (9.3%) | 1351 (19%) | |
| 2 | 5342 (52.1%) | 1135 (15.9%) | |
| Radiation Tx | 7342 (73.1%) | 3290 (47.3%) | <0.001 |
Predictors of undergoing surgical intervention
Results of multivariable logistic regression for modelling the likelihood of undergoing surgery are given in Table 2. After adjustment, increasing age was associated with decreased odds of surgical intervention, as was black race. Female gender and being married were associated with increased odds of surgery. Compared with patients with N0 disease, those with N1 were slightly more likely to undergo surgery, while those with N2 disease were significantly less likely to undergo surgery. Lymph nodes were not retrieved for 813 (11%) patients, and among the remaining patients the median number of nodes retrieved was 8 (IQR: 4, 13).
Table 2:
Adjusted predictors of surgical resection for patients with T3N0–2 non-small-cell lung cancer
| Predictor of surgical intervention | Odds ratio | 95% confidence interval |
P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (per decade) | 0.60 | 0.58 | 0.62 | <0.001 |
| Male gender | 0.86 | 0.80 | 0.93 | <0.001 |
| Race | ||||
| White | Ref. | Ref. | Ref. | Ref. |
| Black | 0.57 | 0.51 | 0.65 | <0.001 |
| Other | 1.20 | 1.03 | 1.40 | 0.022 |
| Nodal status | ||||
| N0 | Ref. | Ref. | Ref. | Ref. |
| N1 | 1.15 | 1.05 | 1.28 | 0.0057 |
| N2 | 0.17 | 0.16 | 0.19 | <0.001 |
| Marital status | ||||
| Married | Ref. | Ref. | Ref. | Ref. |
| Not married | 0.61 | 0.57 | 0.66 | <0.001 |
| Unknown | 0.61 | 0.50 | 0.75 | <0.001 |
| C-index: 0.79 | ||||
Survival analyses
Kaplan–Meier survival curves for patients undergoing surgery compared with those not treated with surgery are shown in Fig. 2A. The 5-year survival rate for patients who had surgery was 29% [95% confidence interval (CI): 28–31%], while that for patients who did not have surgery was 6.8% (95% CI: 6–7%). After Cox proportional hazards adjustment, the surgical group had significantly improved overall survival [odds ratio (OR): 0.43, P < 0.001]. Other factors that predicted improved survival were younger age, female sex, N0 nodal status, being married and use of RT (Table 3).
Figure 2:
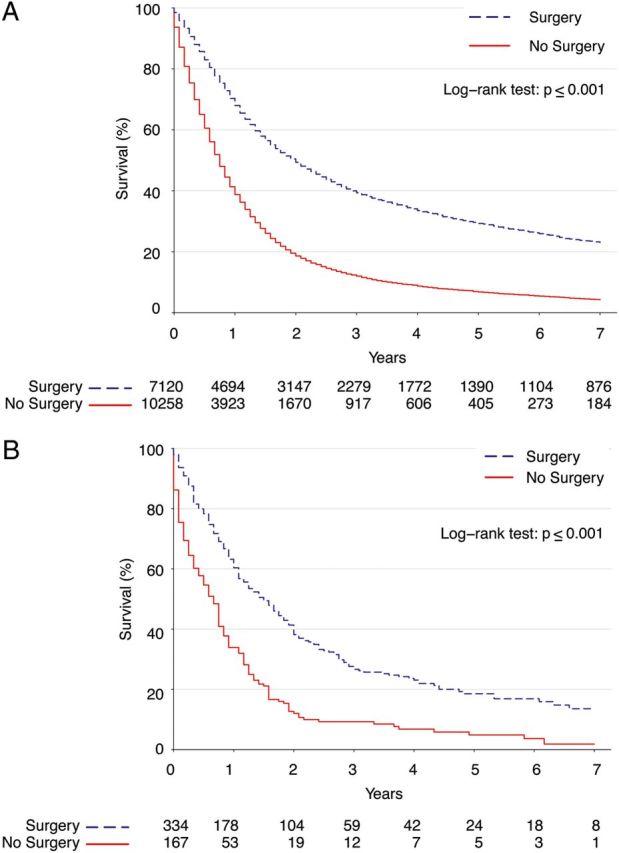
(A) Kaplan–Meier survival estimates for surgery versus no surgery among patients with locally advanced NSCLC. (B) Kaplan–Meier survival estimates among propensity-matched patients.
Table 3:
Risk of death for all patients with T3N0–2 non-small-cell lung cancer
| Predictor | Hazard ratio | 95% confidence interval |
P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Surgical intervention | 0.43 | 0.41 | 0.45 | <0.001 |
| Radiation therapy (any) | 0.65 | 0.63 | 0.68 | <0.001 |
| Nodal status | ||||
| N0 | Ref. | Ref. | Ref. | Ref. |
| N1 | 1.18 | 1.12 | 1.24 | <0.001 |
| N2 | 1.27 | 1.23 | 1.32 | <0.001 |
| Age (per decade) | 1.24 | 1.22 | 1.26 | <0.001 |
| Male sex | 1.15 | 1.11 | 1.20 | <0.001 |
| Race | ||||
| White | Ref. | Ref. | Ref. | Ref. |
| Black | 1.04 | 0.98 | 1.09 | 0.17 |
| Other | 0.91 | 0.84 | 0.98 | 0.012 |
| Marital status | ||||
| Married | Ref. | Ref. | Ref. | Ref. |
| Not married | 1.14 | 1.10 | 1.18 | <0.001 |
| Unknown | 1.00 | 0.90 | 1.10 | 0.97 |
Cox proportional hazards model adjusting for age, surgical intervention, radiation therapy, race, sex, nodal status and marital status.
The results of our confirmatory propensity analysis comparing surgery patients with matched non-operative patients who were surgical candidates but who refused an operation are shown in Fig. 2B, and demonstrated that surgery patients had significantly better long-term survival than matched patients treated non-operatively (OR 0.46, 95% CI: 0.37–0.57, P < 0.001) even after adjustment. As a further sensitivity analysis, we then repeated the propensity analysis, but further limited the non-surgery group to only include patients who were treated with RT, obtaining similar results [OR 0.65 (0.48–0.89), P = 0.0074].
Subgroup analysis of patients treated with surgery
Table 4 summarizes the factors associated with survival in patients who had surgery. After Cox proportional hazards adjustment, patients with N0 disease had significantly improved survival (P < 0.001). Furthermore, in this subgroup of patients who had surgery, younger age and induction RT were associated with improved survival, while adjuvant RT did not have a statistically significant survival benefit. Compared with patients who underwent lobectomy, long-term disease-specific survival was significantly worse both for patients who had sublobar resections (OR 1.28, P < 0.001) and for patients who had a pneumonectomy (OR 1.13, P = 0.0013). Interestingly, while pneumonectomy was associated with only slightly inferior long-term survival, early overall survival compared with lobectomy was substantially worse, both at 3 months (85.3 vs 91.8%) and 6 months (76.6 vs 84.2%).
Table 4:
Adjusted risk of death for patients undergoing surgery for T3N0–2 non-small-cell lung cancer
| Predictor | Hazard ratio | 95% confidence interval |
P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Radiation therapy | ||||
| None | Ref. | Ref. | Ref. | Ref. |
| Induction | 0.79 | 0.72 | 0.87 | <0.001 |
| Adjuvant | 0.96 | 0.91 | 1.03 | 0.25 |
| Both | 0.97 | 0.77 | 1.23 | 0.83 |
| Extent of surgical resection | ||||
| Lobectomy | Ref. | Ref. | Ref. | Ref. |
| Sublobar | 1.21 | 1.11 | 1.32 | <0.001 |
| Pneumonectomy | 1.09 | 1.01 | 1.18 | 0.022 |
| Nodal status | ||||
| N0 | Ref. | Ref. | Ref. | Ref. |
| N1 | 1.34 | 1.24 | 1.44 | <0.001 |
| N2 | 1.53 | 1.42 | 1.65 | <0.001 |
| Age (per decade) | 1.31 | 1.27 | 1.35 | <0.001 |
| Male sex | 1.15 | 1.09 | 1.22 | <0.001 |
| Race | ||||
| White | Ref. | Ref. | Ref. | Ref. |
| Black | 1.10 | 1.00 | 1.22 | 0.051 |
| Other | 0.89 | 0.79 | 1.00 | 0.045 |
| Marital status | ||||
| Married | Ref. | Ref. | Ref. | Ref. |
| Not married | 1.10 | 1.04 | 1.17 | 0.0018 |
| Unknown | 0.87 | 0.73 | 1.04 | 0.12 |
Cox proportional hazards model adjusting for age, surgical intervention, radiation therapy, race, sex, nodal status and marital status.
Subgroup analyses of elderly patients and patients with nodal disease
Because the above analyses showed that increasing age and nodal disease were associated with worse survival after surgery, additional sub-analyses were performed to evaluate the benefit of surgery compared with non-operative management for both elderly patients and patients with nodal disease. First, we conducted an exploratory analysis investigating the benefit of surgery limited to patients who were older than 75 years old. In this cohort, surgery was still associated with superior long-term survival compared with non-operative management (OR 0.49, 95% CI: 0.45–0.53, P < 0.001). Secondly, we performed separate Cox proportional hazard model analyses that were stratified by nodal status and adjusted for age, surgical intervention, RT, race, gender and marital status. Improved survival was associated with surgery regardless of N-stage, though a larger impact was observed for N0 status (OR 0.39, 95% CI: 0.36–0.41, P < 0.001) compared with N1 disease (OR 0.53, 95% CI: 0.48–0.60, P < 0.001) and N2 disease (OR 0.50, 95% CI: 0.47–0.55, P < 0.001). Survival curves stratified by N-stage for the subset of patients treated with surgery are shown in Fig. 3.
Figure 3:
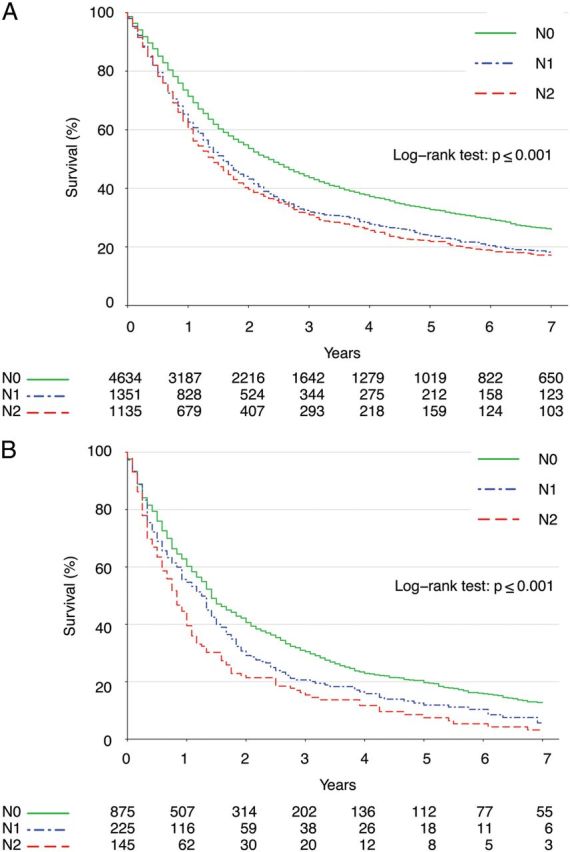
Kaplan–Meier survival estimates among patients treated with surgery, stratified by nodal status for (A) all patients and (B) only elderly patients at least 75 years old.
DISCUSSION
Using population-based SEER data, we demonstrate that a minority of patients with locally advanced but potentially resectable NSCLC undergo surgical resection. Being male, older, black, unmarried and having nodal disease are all characteristics that are associated with surgery not being utilized. However, survival after surgical resection was significantly better compared with non-surgical therapy, even after multivariable adjustment and in subgroup analyses of elderly patients and patients with nodal disease.
Previous studies have established that the primary factors that influence survival after surgery for locally advanced NSCLC are completeness of resection, the extent of local invasion and the presence of regional lymph node metastasis [7, 10–13]. Although our dataset does not contain information on margin status or exact extent of local invasion, our results do confirm the results of these smaller studies with regard to nodal status. Other factors associated with improved survival in patients who had surgery were younger age and having a lobectomy instead of a sublobar resection or a pneumonectomy.
These results can potentially be used to improve the treatment selection process for patients with locally advanced but potentially resectable NSCLC. Our study does show that surgery is utilized in a minority of these patients, and that the use of surgery in this situation has decreased with time. One explanation for this finding is likely that many patients were appropriately not offered surgery due to technically unresectable disease, inadequate pulmonary function or other medical comorbidities contraindicating an operation. However, our propensity-matched analysis suggests that the benefit of surgery persists even when adjusting for issues of operability. Similarly, with recent increases in resolution and accuracy of positron emission tomography imaging, clinicians may be less willing to pursue surgical options in the setting of obvious nodal involvement. However, although our study showed that older age and nodal disease were associated with worse outcomes, improved survival was observed in these subsets of patients when surgery was used instead of non-operative management. Considering the poor prognosis associated with non-surgical management, we advocate for multidisciplinary evaluation in all patients with locally advanced NSCLC, so that short-term perioperative morbidity and mortality risks of surgical resection can be carefully balanced with the potential long-term survival benefits. Patient age and tumour nodal status should be carefully considered in the selection process, but these factors should not necessarily exclude the use of surgery. Another important factor to consider is whether complete resection can be accomplished with a lobectomy.
Interestingly, two non-clinical factors—race and marital status—also had significant associations with patients having surgery. Patients who were unmarried were less likely to have surgery, which might have been related to the support system available to help the patient through the perioperative process. Race has been shown in many studies to influence both treatment and outcomes, and the results of this study are consistent with those of other studies [14–16]. However, clinicians should clearly strive to choose an optimal treatment based on individual clinical and cancer factors alone.
In light of the significantly improved long-term survival associated with surgical resection, consideration should also be given to improving preoperative functional status and optimizing management of comorbidities in patients who are borderline candidates for surgery. Induction chemotherapy has not been shown to improve survival among patients with locally advanced disease, but has been shown to be safe [17]. The use of induction chemotherapy while a patient's operative candidacy is potentially improved by measures such as cardiopulmonary rehabilitation, optimization of management of other medical conditions and smoking cessation warrants serious consideration. If patients do not adequately improve to the point of being able to have surgery, they still would have received oncological treatment during that time period.
These results also show that almost half of patients with T3N0–2 NSCLC who have surgery also are treated with radiation, with about a third of patients who have surgery receiving adjuvant radiation. However, the use of adjuvant radiation was not associated with a survival benefit. Similarly, several studies have shown that, in patients treated with incomplete resection, adjuvant radiation does not significantly improve survival [4, 18–20]. Furthermore, high local recurrence rates of 30–40% are seen when radiation is used to treat positive surgical margins [4, 18, 21]. In addition, the role of induction radiation prior to surgery for T3 tumours larger than 7 cm has been questioned, with Moreno et al. failing to show improved survival compared with surgery alone, while another small single-centre study demonstrated improved survival [22, 23]. Further investigation is needed to delineate the role of adjuvant radiation and surgery in this setting.
The use of the SEER database offers significant advantages over previous smaller studies, being large and widely generalizable to the US population. This study, however, is subject to limitations inherent to all retrospective studies. Most significantly, treatment allocation to surgery or definitive chemoradiation therapy was likely influenced by considerable selection bias. Furthermore, SEER does not include any chemotherapy data, and while many patients who received RT probably also received chemotherapy, this cannot be specifically addressed. We are also limited by a lack of data regarding margin status, and presumably some patients who underwent surgery had incomplete resections. This would likely bias our results against a benefit for surgery; however, as it may be reasonable to assume that survival following complete resection is superior to that described in this study. Also, the SEER dataset does not allow specific identification of why a patient's tumour was T3 and we cannot specifically identify and analyse T3 subgroups such as patients with chest wall invasion. Similarly, the stage recorded in SEER is the pre-treatment clinical stage for patients who have induction therapy or no surgery, and we are unable to confirm whether specific elements of the clinical stage in this setting were confirmed with biopsy or based on imaging characteristics alone. Therefore, patients who had induction therapy or no surgery may have been over- or understaged, which could bias results with surgery both positively and negatively. Perhaps most importantly, the dataset lacks information regarding patients' comorbidities, pulmonary function and overall functional status. This information is clearly very important with regard to both treatment and prognosis. In addition, SEER allows evaluation of short-term survival from the time of diagnosis, but does not allow specific measurement of perioperative mortality for patients who have surgery, and also does not contain information about perioperative morbidity. Therefore, we cannot make any comment on short-term outcomes after surgery.
Despite these limitations, use of the SEER database does allow the assembling and analysis of a very large cohort of patients with relatively homogeneous stage characteristics. Other prospective or even retrospective studies are unlikely to be able to evaluate a cohort of this size. In conclusion, this study used a large population-based database to demonstrate that surgery is associated with improved outcomes, but nonetheless used only in a minority of patients with locally advanced NSCLC. Nodal disease and increasing age are associated with worse survival after surgery, but outcomes after surgery are still better than non-operative management for both patients who are older than 75 and patients with nodal disease. Given the very poor outcomes observed with non-operative management, surgical resection should be carefully considered in all patients with locally advanced NSCLC and should not necessarily be denied because of patient age or nodal disease.
Funding
This work was supported in part by the NIH-funded Cardiothoracic Surgery Trials Network (Mark F. Berry).
Conflict of interest: Thomas A. D'Amico serves as a consultant for Scanlan International, Inc.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute; 2013. http://seer.cancer.gov/csr/1975_2010/ based on November 2012 SEER data submission, posted to the SEER web site, April. [Google Scholar]
- 3.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw. 2013;11:645–53. doi: 10.6004/jnccn.2013.0084. [DOI] [PubMed] [Google Scholar]
- 4.Kozower BD, Larner JM, Detterbeck FC, Jones DR. Special treatment issues in non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e369S–99S. doi: 10.1378/chest.12-2362. [DOI] [PubMed] [Google Scholar]
- 5.Pisters KM, Evans WK, Azzoli CG, Kris MG, Smith CA, Desch CE, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506–18. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 6.Harpole DH, Healey EA, DeCamp MM, Mentzer SJ, Strauss GM, Sugarbaker DJ. Chest wall invasive non-small cell lung cancer: patterns of failure and implications for a revised staging system. Ann Surg Oncol. 1996;3:261–9. doi: 10.1007/BF02306281. [DOI] [PubMed] [Google Scholar]
- 7.Riquet M, Arame A, Le Pimpec Barthes F. Non-small cell lung cancer invading the chest wall. Thorac Surg Clin. 2010;20:519–27. doi: 10.1016/j.thorsurg.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Shah SS, Goldstraw P. Combined pulmonary and thoracic wall resection for stage III lung cancer. Thorax. 1995;50:782–4. doi: 10.1136/thx.50.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoelben E, Ludwig C. Chest wall resection for lung cancer: indications and techniques. Eur J Cardiothorac Surg. 2009;35:450–6. doi: 10.1016/j.ejcts.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Chapelier A, Fadel E, Macchiarini P, Lenot B, Le Roy Ladurie F, Cerrina J, et al. Factors affecting long-term survival after en-bloc resection of lung cancer invading the chest wall. Eur J Cardiothorac Surg. 2000;18:513–8. doi: 10.1016/s1010-7940(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi K, Miyaoka E, Asamura H, Nomori H, Okumura M, Fujii Y, et al. Modern surgical results of lung cancer involving neighboring structures: a retrospective analysis of 531 pT3 cases in a Japanese Lung Cancer Registry Study. J Thorac Cardiovasc Surg. 2012;144:431–7. doi: 10.1016/j.jtcvs.2012.05.069. [DOI] [PubMed] [Google Scholar]
- 12.Magdeleinat P, Alifano M, Benbrahem C, Spaggiari L, Porrello C, Puyo P, et al. Surgical treatment of lung cancer invading the chest wall: results and prognostic factors. Ann Thorac Surg. 2001;71:1094–9. doi: 10.1016/s0003-4975(00)02666-7. [DOI] [PubMed] [Google Scholar]
- 13.Doddoli C, D'Journo B, Le Pimpec-Barthes F, Dujon A, Foucault C, Thomas P, et al. Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg. 2005;80:2032–40. doi: 10.1016/j.athoracsur.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 14.Gadgeel SM, Kalemkerian GP. Racial differences in lung cancer. Cancer Metastasis Rev. 2003;22:39–46. doi: 10.1023/a:1022207917249. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control. 2003;14:761–6. doi: 10.1023/a:1026321923883. [DOI] [PubMed] [Google Scholar]
- 16.Hardy D, Liu CC, Xia R, Cormier JN, Chan W, White A, et al. Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer. 2009;115:2199–211. doi: 10.1002/cncr.24248. [DOI] [PubMed] [Google Scholar]
- 17.Lococo F, Cesario A, Margaritora S, Dall'Armi V, Nachira D, Cusumano G, et al. Induction therapy followed by surgery for T3-T4/N0 non-small cell lung cancer: long-term results. Ann Thorac Surg. 2012;93:1633–40. doi: 10.1016/j.athoracsur.2012.01.109. [DOI] [PubMed] [Google Scholar]
- 18.Albertucci M, DeMeester TR, Rothberg M, Hagen JA, Santoscoy R, Smyrk TC. Surgery and the management of peripheral lung tumors adherent to the parietal pleura. J Thorac Cardiovasc Surg. 1992;103:8–12. discussion 3. [PubMed] [Google Scholar]
- 19.McCaughan BC, Martini N, Bains MS, McCormack PM. Chest wall invasion in carcinoma of the lung. Therapeutic and prognostic implications. J Thorac Cardiovasc Surg. 1985;89:836–41. [PubMed] [Google Scholar]
- 20.Ratto GB, Piacenza G, Frola C, Musante F, Serrano I, Giua R, et al. Chest wall involvement by lung cancer: computed tomographic detection and results of operation. Ann Thorac Surg. 1991;51:182–8. doi: 10.1016/0003-4975(91)90778-o. [DOI] [PubMed] [Google Scholar]
- 21.Nakahashi H, Yasumoto K, Ishida T, Nagashima A, Nishino T, Oka T, et al. Results of surgical treatment of patients with T3 non-small cell lung cancer. Ann Thorac Surg. 1988;46:178–81. doi: 10.1016/s0003-4975(10)65892-4. [DOI] [PubMed] [Google Scholar]
- 22.Moreno AC, Morgensztern D, Yu JB, Boffa DJ, Decker RH, Detterbeck FC, et al. Impact of preoperative radiation on survival of patients with T3N0 >7-cm non-small cell lung cancers treated with anatomic resection using the Surveillance, Epidemiology, and End Results database. J Surg Res. 2013;184:10–18. doi: 10.1016/j.jss.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 23.Shien K, Toyooka S, Kiura K, Matsuo K, Soh J, Yamane M, et al. Induction chemoradiotherapy followed by surgical resection for clinical T3 or T4 locally advanced non-small cell lung cancer. Ann Surg Oncol. 2012;19:2685–92. doi: 10.1245/s10434-012-2302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]