Abstract
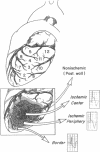
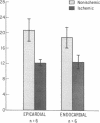
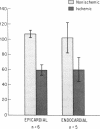
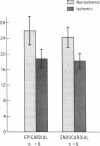
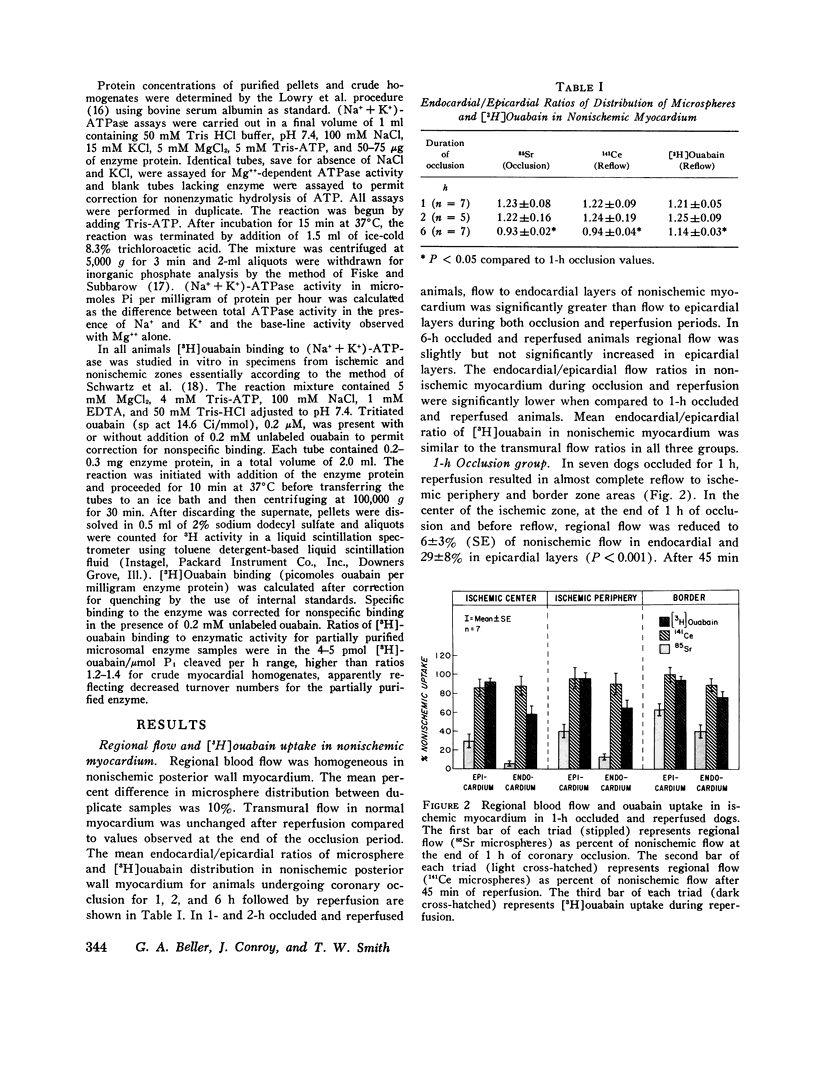
The effects of ischemia on the canine myocardial (Na+ + K+)-ATPase complex were examined in terms of alterations in cardiac glycoside binding and enzymatic activity. Ability of the myocardial cell to bind tritiated ouabain in vivo was assessed after 1, 2, and 6 h of coronary occlusion followed by 45 min of reperfusion, and correlated with measurements of in vitro (Na+ + K+)-ATPase activity and in vitro [3H]ouabain binding after similar periods of ischemia. Regional blood flow alterations during occlusion and reperfusion were simultaneously determined utilizing 15 mum radioactive microspheres to determine the degree to which altered binding of ouabain might be flow related. Anterior wall infarction was produced in 34 dogs by snaring of confluent branches of the left coronary system. Epicardial electrograms delineated ischemic and border zone areas. Coronary reperfusion after 2 and 6 h of occlusion was associated with impaired reflow of blood and markedly impaired uptake of [3H]ouabain in ischemic myocardium. In both groups, in vivo [3H]ouabain binding by ischemic tissue was reduced out of proportion to the reduction in flow. Despite near-complete restoration of flow in seven dogs occluded for 1 h and reperfused, [3H]ouabain remained significantly reduced to 58 +/- 9% of nonischemic uptake in subendocardial layers of the central zone of ischemia. Thus, when coronary flow was restored to areas of myocardium rendered acutely ischemia for 1 or more hours, ischemic zones demonstrated progressively diminished ability to bind ouabain. To determine whether ischemia-induced alteration in myocardial (Na+ + K+)-ATPase might underlie these changes, (Na+ + K+)-ATPase activity and [3H]ouabain binding were measured in microsomal fractions from ischemic myocardium after 1, 2, and 6 h of coronary occlusion. In animals occluded for 6 h, (Na+ + K+)-ATPase activity was significantly reduced by 40% in epicardial and by 35% in endocardial layers compared with nonischemic myocardium. Comparable reductions in in vitro [3H]ouabain binding were also demonstrated. Reperfusion for 45 min after occlusion for 6 h resulted in no significant restoration of enzyme activity when compared to the nonreperfused animals. In six animals occluded for 2 h, a time at which myocardial creatine phosphokinase activity remains unchanged, (Na+ + K+)-ATPase activity was reduced by 25% compared with nonischemic enzyme activity. In five dogs occluded for 1 h, (Na+ + K+)-ATPase activity in ischemic myocardium was unchanged from control levels. We conclude that reduced regional myocardial blood flow, local alterations in cellular milieu, and altered glycoside-binding properties of (Na+ + K+)-ATPase all participate in the reduction of cardiac glycoside binding observed after reperfusion of ischemic myocardium. In addition, after 2 or more hours of severe ischemia, myocardial (Na+ + K+)-ATPase catalytic activity is significantly reduced despite incubation in the presence of optimal substrate concentrations.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Larsen F. S., Brody T. M. Correlation of cardiac sodium- and potassium-activated adenosine triphosphatase activity with ouabain-induced inotropic stimulation. J Pharmacol Exp Ther. 1970 May;173(1):145–151. [PubMed] [Google Scholar]
- Akera T., Larsen F. S., Brody T. M. The effect of ouabain on sodium- and potassium-activated adenosine triphosphatase from the hearts of several mammalian species. J Pharmacol Exp Ther. 1969 Nov;170(1):17–26. [PubMed] [Google Scholar]
- Balasubramanian V., McNamara D. B., Singh J. N., Dhalla N. S. Biochemical basis of heart function. X. Reduction in the Na+-K+-stimulated ATPase activity in failing rat heart due to hypoxia. Can J Physiol Pharmacol. 1973 Jul;51(7):504–510. doi: 10.1139/y73-074. [DOI] [PubMed] [Google Scholar]
- Becker L. C., Ferreira R., Thomas M. Mapping of left ventricular blood flow with radioactive microspheres in experimental coronary artery occlusion. Cardiovasc Res. 1973 May;7(3):391–400. doi: 10.1093/cvr/7.3.391. [DOI] [PubMed] [Google Scholar]
- Becker L. C., Fortuin N. J., Pitt B. Effect of ischemia and antianginal drugs on the distribution of radioactive microspheres in the canine left ventricle. Circ Res. 1971 Feb;28(2):263–269. doi: 10.1161/01.res.28.2.263. [DOI] [PubMed] [Google Scholar]
- Beller G. A., Smith T. W., Hood W. B., Jr Altered distribution of tritiated digoxin in the infarcted canine left ventricle. Circulation. 1972 Sep;46(3):572–579. doi: 10.1161/01.cir.46.3.572. [DOI] [PubMed] [Google Scholar]
- Beller G. A., Smith T. W., Hood W. B., Jr Effects of ischemia and coronary reperfusion on myocardial digoxin uptake. Am J Cardiol. 1975 Dec;36(7):902–907. doi: 10.1016/0002-9149(75)90080-6. [DOI] [PubMed] [Google Scholar]
- Besch H. R., Jr, Allen J. C., Glick G., Schwartz A. Correlation between the inotropic action of ouabain and its effects on subcellular enzyme systems from canine myocardium. J Pharmacol Exp Ther. 1970 Jan;171(1):1–12. [PubMed] [Google Scholar]
- Cobb F. R., Bache R. J., Greenfield J. C., Jr Regional myocardial blood flow in awake dogs. J Clin Invest. 1974 Jun;53(6):1618–1625. doi: 10.1172/JCI107712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech R. J., Hoffman J. I., Noble M. I., Saunders K. B., Henson J. R., Subijanto S. Total and regional coronary blood flow measured by radioactive microspheres in conscious and anesthetized dogs. Circ Res. 1969 Nov;25(5):581–596. doi: 10.1161/01.res.25.5.581. [DOI] [PubMed] [Google Scholar]
- Fortuin N. J., Kaihara S., Becker L. C., Pitt B. Regional myocardial blood flow in the dog studied with radioactive microspheres. Cardiovasc Res. 1971 Jul;5(3):331–336. doi: 10.1093/cvr/5.3.331. [DOI] [PubMed] [Google Scholar]
- Goldman R. H., Deutscher R. N., Schweizer E., Harrison D. C. Effect of a pharmacologic dose of digoxin on inotropy in hyper- and normokalemic dogs. Am J Physiol. 1972 Dec;223(6):1438–1443. doi: 10.1152/ajplegacy.1972.223.6.1438. [DOI] [PubMed] [Google Scholar]
- Hopkins B. E., Taylor R. R., Henderson C., Burrows P. Digoxin distribution in the dog's left ventricle in the presence of coronary artery ligation. J Mol Cell Cardiol. 1973 Apr;5(2):197–203. doi: 10.1016/0022-2828(73)90054-0. [DOI] [PubMed] [Google Scholar]
- JENNINGS R. B., CROUT J. R., SMETTERS G. W. Studies on distribution and localization to potassium in early myocardial ischemic injury. AMA Arch Pathol. 1957 Jun;63(6):586–592. [PubMed] [Google Scholar]
- Kjekshus J. K., Maroko P. R., Sobel B. E. Distribution of myocardial injury and its relation to epicardial ST-segment changes after coronary artery occlusion in the dog. Cardiovasc Res. 1972 Sep;6(5):490–499. doi: 10.1093/cvr/6.5.490. [DOI] [PubMed] [Google Scholar]
- Kjekshus J. K., Sobel B. E. Depressed myocardial creatine phosphokinase activity following experimental myocardial infarction in rabbit. Circ Res. 1970 Sep;27(3):403–414. doi: 10.1161/01.res.27.3.403. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Jennings R. B. The "no-reflow" phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974 Dec;54(6):1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschinsky K., Lüllmann H., van Zwieten P. A. A comparison of the accumulation and release of 3H-ouabain and 3H-digitoxin by guinea-pig heart muscle. Br J Pharmacol Chemother. 1968 Mar;32(3):598–608. doi: 10.1111/j.1476-5381.1968.tb00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer G. A. Effects of digitalis on myocardial ionic exchange. Circulation. 1972 Jul;46(1):180–187. doi: 10.1161/01.cir.46.1.180. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Klaus W. The subcellular basis for the mechanism of inotropic action of cardiac glycosides. Pharmacol Rev. 1971 Sep;23(3):193–261. [PubMed] [Google Scholar]
- Marcus F. I., Kapadia G. G., Goldsmith C. Alteration of the body distribution of tritiated digoxin by acute hyperkalemia in the dog. J Pharmacol Exp Ther. 1969 Jan;165(1):136–148. [PubMed] [Google Scholar]
- Neill W. A., Phelps N. C., Oxendine J. M., Mahler D. J., Sim D. N. Effect of heart rate on coronary blood flow distribution in dogs. Am J Cardiol. 1973 Sep 7;32(3):306–312. doi: 10.1016/s0002-9149(73)80138-9. [DOI] [PubMed] [Google Scholar]
- Puri P. S., Varley K. G., Kim S. W., Barwinsky J., Cohen M., Dhalla N. S. Alterations in energy metabolism and ultrastructure upon reperfusion of the ischemic myocardium after coronary occlusion. Am J Cardiol. 1975 Aug;36(2):234–243. [PubMed] [Google Scholar]
- Rudolph A. M., Heymann M. A. The circulation of the fetus in utero. Methods for studying distribution of blood flow, cardiac output and organ blood flow. Circ Res. 1967 Aug;21(2):163–184. doi: 10.1161/01.res.21.2.163. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Lindenmayer G. E., Allen J. C. The sodium-potassium adenosine triphosphatase: pharmacological, physiological and biochemical aspects. Pharmacol Rev. 1975 Mar;27(01):3–134. [PubMed] [Google Scholar]
- Schwartz A., Wood J. M., Allen J. C., Bornet E. P., Entman M. L., Goldstein M. A., Sordahl L. A., Suzuki M. Biochemical and morphologic correlates of cardiac ischemia. I. Membrane systems. Am J Cardiol. 1973 Jul;32(1):46–61. doi: 10.1016/s0002-9149(73)80085-2. [DOI] [PubMed] [Google Scholar]
- Shell W. E., Kjekshus J. K., Sobel B. E. Quantitative assessment of the extent of myocardial infarction in the conscious dog by means of analysis of serial changes in serum creatine phosphokinase activity. J Clin Invest. 1971 Dec;50(12):2614–2625. doi: 10.1172/JCI106762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. W., Haber E. Digitalis (third of four parts). N Engl J Med. 1973 Nov 15;289(20):1063–1072. doi: 10.1056/NEJM197311152892005. [DOI] [PubMed] [Google Scholar]
- Thompson A. J., Hargis J., Murphy M. L., Doherty J. E. Tritiated digoxin. XX. Tissue distribution in experimental myocardial infarction. Am Heart J. 1974 Sep;88(3):319–324. doi: 10.1016/0002-8703(74)90466-9. [DOI] [PubMed] [Google Scholar]
- Utley J., Carlson E. L., Hoffman J. I., Martinez H. M., Buckberg G. D. Total and regional myocardial blood flow measurements with 25 micron, 15 micron, 9 micron, and filtered 1-10 micron diameter microspheres and antipyrine in dogs and sheep. Circ Res. 1974 Mar;34(3):391–405. doi: 10.1161/01.res.34.3.391. [DOI] [PubMed] [Google Scholar]
- Willerson J. T., Watson J. T., Hutton I., Templeton G. H., Fixler D. E. Reduced myocardial reflow and increased coronary vascular resistance following prolonged myocardial ischemia in the dog. Circ Res. 1975 Jun;36(6):771–781. doi: 10.1161/01.res.36.6.771. [DOI] [PubMed] [Google Scholar]
- Yipintsoi T., Dobbs W. A., Jr, Scanlon P. D., Knopp T. J., Bassingthwaighte J. B. Regional distribution of diffusible tracers and carbonized microspheres in the left ventricle of isolated dog hearts. Circ Res. 1973 Nov;33(5):573–587. doi: 10.1161/01.res.33.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]