Moxifloxacin serum concentrations in children receiving multidrug-resistant tuberculosis treatment following an oral dose of 10 mg/kg were low. Human immunodeficiency virus infection was associated with lower moxifloxacin exposure. Moxifloxacin was generally well tolerated when taken for several months.
Keywords: moxifloxacin pharmacokinetics, moxifloxacin toxicity, children, MDR tuberculosis
Abstract
Background. Moxifloxacin is currently recommended at a dose of 7.5–10 mg/kg for children with multidrug-resistant (MDR) tuberculosis, but pharmacokinetic and long-term safety data of moxifloxacin in children with tuberculosis are lacking. An area under the curve (AUC) of 40–60 µg × h/mL following an oral moxifloxacin dose of 400 mg has been reported in adults.
Methods. In a prospective pharmacokinetic and safety study, children 7–15 years of age routinely receiving moxifloxacin 10 mg/kg daily as part of multidrug treatment for MDR tuberculosis in Cape Town, South Africa, for at least 2 weeks, underwent intensive pharmacokinetic sampling (predose and 1, 2, 4, 8, and either 6 or 11 hours) and were followed for safety. Assays were performed using liquid chromatography–tandem mass spectrometry, and pharmacokinetic measures calculated using noncompartmental analysis.
Results. Twenty-three children were included (median age, 11.1 years; interquartile range [IQR], 9.2–12.0 years); 6 of 23 (26.1%) were human immunodeficiency virus (HIV)-infected. The median maximum serum concentration (Cmax), area under the curve from 0–8 hours (AUC0–8), time until Cmax (Tmax), and half-life for moxifloxacin were 3.08 (IQR, 2.85–3.82) µg/mL, 17.24 (IQR, 14.47–21.99) µg × h/mL, 2.0 (IQR, 1.0–8.0) h, and 4.14 (IQR, 3.45–6.11), respectively. Three children, all HIV-infected, were underweight for age. AUC0–8 was reduced by 6.85 µg × h/mL (95% confidence interval, −11.15 to −2.56) in HIV-infected children. Tmax was shorter with crushed vs whole tablets (P = .047). Except in 1 child with hepatotoxicity, all adverse effects were mild and nonpersistent. Mean corrected QT interval was 403 (standard deviation, 30) ms, and no prolongation >450 ms occurred.
Conclusions. Children 7–15 years of age receiving moxifloxacin 10 mg/kg/day as part of MDR tuberculosis treatment have low serum concentrations compared with adults receiving 400 mg moxifloxacin daily. Higher moxifloxacin dosages may be required in children. Moxifloxacin was well tolerated in children treated for MDR tuberculosis.
The estimated global incidence of multidrug-resistant (MDR) tuberculosis (ie, resistance to at least isoniazid and rifampin) exceeded 450 000 cases in 2012 [1]. The true burden of tuberculosis in children is unknown, but approximately 1 million children are estimated to have developed tuberculosis in 2010, of whom 32 000 had MDR tuberculosis [2]. New diagnostic tools, such as the Xpert MTB/RIF assay, will likely increase the number of MDR tuberculosis cases diagnosed in adults and therefore increase the number of children identified requiring MDR tuberculosis treatment [3]. MDR tuberculosis treatment should typically comprise 4–5 drugs to which the isolate of the child or the source case is susceptible, including an injectable drug for 6 months and a total duration of therapy of 18–24 months [4]. Fluoroquinolones have shown good in vitro and in vivo activity against Mycobacterium tuberculosis [5] and are an essential part of current MDR tuberculosis treatment regimens in adults and children [4, 6]. Moxifloxacin (MFX) has an early bactericidal activity close to that of isoniazid and is currently considered the most potent fluoroquinolone against M. tuberculosis [7, 8]. There is good evidence for its clinical efficacy at a standard dose (400 mg) in antituberculosis treatment in adults [9, 10].
The pharmacokinetics of MFX have been well characterized in adults with tuberculosis [10–15]. Bioavailability after oral intake of MFX is >90%, with little effect of food on absorption [16]. About half of the drug is metabolized in the liver, whereas 45% is excreted unchanged in urine and feces [17]. Following the standard oral dose, MFX pharmacokinetic measures in adults are as follows: maximum serum concentration (Cmax) 4–6 µg/mL, elimination half-life (T1/2) up to 12 hours, and area under the concentration–time curve (AUC) of 40–60 µg × h/mL [8, 12–14, 18]. Coadministration of rifampin lowers MFX serum concentrations by up to 30% [11].
Although there are no prospective randomized trials of fluoroquinolones for the treatment of tuberculosis in children, high-quality observational data underscore their importance in successful MDR tuberculosis treatment in children [19, 20]. Pharmacokinetic data in children are limited to a single case study in a 1-month-old 1000-g former 28-week premature infant treated for Mycoplasma hominis meningitis [21]. Despite its routine use in children with MDR tuberculosis, there are limited data on MFX safety.
The South African treatment guidelines for MDR tuberculosis were revised during 2012 to recommend the use of levofloxacin and MFX both at a dose of 7.5–10 mg/kg instead of the less potent ofloxacin in children, consistent with World Health Organization (WHO) guidelines [4]. For MFX, appropriate dosing is not feasible in children weighing <20 kg (typically <8 years of age) due to the lack of a pediatric formulation. Only the 400-mg tablet formulated for adults is available. Hence, children weighing ≥20 kg routinely receive MFX and children weighing <20 kg receive levofloxacin (which is available in a 250-mg tablet), for the treatment of drug-resistant tuberculosis in most settings.
We characterize the pharmacokinetics and safety of MFX routinely given in combination with individualized optimized background regimen for the treatment of MDR tuberculosis in children >7 years of age and describe the effect of key clinical covariates.
METHODS
Study Design and Setting
This was a prospective intensive pharmacokinetic sampling study among children with MDR tuberculosis in Cape Town, South Africa.
Study Population
From May 2012 through March 2014, human immunodeficiency virus (HIV)-infected and -uninfected children aged 7–15 years routinely started on MDR tuberculosis therapy including MFX were eligible. Children underwent pharmacokinetic sampling if on MDR tuberculosis treatment for 2–8 weeks. HIV-infected children also had to be on combination antiretroviral therapy (cART) for ≥2 weeks. Exclusion criteria were laboratory-documented anemia (hemoglobin <8 g/dL) or lack of informed consent/assent. Pharmacokinetic sampling in children with severe acute illness was deferred until patients were clinically stable.
Diagnosis of Tuberculosis
The diagnosis was made according to consensus clinical research case definitions for pediatric tuberculosis [22]. Bacteriologic confirmation was attempted using sputum and other specimens and Middlebrook 7H9 broth-base (Mycobacterial Growth Indicator Tubes; Becton Dickinson, Sparks, Maryland) culture medium; a commercial molecular line probe assay was used to detect resistance to isoniazid and rifampin (GenoType MTBDRplus assay; Hain Lifescience, Nehren, Germany). In the absence of bacteriologic confirmation, children were treated empirically according to the drug susceptibility test results of the likely source cases' isolates.
Children received individualized treatment regimens, based on WHO recommendations and in accordance with their drug susceptibility test results or that of their source case, where known [4]. Approved tablets of MFX (moxifloxacin hydrochloride 400-mg tablets; Dr Reddy's Laboratories Ltd, Hyderabad, India) were used. All HIV-infected children received trimethoprim-sulfamethoxazole and cART consisting of 2 nucleoside reverse transcriptase inhibitors (lamivudine, stavudine, or abacavir) and either efavirenz (4 of 6 children) or lopinavir/ritonavir (2 of 6 children).
Pharmacokinetic Investigation
Children receiving MFX 7.5–10 mg/kg once daily (according to WHO and South African MDR tuberculosis treatment guidelines) underwent pharmacokinetic sampling 2–8 weeks following initiation of tuberculosis treatment, when MFX would be expected to be at steady state. On the day of sampling, exact 10 mg/kg doses were calculated for each child. Tablets were cut and weighed accordingly (to the nearest 0.1 mg), and given either whole or crushed and suspended in water, according to patient tolerance. All tuberculosis drugs were administered with a small amount of water in the morning after a minimum of 4 hours of fasting; a standard breakfast was offered 1 hour later. HIV-infected children were given cART with breakfast. Blood samples were collected predose (time 0), and at 1, 2, 4, 8, and either 6 or 11 hours after dosing, in ethylenediaminetetraacetic acid–containing vacutainer tubes, centrifuged, and plasma was separated and frozen within 30 minutes at −80°C.
MFX concentrations were determined using a validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) assay, at the Division of Clinical Pharmacology, University of Cape Town. The assay was validated according to US Food and Drug Administration and European Medicines Agency guidelines. Plasma samples were extracted and chromatographic separation achieved on a Gemini-NX 5 µm C18, 50 mm × 2.1 mm analytical column. An AB Sciex API 3000 mass spectrometer was operated at unit resolution in the multiple reaction monitoring mode, monitoring the transition of the protonated molecular ions at mass-to-charge ratio (m/z) 402.2 to the product ions at m/z 384.2 for MFX, and monitoring the transition of the protonated molecular ions at m/z 406.3 to the product ions at m/z 388.2 for the stable isotope-labeled internal standard, MFX-d4. The calibration curve fitted a linear (weighted by 1/concentration) regression over the range 0.0628–16.1 µg/mL.
MFX pharmacokinetic measures were calculated using noncompartmental analysis (NCA). Stata 12.1 SE software (StataCorp 2011, College Station, Texas) was used for NCA and other analyses. Cmax and time until Cmax (Tmax) were taken directly from the concentration-time data. The area under the curve from 0–8 hours (AUC0–8) was calculated using the linear trapezoidal rule. The T1/2 was denoted as ln(2)/Kel, where Kel (elimination rate constant) is the negative slope of the log-linear regression of ≥3 final data points. Kel and T1/2 were not evaluated in patients with <3 concentration data points during the elimination phase. The area under the curve from 0–24 hours (AUC0–24) was calculated using exponential extrapolation from the patient's final concentration data point at either 8 or 11 hours.
Statistical Analysis
Demographic and clinical characteristics were summarized using descriptive statistics. MFX pharmacokinetic measures were reported as medians and interquartile ranges (IQRs). The AUC0–8, Cmax, and Tmax were compared by HIV status, nutritional status (weight-for-age z score [WAZ] ≤ −2 vs WAZ > − 2) using British growth charts, and administration method (crushed vs whole tablets) using the Wilcoxon rank-sum test. A multivariable linear regression model was generated to determine which covariates were associated with AUC0–8. The clinically relevant variables HIV, nutritional status, and age were included in the model. Estimated pharmacodynamic indices (AUC0–24/MIC [minimum inhibitory concentration] and Cmax/MIC) were calculated using an approximate MFX antimicrobial concentration that inhibits growth of 90 % of the strains (MIC90) of 0.5 µg/mL based on published studies [5, 23].
Assessment of Toxicity
All children had clinical safety assessments monthly for the first 6 months of treatment, then every 2 months until treatment was completed. Laboratory assessment was done every 2 months and included blood cell count (for children on cART and linezolid), liver function tests, creatinine, and thyroid function (for children on ethionamide or para-aminosalicylic acid). Toxicity and drug adverse effects were determined using combined data sources including chart and routine clinical review, laboratory investigation, and parental and children's report. Standard Division of AIDS (National Institute of Allergy and Infectious Diseases) criteria were used to grade severity of adverse events. Adverse events were considered MFX-related if they were possibly, probably, or definitely drug-related, and likely due to MFX or if they were not otherwise able to be attributed to another specific drug. Adverse events reported at consecutive follow-up visits without intervening asymptomatic periods were considered a single adverse event. Person time was calculated from the baseline study assessment until treatment completion or at the last available study visit if the child withdrew from the study or was still on treatment at the time of analysis.
A 12-lead electrocardiogram (ECG) was completed on the pharmacokinetic sampling day at 3 hours after drug dosing. ECGs were evaluated using a standard approach by a single pediatric cardiologist, with the corrected QT (QTc) interval calculated using the method described by Fridericia [24]; a QTc >450 ms was classified as prolonged.
Written informed consent was obtained from parents/legal guardians and written informed assent from children. The study was approved by the Health Research Ethics Committees, Stellenbosch University (reference number N11/03/059A) and the University of Cape Town, South Africa (reference number 200/2012).
RESULTS
Twenty-three children, with a median age of 11.1 years (IQR, 9.2–12.0 years) and median weight of 28.9 kg (range, 21.4–66 kg), were included; 6 of 23 (26.1%) were HIV-infected. Three children, all HIV-infected, were underweight for age (UWA). Clinical and demographic features are summarized in Table 1. Pulmonary tuberculosis was predominant (20 children [87%]). Antituberculosis medication used included ethambutol, pyrazinamide, amikacin, kanamycin, MFX, ethionamide, terizidone, para-aminosalicylic acid, high-dose isoniazid, linezolid, clofazimine, amoxicillin-clavulanate, and rifampin. The median number of antituberculosis drugs given was 7 (IQR, 6–7).
Table 1.
Demographic and Clinical Characteristics of Children (n = 23)
| Characteristic | No. (%) |
|---|---|
| Male sex | 9 (39.1) |
| Race | |
| Black | 13 (56.5) |
| Mixed ethnicity | 10 (43.5) |
| Previous tuberculosis episode or treatment | 11 (47.8) |
| Known current tuberculosis source case | 12 (52.2) |
| Certainty of tuberculosis diagnosisa | |
| Bacteriologically confirmed | 20 (87.0) |
| Probable tuberculosis | 3 (13.0) |
| Tuberculosis disease type | |
| PTB only | 14 (60.9) |
| EPTB only | 3 (13.0) |
| PTB and EPTB | 6 (26.1) |
| HIV-infected | 6 (26.1) |
| Weight-for-age z score < − 2.0b | 3 (13.0) |
Abbreviations: EPTB, extrapulmonary tuberculosis; HIV, human immunodeficiency virus; PTB, pulmonary tuberculosis.
a According to Graham et al [22].
b Calculated using 1990 British growth charts.
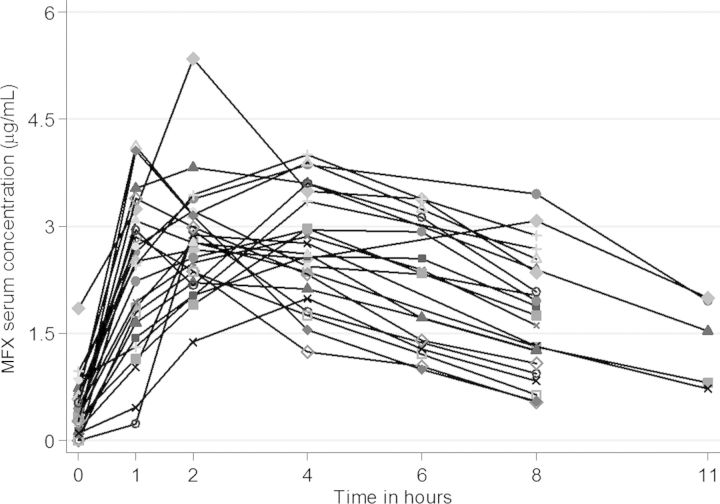
Table 2 shows summary MFX pharmacokinetic measures. The interindividual variability of MFX serum concentrations was high, especially during the absorption phase (Figure 1). MFX concentrations at 0 hour were below the limit of quantification in 3 participants and were taken as zero when generating the NCA parameters. When Kel was available, AUC0–24 was extrapolated. Kel could only be evaluated in 12 of 23 children. These 12 children did not differ from the 11 children without Kel calculation regarding AUC0–8 or Cmax. Tmax occurred faster in children with Kel calculation (data not shown).
Table 2.
Summary Statistics for Pharmacokinetic Measures in Children Following an Oral Moxifloxacin Dose of 10 mg/kg
| Pharmacokinetic Measure | No. | Moxifloxacin |
|---|---|---|
| Cmax, µg/mL | 23 | 3.08 (2.85–3.82) |
| Tmax, h | 23 | 2.0 (1.0–8.0) |
| CL/F, L/h | 12 | 10.53 (7.23–14.14) |
| Vd, L | 12 | 70.61 (57.53–77.70) |
| C0, µg/mL | 12 | 4.27 (3.38–4.86) |
| T1/2, h | 12 | 4.14 (3.45–6.11) |
| AUC0–8, µg × h/mL | 23 | 17.24 (14.47–21.99) |
| AUC0–24, µg × h/mL | 12 | 23.31 (19.24–42.30) |
All pharmacokinetic measures are reported as median and interquartile range, except for Tmax, which is reported as median and range.
Abbreviations: AUC0–8, area under the curve from 0–8 hours; AUC0–24, area under the curve from 0–24 hours; C0, concentration at time 0; CL, clearance; Cmax, maximum serum concentration; F, fraction absorbed; T1/2, half-life; Tmax, time until Cmax; Vd, volume of distribution.
Figure 1.
Individual serum concentrations in children 7–15 years of age following an oral moxifloxacin (MFX) dose of 10 mg/kg/day.
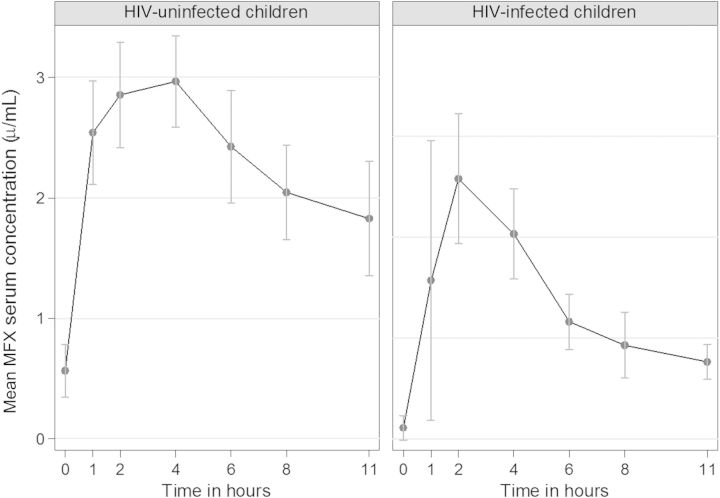
Table 3 shows pharmacokinetic measures stratified by HIV status, nutritional status, and administration method. Serum concentration-time curves following an oral MFX dose of 10 mg/kg stratified by HIV status are presented in Figure 2. HIV infection and UWA were associated with lower MFX AUC0–8, whereas Cmax and Tmax did not differ by nutritional status. There was a nonsignificant trend toward a lower Cmax in HIV-infected children (P = .08). Crushing of tablets was associated with more rapid absorption of MFX, whereas Cmax or AUC did not differ by administration method. Neither sex nor race had an influence on MFX pharmacokinetic measures (data not shown). In a simple linear regression model, AUC0–8 was reduced by 6.85 µg × h/mL (95% confidence interval [CI], −11.15 to −2.56 µg × h/mL) in HIV-infected children, and for each unit decrease in WAZ, AUC was reduced by 2.39 µg × h/mL. In a multivariable regression model, AUC0–8 was reduced by 5.62 µg × h/mL (95% CI, −10.94 to −.30 µg × h/mL) among patients with HIV infection compared with uninfected patients, adjusting for age and nutritional status.
Table 3.
Pharmacokinetic Measures by HIV Status, Nutritional Status, and Administration Method of Moxifloxacin in Children (n = 23)
| Characteristic | Cmax, µg/mL |
Tmax, h |
AUC0–8, µg × h/mL |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Median (IQR) | P Value | No. | Median (Range) | P Value | No. | Median (IQR) | P Value | |
| HIV-status | |||||||||
| HIV-infected | 6 | 2.83 (2.36–2.94) | 6 | 2.0 (1.0–4.0) | 6 | 13.19 (11.89–15.90) | |||
| HIV-uninfected | 17 | 3.21 (2.95–3.82) | .080 | 17 | 4.0 (1.0–8.0) | .334 | 17 | 19.98 (16.71–25.21) | .003 |
| WAZ | |||||||||
| ≥ − 2.0 | 20 | 3.08 (2.87–3.71) | 20 | 2.0 (1.0–8.0) | 20 | 18.76 (16.43–23.60) | |||
| < − 2.0 (UWA) | 3 | 2.78 (1.99–4.06) | .411 | 3 | 2.0 (1.0–4.0) | .594 | 3 | 14.47 (9.95–15.90) | .045 |
| Administration | |||||||||
| Whole | 20 | 2.96 (2.82–3.71) | 20 | 3.0 (1.0–8.0) | 20 | 17.36 (14.93–23.60) | |||
| Crushed | 3 | 3.21 (3.08–4.06) | .294 | 3 | 1.0 (1.0–2.0) | .047 | 3 | 17.24 (14.47–19.53) | .715 |
Abbreviations: AUC0–8, area under the curve from 0–8 hours; Cmax, maximum serum concentration; HIV, human immunodeficiency virus; IQR, interquartile range; Tmax, time until Cmax; UWA, underweight for age; WAZ, weight-for-age z score.
Figure 2.
Effect of human immunodeficiency virus (HIV) on moxifloxacin (MFX) serum concentrations in children aged 7–15 years following an oral dose of 10 mg/kg/day.
Children were followed for a median of 236 days (IQR, 142–541 days). Table 4 shows all adverse events noted as well as adverse effects potentially related to MFX. The main adverse effects potentially related to MFX were gastrointestinal (nausea and vomiting; n = 12), headache (n = 5), elevated alanine aminotransferase (ALT) (n = 4), and arthralgia (n = 4).
Table 4.
Adverse Events in Children Receiving Treatment for Multidrug-Resistant Tuberculosis Including Moxifloxacin
| Adverse Event | All Adverse Events Any Grade (1, 2, 3, 4) |
Adverse Effects Any Grade Possibly, Probably, Definitely Related to Moxifloxacin |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients With Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total No. of Events | Event Rate/PY | No. of Patients With Event | Grade 1 | Grade 3 | Grade 3 | Grade 4 | Total No. of Events | Event Rate/PY | |
| Arthralgia | 5 | 6 | 1 | 0 | 0 | 7 | 0.341 | 4 | 4 | 1 | 0 | 0 | 5 | 0.243 |
| Arthritis | 0 | 0 | 0 | 0 | 0 | 0 | … | 0 | 0 | 0 | 0 | 0 | 0 | … |
| Pain other than traumatic injury | 7 | 9 | 1 | 0 | 0 | 10 | 0.487 | 1 | 1 | 0 | 0 | 0 | 1 | 0.049 |
| Headache | 8 | 10 | 1 | 1 | 0 | 12 | 0.584 | 5 | 4 | 1 | 0 | 0 | 5 | 0.243 |
| Neurosensory alteration | 1 | 1 | 0 | 0 | 0 | 1 | 0.049 | 0 | 0 | 0 | 0 | 0 | 0 | … |
| Insomnia | 0 | 0 | 0 | 0 | 0 | 0 | … | 0 | 0 | 0 | 0 | 0 | 0 | … |
| Fatigue/malaise | 3 | 2 | 1 | 0 | 0 | 3 | 0.146 | 1 | 1 | 0 | 0 | 0 | 1 | 0.049 |
| Nausea | 15 | 22 | 1 | 0 | 0 | 23 | 1.119 | 9 | 9 | 0 | 0 | 0 | 9 | 0.438 |
| Vomiting | 11 | 13 | 1 | 0 | 0 | 14 | 0.681 | 3 | 3 | 0 | 0 | 0 | 3 | 0.146 |
| Anorexia | 2 | 2 | 0 | 0 | 0 | 2 | 0.097 | 0 | 0 | 0 | 0 | 0 | 0 | … |
| Cutaneous reaction | 2 | 2 | 0 | 0 | 0 | 2 | 0.097 | 1 | 1 | 0 | 0 | 0 | 1 | 0.049 |
| Pruritus | 6 | 8 | 0 | 0 | 0 | 8 | 0.389 | 2 | 4 | 0 | 0 | 0 | 4 | 0.195 |
| Acute systemic allergic reaction | 0 | 0 | 0 | 0 | 0 | 0 | … | 0 | 0 | 0 | 0 | 0 | 0 | … |
| Elevated ALT | 6 | 4 | 2 | 1 | 1 | 8 | 0.389 | 4 | 2 | 2 | 1 | 0 | 5 | 0.243 |
| Bilirubin | 1 | 0 | 1 | 0 | 0 | 1 | 0.049 | 1 | 0 | 1 | 0 | 0 | 1 | 0.049 |
Grading of adverse events according to Division of AIDS criteria. 23 patients followed for a median time of 236 days (interquartile range, 142–541 days); total person years = 20.55.
Abbreviations: ALT, alanine aminotransferase; PY, person-years.
In 13 of 23 children, ECG data were available. Mean QTc interval was 403 ms (standard deviation, 30 ms). None had a QTc interval >450 ms.
DISCUSSION
This is the first study to our knowledge investigating the pharmacokinetics and safety of MFX in children with tuberculosis. Exposure to MFX was lower than that achieved with a standard dose of 400 mg in adults receiving MFX for MDR tuberculosis (Supplementary Table 1) despite the slightly higher dose given to children (median dose of 6.7–8 mg/kg in adults vs 10 mg/kg in children) in our study.
A considerably lower AUC in children compared with adults has also been described for levofloxacin, ofloxacin, and many other first- and some second-line antituberculosis agents, and has been attributed to a more rapid elimination of drugs in children [25–28]. In 12 children, in whom it was possible to calculate, the T1/2 of 4 hours was roughly half of that in adults (7–10 hours). However, our data may be biased toward demonstrating more rapid elimination, as the T1/2 could not be calculated in children with a later Tmax and potentially slower elimination. Although drug elimination is expected to be more rapid in children because of allometric scaling [29], the degree of difference from adult values is surprising given that these relatively older children should be metabolically mature and have pharmacokinetics more closely approximating that of adults.
In general, adult activity of phase II enzymes is achieved within the first years of life, although some enzyme isoforms may exceed adult values during childhood [30]. Additionally, the extrapolated AUC0–24 may be underestimated as the extrapolation does not account for a 2-compartment model, which has been described for MFX [13].
In our study, HIV-infected and UWA children had lower MFX concentrations. The multivariate model suggests that this association of UWA with lower exposures may be due to confounding by HIV status. HIV infection has been associated with reduced absorption of antituberculosis agents in adults and children [27, 31] due to drug–drug interactions or gastrointestinal disturbances. In our study, Cmax in HIV-infected children seemed to be lower than in HIV-uninfected children, potential evidence for reduced absorption. Our search did not identify any study investigating drug–drug interactions of antiretrovirals and MFX. MFX is not metabolized by the cytochrome P450 system, but other interactions, such as has been described with rifampin [11], are possible.
Because of the lack of child-friendly formulations for many second-line tuberculosis drugs, it is common practice to crush tablets and administer these with water. The effect of such practices on the bioavailability has not been evaluated for any of the fluoroquinolones. Our data indicate that absorption occurs more rapidly if MFX tablets are crushed (median Tmax, 1.0 hour vs 3.0 hours) with no influence on the AUC; however, the number of participants is small and additional evaluation is warranted. When crushed, MFX tablets are bitter and rarely tolerated by children. Child-friendly formulations of fluoroquinolones are urgently needed.
Fluoroquinolones have concentration-dependent bactericidal activity, with the AUC/MIC ratio most closely associated with in vivo efficacy against M. tuberculosis in mice [7]. Proposed targets for the fluoroquinolones against M. tuberculosis are AUC0–24/MIC ratio of >100 or Cmax/MIC of 8–10 [7, 8]; however, their clinical relevance remains unclear, particularly for children who typically have paucibacillary tuberculosis. In our study, MFX MIC of isolates from the children could not be determined, and the MFX MIC90 of 0.5 µg/mL was taken from published data from the same study setting [23]. Children failed to achieve the proposed pharmacodynamic targets, with a median AUC0–24/MIC of 46.6 (IQR, 38.5–84.6) (n = 12) and a median Cmax/MIC of 6.2 (IQR, 5.7–7.6) (n = 23).
In vitro, M. tuberculosis resistance development depends on the fluoroquinolone concentration, and insufficient exposures may fail to prevent development of resistance [32]. However, the limited clinical data suggest that children treated with currently recommended doses of MFX have better outcomes than adults with MDR tuberculosis [20, 33, 34].
Nevertheless, with increasing numbers of pediatric MDR tuberculosis, especially in the context of HIV, antituberculosis treatment needs be optimized to further improve outcome, shorten the duration of treatment, and to prevent emergence of resistance. Because fluoroquinolones are the backbone of existing MDR tuberculosis therapy and will remain fundamentally important in novel regimens that may be shorter and injectable-sparing, their optimal use in children with MDR tuberculosis is critically important.
Overall, MFX with prolonged dosing was well tolerated. Gastrointestinal intolerance, headache, and mildly elevated ALT were the most frequent adverse effects. Our conservative approach may reflect an overestimation of MFX adverse effects in these children receiving multidrug regimens; adverse effects that may have been associated with other drugs of the antituberculosis treatment, but could not be convincingly attributed to them, were considered possibly MFX related. Except for 1 child with grade 3 ALT elevation, all adverse effects were mild and did not require cessation of therapy.
Use of fluoroquinolones in children has been limited because of their potential to induce arthropathy in juvenile animals [35]; however, no unequivocal documentation of similar fluoroquinolone-induced arthropathy in children has been observed [36, 37]. Five children in our study reported mild arthralgia; none had clinical evidence of arthritis. All resolved spontaneously without adjusting or stopping MFX. Although arthralgia/arthritis requiring cessation of fluoroquinolones has been reported in children with MDR tuberculosis receiving MFX therapy [20, 34], our report adds to a growing body of evidence showing the long-term use of currently recommended doses of fluoroquinolones to be safe [19, 38, 39]. This might partly be due to the low drug exposure in children; safety for higher, adult-equivalent doses should be evaluated.
Fluoroquinolones are known to prolong the QT interval, with MFX having the largest effect [40]. It is reassuring that we did not observe any QTc prolongation; however, we evaluated only a small number of participants and lacked a prefluoroquinolone baseline QTc assessment for comparison. Further evaluation in children is warranted given that in the future, MFX may be combined with novel tuberculosis drugs also known to cause QT prolongation, such as bedaquiline and delamanid.
Our study is limited by the modest number of children and the resulting difficulty in separating the effects of important covariates such as HIV status, nutritional status, or drug administration method as well as the influence of concomitant antituberculosis and antiretroviral medication. We were also limited by the lack of later sampling time points, which precluded our ability to characterize drug elimination in some participants. Future studies with larger study cohorts using population pharmacokinetic modeling techniques could overcome these limitations.
In conclusion, we demonstrate low MFX serum concentrations in children 7–15 years of age with MDR tuberculosis following an oral dose of MFX 10 mg/kg bodyweight. MFX was well tolerated with long-term administration. To approximate serum concentrations found in adults on standard doses, higher dosing of MFX may be required in children. Evaluation of safety and pharmacokinetics with higher doses as well as with child-friendly formulations should be considered.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the clinical research team at the Desmond Tutu Tuberculosis Centre, Stellenbosch University; the clinical paediatric team at Brooklyn Hospital for Chest Diseases; the laboratory team at the Division of Clinical Pharmacology, University of Cape Town; and Professor P. L. van der Merwe from the Faculty of Medicine and Health Sciences, Stellenbosch University, for their dedication in implementing the study. We also thank the children and their parents/legal guardians for participating in this study. We dedicate this research to our esteemed colleague and friend, Dr Klaus Magdorf, in memoriam.
Financial support. This work was supported by the National Institutes of Health (R01 069169-01 to A. C. H.); the German Leprosy and Tuberculosis Relief Association (to S. T.); and the South African National Research Foundation (grant 90729 to H. S. S. and H. M. M.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Geneva, Switzerland: WHO; Global tuberculosis report 2013. WHO/HTM/TB/2013.11. Available at: http://www.who.int/tb/publications/global_report/en/ Accessed 26 June 2014. [Google Scholar]
- 2.Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet. 2014;383:1572–9. doi: 10.1016/S0140-6736(14)60195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raizada N, Sachdeva KS, Nair SA, et al. Enhancing TB case detection: experience in offering upfront Xpert MTB/RIF testing to pediatric presumptive TB and DR TB cases for early rapid diagnosis of drug sensitive and drug resistant TB. PLoS One. 2014;9:e105346. doi: 10.1371/journal.pone.0105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis—2011 update. (WHO/HTM/TB/2011.6). Available at: www.who.int/tb/challenges/mdr/programmatic_guidelines_for_mdrtb/en/ Accessed 26 July 2014. [DOI] [PubMed]
- 5.Rodriguez JC, Ruiz M, Lopez M, Royo G. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int J Antimicrob Agents. 2002;20:464–7. doi: 10.1016/s0924-8579(02)00239-x. [DOI] [PubMed] [Google Scholar]
- 6.Seddon JA, Furin JJ, Gale M, et al. Caring for children with drug-resistant tuberculosis: practice-based recommendations. Am J Respir Crit Care Med. 2012;186:953–64. doi: 10.1164/rccm.201206-1001CI. [DOI] [PubMed] [Google Scholar]
- 7.Shandil RK, Jayaram R, Kaur P, et al. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother. 2007;51:576–82. doi: 10.1128/AAC.00414-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JL, Hadad DJ, Boom WH, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;10:605–12. [PubMed] [Google Scholar]
- 9.Wang JY, Wang JT, Tsai TH, et al. Adding moxifloxacin is associated with a shorter time to culture conversion in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2010;14:65–71. [PubMed] [Google Scholar]
- 10.Pranger AD, van Altena R, Aarnoutse RE, et al. Evaluation of moxifloxacin for the treatment of tuberculosis: 3 years of experience. Eur Respir J. 2011;38:888–94. doi: 10.1183/09031936.00176610. [DOI] [PubMed] [Google Scholar]
- 11.Nijland HM, Ruslami R, Suroto AJ, et al. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin Infect Dis. 2007;45:1001–7. doi: 10.1086/521894. [DOI] [PubMed] [Google Scholar]
- 12.Peloquin CA, Hadad DJ, Molino LP, et al. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52:852–7. doi: 10.1128/AAC.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zvada SP, Denti P, Geldenhuys H, et al. Moxifloxacin population pharmacokinetics in patients with pulmonary tuberculosis and the effect of intermittent high-dose rifapentine. Antimicrob Agents Chemother. 2012;56:4471–3. doi: 10.1128/AAC.00404-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manika K, Chatzika K, Zarogoulidis K, Kioumis I. Moxifloxacin in multidrug-resistant tuberculosis: is there any indication for therapeutic drug monitoring? Eur Respir J. 2012;40:1051–3. doi: 10.1183/09031936.00202411. [DOI] [PubMed] [Google Scholar]
- 15.Ruslami R, Ganiem AR, Dian S, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13:27–35. doi: 10.1016/S1473-3099(12)70264-5. [DOI] [PubMed] [Google Scholar]
- 16.Stass H, Kubitza D. Effects of dairy products on the oral bioavailability of moxifloxacin, a novel 8-methoxyfluoroquinolone, in healthy volunteers. Clin Pharmacokinet. 2001;40(suppl 1):33–8. doi: 10.2165/00003088-200140001-00005. [DOI] [PubMed] [Google Scholar]
- 17.Stass H, Kubitza D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother. 1999;43(suppl B):83–90. doi: 10.1093/jac/43.suppl_2.83. [DOI] [PubMed] [Google Scholar]
- 18.Moxifloxacin. Tuberculosis (Edinb) 2008;88:127–31. doi: 10.1016/S1472-9792(08)70016-7. [DOI] [PubMed] [Google Scholar]
- 19.Seddon JA, Hesseling AC, Godfrey-Faussett P, Schaaf HS. High treatment success in children treated for multidrug-resistant tuberculosis: an observational cohort study. Thorax. 2014;69:458–64. doi: 10.1136/thoraxjnl-2013-203900. [DOI] [PubMed] [Google Scholar]
- 20.Garazzino S, Scolfaro C, Raffaldi I, Barbui AM, Luccoli L, Tovo PA. Moxifloxacin for the treatment of pulmonary tuberculosis in children: a single center experience. Pediatr Pulmonol. 2014;49:372–6. doi: 10.1002/ppul.22755. [DOI] [PubMed] [Google Scholar]
- 21.Watt KM, Massaro MM, Smith B, Cohen-Wolkowiez M, Benjamin DK, Jr, Laughon MM. Pharmacokinetics of moxifloxacin in an infant with Mycoplasma hominis meningitis. Pediatr Infect Dis J. 2012;31:197–9. doi: 10.1097/INF.0b013e31823980c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham SM, Ahmed T, Amanullah F, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis. 2012;205(suppl 2):S199–208. doi: 10.1093/infdis/jis008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirgel FA, Warren RM, Streicher EM, Victor TC, van Helden PD, Bottger EC. gyrA mutations and phenotypic susceptibility levels to ofloxacin and moxifloxacin in clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother. 2012;67:1088–93. doi: 10.1093/jac/dks033. [DOI] [PubMed] [Google Scholar]
- 24.Fridericia LS. The duration of systole in an electrocardiogram in normal humans and in patients with heart disease. 1920. Ann Noninvasive Electrocardiol. 2003;8:343–51. doi: 10.1046/j.1542-474X.2003.08413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thee S, Seddon JA, Donald PR, et al. Pharmacokinetics of isoniazid, rifampin, and pyrazinamide in children younger than two years of age with tuberculosis: evidence for implementation of revised World Health Organization recommendations. Antimicrob Agents Chemother. 2011;55:5560–7. doi: 10.1128/AAC.05429-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaaf HS, Willemse M, Cilliers K, et al. Rifampin pharmacokinetics in children, with and without human immunodeficiency virus infection, hospitalized for the management of severe forms of tuberculosis. BMC Med. 2009;7:19. doi: 10.1186/1741-7015-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham SM, Bell DJ, Nyirongo S, Hartkoorn R, Ward SA, Molyneux EM. Low levels of pyrazinamide and ethambutol in children with tuberculosis and impact of age, nutritional status, and human immunodeficiency virus infection. Antimicrob Agents Chemother. 2006;50:407–13. doi: 10.1128/AAC.50.2.407-413.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thee S, Garcia-Prats AJ, McIlleron HM, et al. Pharmacokinetics of ofloxacin and levofloxacin for prevention and treatment of multidrug-resistant tuberculosis in children. Antimicrob Agents Chemother. 2014;58:2948–51. doi: 10.1128/AAC.02755-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson BJ, Holford NH. Understanding dosing: children are small adults, neonates are immature children. Arch Dis Child. 2013;98:737–44. doi: 10.1136/archdischild-2013-303720. [DOI] [PubMed] [Google Scholar]
- 30.Strolin Benedetti M, Whomsley R, Baltes EL. Differences in absorption, distribution, metabolism and excretion of xenobiotics between the paediatric and adult populations. Expert Opin Drug Metab Toxicol. 2005;1:447–71. doi: 10.1517/17425255.1.3.447. [DOI] [PubMed] [Google Scholar]
- 31.Gurumurthy P, Ramachandran G, Hemanth Kumar AK, et al. Malabsorption of rifampin and isoniazid in HIV-infected patients with and without tuberculosis. Clin Infect Dis. 2004;38:280–3. doi: 10.1086/380795. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, Dong Y, Zhao X, et al. Selection of antibiotic-resistant bacterial mutants: allelic diversity among fluoroquinolone-resistant mutations. J Infect Dis. 2000;182:517–25. doi: 10.1086/315708. [DOI] [PubMed] [Google Scholar]
- 33.Pinon M, Scolfaro C, Bignamini E, et al. Two pediatric cases of multidrug-resistant tuberculosis treated with linezolid and moxifloxacin. Pediatrics. 2010;126:e1253–6. doi: 10.1542/peds.2009-2172. [DOI] [PubMed] [Google Scholar]
- 34.Chauny JV, Lorrot M, Prot-Labarthe S, et al. Treatment of tuberculosis with levofloxacin or moxifloxacin: report of 6 pediatric cases. Pediatr Infect Dis J. 2012;31:1309–11. doi: 10.1097/INF.0b013e318269cc6a. [DOI] [PubMed] [Google Scholar]
- 35.Christ W, Lehnert T, Ulbrich B. Specific toxicologic aspects of the quinolones. Rev Infect Dis. 1988;10(suppl 1):S141–6. doi: 10.1093/clinids/10.supplement_1.s141. [DOI] [PubMed] [Google Scholar]
- 36.Schaad UB. Will fluoroquinolones ever be recommended for common infections in children? Pediatr Infect Dis J. 2007;26:865–7. doi: 10.1097/INF.0b013e3180cc20e4. [DOI] [PubMed] [Google Scholar]
- 37.Grady R. Safety profile of quinolone antibiotics in the pediatric population. Pediatr Infect Dis J. 2003;22:1128–32. doi: 10.1097/01.inf.0000101994.25947.12. [DOI] [PubMed] [Google Scholar]
- 38.Seddon JA, Godfrey-Faussett P, Hesseling AC, Gie RP, Beyers N, Schaaf HS. Management of children exposed to multidrug-resistant Mycobacterium tuberculosis. Lancet Infect Dis. 2012;12:469–79. doi: 10.1016/S1473-3099(11)70366-8. [DOI] [PubMed] [Google Scholar]
- 39.Bamrah S, Brostrom R, Dorina F, et al. Treatment for LTBI in contacts of MDR-TB patients, Federated States of Micronesia, 2009–2012. Int J Tuberc Lung Dis. 2014;18:912–8. doi: 10.5588/ijtld.13.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang J, Wang L, Chen XL, Triggle DJ, Rampe D. Interactions of a series of fluoroquinolone antibacterial drugs with the human cardiac K+ channel HERG. Mol Pharmacol. 2001;59:122–6. doi: 10.1124/mol.59.1.122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.