The intervention tested in the Retention in Care Study demonstrated improvement in retention in human immunodeficiency virus care. Enhanced personal contact with or without additional behavioral skills training significantly improved visit constancy and visit adherence over 12 months.
Keywords: behavioral intervention trial, HIV infection, HIV specialty clinics, randomized controlled trial, retention in care
Abstract
Background. The aim of the study was to determine whether enhanced personal contact with human immunodeficiency virus (HIV)–infected patients across time improves retention in care compared with existing standard of care (SOC) practices, and whether brief skills training improves retention beyond enhanced contact.
Methods. The study, conducted at 6 HIV clinics in the United States, included 1838 patients with a recent history of inconsistent clinic attendance, and new patients. Each clinic randomized participants to 1 of 3 arms and continued to provide SOC practices to all enrollees: enhanced contact with interventionist (EC) (brief face-to-face meeting upon returning for care visit, interim visit call, appointment reminder calls, missed visit call); EC + skills (organization, problem solving, and communication skills); or SOC only. The intervention was delivered by project staff for 12 months following randomization. The outcomes during that 12-month period were (1) percentage of participants attending at least 1 primary care visit in 3 consecutive 4-month intervals (visit constancy), and (2) proportion of kept/scheduled primary care visits (visit adherence).
Results. Log-binomial risk ratios comparing intervention arms against the SOC arm demonstrated better outcomes in both the EC and EC + skills arms (visit constancy: risk ratio [RR], 1.22 [95% confidence interval {CI}, 1.09–1.36] and 1.22 [95% CI, 1.09–1.36], respectively; visit adherence: RR, 1.08 [95% CI, 1.05–1.11] and 1.06 [95% CI, 1.02–1.09], respectively; all Ps < .01). Intervention effects were observed in numerous patient subgroups, although they were lower in patients reporting unmet needs or illicit drug use.
Conclusions. Enhanced contact with patients improved retention in HIV primary care compared with existing SOC practices. A brief patient skill-building component did not improve retention further. Additional intervention elements may be needed for patients reporting illicit drug use or who have unmet needs.
Clinical Trials Registration. CDCHRSA9272007.
For human immunodeficiency virus (HIV)–infected patients to experience the maximal benefits of antiretroviral therapy (ART), they must remain engaged in medical care [1, 2]. Timely initial and continuing engagement in care are critical to allow uninterrupted access to ART and sustained viral suppression, with implications for individual health outcomes and prevention of HIV transmission [3–5]. Despite an increased focus on retention in care by researchers, clinical providers, and policy administrators, many HIV-infected patients fail to maintain effective engagement in care. A recent meta-analysis indicated that for assessment periods of <3 years, 62% of HIV-infected patients were retained in care [6]. Another analysis from data collected in 12 HIV clinics found that 49%–68% of patients were retained in care across 2 years [7]. Other research found that 31% of new patients failed to attend their first scheduled appointment, and a multisite study found that new patients attended a significantly lower percentage of all scheduled primary care visits than established patients [8, 9]. Due to these challenges, the National HIV/AIDS Strategy called for an increase in the percentage of patients in continuous care to 80% by 2015 [10]. No interventions have been shown to improve retention in HIV care in published randomized controlled trials [11].
We examined 2 questions: (1) whether enhanced personal contact between HIV-infected patients and trained project staff improves retention in care compared to clinics' existing standard of care practices, and (2) whether giving patients brief training in behavioral skills relevant to retention (organization, problem solving, and communication with providers) improves retention over and above the effect of enhanced contact. Our target population was patients at historically higher risk for inadequate retention, including established patients with a history of missed visits and patients new to care. This report presents retention-in-care outcomes during the 12-month intervention period.
METHODS
Design Overview
This was a multisite trial in which patients were randomized to 1 of 2 intervention arms or to a standard of care (SOC) arm. In the enhanced contact (EC) arm, we examined whether a dedicated individual, maintaining personal contact with a patient, in combination with basic HIV education, was sufficient to significantly improve clinic attendance for primary care. An enhanced contact plus skills (EC + skills) arm added an additional 1-hour session to deliver support, motivation, and behavioral skills training to patients. The EC + skills arm was based on the assumption that sustained engagement in care may require more than personal contact. The behavioral skills component was informed by the information–motivation–behavioral skills (IMB) model [12, 13]. Applying the IMB model to retention in HIV care, we designed intervention efforts that focused on increasing knowledge about the importance of retention, on promoting motivation to attend HIV primary care visits, and on developing both the self-efficacy to attend appointments and the requisite communication, organization, and problem-solving skills needed to achieve this goal. The tenets of the IMB model have been tested extensively as they relate to HIV-related health behaviors, and have guided successful interventions in the area of HIV prevention [14, 15].
Standard of Care
Appointment reminders have been shown to increase clinic attendance [16–18]; in fact, there is now widespread adoption of patient reminder systems in ambulatory care settings. Despite the benefits of reminder systems, however, patients continue to miss appointments. Given the existing SOC in our study clinics (see Supplementary Table A), we wanted to know if an intervention with continuous personal contacts or personal contacts plus behavior skills trainings would improve retention over and above the SOC. Our clinics all had established SOC reminder systems, but those activities varied by clinic. Appointment reminder calls were SOC at the 6 clinical sites. Manual reminder calls, made by an assigned staff person, were SOC at 3 clinics. Automated reminder calls using a recorded voice were SOC at 4 clinics; 1 clinic used both manual and automated calls.
Setting and Participants
Six academically affiliated HIV clinics participated: Boston University/Boston Medical Center, State University of New York/Downstate Medical Center, Johns Hopkins University Medical Center, University of Alabama at Birmingham, University of Miami/Jackson Memorial Hospital, and Baylor College of Medicine/Thomas Street Health Center. Eligible patients were able to understand and read English or Spanish, able to provide informed consent, ≥18 years of age (19 in Alabama due to consent laws), not planning to move out of the area for 12 months, and not giving hospitalization or incarceration as the reason for a prior missed visit. The study coordinator at each site approached eligible patients who had missed 1 or more visits in the past 12 months, had a gap in care of at least 6 months in the previous year, or were considered new patients. New patients were those completing their first or second visit to the HIV clinic as well as those with a lapse in care of at least 3 years before the study enrollment date. “Established” patients were those past their second primary care visit to the clinic who were inconsistent in clinic attendance in the prior year (as defined above). New patients were oversampled to ensure that approximately one-third of all enrollees were new patients. Each participant provided written informed consent, and the study was approved by the institutional review boards at each participating clinic site.
Randomization
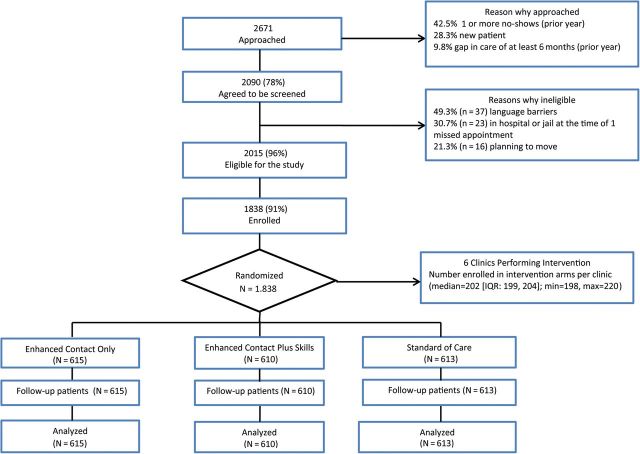
The intervention enrollment goal was 1800 patients, or 300 per clinic; the goal was based on a power calculation for detecting a difference as small as 15% in the percentage attending clinic at least twice in 12 months, with at least 90% power. Consenting patients were randomized to 1 of the 3 arms using a blocking factor of 6. A total of 1838 patients were enrolled, randomized, and included in the preplanned 12-month analysis (Figure 1).
Figure 1.
Flowchart for inclusion of participants in data analysis. Abbreviation: IQR, interquartile range.
Intervention
Interventionists received their training at a 5-day workshop in May 2010, in Atlanta, Georgia. The training consisted of didactic and interactive sessions in which the interventionists practiced using communication and teaching skills for patient interactions, eliciting strengths related to keeping appointments, and delivering skills modules to EC + skills arm participants.
Participants randomized to the EC or EC + skills arms were introduced to an interventionist employed for the project who provided them a brief HIV education module. Enhanced contacts in the EC and EC + skills arms consisted of the following:
Establishing a personal relationship in a face-to-face meeting with the patient.
- Remaining in continuous contact with the patient over the year, through:
- Brief face-to-face meetings with the interventionist at each HIV primary care visit. In these meetings, the interventionist provided positive reinforcement to the patient for keeping the primary care appointment and responded to questions or concerns about the primary care visit.
- Interim contact phone calls approximately halfway between scheduled primary care appointments to maintain contact with patients over the course of the study.
- Personal reminder phone calls at 7 days and 2 days before the next scheduled HIV primary care appointment.
- Phone calls within 24 hours after a missed HIV primary care appointment.
The EC + skills arm had the following unique elements in addition to the above (see Supplementary Figure A):
Patients were scheduled to return within 2 weeks of enrollment to receive one-on-one training on personal organization skills, communication with providers, and problem-solving skills (as prioritized from an unmet needs assessment).
A jointly agreed-to plan to address unmet needs was developed, including referrals to case managers and other supports.
Strengths-based interactions were used to help patients identify problems and use personal strengths to accomplish behavioral goals.
Outcome Measures
Primary Outcomes
The primary outcomes were a binary visit constancy measure (at least 1 kept visit with an HIV primary care provider in 3 consecutive 4-month intervals), and a visit adherence measure (number of kept appointments in 12 months divided by the total number of scheduled appointments, excluding cancellations). Although there is no single gold standard for measuring retention in HIV care [19, 20], prior studies have found that higher levels of missed visits and lower levels of visit constancy are significantly associated with mortality [21, 22], nonsuppressed viral loads [23–25], drug resistance [26], and failure to establish care [8].
Secondary Outcomes
Clinic scheduling efficiency was assessed by contrasting counts per enrollee of kept, missed, and canceled visits for the SOC arm vs each intervention arm. Large increases in canceled visits could be disruptive to the clinic.
Demographic, Clinical, and Behavioral Measures
Enrollees completed an audio computer-assisted self-interview before learning their arm assignment. Participant responses measured perceived unmet support service needs (food, housing, transportation, employment, finances, mental health, or substance abuse treatment), illicit drug use (powder cocaine, crack cocaine, crystal methamphetamine, heroin, prescription pain killers, injected cocaine or heroin), and demographics (age, sex, race/ethnicity, and insurance). Because there were no validated measures of unmet needs available, the unmet needs questions were adapted from the measures by Tobias et al [27]. The illicit drug use questions were adapted from a validated measure known as the Alcohol, Smoking, And Substance Involvement Screening Test [28, 29]. Clinical variables (ART, HIV RNA load, CD4+ T-cell count) were recorded from the medical record. Number and length of successful personal contacts were recorded by the interventionist. Successful contacts were those in which the interventionist talked with the participant.
Statistical Analysis
Visit constancy over 12 months was a yes/no binary variable. Kept appointments percentage (visit adherence) was analyzed as kept visits (events) divided by scheduled visits (trials). For both outcomes, the measure of effect was a risk ratio (relative improvement) relative to the SOC arm, calculated with a log-binomial model. We conducted preplanned tests of interaction for all the variables in Table 1 to determine whether the effect of the intervention was modified by any of these variables. The EC and EC + skills arms were combined for the tests of interaction with variables in Table 1 because each of these 2 arms produced the same magnitude of effect. Secondary outcomes (counts of kept, missed, and canceled visits) were compared by arm with a Poisson regression model. Statistical analyses were performed using SAS software version 9.2 (SAS Institute, Cary, North Carolina).
Table 1.
Baseline Characteristics of the Participants by Intervention Arm, Retention in Care Study, 2010–2012 (N = 1838)
| Variable | Enhanced Contact (n = 615), No. (%) | Enhanced Contact Plus Skills (n = 610), No. (%) | Standard of Care (n = 613), No. (%) | P Value (df) |
|---|---|---|---|---|
| Age group, y | ||||
| 18–29 | 59 (9.6) | 67 (11.0) | 73 (12.0) | .90 (8) |
| 30–39 | 118 (19.2) | 121 (19.8) | 122 (20.0) | |
| 40–49 | 210 (34.2) | 202 (33.1) | 212 (34.7) | |
| 50–59 | 187 (30.5) | 176 (28.9) | 166 (27.2) | |
| ≥60 | 40 (6.5) | 44 (7.2) | 38 (6.2) | |
| Sex | ||||
| Male | 409 (66.3) | 369 (60.4) | 380 (62.0) | .18 (4) |
| Female | 203 (33.2) | 234 (38.4) | 228 (37.2) | |
| Transgender | 3 (0.5) | 7 (1.2) | 5 (0.8) | |
| Race/ethnicity | ||||
| Black | 431 (70.1) | 422 (69.2) | 409 (66.7) | .27 (6) |
| White | 87 (14.1) | 72 (11.8) | 76 (12.4) | |
| Other race | 18 (2.9) | 15 (2.5) | 20 (3.3) | |
| Hispanic | 79 (12.9) | 101 (16.6) | 108 (17.6) | |
| Patient type | ||||
| Newa | 175 (28.1) | 177 (29.0) | 174 (28.4) | .96 (2) |
| Established | 440 (71.9) | 433 (71.0) | 439 (71.6) | |
| Unmet needsb, last 6 mo | ||||
| Yes (any) | 266 (43.2) | 257 (42.1) | 238 (38.8) | .26 (2) |
| No (none) | 349 (56.8) | 353 (57.9) | 375 (61.2) | |
| Any illicit drugc use, last 3 mo | ||||
| Yes | 105 (17.1) | 122 (20.0) | 114 (18.6) | .42 (2) |
| No | 510 (82.9) | 488 (80.0) | 499 (81.4) | |
| On antiretrovirals | ||||
| Yes | 483 (78.5) | 480 (78.7) | 474 (77.3) | .82 (2) |
| No | 132 (21.5) | 130 (21.3) | 139 (22.7) | |
| CD4 count, cells/µL | ||||
| ≥350 | 322 (56.2) | 346 (60.5) | 345 (60.0) | .27 (2) |
| <350 | 251 (43.8) | 226 (39.5) | 230 (40.0) | |
| Viral load | ||||
| Suppressedd | 345 (57.9) | 314 (53.7) | 346 (58.4) | .20 (2) |
| Not suppressed | 251 (42.1) | 271 (46.3) | 246 (41.6) | |
| Insurance | ||||
| Private | 84 (13.9) | 78 (13.0) | 91 (15.1) | .54 (6) |
| Medicare | 111 (18.4) | 116 (19.3) | 133 (22.1) | |
| Medicaid | 256 (42.5) | 246 (41.0) | 234 (38.9) | |
| Other/Ryan Whitee | 152 (24.2) | 160 (26.7) | 144 (23.9) | |
Patients missing data on age, baseline CD4 count, baseline viral load, and insurance were excluded from the table. Clinic visit data, patient type, and insurance originated in the clinic's appointment system. CD4, viral load, and antiretrovirals data came from electronic medical records. A baseline audio computer-assisted self-interview collected patients’ data on age, sex, race/ethnicity, illicit drug use, and unmet needs. Race/ethnicity status was a created variable from race status and ethnicity status; Hispanics were classified as “Hispanic” regardless of race.
a Based on patient's first or second visit, or not seen in ≥3 years.
b Unmet needs were adapted from Tobias et al [27]. The measures included mental health counseling and help with housing, transportation, employment, financial assistance, or substance use.
c Illicit drug use was adapted from the ASSIST instrument [28, 29] and consisted of use of powder cocaine, crack cocaine, crystal methamphetamine, heroin, prescription pain killers, injected cocaine, or heroin. Prescription drugs and painkillers were defined as use of Vicodin, hydroxycodone, Oxycontin/oxycodone, codeine, Percocet, Demerol, Darvon, Dilaudid, or Xanax without a prescription.
d Less than or equal to 200 copies/mL.
e Ryan White CARE Act coverage; “Other” includes university or local charity safety-net programs.
RESULTS
Baseline characteristics by intervention arm for the 1838 enrollees are presented in Table 1. None of the characteristics differed significantly by trial arm, indicating that the random assignment procedure generated comparable arms at baseline. Thus, none of these variables needed to be statistically controlled in the primary analysis examining intervention effects by trial arms.
Primary Outcomes: Test of Intervention Effects
Intervention effects for visit constancy and adherence are shown in Table 2. For the EC and EC + skills arms, visit constancy was 55.8% and 55.6%; it was 45.7% in the SOC arm. Risk ratios showed that visit constancy was 22% higher in the intervention arms than in the SOC arm. For the visit adherence measure, kept visits were 73% for the EC arm, 71% for EC + skills, and 67% for the SOC arm. Risk ratios for visit adherence showed that this outcome was 8% (EC) and 6% (EC + skills) higher than the SOC arm. The increases in visit constancy and visit adherence were significant (P < .01) for each intervention arm compared with the SOC arm. There were no differences between the EC and EC + skills arms on the 2 outcome measures. The ratio of EC to the EC + skills arm was 1.007 for the constancy measure (P = .89), and 1.022 for the visit adherence measure (P = .14). Mean and median numbers of successful enhanced contacts and total minutes per participant are presented in Supplementary Tables B–E. Overall, 47% of telephone contacts were successful (Supplementary Table F).
Table 2.
Retention in Care Outcomes by Intervention Arm, Retention in Care Study, 2010–2012 (N = 1838)
| Study Arm | Visit Constancy, %a | Risk Ratio (95% CI) | Visit Adherence, %b | Risk Ratio (95% CI) |
|---|---|---|---|---|
| Enhanced contact only (n = 615) | 55.8 | 1.22 (1.09–1.36) | 72.5 | 1.08 (1.05–1.11) |
| Enhanced contact plus skills (n = 610) | 55.6 | 1.22 (1.09–1.36) | 70.9 | 1.06 (1.02–1.09) |
| Standard of care (n = 613) | 45.7 | Ref | 67.2 | Ref |
Abbreviation: CI, confidence interval.
a Defined as percentage of participants with a care visit in each of 3 consecutive 4-month intervals.
b Defined as each patient's kept visits divided by scheduled appointments (excluding canceled).
Secondary Outcomes: Examination of Clinic Efficiency During the Intervention
Table 3 compares the EC and EC + skills arms vs the SOC arm for counts of kept, canceled, and no-show visits. Among these 1838 patients, the intervention significantly increased kept visits (for both intervention arms), moderately decreased no-show visits (significant for the EC arm), and moderately increased canceled visits(significant for the EC + skills arm) over the SOC arm.
Table 3.
Analysis of Canceled, Kept, and Missed Visit Counts by Study Arm (N = 1838)
| Study Arm | Canceled Visitsa |
Kept Visits |
Missed Visits |
|||
|---|---|---|---|---|---|---|
| Mean Counts per Person | P Valueb | Mean Counts per Person | P Value | Mean Counts per Person | P Value | |
| Enhanced contact (n = 615) | 1.41 | .12 | 4.12 | <.0001 | 1.56 | .01 |
| Enhanced contact plus skills (n = 610) | 1.49 | .01 | 4.14 | <.0001 | 1.70 | .50 |
| Standard of care (n = 613) | 1.31 | Ref | 3.59 | Ref | 1.75 | Ref |
a Scheduled visits canceled by the clinic or patient ahead of time.
b Log-linear Poisson regression model estimate.
Test of Intervention Effect in Subgroups
Table 4 presents the results of analyses of intervention effects in demographic and clinical subgroups. We tested each risk factor × intervention interaction with the variables in Table 1 and report the significant interaction P values below. Only 2 variables had statistically significant subgroup differences in the intervention effects: unmet needs interaction with the intervention on visit constancy (P = .007) and illicit drug use interaction with the intervention on visit constancy (P = .044). Patients reporting at least 1 unmet need (n = 761) had little benefit from the intervention on either outcome measure, whereas patients without unmet needs (n = 1077) had 2 of the largest effect sizes (risk ratio [RR], 1.36 and 1.10) across these subsets for both outcome measures. The 341 patients reporting a history of illicit drug use failed to show an effect of the intervention on either measure, in contrast to a robust effect of the intervention for patients who reported no history of illicit drug use (RR, 1.28 and 1.08). We additionally tested the clinic site × intervention interactions. Neither was significant (interaction with the intervention on visit constancy, P = .490; interaction with the intervention on visit adherence, P = .102). None of the other variables in Table 4 (age, sex, race/ethnicity, new/established, ART, CD4, viral load, insurance) had significant interaction effects.
Table 4.
Unadjusted Pooled Interventions Versus Standard of Care Risk Ratios for Baseline Characteristics, Retention in Care Study (N = 1838)
| Variable | Visit Constancy, % |
Visit Adherence, % |
||||
|---|---|---|---|---|---|---|
| EC and EC + Skills Intervention Armsa, % (No.) | Standard of Care Arm, % (No.) | Risk Ratio (95% CI) | EC and EC + Skills Intervention Arms, % (No.) | Standard of Care Arm, % (No.) | Risk Ratio (95% CI) | |
| Overall (N = 1838) | 55.7 (1225) | 45.7 (613) | 1.22 (1.10–1.35) | 71.7 (1212) | 67.2 (606) | 1.07 (1.04–1.10) |
| Sex | ||||||
| Male (n = 1158) | 53.4 (778) | 43.0 (380) | 1.24 (1.09–1.42) | 72.5 (770) | 68.9 (375) | 1.05 (1.02–1.09) |
| Female (n = 665) | 59.7 (437) | 50.7 (228) | 1.18 (1.02–1.37) | 70.4 (432) | 65.1 (226) | 1.08 (1.03–1.13) |
| Age group, y | ||||||
| 18–39 (n = 560) | 46.0 (365) | 42.6 (195) | 1.08 (.89–1.32) | 66.7 (850) | 63.9 (410) | 1.04 (.99–1.10) |
| ≥40 (n = 1275) | 59.8 (859) | 47.1 (416) | 1.27 (1.13–1.43) | 73.6 (361) | 68.7 (194) | 1.07 (1.04–1.10) |
| Race/ethnicity | ||||||
| Black (n = 1262) | 55.9 (853) | 44.7 (409) | 1.25 (1.10–1.43) | 70.1 (846) | 65.5 (406) | 1.07 (1.03–1.11) |
| White (n = 235) | 46.5 (159) | 43.4 (76) | 1.07 (.79–1.46) | 75.6 (156) | 72.7 (73) | 1.04 (.96–1.12) |
| Other race (n = 53) | 63.6 (33) | 35.0 (20) | 1.82 (.95–3.48) | 74.5 (32) | 66.0 (20) | 1.13 (.96–1.33) |
| Hispanic (n = 288) | 62.0 (180) | 52.8 (108) | 1.17 (.95–1.44) | 76.1 (178) | 71.3 (107) | 1.07 (1.00–1.14) |
| Patient type | ||||||
| Newa (n = 526) | 50.3 (352) | 43.7 (174) | 1.15 (.94–1.40) | 71.7 (869) | 67.8 (437) | 1.06 (1.00–1.12) |
| Established (n = 1312) | 57.9 (873) | 46.5 (439) | 1.24 (1.11–1.40) | 71.7 (343) | 67.0 (169) | 1.07 (1.04–1.10) |
| Unmet needs, last 6 mo | ||||||
| Yes (any) (n = 761) | 49.1 (523) | 47.5 (238) | 1.04 (.88–1.21) | 69.2 (693) | 67.5 (370) | 1.03 (.98–1.07) |
| No (none) (n = 1077) | 60.5 (702) | 44.5 (375) | 1.36 (1.20–1.54) | 73.6 (519) | 67.0 (236) | 1.10 (1.06–1.14) |
| Any illicit drug use, last 3 mo | ||||||
| Yes (n = 341) | 44.9 (227) | 46.5 (114) | 0.97 (.76–1.23) | 63.6 (224) | 62.8 (113) | 1.01 (.94–1.09) |
| No (n = 1497) | 58.1 (998) | 45.5 (499) | 1.28 (1.15–1.43) | 73.7 (988) | 68.3 (493) | 1.08 (1.05–1.11) |
| On antiretrovirals | ||||||
| Yes (n = 1437) | 57.2 (963) | 46.6 (474) | 1.23 (1.10–1.37 | 72.5 (954) | 67.9 (468) | 1.07 (1.03–1.10) |
| No (n = 401) | 50.0 (262) | 42.5 (139) | 1.18 (.94–1.48) | 68.9 (258) | 65.3 (138) | 1.06 (.99–1.12) |
| Baseline CD4 count, cells/µL | ||||||
| ≥350 (n = 1013) | 58.1 (668) | 49.9 (345) | 1.17 (1.03–1.32) | 73.9 (662) | 69.5 (341) | 1.06 (1.03–1.10) |
| <350 (n = 707) | 55.1 (477) | 43.0 (230) | 1.28 (1.08–1.52) | 69.9 (475) | 66.3 (229) | 1.05 (1.01–1.10) |
| Baseline viral load | ||||||
| Suppressedb (n = 1005) | 58.6 (659) | 50.6 (346) | 1.16 (1.02–1.31) | 74.9 (651) | 71.9 (341) | 1.04 (1.01–1.08) |
| Not suppressed (n = 768) | 51.2 (522) | 40.7 (246) | 1.26 (1.06–1.50) | 67.7 (518) | 62.1 (246) | 1.09 (1.04–1.14) |
| Insurance | ||||||
| Private (n = 253) | 54.3 (162) | 56.0 (91) | 0.97 (.77–1.22) | 79.4 (161) | 77.2 (88) | 1.03 (.96–1.10) |
| Medicare (n = 360) | 61.7 (227) | 42.9 (133) | 1.44 (1.15–1.80) | 74.2 (226) | 64.9 (132) | 1.14 (1.07–1.22) |
| Medicaid (n = 736) | 61.6 (502) | 50.0 (234) | 1.24 (1.07–1.44) | 70.6 (499) | 66.2 (233) | 1.07 (1.02–1.11) |
| Other/Ryan White (n = 456) | 46.4 (312) | 37.5 (144) | 1.21 (.95–1.55) | 68.9 (305) | 67.0 (144) | 1.03 (.97–1.10) |
Cells do not add to arm totals because of missing data on age, sex, CD4 cell count, and baseline viral load.
Abbreviations: CI, confidence interval; EC, enhanced contact.
a Intervention arms combined because of similarity on the study outcomes.
b Less than or equal to 200 copies/mL.
DISCUSSION
Our study provides the first randomized trial evidence of an efficacious intervention for improving retention in HIV care. We have shown that enhanced personal contacts with patients across time improved both visit constancy and visit adherence for primary care. The addition of a brief patient-centered behavioral skills component did not improve the outcomes further. Given that all clinics had either manual or automated reminder calls as SOC practices, we now know that the personal contacts that were part of our intervention yielded measurable increases in retention over and above the existing background of reminder calls. Furthermore, there were no significant differences in the intervention effects across clinics for either of the trial outcomes, and we observed consistent outcomes for many demographic and clinical subgroups. The findings have clear implications for improving health outcomes of persons living with HIV, as well as on achieving the goals of the National HIV/AIDS Strategy [10].
How kept, canceled, and missed visits are affected by a retention-in-care intervention matters because the intervention's impact on the clinic might not be all positive. Considering both intervention arms together, the intervention had a mixed effect on the use of clinic resources, because kept visits increased significantly, whereas missed visits decreased moderately and canceled visits were moderately higher compared with the SOC arm. Canceled visits would negatively affect clinic efficiency when the timing of the cancelation does not allow another patient to be put into that slot, although in some settings the canceled slots could be used to more efficiently handle walk-in and urgent patient visits. We are making the assumption that most canceled visits were caused by the patients, but clinics can also cancel visits and we cannot distinguish between the 2 reasons. We also cannot determine whether a canceled visit was immediately rescheduled, and both issues are limitations for the study. Nevertheless, research has shown that canceled visits are clearly negative for patients, as canceled visits have been shown to be associated with a greater rate of nonsuppressed viral load [30]. However, canceled visits do provide an opportunity to immediately reschedule an appointment and thus keep the patient in the system without additional staff follow-up efforts, and visits canceled in a timely manner allow the clinic to fill that slot with another patient. Missed visits are overwhelmingly negative for clinic efficiency, as well as for the health of patients, as they have been associated with higher rates of deleterious health outcomes [8, 21–26].
The intervention we provided was an enhancement of personal contact with or without additional skills training. Because of the package of EC activities in the intervention arms, we cannot ascribe separate effects to each EC activity (eg, forging a connection with interventionist, face-to-face encounters at clinic visits, missed visit calls, interim calls, or reminder calls). With the randomized design, we are confident that as a package these activities had a significant positive effect on visit constancy and visit adherence. We can be more definite about the lack of additional effects of behavioral skills training because those activities were introduced in the EC + skills arm and showed no additional benefit over and above enhanced contact over 12 months.
Among the few subgroups not demonstrating an intervention effect, several findings are worth noting. In particular, patients with at least 1 unmet need, and patients with a history of illicit drug use benefited little from the intervention. In addition to the interaction results, patients with illicit drug use had the lowest rates of visit constancy and visit adherence among all subgroups. Previous research has found that users of crack cocaine, methamphetamine, or injection drugs were significantly less likely to be linked to HIV care following a linkage intervention [31–33] or be retained in HIV care [21, 27, 34–37]. Our intervention was not designed to increase clinic resources to treat substance users or increase case management, and substance users were not singled out for special attention. Some additional intervention elements over and above the current approach, such as co-located drug treatment services [23], may be required to improve retention among substance users. We also found that patients with at least 1 unmet need benefited little from the intervention. Unmet needs are a big problem because they cut across so many domains that affect patients' ability to remain engaged in care [38]. Having unmet needs may have reduced the patient's receptiveness or ability to respond optimally to the intervention. Other studies have found that as the number of unmet needs increases, the number of clinic visits for primary care decreased [27]. Our intervention components did not directly address unmet needs other than referring enrollees to clinic case managers when interventionists learned that patients had unmet housing, food, transportation, substance use, or mental health needs. Alternative intervention approaches that specifically help patients with unmet needs may be required to improve retention in HIV care in these patients. A concerted effort to help patients resolve unmet needs would provide a context in which a behavioral intervention is more likely to universally improve retention in care.
In summary, this randomized controlled trial presents evidence of an efficacious intervention to improve retention in HIV care. Enhanced personal contacts coupled with basic HIV education, with or without additional behavioral skills training, significantly improved visit adherence and visit constancy during a 12-month intervention period. Patients responded well to the intervention, based on overall consistency in effect for both outcomes. Although widely efficacious across many demographic and clinical subgroups, persons with unmet needs and persons with a history of illicit drug use were less likely to benefit from the intervention and may require additional, more intensive approaches. Concerted efforts to promote better retention in care will take us closer to achieving the engagement in care goals of the National HIV/AIDS Strategy.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are grateful to the interventionists, coordinators, and support staff listed in the Appendix, without whom the study would not have been possible.
Author contributions. L. I. G. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The study protocol is available from L. I. G.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the CDC and the Health Resources and Services Administration (contracts 200-2007-23685, 200-2007-23690, 200-2007-23689, 200-2007-23687, 200-2007-23684, 200-2007-23692).
Potential conflicts of interest. M. S. has received institutional grant support from BMS, Gilead, Merck, ViiV, Janssen, and GSK. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
The Retention in Care Study Group. Boston University/Boston Medical Center: Mari-Lynn Drainoni (principal investigator [PI]), Cintia Ferreira, Lisa Koppelman, Maya McDoom, Michal Naisteter, Karina Osella, Glory Ruiz, Paul Skolnik, and Meg Sullivan (PI). University of Alabama at Birmingham: Scott Batey, Stephanie Gaskin, Michael Mugavero (PI), Jill Murphree, Jim Raper, Michael Saag (PI), Suneetha Thogaripally, James Willig, and Anne Zinski. State University of New York Downstate Medical Center: Sophia Gibbs-Cohen, Elana Desrivieres, Mayange Frederick, Kevin Gravesande, Susan Holman, Harry Johnson, Tonya Taylor, and Tracey Wilson (PI). Baylor College of Medicine, Houston, Texas: Monisha Arya, David Bartholomew, Tawanna Biggs, Hina Budhwani, Jessica Davila, Tom Giordano (PI), Nancy Miertschin, Shapelle Payne, and William Slaughter. Mountain Plains AIDS Education and Training Center: Lucy Bradley-Springer and Marla Corwin. Health Resources and Services Administration: Laura Cheever, Faye Malitz, and Robert Mills. Centers for Disease Control and Prevention: Lytt Gardner, Gary Marks, Jason Craw, and Charles Rose. Centers for Disease Control and Prevention/ICF International: Sonali Girde, and Stacy Muckleroy. Johns Hopkins University: Mollie Jenckes, Jeanne Keruly (PI), Angie McCray, Mary McGann, Richard Moore (PI), Melissa Otterbein, and Liming Zhou. University of Miami/Jackson Memorial Hospital: Carolyn Garzon, Jesline Jean-Simon, Kathy Mercogliano, Lisa Metsch (PI), Allan Rodriguez (PI), Gilbert Saint-Jean, and Marvin Shika.
References
- 1.Cohen S, Van Handel M, Branson B, et al. Vital signs: HIV prevention through care and treatment—United States. MMWR Morb Mortal Wkly Rep. 2011;60:1618–23. [PubMed] [Google Scholar]
- 2.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yehia B, Fleishman J, Metlay J, Moore R, Gebo K. Sustained viral suppression in HIV-infected patients receiving antiretroviral therapy. JAMA. 2012;308:339–42. doi: 10.1001/jama.2012.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giordano TP, White AC, Jr, Sajja P, et al. Factors associated with the use of highly active antiretroviral therapy in patients newly entering care in an urban clinic. J Acquir Immune Defic Syndr. 2003;32:399–405. doi: 10.1097/00126334-200304010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Mugavero M, Amico K, Westfall A, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2012;59:86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks G, Gardner L, Craw J, Crepaz N. Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. AIDS. 2010;24:2665–78. doi: 10.1097/QAD.0b013e32833f4b1b. [DOI] [PubMed] [Google Scholar]
- 7.Fleishman J, Yehia B, Moore R, Korthius P, Gebo K. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012;60:249–59. doi: 10.1097/QAI.0b013e318258c696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mugavero MJ, Lin HY, Allison JJ, et al. Failure to establish HIV care: characterizing the “no show” phenomenon. Clin Infect Dis. 2007;45:127–30. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- 9.Gardner L, Marks G, Craw J, et al. A low-effort clinic-wide intervention improves attendance for HIV primary care. Clin Infect Dis. 2012;55:1124–34. doi: 10.1093/cid/cis623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The White House Office of National AIDS Policy. National HIV/AIDS strategy. Available at: http://www.whitehouse.gov/administration/eop/onap/nhas . Accessed 5 February 2014.
- 11.Higa D, Marks G, Crepaz N, Liau A, Lyles C. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV/AIDS Reports. 2012;9:313–25. doi: 10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher W, Fisher J, Harman J. The information-motivation-behavioral skills model: a general psychological approach to understanding and promoting health behavior. In: Suls J, Wallston K, editors. Social psychological foundations of health and illness. London: Blackwell Press; 2003. pp. 87–206. [Google Scholar]
- 13.Fisher J, Fisher W, Amico K, Harman J. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25:462–73. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 14.Fisher JD, Fisher WA, Shuper PA. The information–motivation–behavioral skills model of HIV preventive behavior. In: Di Clemente RJ, Crosby RA, Kegler MC, editors. Emerging theories in health promotion practices and research. San Francisco, CA: Jossey–Bass Publishers; 2009. pp. 21–63. [Google Scholar]
- 15.Fisher JD, Amico KR, Fisher WA, Harman JJ. The information-motivation-behavioral skills model of antiretroviral adherence and its applications. Curr HIV/AIDS Reports. 2008;5:193–203. doi: 10.1007/s11904-008-0028-y. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell S, Maljanian R, Horowitz S, Pianka M, Cabrera Y, Greene J. Effectiveness of reminder systems on appointment adherence rates. J Health Care Poor Underserved. 2001;12:504–14. doi: 10.1353/hpu.2010.0766. [DOI] [PubMed] [Google Scholar]
- 17.Roberts N, Meade K, Partridge M. The effect of telephone reminders on attendance in respiratory outpatient clinics. J Health Serv Res Policy. 2007;12:69–72. doi: 10.1258/135581907780279567. [DOI] [PubMed] [Google Scholar]
- 18.Parikh A, Gupta K, Wilson A, Fields K, Cosgrove N, Kostis J. The effectiveness of outpatient appointment reminder systems in reducing no-show rates. Am J Med. 2010;123:542–8. doi: 10.1016/j.amjmed.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Mugavero M, Westfall A, Zinski A, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61:574–80. doi: 10.1097/QAI.0b013e318273762f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mugavero M, Davila J, Nevin C, Giordano T. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010;24:607–13. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giordano C, Gifford A, White C, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–9. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 22.Mugavero M, Lin H, Willig J, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48:248–56. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucas G, Chaisson R, Moore R. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–7. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 24.Rastegar D, Fingerhood M, Jasinski D. Highly active antiretroviral therapy outcomes in a primary care clinic. AIDS Care. 2003;15:231–7. doi: 10.1080/0954012031000068371. [DOI] [PubMed] [Google Scholar]
- 25.Valdez H, Lederman MM, Woolley I, et al. Human immunodeficiency virus 1 protease inhibitors in clinical practice: predictors of virological outcome. Arch Intern Med. 1999;159:1771–6. doi: 10.1001/archinte.159.15.1771. [DOI] [PubMed] [Google Scholar]
- 26.Sethi A, Celentano D, Gange S, Moore R, Gallant J. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis. 2003;37:1112–8. doi: 10.1086/378301. [DOI] [PubMed] [Google Scholar]
- 27.Tobias C, Cunningham W, Cabral H, et al. Living with HIV but without medical care: barriers to engagement. AIDS Patient Care STDS. 2007;21:426–34. doi: 10.1089/apc.2006.0138. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. The ASSIST project—Alcohol, Smoking and Substance Involvement Screening Test. Available at: http://www.who.int/substance_abuse/activities/assist/en/ . Accessed 28 April 2014.
- 29.Humeniuk R, Ali R, Babor T, et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST) Addiction. 2008;103:1039–47. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 30.Buscher A, Mugavero M, Westfall A, et al. Extended clinical follow-up intervals in HIV-infected persons with viral suppression. AIDS Patient Care STDS. 2013;27:459–66. doi: 10.1089/apc.2013.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardner L, Metsch L, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19:423–31. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 32.Craw J, Gardner L, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care. J Acquir Immune Defic Syndr. 2008;47:597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 33.Brewer TH, Zhao W, Pereyra M, et al. ARTAS Study Group. Initiating HIV care: attitudes and perceptions of HIV positive crack cocaine users. AIDS Behav. 2007;11:897–904. doi: 10.1007/s10461-007-9210-2. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham C, Sohler N, Berg K, Shapiro S, Heller D. Type of substance use and access to HIV-related health care. AIDS Patient Care STDS. 2006;20:399–407. doi: 10.1089/apc.2006.20.399. [DOI] [PubMed] [Google Scholar]
- 35.Hall H, Gray K, Tang T, Li J, Shouse L, Mermin J. Retention in care of adults and adolescents living with HIV in 13 US areas. J Acquir Immune Defic Syndr. 2012;60:77–82. doi: 10.1097/QAI.0b013e318249fe90. [DOI] [PubMed] [Google Scholar]
- 36.Ulett K, Willig J, Lin H, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23:41–9. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giordano T, Hartman C, Gifford A, Backus L, Morgan R. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials. 2009;10:299–305. doi: 10.1310/hct1005-299. [DOI] [PubMed] [Google Scholar]
- 38.Wohl A, Carlos JA, Tejero J, et al. Barriers and unmet need for supportive services for HIV patients in care in Los Angeles County, California. AIDS Patient Care STDS. 2011;25:525–32. doi: 10.1089/apc.2011.0149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.