Abstract
We evaluated in vivo innate immune responses in monocyte populations from 67 young (aged 21–30 years) and older (aged ≥65 years) adults before and after influenza vaccination. CD14+CD16+ inflammatory monocytes were induced after vaccination in both young and older adults. In classical CD14+CD16– and inflammatory monocytes, production of tumor necrosis factor α and interleukin 6, as measured by intracellular staining, was strongly induced after vaccination. Cytokine production was strongly associated with influenza vaccine antibody response; the highest levels were found as late as day 28 after vaccination in young subjects and were substantially diminished in older subjects. Notably, levels of the anti-inflammatory cytokine interleukin 10 (IL-10) were markedly elevated in monocytes from older subjects before and after vaccination. In purified monocytes, we found age-associated elevation in phosphorylated signal transducer and activator of transcription-3, and decreased serine 359 phosphorylation of the negative IL-10 regulator dual-specificity phosphatase 1. These findings for the first time implicate dysregulated IL-10 production in impaired vaccine responses in older adults.
Keywords: aging, influenza vaccine, monocyte, cytokine, innate immunity
Influenza remains a significant cause of morbidity and mortality in older adults, with 90% of annual influenza-related deaths occurring in individuals older than 65 years [1]. Although the seasonal inactivated vaccine is recommended for prevention, a meta-analysis of 64 studies of influenza vaccine response in older adults (>65 years) revealed an efficacy of 23% for the prevention of influenzalike illness [2]. This poor vaccine response is a result in part of age-associated alterations in immune function [3]. Immunosenescence affects the adaptive immune system, resulting in impairments in B- and T-cell development, signaling, and antigen receptor repertoire [4–8]. The human innate immune system is also affected by aging, with chronic inflammation arising from sources including age-associated herpesvirus reactivation and increased non–cell-associated DNA reflected in elevated levels of cytokines and acute phase reactants; such inflammation contributes to dysregulated innate immune function, particularly of pattern recognition receptors such as Toll-like receptors (TLRs) [9]. However, how aging affects human innate immunity to influenza vaccination remains incompletely understood. Here, we evaluated monocyte function at baseline and after immunization with the inactivated influenza vaccine in a cohort of young and older adults.
METHODS
Clinical Study Design and Recruitment of Participants
Adults (older, aged ≥65 years; younger, aged 21–30 years) were recruited at influenza vaccination clinics organized by the Yale Health Services during the 2011–2012 season. Informed consent was obtained according to a protocol approved by the Human Research Protection Program of the Yale School of Medicine. Participants were evaluated using a questionnaire determining self-reported demographic information, medications, and comorbid conditions. Participants with an acute illness 2 weeks before recruitment were excluded, as were those with primary or acquired immunodeficiency; those receiving immunomodulating medications, including steroids or chemotherapy; and those with a history of cancer (other than localized skin or prostate cancer), cirrhosis, or renal failure requiring hemodialysis. Blood samples were collected immediately before administration of vaccine (day 0) and on days 2, 7, and 28.
Sample Preparation
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood using Histopaque-1077 (Sigma) gradient centrifugation, as described elsewhere [10, 11]. Serum samples were stored at −80°C. Untouched monocytes were isolated using the Dynabeads Untouched Human Monocytes Kit (Life Technologies), following the manufacturer's instructions. Monocytes were ≥80% pure as shown by anti-CD14 staining.
Flow Cytometry
On the day of recruitment, freshly isolated PBMCs were surface stained with anti–CD14- phycoerythrin Texas Red (clone TüK4; Life Technologies), anti–CD16-phycoerythrin–cyanine 7 (clone 3G8; BioLegend), anti–CD11c-allophycocyanin (clone B-ly6; BD Biosciences), and anti–CD11b-allophycocyanin-cyanine 7 (clone ICRF44; Affymetrix eBioscience). Cells were fixed in Cytofix/Cytoperm buffer (BD Biosciences) and stored at −80°C in freezing medium until analysis. On the day of analysis, samples were briefly thawed at 37°C, washed, and permeabilized with BD Perm/Wash buffer. A cocktail of anti–interleukin 6 (IL-6) fluorescein isothiocyanate (clone MQ2–1385; Affimetrix eBioscience), anti–tumor necrosis factor (TNF) α Alexafluor 700 (MAB11; BD Biosciences), and anti–interleukin 10 (IL-10) Pacific Blue (JES3–9D7; Affymetrix eBioscience) in 1X Perm/Wash buffer was used to stain for intracellular cytokines. Samples were washed and analyzed together using a Fortessa instrument (Becton Dickinson) fitted with an automated sampler in 96-well plates and with FlowJo 9.2 software (FlowJo, LLC). For cytokine analysis, files were batch processed using a quadrant drawn on prevaccine baseline samples; the same region was applied to all time points. Intracellular cytokine expression was calculated as percentage of the parent population.
Hemagglutination Inhibition Titer Measurement
Hemagglutination inhibition (HAI) assays were performed as described elsewhere [12] on serum samples collected at day 0 (before vaccination) and day 28 after vaccination to determine antibody titers against each strain in the 2011–2012 vaccine (A/California/7/09 [H1N1], A/Perth/16/2009 [H3N2], and B/Brisbane/60/2008).
Western Blot Analysis
Analysis of PBMCs or purified monocyte lysates harvested at baseline used antibodies against human signal transducer and activator of transcription-3 (STAT3), phosphorylated-STAT3 (p-STAT3) Y705, and p-STAT3 S727 (all from Cell Signaling Technology), dual-specificity phosphatase 1 (DUSP1; Sigma-Aldrich), phospho-DUSP1 (p-DUSP1) S359 (Cell Signaling Technology), and p-DUSP1 S296 (OriGene Technologies). Pixel intensities of specific protein bands and corresponding β-actin bands were estimated using ImageJ software (version 1.46r). The ratio of relative pixel intensity of the protein in question to that of actin was plotted in histograms with Prism 5.0 software (GraphPad).
Statistical Analyses
Descriptive statistics were generated for demographic and health characteristics by age group with Student t test for normally distributed continuous measures. Categorical data were compared between groups with χ2 statistics or Fisher exact test where warranted.
Longitudinal outcomes were analyzed with linear mixed-effect models. Distributions of dependent and independent variables were checked. Longitudinal models address repeated correlated measures within-person by specification of the covariance structure selected based on the lowest Akaike adjusted information criterion [13]. Model fit was assessed by ensuring that studentized residuals met assumptions of independence and normality. An unstructured covariance structure provided the best fit for all longitudinal mixed models. The 3-way interaction term of age group (young or older) by vaccine response (responder or nonresponder) by time points (day 0, 2, 7, or 28) assessed the potential differences among age and vaccine response combinations over time for the various outcomes. Adjustment was made for sex, race (white or nonwhite), number of prescription medications (range, 0–15), and number of comorbid conditions (range, 0–12). To explore the relationship between IL-6 and IL-10 levels across time, this model was reestimated, including terms for IL-10 levels by days since influenza vaccination. Post hoc t tests between age groups at each time period were adjusted for multiple comparisons by the Hochberg method. Longitudinal model effects were 2 sided with an α value of .05 (after Hochberg adjustment) prespecified as significant.
A 2-sided nonparametric Wilcoxon rank sum test was used to test for alterations in STAT3 and DUSP1 phosphorylation. STAT3 phosphorylation in PBMCs was analyzed in a subset of 14 young and 14 older adults from the original cohort for whom sufficient material was available. To assess the association of STAT3 with vaccine response, a Fisher exact test was performed with STAT3 Y705 and S727 divided at their respective medians, resulting in equal-sized groups that had good separation over the observed range. Finally, 6 young and 6 older adults meeting enrollment criteria who were not part of the original cohort were recruited for analyses of purified monocytes (STAT3 and DUSP1 measures) compared with Wilcoxon rank sum tests. Statistical analyses, tables, and graphs were created with SAS (version 9.4; SAS Institute) and GraphPad Prism 5.0 software.
RESULTS
We enrolled 31 young adults (aged 21–30 years) and 36 older adults (aged ≥65 years) before immunization with the seasonal trivalent inactivated influenza vaccine. Samples of peripheral blood were obtained immediately before vaccine administration (day 0) and at days 2, 7, and 28 after vaccination. There were no differences between the older and young adults for sex or race, but not surprisingly they differed by health-associated parameters (Supplementary Table 1). As a result, we used a multivariable statistical model to adjust for these differences. At each time point, freshly isolated PBMCs were subjected to staining for surface lineage markers, then fixed and frozen. Samples were subsequently thawed, intracellular cytokine staining was completed, and flow cytometric analyses of cytokine production carried out for all subjects' samples concurrently.
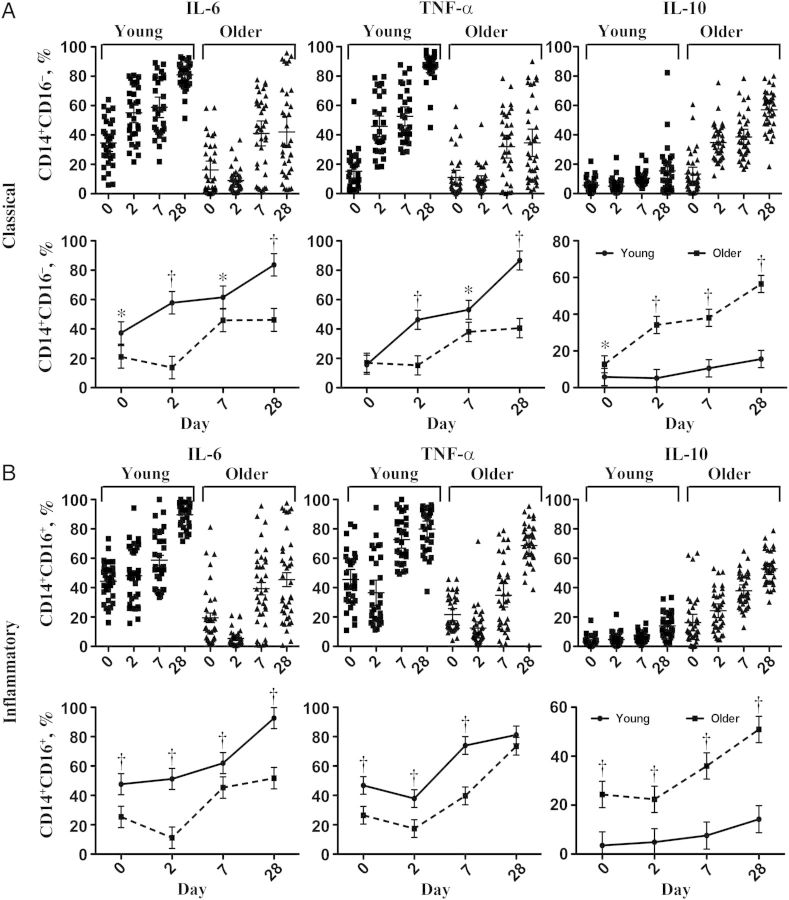
We evaluated monocyte populations at each time point, and found induction of CD14+CD16+ monocytes after influenza vaccination, particularly at days 2 and 7 after vaccination, with resolution toward baseline by day 28 (Figure 1); this induction of CD14+CD16+ so-called inflammatory monocytes did not differ between young and older adults (Figure 1B). We evaluated intracellular cytokine production in both classical CD14+CD16− and inflammatory CD14+CD16+ monocytes and found strong induction of intracellular IL-6 production relative to day 0 in both populations from young subjects at days 2 and 7, with highest levels found at day 28 (Figure 2A and 2B). In monocyte populations from older adults, this induction seemed dysregulated, with diminished expression seen at day 2 and weaker intracellular IL-6 levels in both populations compared with young subjects at days 7 and 28. These differences between young and older adults were statistically significant at days 2 and 28 in classical monocytes and at days 0, 2, and 28 in inflammatory monocytes, in a multivariable statistical model adjusted for within-person correlation of repeated measures and covariates of sex, race, number of prescription medications, and number of comorbid medical conditions (Figure 2).
Figure 1.
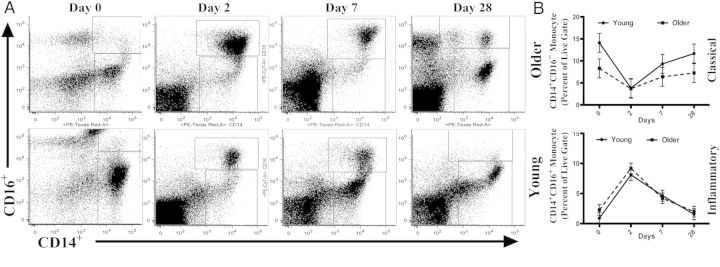
CD14+CD16+ inflammatory monocytes are induced after influenza vaccination. Representative dot plots (A) and (B) graphs depict proportion of CD14+ monocytes in the peripheral blood mononuclear cell population and proportion of CD14+ cells that are classical (CD14+CD16−) or inflammatory (CD14+CD16+) monocytes before vaccination (day 0) and 2, 7, and 28 days after vaccination in young (aged 21–30 years; n = 31) and older (age ≥65 years; n = 36) subjects. Mean and 95% confidence intervals are from a multivariable mixed-effects model adjusted for within-person correlation, sex, race, response to influenza vaccine, number of prescription medications, and number of comorbid medical conditions. Differences between age groups showed a significant increase in percentage of total CD14+ cells (*P = .03) and the proportion of CD14+ cells that were CD14+CD16− (**P = .008) at day 0 in young, compared with older adults, whereas the proportion of CD14+ cells that were CD14+CD16+ monocytes was significantly increased in older adults at day 0, compared with young adults (**P = .008). No other significant differences between age groups were found at other time points.
Figure 2.
Age-associated differences in the induction of cytokine production in classical (CD14+CD16−) (A) and inflammatory (CD14+CD16+) (B) monocytes from young (n = 31) and older (n = 36) subjects after influenza vaccination. Scatterplots represent percentages of classical (A, upper panels) and inflammatory (B, upper panels) monocytes positive for interleukin 6 (IL-6), tumor necrosis factor (TNF) α, and interleukin 10 (IL-10) in young and older subjects at day 0 (before vaccination) and days 2, 7, and 28 after vaccination. Line diagrams depict mean and 95% confidence intervals comparing young and older adults, derived from a longitudinal multivariable mixed model adjusted for within-person correlation, sex, race, number of prescription medications, and number of comorbid medical conditions, shown for classical (A, lower panels) and inflammatory (B, lower panels) monocytes. *P ≤ .05; †P ≤ .001.
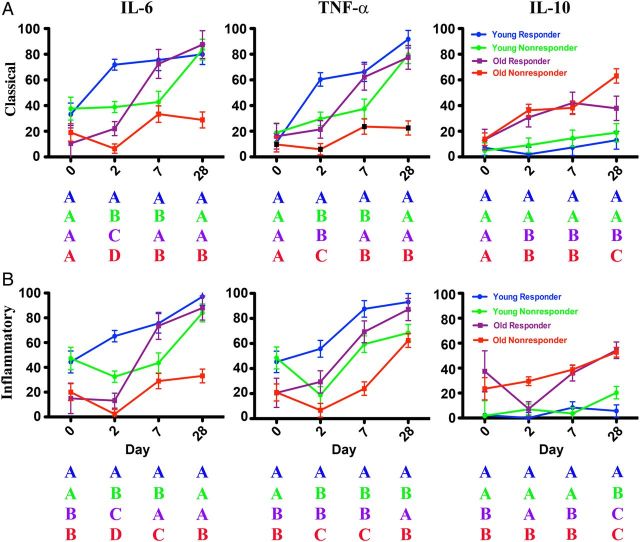
We subsequently classified subjects as influenza vaccine responders (defined as having any 4-fold increase in HAI titer to any of the 3 strains in the vaccine) or nonresponders (having no 4-fold increase in HAI titer to any vaccine strain); the proportions of responders and nonresponders differed between young and older adults (Supplementary Table 1). Based on a multivariable statistical model, P values for differences in cytokine production among vaccine responders and nonresponders are summarized in Table 1 (for classical CD14+CD16− monocytes) and Table 2 (for inflammatory CD14+CD16+ monocytes) and graphically depicted in Figure 3. Notably, we found that the extent of intracellular IL-6 production in classical and inflammatory monocytes was strongly associated with influenza vaccine response in both young and older adults at days 2 and 7 and in older adults at day 28 (Figure 3). For example, at day 2 after vaccination, the highest levels of intracellular IL-6 production in CD14+CD16− monocytes were found in young vaccine responders, with significantly lower levels in young vaccine nonresponders (P < .001). The difference in intracellular IL-6 at day 2 was also significantly lower in older responders and older nonresponders compared with young responders (P < .001 for both comparisons) and in older nonresponders compared with older responders (P = .002). At day 7, the levels of intracellular IL-6 in CD14+CD16− monocytes from young responders were significantly higher than in young nonresponders (P < .001) or older nonresponders (P < .001), and older responders showed higher IL-6 levels than older nonresponders (P < .001) (Figure 3A). Similar patterns were found for inflammatory monocytes at days 2 and 7 (Figure 3B). When these models included the levels of intracellular IL-10 in CD14+CD16− monocytes at each time point, IL-10 levels were significantly (P = .004; type 3 test over all days) associated with the levels of intracellular IL-6 in CD14+CD16− monocytes. A similar association between intracellular IL-10 and IL-6 was also found for inflammatory monocytes (P < .001; type 3 test over all days).
Table 1.
IL-6, TNF-α, and IL-10 Production in Classical Monocytes (CD14+CD16−) From Young and Older Vaccine Responders and Nonresponders at Day 0 (Before Vaccination) and Days 2, 7, and 28 After Vaccination
| Comparison |
P Valueb |
||||||
|---|---|---|---|---|---|---|---|
| Day | Age | Responsea | Age | Responsea | IL-6 | TNF-α | IL-10 |
| 0 | Young | NR | Young | R | NS | NS | NS |
| 0 | Older | NR | Young | NR | .017 | NS | NS |
| 0 | Older | NR | Young | R | NS | NS | NS |
| 0 | Older | R | Young | NR | .008 | NS | NS |
| 0 | Older | R | Young | R | .030 | NS | NS |
| 0 | Older | NR | Older | R | NS | NS | NS |
| 2 | Young | NR | Young | R | <.001 | <.001 | NS |
| 2 | Older | NR | Young | NR | <.001 | <.001 | <.001 |
| 2 | Older | NR | Young | R | <.001 | <.001 | <.001 |
| 2 | Older | R | Young | NR | <.001 | NS | <.001 |
| 2 | Older | R | Young | R | <.001 | <.001 | <.001 |
| 2 | Older | NR | Older | R | <.001 | .001 | NS |
| 7 | Young | NR | Young | R | <.001 | <.001 | NS |
| 7 | Older | NR | Young | NR | NS | NS | <.001 |
| 7 | Older | NR | Young | R | <.001 | <.001 | <.001 |
| 7 | Older | R | Young | NR | .001 | .004 | <.001 |
| 7 | Older | R | Young | R | NS | NS | <.001 |
| 7 | Older | NR | Older | R | <.001 | <.001 | NS |
| 28 | Young | NR | Young | R | NS | NS | NS |
| 28 | Older | NR | Young | NR | <.001 | <.001 | <.001 |
| 28 | Older | NR | Young | R | <.001 | <.001 | <.001 |
| 28 | Older | R | Young | NR | NS | NS | .030 |
| 28 | Older | R | Young | R | NS | NS | <.001 |
| 28 | Older | NR | Older | R | <.001 | <.001 | <.001 |
Abbreviations: IL-6, interleukin 6; IL-10, interleukin 10; NR, nonresponder; NS, not significant; R, responder; TNF, tumor necrosis factor.
a Responders were those having any 4-fold increase in hemagglutination inhibition (HAI) titer to any of the 3 strains in the seasonal vaccine; nonresponders, those with no 4-fold increase in HAI titer to any strain in the vaccine.
b P values shown are for post hoc comparisons of least-square means using a longitudinal multivariable mixed model adjusted for within-person correlation, sex, race (white or nonwhite), number of prescription medications, and number of comorbid conditions. All probabilities are Hochberg adjusted for multiple comparisons.
Table 2.
Comparisons of IL-6, TNF-α, and IL-10 Production in Inflammatory Monocytes (CD14+CD16+) From Young and Older Vaccine Responders and Nonresponders at Day 0 (Before Vaccination) and at Days 2, 7, and 28 After Vaccination
| Comparison |
P Valueb |
||||||
|---|---|---|---|---|---|---|---|
| Day | Age | Responsea | Age | Responsea | IL-6 | TNF-α | IL-10 |
| 0 | Young | NR | Young | R | NS | NS | NS |
| 0 | Older | NR | Young | NR | <.001 | <.001 | .0498 |
| 0 | Older | NR | Young | R | <.001 | <.001 | .0498 |
| 0 | Older | R | Young | NR | <.001 | .004 | .010 |
| 0 | Older | R | Young | R | .002 | .011 | .010 |
| 0 | Older | NR | Older | R | NS | NS | NS |
| 2 | Young | NR | Young | R | <.001 | <.001 | NS |
| 2 | Older | NR | Young | NR | <.001 | NS | <.001 |
| 2 | Older | NR | Young | R | <.001 | <.001 | <.001 |
| 2 | Older | R | Young | NR | <.001 | NS | NS |
| 2 | Older | R | Young | R | <.001 | <.001 | NS |
| 2 | Older | NR | Older | R | .014 | <.001 | <.001 |
| 7 | Young | NR | Young | R | <.001 | <.001 | NS |
| 7 | Older | NR | Young | NR | .04 | <.001 | <.001 |
| 7 | Older | NR | Young | R | <.001 | <.001 | <.001 |
| 7 | Older | R | Young | NR | <.001 | NS | <.001 |
| 7 | Older | R | Young | R | NS | .011 | <.001 |
| 7 | Older | NR | Older | R | <.001 | <.001 | NS |
| 28 | Young | NR | Young | R | NS | <.001 | <.001 |
| 28 | Older | NR | Young | NR | <.001 | NS | <.001 |
| 28 | Older | NR | Young | R | <.001 | <.001 | <.001 |
| 28 | Older | R | Young | NR | NS | .015 | <.001 |
| 28 | Older | R | Young | R | NS | NS | <.001 |
| 28 | Older | NR | Older | R | <.001 | <.001 | NS |
Abbreviations: IL-6, interleukin 6; IL-10, interleukin 10; NR, nonresponder; NS, not significant; R, responder; TNF, tumor necrosis factor.
a Responders were those having any 4-fold increase in hemagglutination inhibition (HAI) titer to any of the 3 strains in the seasonal vaccine; nonresponders, as those with no 4-fold increase in HAI titer to any strain in the vaccine.
b P values shown are for post hoc comparisons of least-square means using a longitudinal multivariable mixed model adjusted for within-person correlation, sex, race (white or nonwhite), number of prescription medications, and number of comorbid conditions. All probabilities are Hochberg adjusted for multiple comparisons.
Figure 3.
Association of influenza vaccine response with postvaccine cytokine production in monocytes. Pre- and postvaccine percentages of classical (A) and inflammatory (B) monocytes expressing interleukin 6 (IL-6), tumor necrosis factor (TNF) α, and interleukin 10 (IL-10) in young (n = 31) and older (n = 36) subjects are stratified by vaccine antibody response. Responders are defined as having any 4-fold increase in hemagglutination inhibition titer to any of the 3 strains in the seasonal vaccine, and nonresponders as those without a 4-fold increase in hemagglutination inhibition titer to any strain in the vaccine. Mean and 95% confidence intervals are derived from a longitudinal multivariable mixed model adjusted for within-person correlation, sex, race, number of prescription medications, and number of comorbid medical conditions. Four color-coded categories are shown: young responders (blue), young nonresponders (green), older responders (purple), and older nonresponders (red) and their cytokine levels are depicted using a 4 letter code to emphasize levels of significance. At each time point, categories with differing letters differ significantly, and categories with the same letter do not. For example, in panel A for IL-6 production in classical monocytes at day 7, young (blue) and older (purple) responders are both depicted by the letter A, and young (green) and older (red) nonresponders by the letter B, indicating no significant differences. However, young responders and young nonresponders have different letters (A vs B), as do young responders vs older nonresponders and older responders vs older nonresponders; all of these differences were statistically significant. See Table 1 and Table 2 for exact P values after Hochberg adjustment for multiple comparisons.
For intracellular TNF-α production, higher levels were found in classical monocytes from young compared with those from older subjects at days 2, 7, and 28 and in inflammatory monocytes from young compared with older subjects at days 0, 2, and 7 (Figure 2). As with intracellular IL-6 production, the levels of intracellular TNF-α were also associated with vaccine response. In classical and inflammatory monocytes, intracellular TNF-α at day 2 was highest in young vaccine responders compared with young nonresponders (P < .001). At day 2, TNF-α production was also significantly higher in older responders than in older nonresponders in classical (P = .001) and inflammatory monocytes (P < .001). At day 7, intracellular TNF-α in both monocyte subsets was significantly higher in young responders than in young nonresponders (P < .001) or older nonresponders (P < .001) (Figure 3). Thus, these results indicate that influenza vaccination induces the production of CD14+CD16+ inflammatory monocytes; moreover, the extent of intracellular IL-6 and TNF-α production in classical and inflammatory monocytes is altered in older compared with young adults and is strongly correlated with antibody vaccine response, as manifested by HAI titer.
In view of these age-associated decreases in proinflammatory cytokine production, we evaluated the production of the anti-inflammatory cytokine IL-10 in monocyte populations after influenza vaccination. Notably, the levels of intracellular IL-10 were markedly elevated in classical and inflammatory monocytes from older subjects at all time points, compared with those from young subjects. In classical monocytes (Figure 2A), intracellular IL-10 increased progressively to highest levels at day 28 after vaccination, whereas in CD14+CD16+ monocytes IL-10 levels seemed stable between days 0 and 2 before increasing at days 7 and 28 (Figure 2B). The extent of IL-10 production was not generally associated with influenza vaccine antibody response and seemed to largely reflect age-associated differences (Figure 3). However, significant increases in IL-10 levels were found in older vaccine nonresponders compared with older responders at day 28 in classical monocytes (P < .001) and at day 2 in inflammatory monocytes (P < .001). These findings indicate that IL-10 levels are increased in monocytes from older subjects, compared with those from young subjects, a potential contributing factor to age-associated alterations in influenza vaccine response.
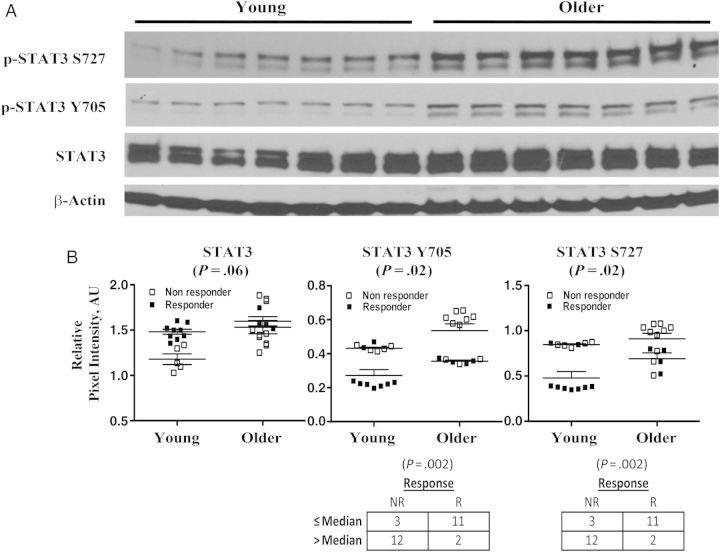
Because both IL-6 and IL-10 signal transduction use STAT3-dependent pathways, we assessed total and p-STAT3 protein in Western blot analyses of PBMC lysates from young and older adults (Figure 4A). In a subset of 14 young and 14 older adults from the original cohort, we found unchanged levels of total STAT3 protein but a significant baseline increase in STAT3 Y705 and S727 phosphorylation in older compared with young subjects (Figure 4B). We did not observe a change in STAT3 phosphorylation after vaccination for a limited number of available samples from young and older adults (data not shown). However, the baseline level of STAT3 Y705 or S727 phosphorylation showed significant inverse associations with influenza vaccine response in this subset (Figure 4B).
Figure 4.
Age-associated alteration in signal transducer and activator of transcription-3 (STAT3) phosphorylation. Total and phosphorylated STAT3 (p-STAT3) protein in Western blot analyses of peripheral blood mononuclear cell lysates from young (n = 14) and older (n = 14) subjects at baseline. A, p-STAT3 S727, p-STAT3 Y705, STAT3, and β-actin as loading control for a representative panel of 7 young and 7 older subjects. B, Quantitation (relative pixel intensity in arbitrary units [AU]) of STAT3, p-STAT3 Y705, and p-STAT3 S727 protein levels normalized to β-actin. Older subjects had significantly higher levels of p-STAT3Y705 (P = .02) and p-STAT3S727 (P = .02) (mean ± standard error of the mean; Wilcoxon rank sum test, 2-sided probability). Symbols in graphs indicate whether a person was a responder (R) or nonresponder (NR) to influenza vaccine. However, cutoff points for normalized STAT3 Y705 and S727 phosphorylation levels were medians selected regardless of age or response. The association between STAT3-Y705 phosphorylation and vaccine response was highly significant (P < .002; Fisher exact test). Those with a normalized STAT3 Y705 level no higher than the median level of 0.4 (pixel intensity in AU of phosphorylated Y705 normalized to β-actin) are more likely to be vaccine responders, whereas those with a level >0.4 are much more likely to be NRs. Similarly, for STAT3 S727 phosphorylation, those with a level no higher than the median of 0.8 are more likely to be vaccine responders, and those with a level >0.8 are very likely to be nonresponders (P < .002; Fisher exact test).
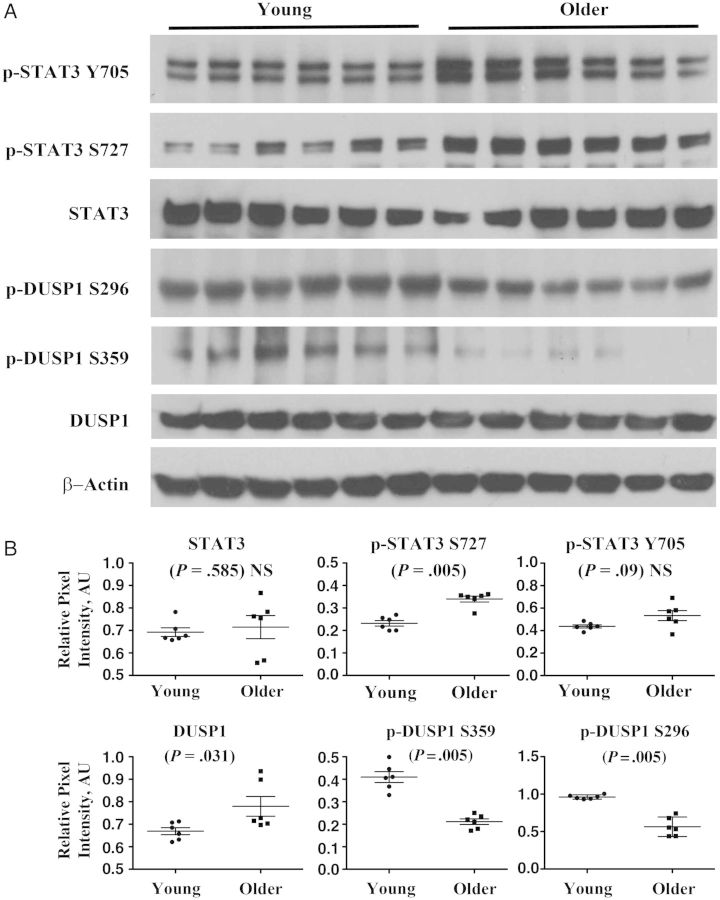
We subsequently studied monocytes purified via negative magnetic bead sorting in 12 newly recruited young and older adults, and we also found significantly increased levels of p-STAT3 S727 in monocytes from older adults, compared with those from young adults (Figure 5). Finally, we used Western blot analysis to measure total and phosphorylated 1 DUSP1, a negative regulator of IL-10 production, in monocytes (Figure 5A). DUSP1 phosphorylation at serine 296 is associated with targeting to the proteasome [14, 15], whereas phosphorylation at serine 359 is associated with increased DUSP1 stability [16, 17]. We found a slight elevation in total DUSP1 (P = .03) in older versus young subjects that was associated with decreased phosphorylation at S296 (P = .005). However, we found a substantial decrease in S359 phosphorylated DUSP1 in monocytes from older subjects (P = .005), suggesting a basis for an age-associated increase in anti-inflammatory IL-10 production (Figure 5B).
Figure 5.
Age-associated alterations in phosphorylated STAT3 (p-STAT3) and dual-specificity phosphatase 1 (DUSP1) in monocytes. A, Total and phosphorylated (p-STAT3 Y705 and p-STAT3 S727) STAT3 and total and phosphorylated (S296 and S359) DUSP1 protein levels and β-actin as loading control are depicted in Western analyses of monocytes isolated by negative magnetic bead sorting from young (n = 6) and older (n = 6) subjects at baseline. B, Quantitation of protein expression with P values indicated (Wilcoxon rank sum test, 2-sided probability). Abbreviations: AU, arbitrary units; NS, not significant.
DISCUSSION
To better understand the effects of aging innate immune responses to the trivalent inactivated influenza vaccine, we evaluated human monocyte function before vaccination and at days 2, 7 and 28 after vaccination in young (aged 21–30) and older (aged ≥65) adults. We found induction of CD14+CD16+ inflammatory monocytes after vaccination at days 2 and 7, with a return to baseline by day 28. The extent of CD14+CD16+ monocyte increase seemed comparable in young and older subjects. Increases in such inflammatory monocytes have been reported in the context of inflammatory conditions (eg, sepsis), chronic viral infections (eg, human immunodeficiency virus infection), and myocardial infarction [18]; however, to our knowledge, this is the first report of human CD14+CD16+ monocyte induction after immunization with a nonadjuvanted inactivated vaccine.
Although similar levels of CD14+CD16+ monocytes were seen in young and older subjects, we found substantial age-associated differences in cytokine production, as assessed with intracytoplasmic staining, in both classical CD14+CD16− and inflammatory CD14+CD16+ monocytes. Intracellular IL-6 and TNF-α levels increased progressively after vaccination in both classical and inflammatory monocytes, with highest levels found at day 28 in young subjects. This induction of cytokine production was markedly blunted in monocytes from older adults, though an increase from baseline was also seen at day 28 for both TNF-α and IL-6. These findings highlight age-associated differences in the in vivo innate immune response to influenza vaccine. They also provide evidence for sustained innate immune engagement after immunization that extends until at least day 28. The basis for this sustained increase in monocyte cytokine production is unclear, but in view of evidence for antigen persistence after respiratory virus infection or immunization in mice [19, 20], these findings suggest that such persistence also occurs in the response to a nonadjuvanted inactivated vaccine in humans.
Notably, the extent of intracellular TNF-α and IL-6 production after influenza vaccination was strongly associated with vaccine response, as measured by HAI titer at day 28. Young subjects showing a seroconversion (≥4-fold antibody response to ≥1 vaccine strain) had increased levels of TNF-α and IL-6 in both classical and inflammatory monocytes at days 2 and 7 after vaccination, compared with young vaccine nonresponders. Older responders to vaccine also had increased levels of TNF-α and IL-6 in both monocyte populations at days 2, 7, and 28, compared with older nonresponders. Age-associated differences were most apparent at day 2 for IL-6 production, with the highest levels found in young responders, followed by young nonresponders, older responders, and older nonresponders. Although the lowest postvaccine levels of TNF-α and IL-6 were uniformly found in older nonresponders, older responders to vaccine seem to have a delayed increase in cytokine production at day 7, to levels comparable to those in young responders. These findings remained significant in a longitudinal multivariable statistical model after adjustment for within-person correlation, sex, race, number of prescription medications, and number of comorbid medical conditions. Our previous studies revealed an association between influenza vaccine antibody response and both TLR-induced cytokine production in dendritic cells [11] and TLR-induced expression of B7 costimulatory proteins in monocytes [12]. By contrast, our study aimed to elucidate the in vivo effects of influenza vaccination itself, in the absence of in vitro stimulation. These results indicate that the postvaccine production of TNF-α and IL-6 in monocytes is strongly associated with antibody response to influenza vaccine and provide further evidence for the critical role of innate immunity in vaccine response.
To understand the basis for these age-associated alterations, we evaluated the intracellular expression of IL-10, which has an anti-inflammatory function in monocytes [21]. We found a marked age-associated increase in IL-10 production across all time points in classical and inflammatory monocytes from older compared with young subjects. Older adults had generally elevated levels of IL-10 regardless of responder or nonresponder status. Nonetheless, this increased IL-10 is likely to contribute to the impaired monocyte IL-6 and TNF-α production associated with impaired vaccine responses to influenza vaccine in older adults. In this regard, we evaluated STAT3 phosphorylation, because STAT3 is a downstream target of both IL-10 and IL-6 signaling. We found substantially elevated baseline levels of p-STAT3 protein in both PBMCs and purified monocytes from older versus young adults. Our sample size was not sufficient to determine whether a sex-specific difference in p-STAT3 protein was present, as has been recently reported in gene expression microarray analyses [22]. However, even within this convenience subset of our cohort, we found a significant inverse correlation between STAT3 Y705 or S727 phosphorylation and vaccine response.
Although IL-6 signaling seems to result in transient STAT3 activation, we speculate that our findings reflect sustained STAT3 activation after IL-10 receptor signal transduction and the critical role of STAT3 in the IL-10–mediated anti-inflammatory response [23–25]. We also evaluated the expression of DUSP1, a mitogen-activated protein (MAP) kinase phosphatase that is a negative regulator of IL-10 expression [26–29]. We found a modest decrease in total DUSP1 protein that may result in part from decreased DUSP1 phosphorylation of S296, a site whose phosphorylation is associated with DUSP1 proteasomal degradation [14, 15]. We also found significantly diminished baseline expression of phosphorylated DUSP1 S359 in purified monocytes from older subjects, compared with those from young subjects. DUSP1 phosphorylation at serine 359 or 364 seems to enhance its stability and is associated with increased anti-inflammatory function [16, 17]; our finding of reduced p-DUSP1 may contribute to markedly increased levels of IL-10 in monocyte populations, and by extension, to the diminished response to influenza vaccination in older adults.
DUSP1 expression is induced by TLR activation and may also be influenced by resultant PI3 kinase signaling [17, 26]. In this context, age-associated alterations in human TLR function could contribute to dysregulated DUSP1 expression [10, 11]. The extent of TLR activation by the inactivated influenza vaccine used in humans remains unclear, although there is evidence for TLR engagement by residual viral RNA in the vaccine [30]. It is also possible that DUSP1-mediated regulation of cytokine production via its modulation of MAP kinase phosphorylation could occur as a result of TLR-independent MAP kinase signaling. In this regard, murine DUSP1 has been reported to be critical for T-cell activation and in vivo responses to influenza infection [31]. Future studies will examine the basis for age-associated diminished DUSP1 function. Notably, the expression of other DUSP proteins—DUSP4 and DUSP6—is increased with age in human CD4 T cells and results in impaired T-dependent B-cell responses and T-cell receptor–dependent extracellular signal-regulated kinase activation, respectively [32, 33]. Our findings of decreased DUSP1 phosphorylation with age in human monocytes reemphasize the importance of MAP kinase regulation in age-dependent immunity.
To our knowledge, our results represent the first evidence for induction of inflammatory monocytes, prolonged innate immune activation with impaired response in older adults, and age-associated dysregulation of IL-10 production in the human innate immune response to influenza vaccine. It will be of interest to determine whether the impaired activation of IL-6 and TNF-α expression and increase in IL-10 expression are affected by high-dose [34, 35] or adjuvanted [36] influenza vaccines that are associated with improved HAI titers in older adults. These findings also raise the possibility that modulating the anti-inflammatory function of IL-10, via the IL-10 signaling pathway itself or IL-10 regulators such as DUSP1, could improve outcomes from vaccination or infection in older adults.
Note added in proof. Expansion of CD14+ CD16+ inflammatory monocytes was also recently reported in the context of dengue virus infection [37].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank participants in this study, Ann Marie Cirkot, RN, and Michael Rigsby, MD of the Yale University Health Services for their support of this research.
Financial support. This work was supported by the National Institute on Aging (grant K24 AG042489 to A. C. S.), the Yale Claude D. Pepper Older Americans Independence Center (grant P30 AG021342), and the National Institute of Allergy and Infectious Diseases (grant U19 AI089992 to D. A. H., S. H. K., and A. C. S.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Rivetti D, Jefferson T, Thomas R, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2006;19:CD004876. doi: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Haq K, McElhaney JE. Immunosenescence: influenza vaccination and the elderly. Curr Opin Immunol. 2014;29C:38–42. doi: 10.1016/j.coi.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Frasca D, Blomberg BB. Aging affects human B cell responses. J Clin Immunol. 2011;31:430–5. doi: 10.1007/s10875-010-9501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frasca D, Blomberg BB. B cell function and influenza vaccine responses in healthy aging and disease. Curr Opin Immunol. 2014;29C:112–8. doi: 10.1016/j.coi.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–36. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynes L, Swain SL. Aged-related shifts in T cell homeostasis lead to intrinsic T cell defects. Semin Immunol. 2012;24:350–5. doi: 10.1016/j.smim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolich-Zugich J, Li G, Uhrlaub JL, Renkema KR, Smithey MJ. Age-related changes in CD8 T cell homeostasis and immunity to infection. Semin Immunol. 2012;24:356–64. doi: 10.1016/j.smim.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–87. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Duin D, Mohanty S, Thomas V, et al. Age-associated defect in human TLR-1/2 function. J Immunol. 2007;178:970–5. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 11.Panda A, Qian F, Mohanty S, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–27. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Duin D, Allore HG, Mohanty S, et al. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J Infect Dis. 2007;195:1590–7. doi: 10.1086/516788. [DOI] [PubMed] [Google Scholar]
- 13.Panda A, Chen S, Shaw AC, Allore HG. Statistical approaches for analyzing immunologic data of repeated observations: a practical guide. J Immunol Methods. 2013;398–399:19–26. doi: 10.1016/j.jim.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin YW, Chuang SM, Yang JL. ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J Biol Chem. 2003;278:21534–41. doi: 10.1074/jbc.M301854200. [DOI] [PubMed] [Google Scholar]
- 15.Lin YW, Yang JL. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J Biol Chem. 2006;281:915–26. doi: 10.1074/jbc.M508720200. [DOI] [PubMed] [Google Scholar]
- 16.Brondello JM, Pouyssegur J, McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286:2514–7. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- 17.Gunzl P, Bauer K, Hainzl E, et al. Anti-inflammatory properties of the PI3K pathway are mediated by IL-10/DUSP regulation. J Leukoc Biol. 2010;88:1259–69. doi: 10.1189/jlb.0110001. [DOI] [PubMed] [Google Scholar]
- 18.Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 19.Kim TS, Hufford MM, Sun J, Fu YX, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207:1161–72. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamburini BA, Burchill MA, Kedl RM. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat Commun. 2014;5:3989. doi: 10.1038/ncomms4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabat R, Grutz G, Warszawska K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–44. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Furman D, Hejblum BP, Simon N, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111:869–74. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun DA, Fribourg M, Sealfon SC. Cytokine response is determined by duration of receptor and signal transducers and activators of transcription 3 (STAT3) activation. J Biol Chem. 2013;288:2986–93. doi: 10.1074/jbc.M112.386573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda K, Clausen BE, Kaisho T, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 25.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567–76. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 26.Chi H, Barry SP, Roth RJ, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–9. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammer M, Mages J, Dietrich H, et al. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176:1899–907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Q, Wang X, Nelin LD, et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–40. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeisy-Scott V, Kim JH, Davis WG, Cao W, Katz JM, Sambhara S. TLR7 recognition is dispensable for influenza virus A infection but important for the induction of hemagglutinin-specific antibodies in response to the 2009 pandemic split vaccine in mice. J Virol. 2012;86:10988–98. doi: 10.1128/JVI.01064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Reynolds JM, Chang SH, et al. MKP-1 is necessary for T cell activation and function. J Biol Chem. 2009;284:30815–24. doi: 10.1074/jbc.M109.052472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li G, Yu M, Lee WW, et al. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18:1518–24. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu M, Li G, Lee WW, et al. Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proc Natl Acad Sci U S A. 2012;109:E879–88. doi: 10.1073/pnas.1109797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine. 2007;25:7656–63. doi: 10.1016/j.vaccine.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200:172–80. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 36.Seo YB, Choi WS, Lee J, Song JY, Cheong HJ, Kim WJ. Comparison of the immunogenicity and safety of the conventional subunit, MF59-adjuvanted, and intradermal influenza vaccines in the elderly. Clin Vaccine Immunol. 2014;21:989–96. doi: 10.1128/CVI.00615-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwissa M, Nakaya HI, Onlamoon N, et al. Dengue virus infection induces expansion of a CD14+ CD16+ monocyte population that stimulates plasmablast differentiation. Cell Host Microbe. 2014;16:115–27. doi: 10.1016/j.chom.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.