Abstract
Background. Cathelicidin is a proposed defender against infection of the urinary tract via its antimicrobial properties, but its activity has not been delineated in a dedicated cystitis model.
Methods. Female C57Bl/6 mice, wild type or deficient in cathelin-related antimicrobial peptide (CRAMP; an ortholog of the sole human cathelicidin, LL-37), were infected transurethrally with the cystitis-derived uropathogenic Escherichia coli (UPEC) strain UTI89. Infection course was evaluated by bladder titers, intracellular bacterial community quantification, and histological analysis. Immune responses and resolution were characterized through cytokine profiling, microscopy, and quantitation of epithelial recovery from exfoliation.
Results. CRAMP-deficient mice exhibited significantly lower bladder bacterial loads and fewer intracellular bacterial communities during acute cystitis. Although differences in bacterial titers were evident as early as 1 hour after infection, CRAMP-deficient mice showed no baseline alterations in immune activation, uroepithelial structure, apical expression of uroplakins (which serve as bacterial receptors), or intracellular bacterial growth rate. CRAMP-deficient hosts demonstrated less intense cytokine responses, diminished neutrophil infiltration, and accelerated uroepithelial recovery.
Conclusions. Mice lacking the antimicrobial peptide cathelicidin experienced less severe infection than wild-type mice in a well-established model of cystitis. Although CRAMP exhibits in vitro antibacterial activity against UPEC, it may enhance UPEC infection in the bladder by promoting epithelial receptivity and local inflammation.
Keywords: cathelicidin, CRAMP, uropathogenic Escherichia coli, cystitis, intracellular bacterial community, innate immunity, neutrophil, uroepithelium, immune response
Urinary tract infections (UTIs) are common worldwide, predominantly affecting young, premenopausal women. Over 80% of UTIs are caused by uropathogenic Escherichia coli (UPEC), among which many strains have acquired significant antibiotic resistance [1]. A thorough understanding of both host and bacterial factors affecting the progression of UTIs is necessary to devise new treatment methods. Complicating this effort, UPEC comprise an array of strains that, by virtue of different genetic attributes and virulence factors, are adapted to colonization and virulence in distinct niches within and outside the urinary tract [2]. For example, the cystitis-derived UPEC strain UTI89 attaches via type 1 pili to mannosylated uroplakins on the lumenal surface of bladder epithelial cells and is internalized [3, 4]. Intracellular organisms, protected from immune effectors and antibiotic therapies, replicate into biofilm-like structures termed intracellular bacterial communities (IBCs) [5, 6]. Exfoliation of the superficial epithelium is one host strategy to eliminate invasive bacteria, while a cytokine response recruits phagocytes (predominantly neutrophils and macrophages) to the infected bladder [3, 7–10]. Host strain–dependent attributes specify additional influences on the progress and outcomes of experimental infection. In healthy C57Bl/6 mice, UPEC undergo multiple IBC cycles within the first 24 hours, but pathogen control and diminishing bacterial titer are evident by 48 hours after infection [11]. In other susceptible host strains (eg, C3H/HeN), a dose-dependent proportion of infected animals maintains high-titer chronic cystitis for weeks following inoculation [12].
While exfoliation and urine flow represent mechanical defenses that protect the bladder, molecules including lysozyme, lipocalin, lactoferrin, and antimicrobial peptides also make the lumenal environment less hospitable for pathogens [13]. Antimicrobial peptides compose a diverse group of 12–50 amino acid chains produced by a wide variety of organisms. In humans, the chief classes of antimicrobial peptides are cathelicidins and defensins; both have been implicated in defense of epithelial surfaces against infectious agents [14]. Humans and mice each express a single cathelicidin, termed “LL-37” in humans and “cathelin-related antimicrobial peptide” (CRAMP) in mice [14, 15]. Although their antimicrobial domains vary in primary sequence, both form amphipathic α-helices with activity against gram-positive and gram-negative bacteria, fungi, and viruses [14]. Cathelicidin is constitutively expressed at low levels by epithelial cells in many tissues, including the urogenital tract, skin, lungs, and gut; pathogen recognition can rapidly induce local production of the peptide [15–18]. Beyond its antimicrobial properties, cathelicidin can recruit immune cells to sites of infection and stimulate production of proinflammatory cytokines [14, 19]. Recruited neutrophils can also produce cathelicidin to aid in controlling infection [15, 20].
Uroepithelial expression of cathelicidin has been described as an important innate defense mechanism in the urinary tract. Higher cathelicidin levels are detected in urine during human UTI, while postinfection levels fall below those of controls, suggesting that patients with naturally lower levels of cathelicidin may be more susceptible to UTI [18]. Chromek et al examined UTI in CRAMP-deficient mice on a 129/SvJ genetic background, using a UPEC strain, CFT073, isolated from the blood of a patient with pyelonephritis [16]. These authors observed more severe infections in CRAMP-deficient mice, evidenced by augmented bacterial binding to renal proximal tubule cells and higher kidney bacterial loads 48 hours after infection [16].
To further specify the role of cathelicidin during the most common form of human UTI, cystitis, we used the cystitis-derived UPEC strain UTI89 and CRAMP-deficient C57Bl/6 mice. Although CRAMP effectively limited UPEC growth in vitro, its role during UTI was revealed as more complex than previously appreciated. Contrary to expectation, we found that CRAMP-deficient mice demonstrated lower bladder bacterial loads at multiple time points and recovered more quickly from cystitis. CRAMP-deficient mice exhibited an attenuated immune response to infection and less tissue damage, correlating with accelerated epithelial restoration. Our data indicate that CRAMP may confer benefits to both host and pathogen in distinct ways within the urinary tract.
MATERIALS AND METHODS
Bacterial Strains and Culture
For infections, the cystitis-derived UPEC isolate UTI89 [21] was inoculated into Luria-Bertani broth (LB; 20 mL) directly from frozen stock and grown statically at 37°C for 16 hours. Overnight cultures were centrifuged at 5000 × g for 10 minutes, resuspended in sterile phosphate-buffered saline (PBS) to OD600nm of 1.0, and diluted 1:1 in PBS for inoculation.
Mice and Infections
Female mice aged 8–10 weeks were used for all experiments; animal procedures were approved in advance by the Animal Studies Committee at Washington University. Homozygous CRAMP-deficient mice on a C57BL/6 background (a kind gift from R. Gallo [22]) were bred on site. Mice were genotyped with PCR on tail DNA with primers specific to the third and fourth exons of the Cnlp gene [23]. C57Bl/6J mice (bred on site and purchased from Jackson Laboratories, Bar Harbor, ME) were used as comparators. Cystitis was induced by transurethral inoculation of 50 µL of bacterial suspension in PBS (approximately 107 colony-forming units [CFU]) as previously described [3].
Organ and Urine Bacterial Titers
Urine collected from mice at 1 hour after infection was serially diluted and plated on LB agar. Bladders and kidneys were harvested by a sterile technique at specific time points (1, 6, 16, 24, and 48 hours and 2 weeks after infection), homogenized in sterile PBS (Bullet Blender, Next Advance, Averill Park, NY), and plated on LB agar. Remaining homogenates were stored at −80°C for use in uroplakin immunoblotting and soluble analyses as described below.
Confocal Microscopy and IBC Volume Determination
Mice were infected with UTI89/pcomGFP [24] and bladders harvested at 16 hours after infection. Bladders were bisected, stretched with fine forceps and mounting pins, fixed in 2.5% paraformaldehyde, and stained with Syto 61 (1:1000; Life Technologies, Grand Island, NY). Bladder halves were imaged using a Zeiss LSM510 inverted confocal microscope; Z-stacks of individual IBCs were collected and volumes quantified using Volocity 6.3 software (PerkinElmer, Shelton, CT).
IBC Enumeration
Bladders were harvested at 16 hours after infection, bisected, stretched, gently washed twice with PBS, fixed for 1 hour at 4°C (0.2% glutaraldehyde, 50 mM ethylene glycol tetraacetic acid, and 100 mM MgCl2 in PBS), and stained as previously described [25]. Tissues were washed 3 times with PBS and stored at 4°C until IBCs were counted under a dissecting microscope [25].
In Vivo Binding and Invasion Assays
Bladders were harvested 1 hour after infection, partially bisected, and washed three times with 500 µL PBS with gentle rocking; washes were plated to LB agar to enumerate lumenal bacteria. Washed bladders were incubated in 100 µg/mL gentamicin at 37°C for 90 minutes; this gentamicin wash was plated to confirm death of extracellular bacteria. Bladders were then homogenized and plated to enumerate intracellular bacteria.
Electron Microscopy of CRAMP-Treated Cultures
Overnight bacterial cultures were pelleted, resuspended to OD600nm of 1.0, and treated with 10 µg/mL CRAMP or PBS for 1 hour at 37°C. Cultures were fixed (1% glutaraldehyde) before bacteria were adsorbed to glow-discharged formvar/carbon-coated copper grids for 2 minutes. Grids were washed in water and stained for 1 minute with 1% aqueous uranyl acetate (Ted Pella, Redding, CA). Air-dried samples were viewed on a JEOL 1200EX transmission electron microscope (JEOL USA, Peabody, MA).
Immunoblotting
Bladder homogenates were thawed, vortexed, and separated on 12% sodium dodecyl sulfate polyacrylamide gels. Proteins were transferred to polyvinylidene fluoride membranes and blocked in 2% powdered milk plus 2% bovine serum albumin (BSA) in blocking buffer (0.5% Tween-20, 0.5 M NaCl, and 0.01 M Tris base; pH 8.2). Membranes were incubated with either rabbit anti-uroplakin IIIa (UPIIIa; 1:5000, sc-33570, Santa Cruz Biotechnology, Dallas, TX) or goat anti-uroplakin Ia (UPIa; 1:250; sc-15173, Santa Cruz Biotechnology), and the control rabbit anti-CoxIV (1:2000; no. 4844, Cell Signaling Technology, Danvers, MA). Alkaline phosphatase–conjugated anti-rabbit immunoglobulin G (1:2500; Sigma, A3812) or anti-goat IgG (1:2000; Sigma, A4187) was used as secondary antibody. Blots were incubated with Tropix CDP-STAR (Life Technologies) and exposed to film. Band densities were quantified using ImageJ, and uroplakin levels were normalized to the loading control CoxIV. All homogenates (3 uninfected mice/strain and 5 infected mice/strain/time point) were run adjacently on 3 separate gels and blots, with independent quantification.
cAMP Quantification
Bladders were harvested sterilely 30 minutes after infection, everted (with 2 pairs of fine-tipped forceps, one securing the superior end and the other everting the tissue from the cut inferior end), and incubated in 0.1 M HCl for 30 minutes at room temperature. Lysates were cleared by centrifugation at 20 000 × g for 10 minutes and stored at −20°C, acetylated, and used in the cAMP Enzyme Immunoassay Kit (Sigma, CA201) according to manufacturer's instructions. Comparator C57Bl/6 mice were administered 10 mg/kg forskolin (Sigma) intraperitoneally plus 100 µM forskolin transurethrally [26], or dimethyl sulfoxide vehicle control 30 minutes before processing.
Tissue Histological Analysis
The bladder and left kidney of 2 (C57Bl/6) or 3 (CRAMP-deficient) mice were harvested, fixed in methacarn (a 6:3:1 solution of methanol:chloroform:glacial acetic acid), and set in 2% agar. Agar blocks were embedded in paraffin, and 5-µm sections were stained with hematoxylin and eosin and then imaged using an Olympus DP25 camera and BX40 light microscope.
Immunofluorescence Microscopy
Unstained sections from uninfected C57Bl/6 and CRAMP-deficient bladders were deparaffinized in mixed xylenes, rehydrated in isopropanol, washed in water, and boiled 30 minutes in 10 mM sodium citrate. Sections were blocked for 1 hour in PBS with 1% BSA and 0.3% Triton X-100 at room temperature and incubated with rabbit anti-UPIIIa (1:100) overnight at 4°C. Alexa Fluor 488–conjugated goat anti-rabbit IgG (1:500; Life Technologies) and Syto 61 (1:10 000) were added for 1 hour at room temperature before mounting and visualization.
Cytokine Analysis
Cytokines in bladder homogenates were quantified using a 23-plex magnetic bead cytokine array (Bio-Plex Pro, Mouse Group I; Bio-Rad, Hercules, CA). Homogenates were thawed on ice and cleared by centrifugation at 20 000 × g for 10 minutes. Individual samples (3 uninfected mice/strain and 5 infected mice/strain/time point) were assayed in duplicate, and data were analyzed per the manufacturer's instructions.
Tissue Myeloperoxidase (MPO) Assay
MPO activity was quantified as a measure of neutrophils present in bladder tissue [27]. Cleared lysates from bladder homogenates were analyzed with the Fluoro MPO kit (Cell Technology, Mountain View, CA) per the manufacturer's instructions.
Statistical Analysis
All data were analyzed using the Mann–Whitney U test in GraphPad Prism software. P values of < .05 were considered statistically significant.
RESULTS
CRAMP-Deficient Mice Display Less Severe Cystitis
We confirmed the in vitro antimicrobial properties of CRAMP against the cystitis-derived UPEC strain UTI89 (Supplementary Figure 1). We therefore expected our CRAMP-deficient mice to display more extensive infection in the urinary tract. Instead, CRAMP-deficient mice exhibited significantly lower bladder bacterial loads as early as 1 hour after infection and at all time points through 48 hours after infection (P < .005 at 1, 6, and 48 hours after infection; P < .05 at 16 and 24 hours after infection; Figure 1A). Kidney infection was infrequently observed in either host strain (data not shown). Wild-type C57Bl/6 mice rarely progress to chronic high-titer cystitis [12], and similarly, CRAMP-deficient bladders did not harbor elevated bacterial loads 2 weeks after infection (Figure 1B).
Figure 1.
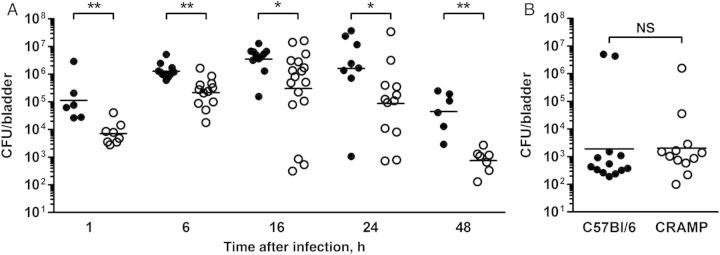
A, Bladder bacterial loads in wild-type C57Bl/6 mice (filled circles) were significantly higher than in cathelin-related antimicrobial peptide (CRAMP)–deficient mice (open circles) at 1, 6, 16, 24, and 48 hours after infection (*P < .05 and **P < .005). Bar represents the geometric mean. B, C57Bl/6 and CRAMP-deficient mice show equivalent and minimal bacterial loads at 2 weeks after infection. Abbreviations: CFU, colony-forming units; NS, not significant.
Intracellular Growth and IBC Formation Is Unaffected in the Absence of CRAMP
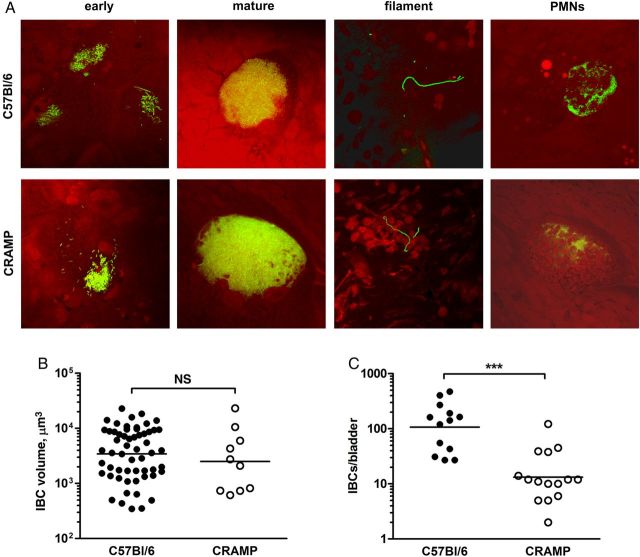
The IBC represents a key phenotypic stage in the development of murine cystitis [5, 6, 28, 29]. Thus, we asked whether defective IBC formation in CRAMP-deficient mice might correlate with the lower bladder titers observed in these hosts. Confocal microscopy revealed early and mature IBCs, as well as filamentous forms, in C57Bl/6 and CRAMP-deficient mice (Figure 2A). Neutrophils properly located infected epithelial cells in both strains of mice (Figure 2A). Quantification of IBC volumes did not reveal differences (Figure 2B), but bladders from CRAMP-deficient mice bore significantly fewer IBCs than those of wild-type mice (P = .0002; Figure 2C). Together, these data indicate that, while the IBC pathway is intact in CRAMP-deficient mice, diminished IBC numbers point to an early alteration in the infectious process (eg, impaired initiation of infection or accelerated clearance) in these hosts.
Figure 2.
A, Early and mature intracellular bacterial communities (IBCs) can be found in the bladder epithelial cells of both C57Bl/6 and cathelin-related antimicrobial peptide (CRAMP)–deficient mice after infection with green fluorescent protein–expressing UTI89. Filamentous bacteria were also identified in both host strains of mice, as well as neutrophils that localize to IBC-bearing epithelial cells. Representative pictures are shown. B, IBC volumes were equivalent between the 2 host strains, as quantified by Volocity software (all IBCs from 3 C57Bl/6 and 4 CRAMP-deficient mice). C, IBC numbers, measured by lacZ staining, were significantly higher in wild-type mice (***P < .001; n = 13–15 per strain). Bars in panels B and C represent the geometric mean. Abbreviation: PMN, polymorphonuclear cell.
Epithelial Binding and Invasion Are Diminished in CRAMP-Deficient Mice
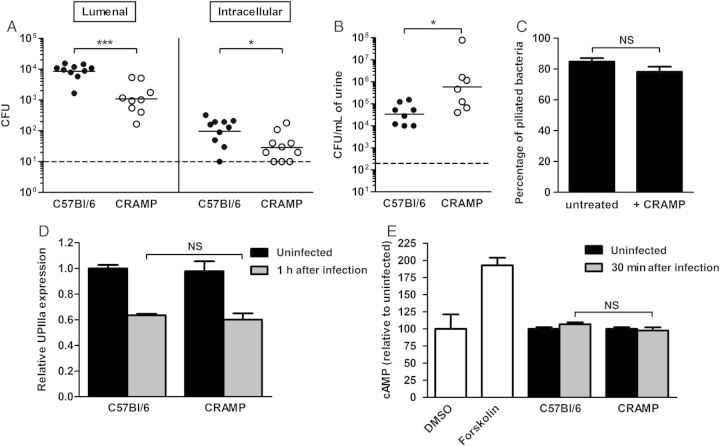
Given that bladder bacterial loads differed as early as 1 hour after infection, we investigated binding, invasion, and other processes occurring within this interval. Using in vivo binding and invasion assays, we recovered significantly fewer bacteria from both the lumenal and intracellular compartments 1 hour after infection in CRAMP-deficient mice (P < .001 and P < .05, respectively; Figure 3A), implicating bacterial binding and invasion in the defective pathogenesis observed in these hosts. Corroborating this finding, CRAMP-deficient mice liberated significantly more bacteria in the urine 1 hour after infection (P < .05; Figure 3B).
Figure 3.
A, Bacterial titers in the lumenal and intracellular (gentamicin-protected) compartments at 1 hour after infection were both significantly lower in cathelin-related antimicrobial peptide (CRAMP)–deficient mice (*P < .05 and ***P < .001; n = 5 mice/strain/experiment). B, CRAMP-deficient mice liberate increased numbers of bacteria in the urine 1 hour after infection (*P < .05). Dashed line represents the limit of detection, and bars represent the geometric mean in A and B. C, Proportion of bacteria, either untreated or after 1 hour of exposure to 10 µg/mL CRAMP, that were piliated, as determined by electron microscopy (n = 100/condition × 5 separate experiments). D, Densitometric quantification by immunoblot of uroplakin IIIa (UPIIIa) in bladder homogenates (uninfected and 1 hour after infection) as a measure of epithelial exfoliation showed no difference between host strains. Mitochondrial enzyme CoxIV was used to normalize the mount of protein in each sample. E, No significant difference in bladder epithelial cAMP expression 30 minutes after infection was found between wild-type and CRAMP-deficient mice (n = 6–7 mice/strain). Negative and positive controls, respectively (white bars), included vehicle-only (dimethyl sulfoxide [DMSO]) and forskolin-treated C57Bl/6 bladders (n = 2 mice/treatment). Mean and standard errors of the mean are indicated in panels C–E. Abbreviations: CFU, colony-forming units; NS, not significant.
Because type 1 pili represent the primary determinant of UPEC binding to bladder epithelium, we tested whether CRAMP might represent a stimulus for bacterial expression of these organelles. However, no alteration in piliation was observed by electron microscopy upon CRAMP exposure in vitro (84.8% vs 78.2%; Figure 3C). Bladder UPIIIa content (evaluated by immunoblot of tissue homogenates) was also equivalent between wild-type and CRAMP-deficient mice at 1 hour after infection, indicating that accelerated uroepithelial exfoliation does not underlie decreased bacterial loads in CRAMP-deficient bladders (Figure 3D).
Abraham et al have shown that Toll-like receptor 4 (TLR4)–dependent surges in epithelial cell cAMP result in expulsion of internalized UPEC [26, 30, 31]. In addition, cathelicidin may bind to and mask recognition of bacterial lipopolysaccharide (LPS), thereby downregulating TLR4-dependent immune signaling [32]. Consequently, we hypothesized that, in CRAMP-deficient mice, TLR4 hyperactivation due to unmasked LPS may drive increased epithelial expulsion of internalized bacteria. However, cAMP activation in superficial epithelium in response to UTI89 infection was equivalent in wild-type and CRAMP-deficient mice (Figure 3E).
CRAMP Deficiency Does Not Impart Inherent Epithelial Changes
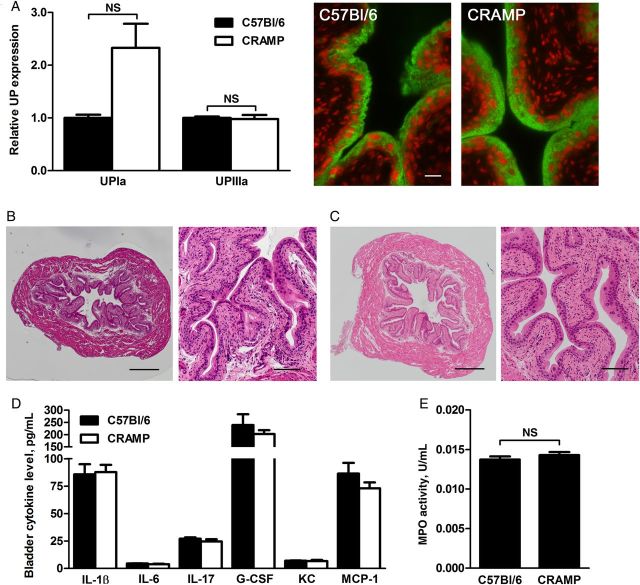
In further considering attenuated UPEC binding and invasion in CRAMP-deficient mice, we reasoned that these hosts might show baseline differences in epithelial structure or surface receptors (eg, uroplakins). Immunoblots revealed that UPIa and UPIIIa were equivalently expressed in bladder homogenates from the 2 mouse strains; similar expression and uroepithelial localization were also shown by immunofluorescence microscopy for UPIIIa of uninfected bladder sections (Figure 4A). Histological analysis found that uninfected bladders of wild-type and CRAMP-deficient mice were similar in size and in tissue structure and organization (Figure 4B and 4C).
Figure 4.
A, Densitometric analysis of Western blots for uroplakin Ia (UPIa) and UPIIIa in uninfected bladder homogenates showed no significant differences (UPIa, P = .1354; UPIIIa, P = .1000). Mitochondrial enzyme CoxIV was used to normalize the amount of protein in each sample. Mean and standard errors of the mean (SEM) are indicated. At right are representative fluorescence images of uninfected bladder sections (green, UPIIIa; red, nuclei; scale bar, 20 µm). B and C, Sections of uninfected C57Bl/6 (B) and cathelin-related antimicrobial peptide (CRAMP)–deficient (C) bladders were stained with hematoxylin and eosin for morphological analysis. Whole bladders are shown on the left (scale bar, 400 µm), and magnified epithelial views are shown on the right (scale bar, 100 µm). D, No significant differences were seen in cytokine profiles of uninfected bladder homogenates from C57Bl/6 and CRAMP-deficient mice. E, Myeloperoxidase (MPO) activity, a surrogate for neutrophil content, was also similar in uninfected whole bladder homogenates from the 2 host strains (n = 6 C57Bl/6 mice and 11 CRAMP-deficient mice). Mean and SEM are indicated in panels D and E. Abbreviations: G-CSF, granulocyte colony-stimulating factor; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-17, interleukin 17; KC, keratinocyte chemoattractant; MCP-1, monocyte chemoattractant protein 1; NS, not significant.
Cathelicidin expression can stimulate cytokine release from epithelial cells and promote immune cell recruitment to infected tissues [19, 33]. To investigate whether CRAMP influences soluble and cellular immune constituents in the bladder environment at baseline, we compared expression of 23 cytokines in uninfected C57Bl/6 and CRAMP-deficient bladders, finding no significant differences; 6 representative cytokines relevant to host response in cystitis are shown (Figure 4D; complete data are shown in Supplementary Table 1). We also found no difference in bladder neutrophil content (as measured on the basis of MPO activity) in the 2 uninfected host strains (Figure 4E). These data indicate that UPEC pathogenicity in the CRAMP-deficient host is not influenced by preinfection alterations in the resting immunologic state of the bladder.
CRAMP-Deficient Mice Show Attenuated Inflammatory Responses to Infection
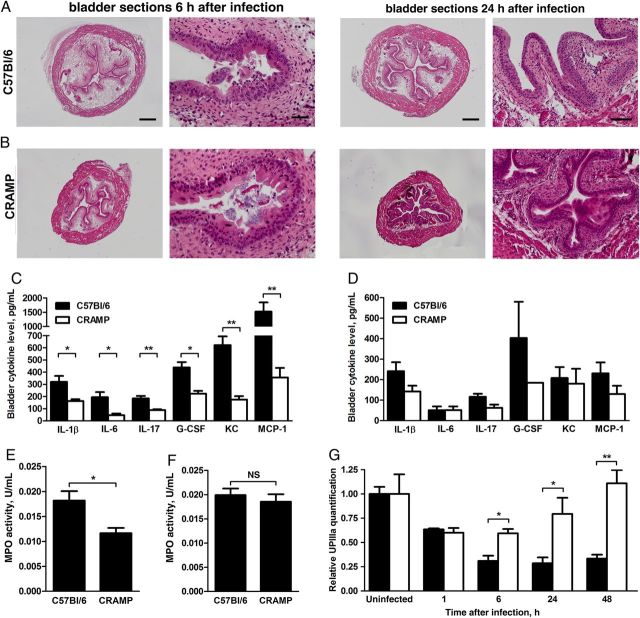
We next investigated whether CRAMP deficiency altered the inflammatory response to UPEC introduction into the bladder. Histological analysis detected lumenal bacteria and areas of epithelial exfoliation in both host strains by 6 hours after infection. Bladders from both hosts displayed mild edema and inflammatory cellular infiltrates (Figure 5A and 5B). While histological differences at this time point were modest, analysis of bladder homogenates 6 hours after infection revealed significantly higher levels of 16 inflammatory cytokines in wild-type mice, compared with CRAMP-deficient mice (Figure 5C; complete data are shown in Supplementary Table 1). As inflammation and associated tissue damage may promote sustained UPEC infection [2, 11, 12], we repeated these analyses 24 hours after infection; at this subsequent interval, bladders of CRAMP-deficient mice displayed epithelial restoration and minimal edema, while wild-type bladders remained notably edematous, with widespread epithelial exfoliation (Figure 5A and 5B). Meanwhile, tissue cytokines remained generally higher in wild-type mice 24 hours after infection, although these differences did not retain statistical significance (Figure 5D and Supplementary Table 1). Similarly, bladder MPO content was significantly higher in CRAMP-deficient hosts at 6 hours after infection (P = .016; Figure 5E), but this difference had resolved by 24 hours after infection (Figure 5F).
Figure 5.
Sections of C57Bl/6 (A) and cathelin-related antimicrobial peptide (CRAMP)–deficient bladders (B) 6 and 24 hours after infection were stained with hematoxylin and eosin for morphological analysis. Whole bladder sections (left panels; scale bar, 400 µm) show substantially increased edema in wild-type mice at both time points. Acute cystitis is evident in both host strains 6 hours after infection (right panels; scale bar, 40 µm). However, at 24 hours after infection, the infected wild-type bladder remains edematous and completely exfoliated, while CRAMP-deficient bladders reflect resolved inflammatory changes and recovery of the superficial epithelial layer. C, Expression of 6 representative cytokines is significantly lower in CRAMP-deficient bladder homogenates 6 hours after infection (*P < .05 and **P < .01). D, At 24 hours after infection, only granulocyte macrophage colony-stimulating factor expression was significantly lower in CRAMP-deficient mice, among 23 cytokines measured (*P = .032; data not shown). E and F, Whole-bladder myeloperoxidase (MPO) activity is significantly lower in CRAMP-deficient mice 6 hours after infection (E); this difference was no longer significant at 24 hours after infection (F). G, Densitometric analysis of bladder UPIIIa expression indicates more rapid epithelial recovery in CRAMP-deficient mice (white bars) than in wild-type mice (black bars; *P < .05 and **P < .01). Mean and standard errors of the mean are indicated in panels C–G. Abbreviations: G-CSF, granulocyte colony-stimulating factor; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-17, interleukin 17; KC, keratinocyte chemoattractant; MCP-1, monocyte chemoattractant protein 1; NS, not significant.
These contrasts in inflammatory response were also reflected in recovery of the superficial epithelium. Although bladder uroplakin content at baseline and 1 hour after infection was equivalent between host strains (Figure 3D), at 6 and 24 hours after infection significantly more exfoliation was evident in wild-type mice (P = .0317; Figure 5G). More striking, by 48 hours after infection, infected CRAMP-deficient mice had restored the superficial epithelium fully, while wild-type bladder UPIIIa levels remained low, at about one third of baseline levels (P = .0079; Figure 5G). In combination, these data suggest significant alterations in the immune response to UPEC cystitis in CRAMP-deficient mice.
DISCUSSION
Cathelicidin antimicrobial peptides have been implicated in the defense of multiple epithelial surfaces, including the skin, lungs, and urinary tract. Within the murine urinary tract, the expression and protective capacity of cathelicidin have been described in relation to kidney infection in 129/SvJ mice inoculated with 108 CFU of UPEC strain CFT073 [16]. Here, we used an established murine cystitis model, notably different from prior studies in host strain, bacterial strain, infectious dose, and the organ of focus. Our findings in this model system suggest that CRAMP exerts a complex influence on the progression and outcome of infection.
Although in vitro assays showed antimicrobial activity of CRAMP against UTI89, CRAMP was not essential for uroepithelial defense in our in vivo cystitis model. Instead, as indicated by significantly lower IBC numbers and bladder bacterial loads throughout infection, hosts lacking CRAMP resolved infections more efficiently. Diminished IBC formation in CRAMP-deficient hosts resulted from very early alterations in pathogenesis, specifically in epithelial binding and invasion by UPEC; however, we found no differences in several molecular determinants of these processes. Of note, cathelicidin has also been shown to enhance the bactericidal capacity of human and murine neutrophils by stimulating reactive oxygen species production in response to bacterial components and by augmenting bacterial engulfment. Specifically, bone marrow–derived neutrophils from CRAMP-deficient 129/SvJ mice showed diminished killing of gram-negative and gram-positive bacteria, compared with wild-type neutrophils [34], and cathelicidin may alter gene expression in neutrophils [35]. In contrast, we found normal UPEC-killing ability in bone marrow–derived neutrophils from CRAMP-deficient C57Bl/6 mice (Supplementary Figure 2). In total, our data instead indicate important activities for cathelicidin during UTI that are independent of its direct antimicrobial activity and support of phagocyte killing. Future studies of cathelicidin in epithelial defense should account for these multiple biological activities during host-pathogen interactions, as well as examine both epithelial and hematopoietic sources of the peptide.
Consonant with recent transcriptional profiling data [36, 37] and other prior studies [7, 38], we observed a sharp proinflammatory cytokine response in infected wild-type C57BL/6 mice by 6 hours after infection, with subsequent resolution. However, CRAMP-deficient hosts exhibited significantly reduced proinflammatory cytokine expression, edema, and neutrophil influx by 6 hours after infection (Figure 5 and Supplementary Table 1). Our findings support a model in which CRAMP participates in activation and development of the host immune response to UPEC within the bladder, a response that helps to control but may also facilitate UPEC infection [2]. Of note, a similar function for cathelicidin has been demonstrated in studies with cultured human lung epithelial cells and with human and murine peripheral monocytes [39, 40]. Consistent with an immunostimulatory role, cathelicidin concentrations during human UTI are on the order of other immunomodulators but well below concentrations necessary for bactericidal activity [16, 18] (Supplementary Figure 1). While the bactericidal activity of antimicrobial peptides may be impaired in high-salt conditions, it is unknown how such conditions (as may occur in urine) may influence its immunostimulatory properties. During experimental cystitis, absence of CRAMP may mitigate inflammatory cytokine responses and exfoliation, processes that, in wild-type hosts, produce the significant epithelial damage that offers UPEC access to naive cells in deeper uroepithelial layers [41]. Although bladder instillation of concentrated cathelicidin can elicit edema and leukocytic infiltrates [42], enhanced IBC formation in wild-type mice may also drive augmented inflammation. Indeed, multiple studies indicate that the degree of intracellular invasion and IBC formation predicts the intensity of inflammatory responses in the bladder and that increased inflammation in turn facilitates persistence of UPEC infection [2, 11, 12]. Thus, the development and outcome of uroepithelial infection depend on ongoing interplay between invasive bacterial populations and the soluble and cellular immune components of the host milieu.
Finally, cathelicidin is among multiple antimicrobial peptides that could influence the progression of UTIs. For example, α-defensins produced by infiltrating neutrophils, as well as renally expressed β-defensins, function to kill uropathogens and promote innate responses in infected tissue [43]. Uroepithelial expression of RNase 7 also increases during infection to yield urinary concentrations inhibitory to uropathogens [44, 45]. Further, antimicrobial peptides have been shown to signal to other types of epithelial cells through surface receptors or upon internalization [39, 46]. Beyond potential signaling roles in host epithelial or hematopoietic cells, cathelicidin and other antimicrobial peptides might also elicit transcriptional programs (eg, envelope stress responses) in bacteria. Cross-talk and/or redundancy likely occur among urinary antimicrobial peptides and would complicate the development of a complete model detailing the effects of individual antimicrobial peptides on the course of UTI. Our data also do not exclude the possibility that CRAMP-deficient mice might have alterations in intracellular events that follow bacterial internalization. However, the field presently lacks detailed molecular and cell-biological knowledge of these events, including a mechanism for presumed UPEC escape from the endocytic vacuole into the epithelial cell cytoplasm. Further exploration of potential influences of cathelicidin on UTI pathogenesis and accompanying immune responses may reveal avenues for modulating these host responses, to mitigate progression, chronic infection, and recurrence.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Kristin Tiemann, Jeff Elsner, Wandy Beatty, and the Washington University Department of Developmental Biology Histology Core, for technical assistance; and Michael Caparon and James Fleckenstein, for critical review of the manuscript.
Financial support. This work was supported by the National Institutes of Health (grants DK064540, DK082546, and DK080752 to D. A. H.) and the National Science Foundation (graduate research fellowship DGE-1143954 to E. S. D.).
Potential conflicts of interest. D. A. H. serves on the scientific advisory board of BioVersys. E. S. D. certifies no potential conflicts of interest.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–60. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 2.Ulett GC, Totsika M, Schaale K, Carey AJ, Sweet MJ, Schembri MA. Uropathogenic Escherichia coli virulence and innate immune responses during urinary tract infection. Curr Opin Microbiol. 2013;16:100–7. doi: 10.1016/j.mib.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Mulvey MA, Lopez-Boado YS, Wilson CL, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–7. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 4.Zhou G, Mo WJ, Sebbel P, et al. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J Cell Sci. 2001;114:4095–103. doi: 10.1242/jcs.114.22.4095. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–7. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 6.Hunstad DA, Justice SS. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol. 2010;64:203–21. doi: 10.1146/annurev.micro.112408.134258. [DOI] [PubMed] [Google Scholar]
- 7.Hedges S, Anderson P, Lidin-Janson G, de Man P, Svanborg C. Interleukin-6 response to deliberate colonization of the human urinary tract with gram-negative bacteria. Infect Immun. 1991;59:421–7. doi: 10.1128/iai.59.1.421-427.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McTaggart LA, Rigby RC, Elliott TS. The pathogenesis of urinary tract infections associated with Escherichia coli, Staphylococcus saprophyticus and S. epidermidis. J Med Microbiol. 1990;32:135–41. doi: 10.1099/00222615-32-2-135. [DOI] [PubMed] [Google Scholar]
- 9.Samuelsson P, Hang L, Wullt B, Irjala H, Svanborg C. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect Immun. 2004;72:3179–86. doi: 10.1128/IAI.72.6.3179-3186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahin RD, Engberg I, Hagberg L, Svanborg Eden C. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J Immunol. 1987;138:3475–80. [PubMed] [Google Scholar]
- 11.Schilling JD, Lorenz RG, Hultgren SJ. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect Immun. 2002;70:7042–9. doi: 10.1128/IAI.70.12.7042-7049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010;6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weichhart T, Haidinger M, Horl WH, Saemann MD. Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur J Clin Invest. 2008;38(suppl 2):29–38. doi: 10.1111/j.1365-2362.2008.02006.x. [DOI] [PubMed] [Google Scholar]
- 14.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–25. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Gallo RL, Kim KJ, Bernfield M, et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272:13088–93. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 16.Chromek M, Slamova Z, Bergman P, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–41. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 17.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen KL, Dynesen P, Larsen P, Jakobsen L, Andersen PS, Frimodt-Møller N. Role of urinary cathelicidin LL-37 and human β-defensin 1 in uncomplicated Escherichia coli urinary tract infections. Infect Immun. 2014;82:1572–8. doi: 10.1128/IAI.01393-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock RE. Impact of LL-37 on anti-infective immunity. J Leukoc Biol. 2005;77:451–9. doi: 10.1189/jlb.0704380. [DOI] [PubMed] [Google Scholar]
- 20.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–93. [PubMed] [Google Scholar]
- 21.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–9. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchau AS, Morizane S, Trowbridge J, et al. The host defense peptide cathelicidin is required for NK cell-mediated suppression of tumor growth. J Immunol. 2010;184:369–78. doi: 10.4049/jimmunol.0902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 24.Valdivia RH, Hromockyj AE, Monack D, Ramakrishnan L, Falkow S. Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene. 1996;173:47–52. doi: 10.1016/0378-1119(95)00706-7. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson TF, Watts KM, Hunstad DA. OmpA of uropathogenic Escherichia coli promotes postinvasion pathogenesis of cystitis. Infect Immun. 2009;77:5245–51. doi: 10.1128/IAI.00670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song J, Bishop BL, Li G, Duncan MJ, Abraham SN. TLR4-initiated and cAMP-mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe. 2007;1:287–98. doi: 10.1016/j.chom.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loughman JA, Hunstad DA. Attenuation of human neutrophil migration and function by uropathogenic bacteria. Microbes Infect. 2011;13:555–65. doi: 10.1016/j.micinf.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Justice SS, Hung C, Theriot JA, et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101:1333–8. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–30. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- 31.Song J, Bishop BL, Li G, Grady R, Stapleton A, Abraham SN. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc Natl Acad Sci U S A. 2009;106:14966–71. doi: 10.1073/pnas.0900527106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song J, Abraham SN. TLR-mediated immune responses in the urinary tract. Curr Opin Microbiol. 2008;11:66–73. doi: 10.1016/j.mib.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovach MA, Ballinger MN, Newstead MW, et al. Cathelicidin-related antimicrobial peptide is required for effective lung mucosal immunity in Gram-negative bacterial pneumonia. J Immunol. 2012;189:304–11. doi: 10.4049/jimmunol.1103196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alalwani SM, Sierigk J, Herr C, et al. The antimicrobial peptide LL-37 modulates the inflammatory and host defense response of human neutrophils. Eur J Immunol. 2010;40:1118–26. doi: 10.1002/eji.200939275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinheiro da Silva F, Medeiros MC, Dos Santos AB, et al. Neutrophils LL-37 migrate to the nucleus during overwhelming infection. Tissue Cell. 2013;45:318–20. doi: 10.1016/j.tice.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Duell BL, Carey AJ, Tan CK, et al. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J Immunol. 2012;188:781–92. doi: 10.4049/jimmunol.1101231. [DOI] [PubMed] [Google Scholar]
- 37.Tan CK, Carey AJ, Cui X, et al. Genome-wide mapping of cystitis due to Streptococcus agalactiae and Escherichia coli in mice identifies a unique bladder transcriptome that signifies pathogen-specific antimicrobial defense against urinary tract infection. Infect Immun. 2012;80:3145–60. doi: 10.1128/IAI.00023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agace W, Hedges S, Ceska M, Svanborg C. Interleukin-8 and the neutrophil response in mucosal Gram-negative infections. J Clin Invest. 1993;92:780–5. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–91. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 40.Tjabringa GS, Aarbiou J, Ninaber DK, et al. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 41.Blango MG, Ott EM, Erman A, Veranic P, Mulvey MA. Forced resurgence and targeting of intracellular uropathogenic Escherichia coli reservoirs. PLoS One. 2014;9:e93327. doi: 10.1371/journal.pone.0093327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oottamasathien S, Jia W, McCoard L, et al. A murine model of inflammatory bladder disease: cathelicidin peptide induced bladder inflammation and treatment with sulfated polysaccharides. J Urol. 2011;186:1684–92. doi: 10.1016/j.juro.2011.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–7. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 44.Spencer JD, Schwaderer AL, Wang H, et al. Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int. 2013;83:615–25. doi: 10.1038/ki.2012.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zasloff M. The antibacterial shield of the human urinary tract. Kidney Int. 2013;83:548–50. doi: 10.1038/ki.2012.467. [DOI] [PubMed] [Google Scholar]
- 46.Lau YE, Rozek A, Scott MG, Goosney DL, Davidson DJ, Hancock RE. Interaction and cellular localization of the human host defense peptide LL-37 with lung epithelial cells. Infect Immun. 2005;73:583–91. doi: 10.1128/IAI.73.1.583-591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.