Abstract
Biofunctionalized polyethylene glycol maleimide (PEG-MAL) hydrogels were engineered as a platform to deliver pancreatic islets to the small bowel mesentery and promote graft vascularization. VEGF, a potent stimulator of angiogenesis, was incorporated into the hydrogel to be released in an on-demand manner through enzymatic degradation. PEG-MAL hydrogel enabled extended in vivo release of VEGF. Isolated rat islets encapsulated in PEG-MAL hydrogels remained viable in culture and secreted insulin. Islets encapsulated in PEG-MAL matrix and transplanted to the small bowel mesentery of healthy rats grafted to the host tissue and revascularized by 4 weeks. Addition of VEGF release to the PEG-MAL matrix greatly augmented the vascularization response. These results establish PEG-MAL engineered matrices as a vascular-inductive cell delivery vehicle and warrant their further investigation as islet transplantation vehicles in diabetic animal models.
Keywords: Islet, PEG, matrix, vascularization, angiogenesis, mesentery, VEGF
Introduction
One in every 400 to 600 children and adolescents in the U.S. suffers from type 1 diabetes mellitus T1DM [1], a condition that results from the autoimmune destruction of the insulin-producing beta cells in the pancreas. Successful transplantation of either a whole pancreas or pancreatic islets can replace the burden of exogenous insulin injections with a self-regulating insulin source. However, islet cell transplantation is less invasive. The current clinical standard for islet transplantation involves infusing donor islets into the recipient’s hepatic portal vein where they become lodged and engraft in the vascular system of the liver [2]. While still an experimental treatment, the short-term outcomes are promising with 44% of patients receiving islet transplantation, from 2007–2010, remaining insulin independent three years later [3]. However, only about 20% of islet transplant recipients maintain exogenous insulin independence at 5 years [4–7, 3]. Furthermore, islet infusions from multiple donors are often required to achieve insulin independence, stressing an already restricted supply of islets, and limiting wide-spread adoption of islet transplantation therapy. Other factors reducing successful islet engraftment are instant blood-mediated inflammatory reaction [8], toxic responses to immunosuppressive drugs [9], and loss of islet viability/function during isolation [10]. Additionally, inadequate vascularization following transplantation remains a major issue for long-term islet health [11, 12].
Pancreatic islets have higher densities of blood vessels compared to the surrounding exocrine tissue and receive 15–20% of pancreatic blood supply despite comprising only 1–2% of pancreas mass [13, 14]. A dense network of capillaries maintains blood flow through the islet cell mass. Following islet isolation and transplantation the post-engraftment vasculature exhibits much lower vessel density than in native islets [15–17]. The resultant reduced blood flow and lower oxygen tension produce ischemia compared to native islets [18, 14] that is a major cause of reduced islet viability, function, and engraftment [19–21].
Previous strategies to enhance vascularization in transplanted islets include delivery of angiogenic genes, growth factors, and vascular-inductive cell types. Gene transfer studies have shown that activation of angiogenic signaling through either increased VEGF [22–27], angiopoeietin-1 [28], or thrombospondin-1 [29] results in improved revascularization of transplanted islets in rodents. Despite these promising results, clinical implementation of angiogenic gene therapy for islet transplantation is complicated by the additional safety considerations associated with gene transfer. In addition, overexpression of angiogenic genes in islets has been shown to be detrimental to beta cell function through hypervascularization and increased inflammation [30, 31]. Co-delivery of pro-angiogenic cell types, such as mesenchymal stem cells [32] which also function to temper islet graft inflammation [33, 34], or as direct contributors to vascular endothelium in the case of endothelial cells and progenitors [35–37], have also resulted in improved islet vascularization. The drawbacks of this strategy are the increased costs and greater clinical complexity of delivering cells from multiple sources.
In contrast, protein-based angiogenic growth factor delivery provides a simple and safe means of achieving therapeutic vascularization [38–40]. This strategy is attractive for islet transplantation because proteins can be easily co-delivered with islets in a biomaterial scaffold or hydrogel with few safety concerns. Protein-based therapeutic angiogenesis, mostly through bolus delivery, has shown limited effectiveness in clinical trials to treat myocardial and chronic limb ischemia [41], prompting researchers to investigate alternative sources of angiogenic factors such as gene therapy [42]. These results also highlight the need for more sophisticated controlled release protein delivery strategies. In islet transplantation, a constant source of angiogenic factors may be unnecessary as the goal is to achieve a simple initial vascularization response and not treat a chronic ischemia injury.
Beta cells are highly sensitive to oxidative or inflammatory environments, and will easily undergo apoptosis when stressed [43]. Anti-inflammatory therapy has been investigated as a means of preventing beta cell loss in islet grafts [44]. Major sources of early islet graft inflammation include instant blood-mediated inflammatory reaction initiated by the direct islet to blood contact during the portal vein injection procedure [45] and Kupffer cell-mediated oxidation in the liver [46]. Localized ischemia from vessel occlusions where islets lodge in the liver also induces inflammation. In alternative transplantation sites to liver, tissue damage from the biomaterial or islet implantation surgery is an additional source of inflammation. Anti-inflammatory therapy may help reduce immunological rejection from either underlying autoimmunity or de novo allo-immunity [8]. While controlling inflammation is a critical component of islet transplantation necessary for clinically successful treatments for type 1 diabetes, vascularization of transplanted islets plays an equally important roles in islet health, survival, and function. In this study, our goal was to focus on the role of vascularization in islet survival decoupled from immune-mediated sources of islet death. We sought to give the transplanted islets the best possible chance for survival by grafting them on the surface of tissue with a bioadhesive hydrogel where the transplanted cells did not contact the blood stream directly and no localized tissue injuries were created by the procedure itself. In this way we sought to achieve an initially low inflammation environment without the additional complexity of incorporating anti-inflammatory therapeutics, although this could be a potential synergistic strategy to employ in future studies, especially with allogeneic islet grafts.
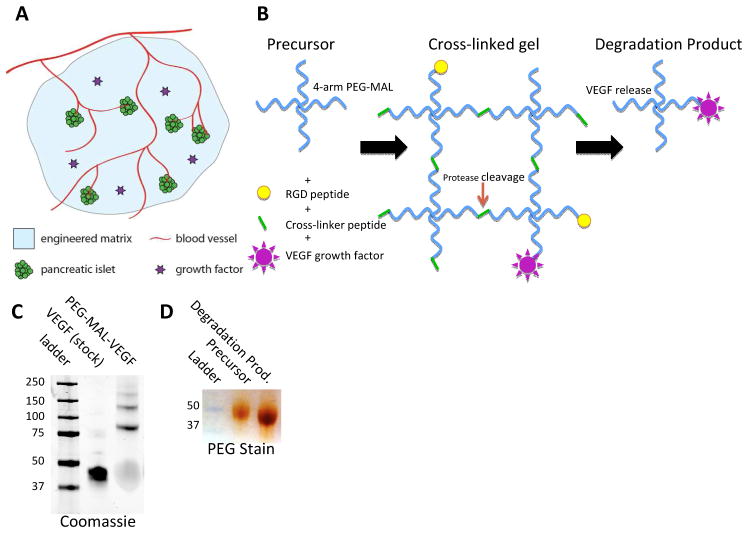
Here we describe a method for delivering pancreatic islets in a biologically active engineered matrix, designed to promote beta cell survival and trigger a host vascularization response through growth factor delivery (Fig. 1A). Other studies have shown that PEG-based hydrogels are suitable materials for islet delivery. PEG-hydrogels have been designed to support islet cell survival in vitro [47] and promote pancreatic beta cell and whole islet activity [48–53]. In the present study, we engineered a PEG-based hydrogel for islet transplantation that utilizes a mild maleimide-thiol cross-linking reaction for preserving viability while maintaining a rapid gelation time for feasible in situ delivery. We incorporated bioactive RGD peptide motifs to allow for cell adhesion and the angiogenic growth factor VEGF to promote vascular invasion. VEGF-A, is a primary driver for initiating and maintaining the intra-islet capillary bed during development and islet transplantation [25, 18, 54]. Pancreas specific knock out of VEGF-A in mice reduces islet perfusion, insulin secretion, and glucose tolerance [18]. We selected a fast-degrading peptide, GCRDVPMSMRGGDRCG [55, 56], to cross-link the hydrogel to allow for rapid in vivo degradation of the PEG-MAL matrix and promote vascular invasion.
Fig. 1.
Michael-type addition hydrogel reaction scheme. A Transplanted pancreatic islets are embedded in a PEG-MAL hydrogel and vascularized by invading angiogenesis from the surrounding host tissue. B 4-arm PEG-macromers are first functionalized with RGD adhesive ligand and VEGF followed by cross-linking with a thiol-flanked enzyme-degradable peptide. Bound VEGF is released upon proteolytic degradation of the hydrogel. C Coomassie stain of pure VEGF and PEG-MAL conjugated VEGF showing VEGF PEGylation. D Barium-Chloride/Iodine PEG stain after SDS-PAGE, precursor 4-arm macromer and gel degradation product are similar molecular weight.
Previous studies indicate that sustained delivery of VEGF from PEG hydrogel induced growth of stable vasculature in vivo [57, 58]. We hypothesized that controlled presentation of angiogenic cues within this bioartificial matrix would enhance the vascularization of transplanted islets (Fig. 1A). This strategy is fundamentally different from other biomaterial approaches to islet transplantation [59, 60] focusing on coatings for immunoprotection [61, 53, 52, 62] and simple matrix carriers that serve as growth factor reservoirs [22, 24, 23, 38, 63, 39] but do not control the delivery profile of bio-therapeutics.
Naturally derived matrices may offer many advantages for supporting cellular activities over synthetic hydrogels such as nano-topography, growth-factor binding sites, and an underlying heterogeneity which help to direct complex cell behaviors. While naturally derived matrices such as alginate, collagen, and fibrin can be useful for tissue regeneration, a drawback to their use is the potential for unintended and uncontrollable properties such as cryptic binding sites, antigenicity, and batch to batch variations which increase the system complexity. While synthetic matrices do not achieve the full bioactivity or complexity of natural ECM, their usefulness lies in the versatility and simplicity to implement “plug-and-play” design variations for easy modification of incorporated bioactive components such as adhesive ligands and degradation kinetics on a highly inert background material. This high signal to noise ratio provides a controlled environment within which to test hypotheses about the action of specific sets of biomolecules. The concept of synthetic extracellular matrices for tissue engineering is covered in depth in a review by Lutolf and Hubbell [64], and the use of synthetic extracellular matrices for islet transplantation specific applications is reviewed by Borg and Bonifacio [65]. In this study we chose to employ a synthetic hydrogel over a naturally derived matrix for the benefits of a simple design where we could study the effects of a rationally designed bioactive component against a low-adhesion background while maintaining control over the degradation profile and release kinetics.
Results
PEG-MAL hydrogel formulation
Biofunctionalized PEG-MAL hydrogels were formed by pre-incubating a 4-arm PEG-MAL macromer with c-terminal cysteine RGD peptide to provide a ligand for cell adhesion (Fig. 1B). We previously showed that this RGD peptide reacts with PEG-MAL to nearly 100% incorporation [66]. Conjugation of VEGF to PEG-MAL was confirmed by molecular weight increase of the VEGF protein band on SDS-PAGE (Fig. 1C). Functionalized PEG-MAL macromer was cross-linked into a hydrogel by addition of a cysteine-flanked protease degradable peptide sequence, previously reported to facilitate rapid angiogenic infiltration [55, 56]. To confirm that the gel degradation process was consistent with the model proposed in Figure 1, PEG-MAL gels were incubated overnight in collagenase solution to ensure complete cleavage of all peptide cross-linkers and dissolution of the gel. Gel degradation products were run on SDS-PAGE and stained for PEG. The PEG-MAL gel degradation product had comparable molecular weight to the precursor PEG-MAL macromer (Fig. 1D), demonstrating that the cross-linking process results in a predictable matrix lattice structure with a homogeneous and low molecular weight degradation product.
PEG-MAL delivery of VEGF by proteolytic gel degradation
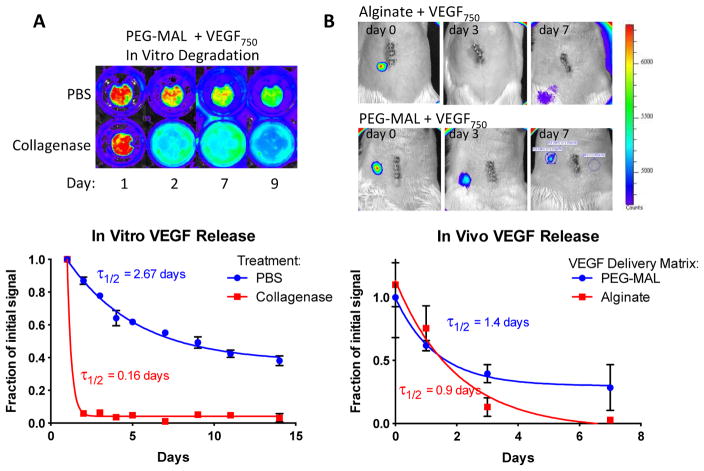
We next examined the release profile of infrared-tagged VEGF from PEG-MAL hydrogel by collagenase digestion in vitro. Collagenase-treated samples degraded completely overnight and released all of their VEGF into the media. Control samples in PBS retained a strong IR signal confined to the implant volume with 40% signal remaining at 14 days (τ1/2 = 2.67 days) (Fig. 2A). VEGF release from the hydrogel was also measured in vivo. VEGF was released over time with signal persisting in vivo for 7 days (τ1/2 = 1.4 days). VEGF delivered by calcium cross-linked alginate gels had no signal remaining after 3 days in vivo (τ1/2 = 0.9 days) (Fig. 2B). The short half-life of the VEGF signal in alginate is indicative of a rapid and passive release while the PEG-MAL hydrogel delivery extended the release profile to at least 7 days. Direct visualization of the small bowel mesentery in animals euthanized at 14 days revealed a strong residual VEGF signal in the PEG-MAL implant site but no signal was observed in alginate, indicating that PEG-MAL delivery extends VEGF signal beyond the 7 days that were seen through trans-abdominal measurements.
Fig. 2.
VEGF release profile from PEG-MAL hydrogel. A PEG-MAL gels functionalized with IR-750 labeled VEGF (VEGF750) were incubated in either PBS or collagenase type 1 enzyme. Mean fluorescence in the gel was measured with a Xenogen IVIS machine over 14 days. B PEG-MAL and alginate gels functionalized with VEGF750 were implanted in the small bowel mesentery of rats. Trans-abdominal measurements of gel fluorescence were measured over 7 days.
PEG-MAL hydrogel cyto-compatibility with encapsulated islets
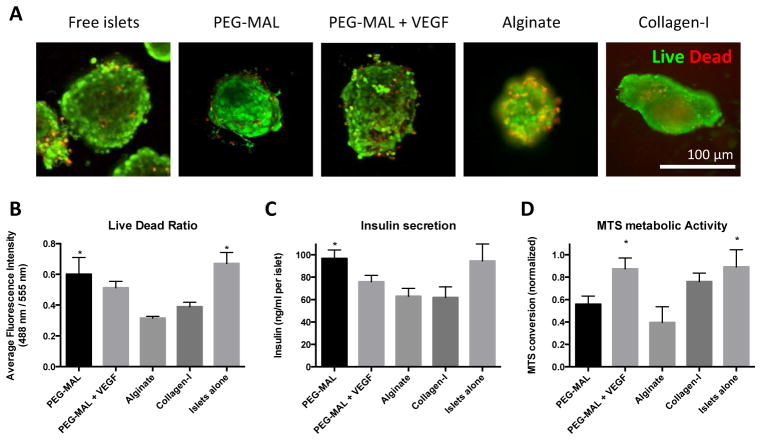
Viability was assessed in islets encapsulated in PEG-MAL and compared to alginate and Collagen-I (Fig. 3A). Alginate hydrogels were somewhat opaque resulting in less clear images than other groups. While many cells remained viable after alginate and collagen encapsulation, there were statistically twice as many live cells in PEG-MAL hydrogel than in alginate or collagen I (Fig. 3B).
Fig. 3.
In vitro characterization of PEG-MAL islet cytocompatibility in PEG-MAL compared to alginate and collagen-I hydrogel. A Isolated rat pancreatic islets were live/dead stained 24 hours after encapsulation in PEG-MAL hydrogel. B Fluorescence intensity ratio of live to dead signal in islets structures. C ELISA measurement of insulin secretion after 24 hours in encapsulated islets. D MTS measurement of metabolic activity after 24 hours in encapsulated islets. * Indicates significantly different from alginate, p < 0.05.
We assessed insulin secretion from gel-encapsulated islets into the medium over 24 hours post-encapsulation. While islets produced insulin across all groups, insulin output in PEG-MAL gels was 25% higher than alginate (Fig. 3C). Islets alone and islets in PEG-MAL + VEGF had double the levels of MTS metabolism than islets in alginate (Fig. 3D). These results indicate that incorporation of VEGF may be beneficial for islet metabolism and survival. VEGF has been previously shown to boost MTT metabolism and viability in isolated islets [67].
Engraftment and vascularization of islets transplanted to small bowel mesentery
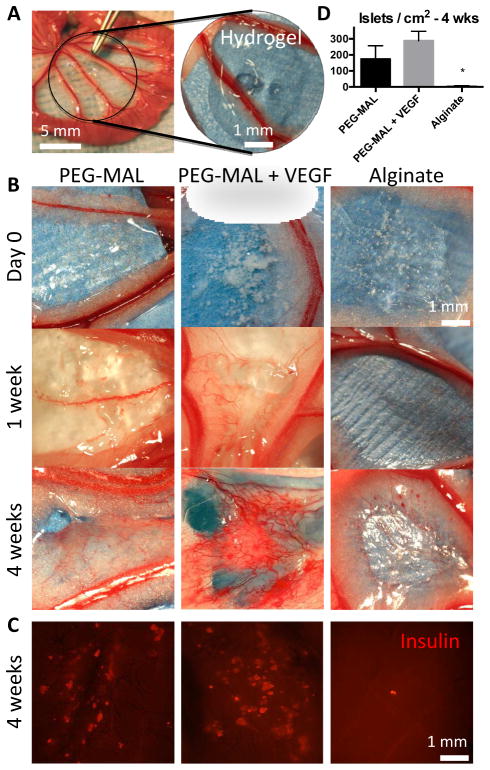
We next investigated the in vivo vascularization and grafting of pancreatic islets delivered via PEG-MAL hydrogels to the small bowel mesentery. We chose to deliver islets to the small bowel mesentery in contrast to other commonly used implant sites such as subcutaneous space, epididymal fat pad, and renal subcapsule [68, 69]. The small bowel mesentery is a strong candidate for islet transplantation due to significant vascularization potential, accessibility, easy visualization, and direct blood supply to the hepatic portal vein (Fig. 4A).
Fig. 4.
Islet transplantation to the small bowel mesentery with PEG-MAL and alginate. A Hydrogels were cross-linked directly onto the tissue surface of the mesentery. B Macroscopic images of implant site at 0, 1, and 4 weeks. C Whole mount immunostain for insulin of explanted hydrogel at 4 weeks. D Quantification of islets in immunostained explants.
By one week new vessels could be clearly seen growing into the PEG-MAL hydrogels from surrounding tissue (Fig. 4B). Alginate gels completely dissipated with little to no vascularization response. At 4 weeks, a strong and dense vascular in-growth response was evident only in the PEG-MAL and PEG-MAL + VEGF groups. Explanted tissue samples from the PEG-MAL hydrogels were stained as whole mounts for insulin and imaged on a fluorescent stereoscope to examine overall islet survival. On average 288 independent insulin-positive structures per cm2 were visible in PEG-MAL + VEGF gels, 174 insulin-positive structures/cm2 for PEG-MAL without growth factor, and 6 insulin-positive structures/cm2 for alginate (Fig. 4C, D).
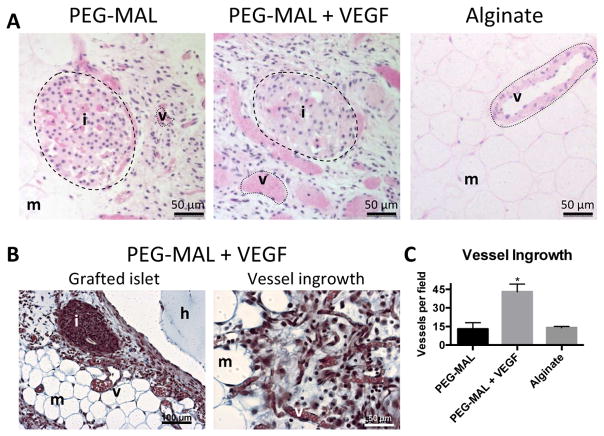
Engrafted islets were clearly visualized in H&E sections of PEG-MAL islet explants with erythrocyte-filled vascular structures growing in and around the islets themselves (Fig. 5A, B). Islets in both PEG-MAL and PEG-MAL + VEGF were infiltrated with vessels but the tissue surrounding islets in PEG-MAL + VEGF included larger and more vessels (Fig. 5C). No islets were seen in sections of alginate implants and the implant zone resembled the sparsely vascularized tissue of normal mesentery with infrequent yet large and mature pre-existing mesenteric vessels. Closer examination of the induced vasculature in the PEG-MAL VEGF group by trichrome staining revealed a dense collagen fibril network in the implant zone with many small vessels on the order of 10 μm. Taken together, these results demonstrate successful islet grafting and revascularization to the small bowel mesentery.
Fig. 5.
Histology of explanted hydrogel transplants at 4 weeks. A Hematoxylin and eosin staining shows revascularization of islets. B Trichrome staining of PEG-MAL + VEGF group. C Quantification of erythrocyte-positive tubular structures. i = islet, v = vessel, m = mesentery, h = hydrogel
Discussion
In this study, we engineered bio-functionalized hydrogels to promote islet engraftment and vascularization in the small bowel mesentery. The hydrogel was designed to support encapsulation of sensitive islet beta cells while eliciting a specific response from the host tissue at the implantation site. The small bowel mesentery is a strong candidate for islet transplantation due to significant vascularization potential, accessibility, easy visualization, and direct blood supply to the hepatic portal vein. The hepatic portal system feeds into the liver, a significant sink of physiologically produced insulin where circulating blood glucose is quickly converted to stored glycogen. By transplanting islets in a vascularized bed located upstream from the liver, insulin secreted directly into the hepatic portal system will be more concentrated when it reaches the liver and may have a greater effect on reducing blood sugar than islets transplanted to more remote sites.
Islets encapsulated in PEG-MAL hydrogel maintained viability and secreted insulin similarly to free islets. High islet viability in PEG-MAL hydrogel is likely due to mild cross-linking reaction of the PEG-MAL hydrogel. Less robust islet survival in alginate may be due to a lack of extracellular matrix and cell-cell interactions. Anseth and colleagues showed reduced beta cell viability after encapsulation in photo-polymerized PEG hydrogel due to a lack of ECM and cell-cell interactions [70], but these cells recovered their functionality when RGD peptide and ephrin bioactive domains were added to the matrix. The RGD adhesive ligands in our PEG-MAL hydrogel may similarly provide important matrix adhesion signaling leading to improved islet survival in a 3D matrix environment. Islets also secreted lower levels of insulin in Collagen-I gels than in PEG gels or islets alone. Collagen-I may be a poor ECM for islets and in fact may negatively affect beta cells insulin secretion. In a different study by Anseth and colleagues, beta cell insulin secretion was measured in cells encapsulated in PEG gels incorporating different ECM proteins [49]. In the cited study, beta cells encapsulated in PEG gels incorporating Collagen-I showed less insulin secretion than gels with albumin or no ECM. This result supports the conclusion that Collagen-I may be a poor ECM matrix for supporting beta cell insulin secretion.
Our results suggest VEGF may boost metabolism and viability, but we measured slightly lower insulin secretion when VEGF was incorporated although this difference was not significant. In principle, we expect the main beneficial effect of VEGF on islets to be seen in vivo resulting from increased angiogenesis and blood flow, while VEGF may have little effect on insulin secretion in vitro in the context of the PEG-MAL hydrogel system. While most studies from the literature have indicated that angiogenic growth factors improve insulin secretion in vivo by improving blood flow to islets, there is some evidence that exogenously high concentrations of VEGF could have a negative effect on beta cell insulin secretion acting through inflammation or other means [30, 31].
Our control condition placed islets in bulk alginate gel, not microbeads. Cells are routinely encapsulated in alginate microbeads with high viability, but bulk alginate gel is less robustly cross-linked than microbeads due to diffusion limitations of ions within the surgical time constraints of our in situ gelation model. Barium ions are known to provide stronger ionic bonds than calcium in alginate hydrogel and barium cross-linked alginate is more resistant to dissolution of the gel in vivo [71]. However, barium cross-linking is not suitable for in situ delivery due to the high toxicity of free barium. A combination of lack of bioactive ligands in alginate and less robust cross-linking may explain the poorer performance of alginate as a delivery matrix in this model when compared to PEG-MAL.
The release profile of VEGF from the hydrogel as measured by IR fluorescence of labeled protein indicates that the growth factor is sequestered within the hydrogel and the predominant mode of release is through proteolytic degradation of the gel and not by passive release. The signal reduction seen in gels incubated in PBS over the two week measurement window could be due to several factors including hydrolytic degradation of the NHS ester dye linkage or of the bulk PEG gel itself. Alternatively, a percentage of PEG-MAL linked VEGF that failed to completely cross-link to the gel network may leach out over time. Notably, PEG-MAL with VEGF-750 asymptotically approached 40% signal retention after 14 days and did not trend towards zero. This measurement indicates long-term retention on the scale of weeks of a large percentage of the initially incorporated VEGF. When implanted in vivo, tissue-remodeling processes degrading the PEG-MAL implant and released the majority of the VEGF over a period of 7 days coinciding with the rapid ingrowth of vessels seen on images of explanted samples, while alginate released the VEGF over 3 days. The in vitro degradation experiment was performed under a non-physiological concentration of a single collagenase enzyme (100 μg/mL) while the concentrations of proteases in vivo are likely lower, contain a variety of different enzymes, and degradation may experience a lag time before the onset of active tissue remodeling processes. These multiple reasons are likely responsible for the differences observed between in vitro and in vivo degradation rates.
Many biomaterial strategies for protein delivery aim to achieve sustained levels of growth factor release over weeks or months. Here, our goal was to achieve rapid vascularization of transplanted islets over a short time frame by a delivering an initial sustained influx of growth factor but not artificially inflate VEGF to chronically high levels. This strategy mimics endogenous wound healing where the initial high VEGF signaling only lasts a few days and subsides after vessel ingrowth. To achieve this controlled degradation and release in vivo, we selected a cross-linker peptide with a fast kinetic reaction rate compatible with a wide range of collagenase enzymes. Because of the modular nature of the PEG-MAL system, slower release rates could easily be achieved by using different cross-linker peptides with slower degradation kinetics.
We attribute the stark contrast between the ability of islets to graft in PEG-MAL over alginate gel to the engineered design of the PEG-MAL hydrogel. We designed the PEG-MAL matrix to rapidly degrade in vivo to facilitate controlled VEGF release and rapid islet vascularization. Importantly the gel degrades quickly in the presence of proteolytic enzymes but remains intact for weeks in buffer. This PEG matrix design, which also incorporates RGD adhesive peptides, facilitates easy invasion by surrounding tissue and vasculature. We hypothesize that VEGF-stimulated angiogenic remodeling of the PEG-MAL matrix releases additional VEGF in an on-demand manner as the material is degraded, thus creating a simple positive feedback mechanism to promote further vascular invasion. Small molecular weight PEG macromers resulting from proteolytic degradation are easily cleared from the implant site [72]. One could speculate that a similarly functionalized alginate matrix with RGD and VEGF may provide similar support for islet survival and vascularization as the PEG-MAL hydrogel. However, the degradation mechanism of alginate is quite different from the proteolytically cleavable cross-links of the PEG-MAL hydrogel, relying on resorption of the ionic bond cross-links to allow tissue remodeling and growth-factor release. Furthermore, alginate, while tissue-adhesive during cross-linking, lacks the cysteine-bonding properties of the maleimide reactive group which make PEG-MAL such an effective tissue-bonding material for delivering cells and therapeutics hydrated tissue surfaces in vivo. We previously reported on the tissue bonding properties of PEG-MAL hydrogel and observed a 50 μm penetration depth of in situ cross-linked PEG-MAL onto myocardium [66]. This tissue bonding effect keeps the transplanted islets anchored to the graft site long enough for tissue remodeling to occur. Applied as bulk matrices, cross-linked to tissue surfaces, alginate gels either delaminated from the graft site or more likely dissipated within one week as no alginate gels were found ectopically from the implant site during necropsy.
Our results showed remarkable vascularization of PEG-MAL hydrogels visualized in macroscopic images of the implant site. We attribute the growth of these vessels as a host response to both the intended effect of the PEG-MAL incorporated RGD and VEGF and intrinsic angiogenic signaling from the islets themselves. Previous studies have shown that isolated islets secrete a variety of growth factors and cytokines to initiate an intrinsic revascularization and engraftment response [18]. It is likely that this intrinsic islet signaling leads to a degree of vascularization. Addition of VEGF to the implant greatly enhanced the vascularization response in the immediate vicinity of grafted islets. Data from the literature suggests that augmenting islet vascularization with VEGF is beneficial in achieving better long-term islet survival and tighter control of blood glucose homeostasis [73].
Conclusion
Inadequate vascularization remains an important barrier to successful islet transplantation therapy. Accelerating post-transplant islet engraftment would reduce the required transplant cell mass and conceivably expand islet transplantation as an available treatment for T1DM. Our biomaterial delivery strategy in this study could potentially reduce the effective number of islets required to achieve independence from exogenous insulin therapy as well as improve the long-term survival rate and function of the grafted tissue. A key aspect of our strategy is to avoid injection of islets into the blood stream where they are exposed to instant blood-mediated inflammatory reaction, circulating antibodies against auto-antigens, and ischemia and fibrosis upon lodging in the liver. Delivering islets to the mesentery using biomaterial delivery matrices is ultimately far less stressful on the islet cells than hepatic portal transplantation yet maintains the critical access to portal vein blood flow for insulin metabolism. Here we incorporated bioactivity in the PEG-MAL matrix designed to induce angiogenesis.
In the future, PEG-MAL could be used to investigate incorporation of different bioadhesive ligands that are tuned to support islet activities and optimize insulin secretion. Different matrix components such as laminin peptides and islet specific signaling peptides such as Glucagon-like peptide-1 could easily be incorporated. As a modular delivery platform, PEG-MAL hydrogel provides an engineering tool to test the effects of delivery of bioactive component on islet survival and engraftment.
Materials and methods
PEG-MAL Hydrogel Preparation
PEG-based hydrogels were cross-linked by maleimide Michael-type addition chemistry [66]. To form bioactive hydrogels, a 20,000 MW 4-arm PEG-maleimide macromer (Laysan Bio) was first reacted with the cell adhesive peptide GRGDSPC (AAPPTEC) for 1 hour in 4mM triethanolamine in PBS. Human VEGF165 (Invitrogen), a homodimer protein with C-terminal unpaired cysteine, was physically incorporated into the gel by pre-incubation with the PEG-MAL macromer prior to cross-linking. Conjugations of RGD and VEGF to PEG-MAL were confirmed by increases in molecular weight on SDS-PAGE gels. The precursor solution was cross-linked into a gel with addition of a cysteine-flanked proteolytically degradable peptide sequence GCRDVPMSMRGGDRCG (AAPPTEC) at stoichiometrically balanced 1:1 cysteine to remaining maleimide reactive group molar ratio. PEG-MAL gel precursors and degradation products were run on a 4–12% Bis-Tris SDS-PAGE gel (Invitrogen) and stained for PEG with 4% Barium chloride in 0.1 N perchloric acid and 0.1% Iodine in water.
Alginate and Collagen Hydrogel Preparation
For alginate implants, 100 μL 4% Pronova UP LVM alginate (NovaMatrix) in HEPES buffered saline was mixed with 1 μg VEGF and cross-linked by overlay of 500 μL 1.8 mM CaCl2 for 10 minutes. Collagen I gels (3 mg/mL) were formed from dilution and pH stabilization of 10 mg/mL solubilized rat tail Collagen I (BD).
VEGF Labeling
VEGF was solubilized at 1 mg/mL in PBS. VivoTag-S 750 (Perkin Elmer) was solubilized in DMSO. 50 molar excess of VivoTag-S 750 was added to the VEGF and incubated at room temperature for 1 hour. Labeled protein was purified from excess dye by 3 successive purification rounds on Zeba Spin Desalting Columns 7,000 MWCO (Pierce). Dye concentration in the purified conjugated protein sample was measured on a NanoDrop 1000 system using dye extinction coefficient of 240,000 M−1cm−1, absorbance of 750 ± 5 nm, and MW of 1183 g/mol. VEGF was conjugated to PEG-MAL by incubation for 1 hour in 10% (wt/vol) PEG-MAL in PBS + 4 mM triethanolamine prior to addition to gels. To confirm protein labeling and ability of labeled VEGF to react with PEG-MAL, labeled VEGF was run on a 4–12% Bis-Tris SDS-PAGE gel (Invitrogen) along with PEG-MAL conjugated labeled VEGF, unlabeled VEGF and imaged on a LI-COR Odyssey infrared imaging system.
In vitro VEGF release
Gels of PEG-MAL macromer (100 μL) pre-functionalized with 1 μg IR-labeled VEGF and cross-linked by degradable peptide were cast in 9 mm × 1 mm cylindrical silicon molds. Gels were floated in baths of PBS and incubated at 37°C for 24 hours to fully swell. Type I collagenase (0.1 mg/mL) was added to half the samples. Samples were imaged daily on a 700 series Xenogen IVIS machine at 710 nm excitation, 780 nm emission and 60 s exposure time. Images were quantified for background subtracted average photon counts per ROI gated over the gel volume.
In vivo fluorescence imaging
PEG-MAL macromer (100 μL) pre-functionalized with 1 μg IR-labeled VEGF was mixed with cross-linker and polymerized directly onto the small bowel mesentery of Lewis rats. For alginate implants, 100 uL 4% Pronova UP LVM alginate (NovaMatrix) in HEPES buffered saline was mixed with 1 μg IR-labeled VEGF, pipetted onto the mesentery surface and cross-linked in place by bathing in 1.8 mM CaCl2. Rats were fed a low-alfalfa diet to minimize tissue auto-fluorescence. Anesthetized rats were imaged at 710 nm excitation, 780 nm emission, and 60 s exposure time in a 700 series Xenogen IVIS machine a 0, 2, and 7 days post-transplant. Images were analyzed for background-subtracted average photon counts within an ROI gated over the implant site.
Islet isolation
Islets were isolated according to standard methods adopted from Carter et al [74]. Rats were anesthetized with isoflurane and euthanized by exsanguination by severing the inferior vena cava. The distal common bile duct was clamped at the insertion point into the small intestine. Ten mL of ice cold Liberase TL (Roche) at 0.2 mg/mL in HBSS was injected into the proximal common bile duct through a catheter pulled from PE-50 polyethylene tubing attached to a 10 mL syringe. The inflated pancreas was dissected from surrounding tissue and placed in a container on ice. Vials containing pancreases were incubated for 14 minutes in a 37°C water bath with intermediate shaking. Tissue was broken up by pipetting up and down and then incubated for a further 7 minutes. Collagenase action was halted by addition of 40 mL ice cold HBSS + 10% FBS. Each sample was filtered through a 1 mm mesh strainer to remove undigested tissue. Islets were separated by density gradient centrifugation on a three layer Islet Gradient (Cellgro) of densities 1.108, 1.096, and 1.037. Separated islets were picked from between the 1.096 and 1.037 layers with a sterile transfer pipette. Islets were cultured in RPMI-1640 + 10% FBS + 1% Penicillin-Streptomycin in a 37°C, 5% CO2 incubator.
Islet culture and in vitro assays
For in vitro studies, islets were isolated the day before and allowed to recover for 24 hours before encapsulation in hydrogel. Fifty microliter gels of 4% (wt/vol) PEG-MAL pre-functionalized with 2 mM RGD and with or without 500 ng VEGF were cast in the bottom of 8 well Lab-Tek Chambered Coverglasses (Nunc). Approximately 25–50 islets were encapsulated per 50 μL gel. Gels were cultured with 500 μL media (RPMI-1640 + 10% FBS + 1% Penicillin-Streptomycin) changed daily. Collagen I gels (3 mg/mL) were formed from dilution and pH stabilization of 10 mg/mL solubilized rat tail Collagen I (BD). Alginage gels were formed from 4% Pronova UP LVM alginate (NovaMatrix) in HEPES buffered saline was mixed with islets, cross-linked by bathing in 1.8 mM CaCl2. Samples were Live/Dead stained with 2 μM calcein AM and 4 μM ethidium homodimer (Invitrogen) and imaged with a 20X objective on a Nikon-C1 laser scanning confocal microscope. Insulin release to the media from encapsulated islets was measured by ELISA with a high range rat insulin ELISA kit (Mercodia). Islet metabolic activity was measured by CellTiter 96 Aqueous MTS assay (Promega). Twenty μL of MTS reagent in 500 μL media was added per hydrogel sample and incubated for 4 hours. MTS incubation media was transferred to a new plate for absorbance reading at 490 nm.
Islet Transplantation
Isoflurane anesthetized male Lewis rats (10 weeks) prepared for sterile surgery were laparotomized with a 1 cm two-layer incision through the skin and abdominal muscle along the linea alba. A small section of small bowel and associated mesentery were gently exteriorized and placed on gauze moistened with warm sterile saline. PEG-MAL macromer (100 uL) pre-functionalized with 2 mM RGD and ± 1 μg IR-labeled VEGF was mixed with 1500 islets freshly isolated 24 hours previously and peptide cross-linker. The mixture was immediately pipetted onto the small bowel mesentery and allowed to cross-link for 10 minutes. For alginate implants, 100 uL 4% Pronova UP LVM alginate (NovaMatrix) in HEPES buffered saline was mixed with islets, pipetted onto the mesentery surface, and cross-linked in place by bathing in 1.8 mMCaCl2. The implant site was marked with a single loop of #6 silk suture placed proximal to the hydrogel. After hydrogel formation the small bowel was reinserted into the abdomen and the animal closed. Animals were sacrificed at 1 and 4 weeks post-transplantation for examination of the graft site. Histology and immunofluorescence was only performed on grafts from animals sacrified at 4 weeks. All animal experiments were performed with the approval of the Georgia Tech Animal Care and Use Committee (IACUC) under the supervision a research veterinarian and within the guidelines of the national guide for the care and use of laboratory animals.
Histology
Explanted samples containing hydrogel, islets, and tissue were fixed overnight in 10% neutral buffered formalin at 4°C. Samples were pre-stained (whole mount) for insulin by overnight permeabilization/blocking in 1% HD-BSA with 0.1% Triton-X-100 in PBS, overnight incubation with chicken anti-insulin primary antibody (Abcam) in PBS, overnight incubation with Alexa Fluor 555 goat anti-chicken secondary antibody (Invitrogen). Whole mount stains were imaged on a Zeiss fluorescent stereoscope for insulin staining before embedding for histology sections. Samples were dehydrated in successive changes of ethanol and embedded in Immuno-Bed GMA polymer (Polysciences). GMA blocks were sectioned at 2 μm thickness, deplasticized with a solvent exchange routine of xylene, acetone, ethanol, and water. Routine hematoxylin and eosin and trichrome were performed on deplasticized sections.
Statistics
Statistics were performed in Graphpad Prism software. Multiple group comparison analysis was analyzed by ANOVA with Tukey post-hoc comparisons. Sample sizes were n=4 for in vivo experiments and n=6 for in vitro experiments.
Acknowledgments
Funding for this work was provided by:
NIH (R01-EB004496), Georgia Tech/Emory Center (GTEC) for the Engineering of Living Tissues and the Atlanta Clinical and Translational Science Institute (ACTSI) supported in part by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources, National Science Foundation under the Science and Technology Center Emergent Behaviors of Integrated Cellular Systems (EBICS) Grant No. CBET-0939511; AHA predoctoral fellowship (E.A.P.)
References
- 1.National diabetes fact sheet. Centers for Disease Control and Prevention; 2011. [Accessed 11 Mar 2013]. http://www.cdc.gov/DIABETES//pubs/pdf/ndfs_2011.pdf. [Google Scholar]
- 2.Shapiro AM. State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr Diab Rep. 2011;11(5):345–54. doi: 10.1007/s11892-011-0217-8. [DOI] [PubMed] [Google Scholar]
- 3.Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes care. 2012;35(7):1436–45. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rickels MR, Schutta MH, Mueller R, Markmann JF, Barker CF, Naji A, et al. Islet cell hormonal responses to hypoglycemia after human islet transplantation for type 1 diabetes. Diabetes. 2005;54(11):3205–11. doi: 10.2337/diabetes.54.11.3205. [DOI] [PubMed] [Google Scholar]
- 5.Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350(7):694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 6.Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51(7):2148–57. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 7.Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50(4):710–9. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 8.Azzi J, Geara AS, El-Sayegh S, Abdi R. Immunological aspects of pancreatic islet cell transplantation. Expert Rev Clin Immunol. 2010;6(1):111–24. doi: 10.1586/eci.09.67. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JD, Ao Z, Ao P, Li H, Dai LJ, He Z, et al. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 2009;18(8):833–45. doi: 10.3727/096368909X471198. [DOI] [PubMed] [Google Scholar]
- 10.Negi S, Jetha A, Aikin R, Hasilo C, Sladek R, Paraskevas S. Analysis of beta-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. PloS One. 2012;7(1):e30415. doi: 10.1371/journal.pone.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakey JR, Mirbolooki M, Shapiro AM. Current status of clinical islet cell transplantation. Methods Mol Biol. 2006;333:47–104. doi: 10.1385/1-59745-049-9:47. [DOI] [PubMed] [Google Scholar]
- 12.Vaithilingam V, Sundaram G, Tuch BE. Islet cell transplantation. Curr Opin Organ Transplant. 2008;13(6):633–8. doi: 10.1097/MOT.0b013e328317a48b. [DOI] [PubMed] [Google Scholar]
- 13.Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 2007;31(4):705–14. doi: 10.1007/s00268-006-0719-8. [DOI] [PubMed] [Google Scholar]
- 14.Brissova M, Powers AC. Revascularization of transplanted islets: can it be improved? Diabetes. 2008;57(9):2269–71. doi: 10.2337/db08-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50(3):489–95. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]
- 16.Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51(5):1362–6. doi: 10.2337/diabetes.51.5.1362. [DOI] [PubMed] [Google Scholar]
- 17.Henriksnas J, Lau J, Zang G, Berggren PO, Kohler M, Carlsson PO. Markedly decreased blood perfusion of pancreatic islets transplanted intraportally into the liver: disruption of islet integrity necessary for islet revascularization. Diabetes. 2012;61(3):665–73. doi: 10.2337/db10-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, et al. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55(11):2974–85. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 19.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77(5):587–97. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 20.Emamaullee JA, Shapiro AM. Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant. 2007;16(1):1–8. doi: 10.3727/000000007783464461. [DOI] [PubMed] [Google Scholar]
- 21.Linn T, Schmitz J, Hauck-Schmalenberger I, Lai Y, Bretzel RG, Brandhorst H, et al. Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin Exp Immunol. 2006;144(2):179–87. doi: 10.1111/j.1365-2249.2006.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng K, Fraga D, Zhang C, Kotb M, Gaber AO, Guntaka RV, et al. Adenovirus-based vascular endothelial growth factor gene delivery to human pancreatic islets. Gene Ther. 2004;11(14):1105–16. doi: 10.1038/sj.gt.3302267. [DOI] [PubMed] [Google Scholar]
- 23.Narang AS, Cheng K, Henry J, Zhang C, Sabek O, Fraga D, et al. Vascular endothelial growth factor gene delivery for revascularization in transplanted human islets. Pharm Res. 2004;21(1):15–25. doi: 10.1023/b:pham.0000012147.52900.b8. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y, Liu YF, Zhang JL, Li TM, Zhao N. Elevation of vascular endothelial growth factor production and its effect on revascularization and function of graft islets in diabetic rats. World J Gastroenterol. 2007;13(20):2862–6. doi: 10.3748/wjg.v13.i20.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, et al. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53(4):963–70. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 26.Langlois A, Bietiger W, Seyfritz E, Maillard E, Vivot K, Peronet C, et al. Improvement of rat islet viability during transplantation: validation of pharmacological approach to induce VEGF overexpression. Cell Transplant. 2011;20(9):1333–42. doi: 10.3727/096368910X557182. [DOI] [PubMed] [Google Scholar]
- 27.Shimoda M, Chen S, Noguchi H, Matsumoto S, Grayburn PA. In vivo non-viral gene delivery of human vascular endothelial growth factor improves revascularisation and restoration of euglycaemia after human islet transplantation into mouse liver. Diabetologia. 2010;53(8):1669–79. doi: 10.1007/s00125-010-1745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su D, Zhang N, He J, Qu S, Slusher S, Bottino R, et al. Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes. 2007;56(9):2274–83. doi: 10.2337/db07-0371. [DOI] [PubMed] [Google Scholar]
- 29.Olerud J, Johansson M, Lawler J, Welsh N, Carlsson PO. Improved vascular engraftment and graft function after inhibition of the angiostatic factor thrombospondin-1 in mouse pancreatic islets. Diabetes. 2008;57(7):1870–7. doi: 10.2337/db07-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agudo J, Ayuso E, Jimenez V, Casellas A, Mallol C, Salavert A, et al. Vascular endothelial growth factor-mediated islet hypervascularization and inflammation contribute to progressive reduction of beta-cell mass. Diabetes. 2012;61(11):2851–61. doi: 10.2337/db12-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Q, Brissova M, Reinert RB, Pan FC, Brahmachary P, Jeansson M, et al. Enhanced expression of VEGF-A in beta cells increases endothelial cell number but impairs islet morphogenesis and beta cell proliferation. Dev Biol. 2012;367(1):40–54. doi: 10.1016/j.ydbio.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavallari G, Olivi E, Bianchi F, Neri F, Foroni L, Valente S, et al. Mesenchymal stem cells and islet cotransplantation in diabetic rats: improved islet graft revascularization and function by human adipose tissue-derived stem cells preconditioned with natural molecules. Cell Transplant. 2012;21(12):2771–81. doi: 10.3727/096368912X637046. [DOI] [PubMed] [Google Scholar]
- 33.Sakata N, Goto M, Yoshimatsu G, Egawa S, Unno M. Utility of co-transplanting mesenchymal stem cells in islet transplantation. World J Gastroenterol. 2011;17(47):5150–5. doi: 10.3748/wjg.v17.i47.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeung TY, Seeberger KL, Kin T, Adesida A, Jomha N, Shapiro AM, et al. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PloS One. 2012;7(5):e38189. doi: 10.1371/journal.pone.0038189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta R, Sefton MV. Application of an endothelialized modular construct for islet transplantation in syngeneic and allogeneic immunosuppressed rat models. Tissue Eng Part A. 2011;17(15–16):2005–15. doi: 10.1089/ten.tea.2010.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman-Francis K, Koffler J, Weinberg N, Dor Y, Levenberg S. Engineered vascular beds provide key signals to pancreatic hormone-producing cells. PloS One. 2012;7(7):e40741. doi: 10.1371/journal.pone.0040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MC, et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant. 2012;21(6):1305–20. doi: 10.3727/096368911X627534. [DOI] [PubMed] [Google Scholar]
- 38.Sigrist S, Mechine-Neuville A, Mandes K, Calenda V, Braun S, Legeay G, et al. Influence of VEGF on the viability of encapsulated pancreatic rat islets after transplantation in diabetic mice. Cell Transplant. 2003;12(6):627–35. doi: 10.3727/000000003108747109. [DOI] [PubMed] [Google Scholar]
- 39.Stendahl JC, Wang LJ, Chow LW, Kaufman DB, Stupp SI. Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation. Transplantation. 2008;86(3):478–81. doi: 10.1097/TP.0b013e3181806d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow LW, Wang LJ, Kaufman DB, Stupp SI. Self-assembling nanostructures to deliver angiogenic factors to pancreatic islets. Biomaterials. 2010;31(24):6154–61. doi: 10.1016/j.biomaterials.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Said SS, Pickering JG, Mequanint K. Advances in growth factor delivery for therapeutic angiogenesis. J Vasc Res. 2013;50(1):35–51. doi: 10.1159/000345108. [DOI] [PubMed] [Google Scholar]
- 42.Giacca M, Zacchigna S. VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Ther. 2012;19(6):622–9. doi: 10.1038/gt.2012.17. [DOI] [PubMed] [Google Scholar]
- 43.Papa FR. Endoplasmic reticulum stress, pancreatic beta-cell degeneration, and diabetes. Cold Spring Harb Perspect Med. 2012;2(9):a007666. doi: 10.1101/cshperspect.a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorelle JA, Itoh T, Peng H, Kanak MA, Sugimoto K, Matsumoto S, et al. Withaferin A inhibits pro-inflammatory cytokine-induced damage to islets in culture and following transplantation. Diabetologia. 2013 doi: 10.1007/s00125-012-2813-9. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant. 2011;16(6):620–6. doi: 10.1097/MOT.0b013e32834c2393. [DOI] [PubMed] [Google Scholar]
- 46.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Bio. 2005;77(5):587–97. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 47.Cruise GM, Hegre OD, Scharp DS, Hubbell JA. A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnol Bioeng. 1998;57(6):655–65. doi: 10.1002/(sici)1097-0290(19980320)57:6<655::aid-bit3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 48.Lin CC, Anseth KS. Glucagon-like peptide-1 functionalized PEG hydrogels promote survival and function of encapsulated pancreatic beta-cells. Biomacromolecules. 2009;10(9):2460–7. doi: 10.1021/bm900420f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A. 2008;14(12):1959–68. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber LM, He J, Bradley B, Haskins K, Anseth KS. PEG-based hydrogels as an in vitro encapsulation platform for testing controlled beta-cell microenvironments. Acta Biomater. 2006;2(1):1–8. doi: 10.1016/j.actbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Weber LM, Lopez CG, Anseth KS. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J Biomed Mater Res A. 2009;90(3):720–9. doi: 10.1002/jbm.a.32134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber LM, Cheung CY, Anseth KS. Multifunctional pancreatic islet encapsulation barriers achieved via multilayer PEG hydrogels. Cell Transplant. 2008;16(10):1049–57. [PubMed] [Google Scholar]
- 53.Weber LM, Anseth KS. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol. 2008;27(8):667–73. doi: 10.1016/j.matbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konstantinova I, Lammert E. Microvascular development: learning from pancreatic islets. BioEssays. 2004;26(10):1069–75. doi: 10.1002/bies.20105. [DOI] [PubMed] [Google Scholar]
- 55.Patterson J, Hubbell JA. SPARC-derived protease substrates to enhance the plasmin sensitivity of molecularly engineered PEG hydrogels. Biomaterials. 2011;32(5):1301–10. doi: 10.1016/j.biomaterials.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 56.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31(30):7836–45. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 57.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17(15):2260–2. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 58.Phelps EA, Landazuri N, Thule PM, Taylor WR, Garcia AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci U S A. 2010;107(8):3323–8. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narang AS, Mahato RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacol Rev. 2006;58(2):194–243. doi: 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- 60.Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant. 2009;18(1):1–12. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yun LD, Hee NJ, Byun Y. Functional and histological evaluation of transplanted pancreatic islets immunoprotected by PEGylation and cyclosporine for 1 year. Biomaterials. 2007;28(11):1957–66. doi: 10.1016/j.biomaterials.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 62.Wilson JT, Cui W, Chaikof EL. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett. 2008;8(7):1940–8. doi: 10.1021/nl080694q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hiscox AM, Stone AL, Limesand S, Hoying JB, Williams SK. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng Part A. 2008;14(3):433–40. doi: 10.1089/tea.2007.0099. [DOI] [PubMed] [Google Scholar]
- 64.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 65.Borg DJ, Bonifacio E. The use of biomaterials in islet transplantation. Curr Diab Rep. 2011;11(5):434–44. doi: 10.1007/s11892-011-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, et al. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater. 2012;24(1):64–70. 2. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cross SE, Richards SK, Clark A, Benest AV, Bates DO, Mathieson PW, et al. Vascular endothelial growth factor as a survival factor for human islets: effect of immunosuppressive drugs. Diabetologia. 2007;50(7):1423–32. doi: 10.1007/s00125-007-0670-8. [DOI] [PubMed] [Google Scholar]
- 68.Merani S, Toso C, Emamaullee J, Shapiro AM. Optimal implantation site for pancreatic islet transplantation. Br J Surg. 2008;95(12):1449–61. doi: 10.1002/bjs.6391. [DOI] [PubMed] [Google Scholar]
- 69.van der Windt DJ, Echeverri GJ, Ijzermans JN, Cooper DK. The choice of anatomical site for islet transplantation. Cell Transplant. 2008;17(9):1005–14. doi: 10.3727/096368908786991515. [DOI] [PubMed] [Google Scholar]
- 70.Lin CC, Anseth KS. Cell-cell communication mimicry with poly(ethylene glycol) hydrogels for enhancing beta-cell function. Proc Natl Acad Sci U S A. 2011;108(16):6380–5. doi: 10.1073/pnas.1014026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui H, Tucker-Burden C, Cauffiel SM, Barry AK, Iwakoshi NN, Weber CJ, et al. Long-term metabolic control of autoimmune diabetes in spontaneously diabetic nonobese diabetic mice by nonvascularized microencapsulated adult porcine islets. Transplantation. 2009;88(2):160–9. doi: 10.1097/TP.0b013e3181abbfc1. [DOI] [PubMed] [Google Scholar]
- 72.Yamaoka T, Tabata Y, Ikada Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J Pharm Sci. 1994;83(4):601–6. doi: 10.1002/jps.2600830432. [DOI] [PubMed] [Google Scholar]
- 73.Vernon RB, Preisinger A, Gooden MD, D’Amico LA, Yue BB, Bollyky PL, et al. Reversal of Diabetes in Mice with a Bioengineered Islet Implant Incorporating a Type I Collagen Hydrogel and Sustained Release of Vascular Endothelial Growth Factor. Cell Transplant. 2012;21(10):2099–110. doi: 10.3727/096368912X636786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online. 2009;11:3–31. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]