Abstract
The reliable neuroimaging finding that older adults often show greater activity (over-recruitment) than younger adults is typically attributed to compensation. Yet, the neural mechanisms of over-recruitment in older adults (OAs) are largely unknown. Rodent electrophysiology studies have shown that as number of afferent fibers within a circuit decreases with age, the fibers that remain show higher synaptic field potentials (less wiring, more firing). Extrapolating to system-level measures in humans, we proposed and tested the hypothesis that greater activity in OAs compensates for impaired white-matter connectivity. Using a neuropsychological test battery, we measured individual differences in executive functions associated with the prefrontal cortex (PFC) and memory functions associated with the medial temporal lobes (MTLs). Using event-related functional magnetic resonance imaging, we compared activity for successful versus unsuccessful trials during a source memory task. Finally, we measured white-matter integrity using diffusion tensor imaging. The study yielded 3 main findings. First, low-executive OAs showed greater success-related activity in the PFC, whereas low-memory OAs showed greater success-related activity in the MTLs. Second, low-executive OAs displayed white-matter deficits in the PFC, whereas low-memory OAs displayed white-matter deficits in the MTLs. Finally, in both prefrontal and MTL regions, white-matter decline and success-related activations occurred in close proximity and were negatively correlated. This finding supports the less-wiring-more-firing hypothesis, which provides a testable account of compensatory over-recruitment in OAs.
Keywords: DTI, elderly, fMRI, frontal, MTL, PFC
Introduction
In functional neuroimaging studies, older adults (OAs) tend to show greater activity (over-recruitment) than younger adults, an effect that is often attributed to functional compensation (Cabeza 2002; Hedden and Gabrieli 2004; Park and Reuter-Lorenz 2009). Despite the popularity of this compensation account, the underlying neural mechanisms of over-recruitment in OAs are largely unknown. Here, we propose and test a hypothesis inspired by rodent electrophysiological evidence showing that when number of afferent fibers within a circuit decreases with age, the fibers that remain show an increase in synaptic field potentials (Barnes and McNaughton 1980; Burke and Barnes 2010). This finding suggests that neurons compensate for an age-related loss of local connectivity with greater synaptic responsiveness (i.e., “less wiring, more firing”). Extrapolating these animal findings to system-level measures in humans, we hypothesized that greater activity in older OAs may compensate for a decline in white-matter integrity in specific brain circuits. To test this hypothesis, we measured neural activity using functional MRI (fMRI), assessed white-matter integrity using diffusion tensor imaging (DTI), and looked for negative correlations between these DTI and fMRI measures (i.e., as white-matter integrity declines, fMRI activity should be greater).
To specify the location of these negative fMRI-DTI correlations, we focused on a cognitive task, source memory, which declines with aging and has been associated with the deterioration of 2 principal brain regions, the prefrontal cortex (PFC) and the medial temporal lobes (MTL) (Daselaar and Cabeza 2008). Importantly, the relative contributions of these 2 regions to source memory deficits in OAs show large individual differences, which can be detected with neuropsychological tests. Factor analyses of neuropsychological test batteries have shown that variability in age-related source memory decline can be explained by 2 quasi-orthogonal factors: an “executive” factor linked to PFC function, and a “memory” factor likely to reflect MTL function (Glisky et al. 1995; Mather et al. 1999; Prull et al. 2006; Glisky 2007). Thus, we expected that low-executive OAs would show DTI-fMRI links mainly within PFC, and low-memory OAs, mainly within MTL.
To specify the notion of “compensation,” we focused on activations that could be directly associated with successful source memory performance. Using event-related fMRI analyses, we measured “retrieval success activity” (RSA) defined as greater activity for source memory hits than source memory misses. Then, we linked differences in RSA to differences in the DTI measure fractional anisotropy (FA), which is reduced with aging and indicative of white-matter decline.
We made 3 main predictions: 1) Low-executive OAs would show greater RSA mainly within PFC, and low-memory OAs, mainly within MTL; 2) low-executive OAs would show reduced FA mainly within PFC, and low-memory OAs, mainly within MTL; and 3) Linking DTI and fMRI findings, smaller FA values would occur in white-matter regions connected to the gray-matter areas showing increased RSA (over-recruitment), namely PFC for low-executive OAs and MTL for low-memory OAs. Moreover, we predicted that the magnitude of FA and RSA would be negatively correlated across OAs within the executive and memory brain circuits (i.e., less wiring, more firing).
Materials and Methods
Participants
Forty-nine OAs—screened for contraindications to MRI—participated in the study. Four participants were excluded because of scanner issues, leaving 45 remaining participants (20 males, 25 females). Their average age was 69.7 (SD = 6.3), and average years of educations was 17.1 (SD = 2.1). Participants gave written informed consent and received financial compensation. All experimental procedures were approved by the Duke University institutional review board.
Neuropsychological Assessment of Executive and Memory Functions
We used a variety of tests from the CANTAB (http://www.cambridgecognition.com) to create composite scores of executive function (EF; De Luca et al. 2003; Saunders and Summers 2010; Chamberlain et al. 2011) and memory function (Wood et al. 2002; de Rover et al. 2011). In keeping with factor analyses distinguishing executive and memory factors (Glisky et al. 1995; Glisky 2007), our composite executive score combined working memory, task switching, and processing speed measures and our composite memory score combined recognition and recall episodic memory tests. To assess EF, we used the intraextra dimensional set shift (IED), spatial span (SSP), spatial working memory (SWM), reaction time (RTI), and rapid visual information processing (RVP) test scores. For assessing memory function, we used the pattern recognition memory (PRM) and paired associates learning (PAL) test scores. Composite executive and memory scores were created by generating z-scores based on the performance on executive tasks (SWM total errors, SSP span length, IED pre-EDS errors, IED EDS errors, RTI 5-choice RTI, RVP total hits) and memory scores (PRM % correct; PAL total errors) tests (inverse for negative performance measures) across participants, and subsequently averaging these z-scores for each individual participant. The resulting composite EF scores ranged from z = −1.13 to z = 0.78, with a standard deviation of 0.46. The composite memory function (MF) scores ranged from z = −3.4 to z = 1.09, with a standard deviation of 0.92.
Source Memory Task
Materials
Stimuli consisted of 440 English words with normative word frequencies in the lexicon of 5–15 per million, M = 8.8 (3.1), and had a mean length of M = 7.1 (2.3) letters (Kučera and Francis 1967). Unique study and test lists were randomly generated for each participant and words were assigned to the following conditions: item (180), source (180), or item lures (80 words—presented only at retrieval). There were 4 encoding lists, each composed of 260 trials (50 words presented twice, and 40 words presented 4 times). At retrieval, there were 4 item test lists, each consisting of 45 targets (studied words) and 20 lures (nonstudied words), and 4 source test lists, each consisting of 45 studied words.
Encoding
Participants studied words outside the scanner. Words were presented on a 19-inch computer monitor in black font on a gray background for 3 s with a 1-s intertrial interval using a PC with Cogent (http://www.vislab.ucl.ac.uk/cogent_2000.php), a stimulus presentation toolbox within MatLab (www.mathworks.com/products/matlab). Participants made a “pleasant/unpleasant” judgment for half of the trials, and a “bigger/smaller than a shoebox” judgment for the other half. For the purpose of the present study, words that were encoded 2 or 4 times were collapsed into one condition for the subsequent fMRI analysis.
Retrieval
Approximately 15 min after the encoding phase, participants were placed in the MRI scanner and tested for their memory of the studied items. There were 2 retrieval conditions, item and source retrieval. During item retrieval scans, participants made old/new responses on a 4-point confidence scale: definitely old, probably old, probably new, and definitely new. During source retrieval scans, participants made source judgments on a 4-point scale: definitely pleasant/unpleasant, probably pleasant/unpleasant, probably bigger/smaller, and definitely bigger/smaller. Four item and 4 source runs were presented in consecutive blocks to minimize the effects of task switching. Retrieval stimuli were presented for 3 s, with a white crosshair presented for fixation during the intertrial interval. Stimulus order and intertrial jitter (range: 1–7 s) were determined by a genetic algorithm designed to maximize statistical efficiency and facilitate deconvolution of the hemodynamic response (Wager and Nichols 2003). Stimuli were presented via a mirror in the scanner head coil and a rear projection system using a PC computer with Cogent. For the purpose of the present study, only results from the source memory runs are reported.
Brain Measures
MRI Scanning
Participants were scanned on a 3-T gradient-echo scanner (General Electric 3.0 Tesla Signa Excite HD short bore scanner, equipped with an 8-channel head coil). Coplanar functional images were acquired using an inverse spiral sequence (64 × 64 matrix, time repetition [TR] = 1700 ms, time echo [TE] = 31 ms, field of view [FOV] 240 mm, 37 slices, 3.8-mm slice thickness, 254 images). Using a spiral-in gradient-echo sequence: slice order = interleaved, matrix = 642, FOV = 24 cm, TR = 2000 ms, TE = 27 ms, sections = 34, thickness = 3.8 mm, interscan spacing = 0, flip angle = 60, SENSE reduction factor = 2). Following functional imaging, a high-resolution SPGR series (1-mm sections covering whole brain, interscan spacing = 0, matrix = 2562, flip angle = 30, TR = 22 ms, TE = min full, FOV = 19.2 cm) was collected. Finally, DT MRI data were collected using a single-shot echo-planar imaging sequence (TR = 1700 ms, slices = 50, thickness = 2.0 mm, FOV = 256 × 256 mm2, matrix size 128 × 128, voxel size = 2 mm3, b value = 1000 s/mm2, diffusion-sensitizing directions = 25, total images = 960, total scan time = 5 min). The anatomical MRI was acquired using a 3D T1-weighted echo-planar sequence (matrix = 2562, TR = 12 ms, TE = 5 ms, FOV = 24 cm, slices = 68, slice thickness = 1.9 mm, sections = 248). Scanner noise was reduced with ear plugs, and head motion was minimized with foam pads. Total scan time, including breaks and structural scans, was approximately 1 h 40 min. Behavioral responses were recorded with a 4-key fiber-optic response box (Resonance Technology, Inc.), and when necessary, vision was corrected using MRI-compatible lenses that matched the distance prescription used by the participant.
fMRI Analysis
Functional scans were processed using SPM5. The first 4 images were discarded to allow for scanner equilibrium. Images were corrected for asynchronous slice acquisition (slice timing: reference slice = 17, TA = 1.97) and realigned to the first functional image within the series to correct for head motion. For normalization, we used a study-specific template created using unified segmentation and diffeomorphic image registration (DARTEL) in SPM5 (Ashburner 2007). First, each subject's image was segmented into gray-matter, white-matter, and cerebral spinal fluid probabilistic images. The segmented gray matter images were then normalized to MNI space using the DARTEL procedure integrated in SPM5, involving diffeomorphic registration (Ashburner 2007), and resliced to a resolution of 3.75-mm isotropic resolution. DARTEL creates a template that is representative of the brain size and shape of all the participants (Harris et al. 2009). Normalized images were subsequently smoothed with an 8-mm Gaussian kernel.
The hemodynamic response for each trial was modeled using the canonical hemodynamic response function. Data were high-pass filtered using a cutoff of 128 s. Each trial type (hit/miss, high/low confidence, no response) was modeled separately. To identify regions related to successful retrieval, high confidence hits (source hits) were contrasted to retrieval misses (source misses) at an uncorrected threshold of P < 0.001, and a minimum cluster size of 10 voxels (3.75 mm3) using a random effects (RFX) analysis. EF and MF scores were also modeled as independent variables in the RFX analysis to distinguish and investigate individual differences in successful retrieval activity related to MFs and EFs.
DTI Analysis
Diffusion-weighted data were analyzed using the University of Oxford's center for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL) package (www.fmrib.ox.ac.uk/fsl). DTI data were corrected for eddy currents, and the first volume was used to generate a binary brain mask with the Brain Extraction Tool (BET). DTIfit was used to fit a diffusion tensor (a mathematical description of magnitude and directionality of the water molecule movement in 3D space) to each voxel included in the brain mask. Diagonalization of the tensor yielded 3 voxel-specific eigenvalues (λ1 > λ2 > λ3), which represent diffusivities along the 3 principal directions of the tensor. Voxelwise maps of FA were yielded by this step, and were brought into MNI space using Tract-Based Spatial Statistics (TBBS; Smith et al. 2006). In accordance with the standard TBSS pipeline, FA maps from all 45 subjects were nonlinearly normalized to the 1 × 1 × 1 mm3 FMRIB58_FA standard space white-matter template. Next, individually transformed FA images were spatially smoothed with a 6-mm3 Gaussian filter.
In order to investigate the relation between individual differences in EF, memory function, and white matter connectivity, a voxelwise analysis of the normalized FA maps, after masking with the white matter template, was completed in a Linear Regression model in SPM5 using executive and memory scores as independent variables together in the same model similar to the fMRI analysis and the FA maps as dependent variable using a whole-brain threshold of P < 0.001, uncorrected, and a cluster size of 50 1-mm3 voxels.
Results
Behavioral Results
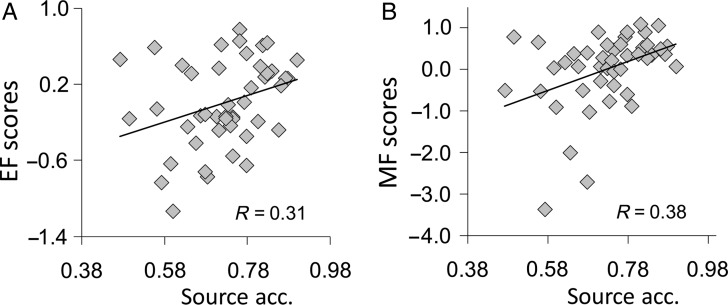
Source memory accuracy was 0.73 ± 0.015, and RTs were 2.29 s ± 0.59 for source hits, and 2.46 ± 68 for source misses. EF and MF scores were correlated (R = 0.39; P = 0.008). Importantly, and as expected, both EF (R = 0.31, P = 0.04), and MF (R = 0.38, P = 0.02) scores were significantly correlated with source memory accuracy across participants. Figure 1 shows the scatter plots illustrating the correlations. This finding confirms the joint contribution of executive and memory factors to explaining individual differences in episodic memory performance (Glisky et al. 1995; Glisky 2007).
Figure 1.
(A) Positive correlation between executive function (EF) scores and source memory accuracy. (B) Correlation between memory function (MF) scores and source memory accuracy.
fMRI: Low-Executive OAs Showed Greater RSA in PFC, low-Memory OAs, in MTL
Table 1 lists all regions showing negative correlations between EF and MF scores and RSA (source hits > source misses) in terms of their T values in the fMRI analysis. Both EF and MF factors were modeled together, parsing out their common variance.
Table 1.
Regions showing correlations between neuropsychological scores and retrieval success activity
| Region | Side | BA | MNI |
RSA correlation | ||
|---|---|---|---|---|---|---|
| X | Y | Z | T value | |||
| EF score correlations | ||||||
| Dorsolateral PFC | Left | 9 | −30 | 11 | 34 | 3.90 |
| Ant. cingulate | Left | 32 | −8 | 11 | 42 | 4.45 |
| Insula | Left | 13 | −34 | −4 | 19 | 3.87 |
| MF score correlations | ||||||
| Hippocampus | Left | – | −23 | −19 | −8 | 4.23 |
| Thalamus | Left | – | −8 | −26 | 4 | 4.75 |
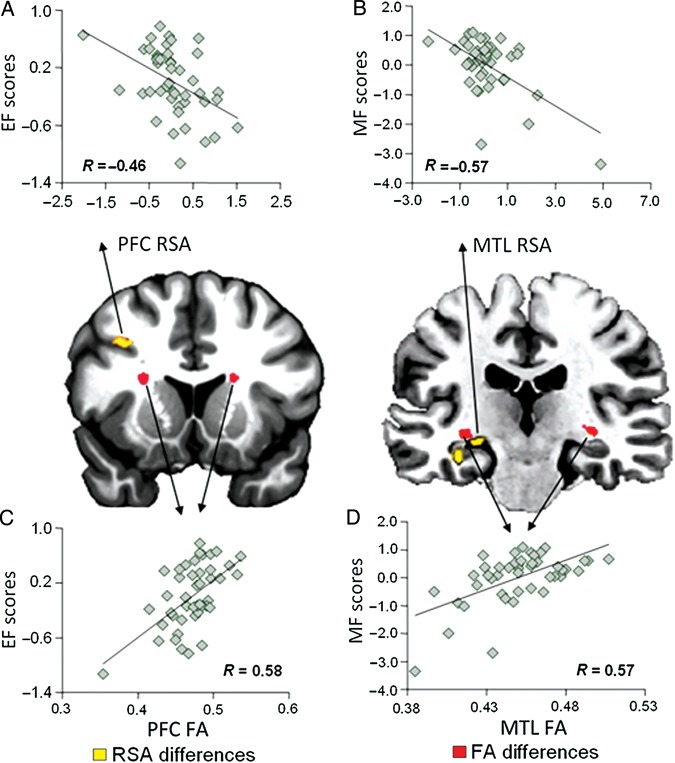
No region showed positive correlations between these measures. Consistent with our “first prediction,” low-executive OAs showed greater RSA in PFC regions whereas low-memory OAs showed greater RSA in MTL regions. Figure 2A,B displays the actual correlations for each factor separately. As shown in Figure 2A, EF scores were negatively correlated (R = −0.46; P = 0.0015) with RSA in left dorsolateral PFC (BA 9). As displayed by Figure 2B, MF scores were negatively correlated (R = −0.57; P = 0.00044) with RSA in the left hippocampus. Table 1 also lists other regions showing negative RSA correlations with EF scores, including left anterior cingulate and insular regions, and, the left thalamus with MF scores. Anterior cingulate and insular regions have been associated with EFs (Carter et al. 1999; Miller and Cohen 2001; Uddin et al. 2010) and the thalamus with memory functions (Aggleton et al. 2010). Thus, even though these additional regions were not part of our predictions, they support the validity of our executive (EF) and memory (MF) composite scores.
Figure 2.
(A) Negative correlation between EF scores and retrieval success activity (RSA) in prefrontal cortex (PFC). (B) Negative correlation between MF scores and RSA in the medial temporal lobe (MTL). (C) Positive correlation between EF scores and fractional anisotropy (FA) values in PFC. (D) Positive correlation between MF scores and FA values in MTL.
We should note that the correlations in PFC and MTL are also driven by negative effects as shown in Figure 2A,B, which seems incompatible with successful compensatory strategies. However, these regions not only show retrieval success effects (hit>miss), but also novelty effects (new>old) involved in memory encoding, which occur relatively automatically (Martin 1999). When an item seems completely unfamiliar, these novelty effects may come to the fore. Thus, a reverse RSA (miss>hit) may be interpreted as novelty response due to a complete lack of familiarity with the item itself, which reflects even worse memory as compared to a source miss.
DTI: Low-Executive OAs Showed Reduced White-Matter Integrity in PFC, Low-Memory OAs in MTL
Consistent with our second prediction, low-executive OAs showed reduced FA in PFC white-matter and low-memory OAs, in MTL white-matter. The RFX analysis with FA as dependent variable and EF and MF scores as independent variables showed significant effects. Low EF scores were associated with low FA in left (MNI xyz = −23, 8, 14; mean cluster T value = 3.54) and right (MNI xyz = 22, 13, 15; mean cluster T value = 3.47) “frontal lobe tracts.” The correlation between EF scores and FA values in these PFC white matter (combined over left and right) was highly significant (R = 0.58, P = 0.00003; Fig. 2C). Similarly, low MF scores were associated with reduced FA in left (MNI xyz = −31, −21, −5; mean cluster T value = 3.40) and right (MNI xyz = 32, −20, −4; mean cluster T value = 3.46) “temporal lobe tracts.” The correlation between MF scores and FA values in these temporal regions (combined over left and right) was also highly significant (R = 0.57; P = 0.000044; Fig. 2D). Critically, we did not find any other white matter region associated with low (or high) executive or memory scores. Thus, low-executive and low-memory scores were selectively associated with PFC and MTL white-matter tracts identified in Figure 2C,D. The fact that we found the same pattern in both hemispheres further attests to the robustness of our findings.
The voxels from the Frontal FA tract predominantly represented the anterior thalamic radiation (63% of all tract voxels) and to a lesser extent the superior longitudinal fasciculus (21%), and the forceps minor (21%). For the MTL FA tract, voxels predominantly represented the inferior longitudinal fasciculus (ILF, 55% of all tract voxels), as well as the uncinate fasciculus (UF, 20%).
Linking DTI and fMRI: In Both PFC and MTL, FA was Negatively Correlated with RSA
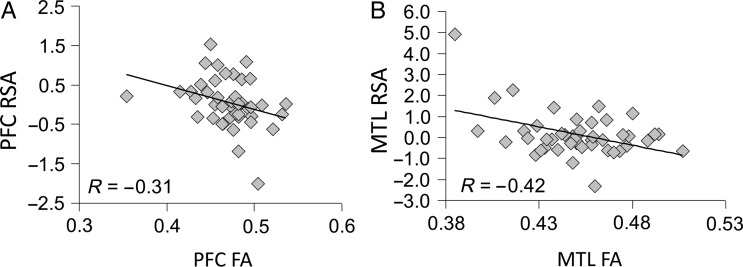
Finally, consistent with our “third prediction,” in both PFC and MTL, the reduction in white-matter integrity (FA) was negatively correlated with the extent of success-related activity (RSA). As illustrated by Figure 3A, FA in the frontostriatal white-matter impaired low-executive OAs (Fig. 2A) was negatively correlated (R = −0.31; P = 0.019) with RSA in the dorsolateral PFC over-recruited by this group (Fig. 2C). Likewise, as shown by Figure 3B, FA in the MTL white-matter region impaired in low-memory OAs (Fig. 2B) was negatively correlated (R = −0.42; P = 0.0021) with RSA in the hippocampal region over-recruited by this group (Fig. 2D). Thus, in line with animal findings of brain connectivity (Barnes and McNaughton 1980; Burke and Barnes 2010), our findings indicate that age-related over-recruitment in specific brain regions, PFC and MTL, reflects successful compensation for reduced white-matter connectivity within corresponding brain circuits, frontal, and temporal respectively (less wiring, more firing).
Figure 3.
(A) Negative correlation between FA values and RSA in PFC. (B) Negative correlation between FA values and RSA in MTL.
Discussion
In the current study, we investigated the hypothesis inspired by rodent electrophysiology data (Barnes and McNaughton 1980; Burke and Barnes 2010) that over-recruitment in OAs may compensate for white matter decline (less wiring, more firing). In our OA participants, we measured executive and memory abilities using a neuropsychological battery, white-matter integrity using DTI, and success-related memory activity using event-related fMRI. The study yielded 3 main findings. First, low-executive OAs showed enhanced RSA in PFC, and low-memory OAs, in MTL. Second, low-executive OAs displayed impaired white matter in PFC, and low-memory OAs, in MTL. Finally, consistent with the less-wiring-more-firing hypothesis, in both PFC and MTL, white matter integrity and RSA were negatively correlated. These 3 findings are discussed in separate sections below.
fMRI: Low-Executive OAs Showed Greater RSA in PFC, Low-Memory OAs, in MTL
Our first finding was that low-executive OAs showed greater success-related activity in PFC activity whereas low-memory OAs showed greater success-related activity in MTL. Executive scores were negatively correlated with RSA in left dorsolateral PFC (Fig. 2A), while memory scores were negatively correlated with RSA in the left hippocampus (Fig. 2B). This finding is consistent with previous fMRI studies showing over-recruitment in low-performing OAs (Bookheimer et al. 2000; Daselaar et al. 2003; Dickerson et al. 2004; Duverne et al. 2009; Wang et al. 2009). In some of these studies, over-recruitment was found in individuals with genetic markers of dementia (e.g., Bookheimer et al. 2000) or mild cognitive impairment (Dickerson et al. 2004), and was interpreted as reflecting compensation for incipient pathology. In contrast, in our study, as well as in other studies (Daselaar et al. 2003; Duverne et al. 2009), the greater activations were observed in OAs within the low-normal range of performance suggesting that the compensatory mechanism is not limited to clinical populations.
Our first finding goes beyond available evidence in 2 important ways. First, we found PFC and MTL over-recruitment not just for overall activity but for success-related activity. Whereas greater overall activity could reflect a response to task demands, the augmented RSA we observed demonstrate an additional contribution of PFC and MTL to successful source memory performance in low-performing OAs. The effect cannot be explained by differences in RTs because the contrast performed within-participants was between hits and misses and the former were faster than for the latter. This finding challenges the common assumption in blocked positron emission tomography (PET) and fMRI studies that over-recruitment in high-performing OAs reflected beneficial processes whereas over-recruitment in low-performing OAs reflected detrimental processes (e.g., dedifferentiation). By combining the study of individual differences with event-related fMRI, we were able to show that over-recruitment in low-performing OAs is not necessarily detrimental and can be actually beneficial for performance in this group.
Second, whereas previous PET/fMRI studies on individual differences among OAs generally focused on a single cognitive dimension, we measured 2 dimensions, executive and memory functions. We found a dissociation between the regions showing over-recruitment, PFC or MTL. Many studies have reported age-related over-recruitment in PFC (e.g., Daselaar et al. 2003; Morcom et al. 2003; Grady et al. 2005) and a few have reported it in MTL (Maguire and Frith 2003; Cabeza et al. 2004; Daselaar et al. 2006). However, this is the first study showing that whether one finds PFC or MTL over-recruitment depends on whether one focuses on individual differences in executive or memory functions. This finding is consistent with a multi-factorial view of neurocognitive aging, which is also supported by our second finding.
Before turning to the second finding, it is worth mentioning that the concept of “compensation” is ambiguous and can lead to incongruent predictions. As compensatory activity should occur in those who need compensation the most, it is reasonable to predict over-recruitment in low-performing OAs (Bookheimer et al. 2000; Daselaar et al. 2003; Dickerson et al. 2004; Duverne et al. 2009; Wang et al. 2009). Yet, since compensatory activity is assumed to enhance performance, it is also reasonable to predict over-recruitment in high-performing OAs (Cabeza et al. 2002; Rosen et al. 2002). To avoid these contradictory predictions, we have proposed the distinction between attempted and successful compensation (Dennis and Cabeza 2012). “Attempted compensation” refers to effort to counteract insufficient neural resources, and hence, it should be greater for low- than high-performing OAs. In contrast, “successful compensation” refers to a positive effect on performance, and hence, it should be greater for successful than unsuccessful trials. We now believe successful compensation should not be defined in terms of individual differences (e.g., Cabeza et al. 2002) because an activation may enhance performance but the effect may not be powerful enough to offset the original deficit and alter the position of an individual within the group. Taking advantage of event-related designs, we believe it is better to define successful compensation in terms of successful versus unsuccessful trials within the same participant. That way it is possible to identify brain regions associated with successful compensation also in low-performing OAs, as in the current study.
DTI: Low-Executive OAs Showed Impaired White-Matter in PFC, low-Memory OAs in MTL
Our second finding was that low-executive OAs showed impaired white matter within PFC, whereas low-memory OAs showed white matter within MTL. Executive scores were positively correlated with FA in a frontostriatal white matter tract (Fig. 2C), while memory scores were positively correlated with FA in MTL tracts (inferior longitudinal fasciculi) in the vicinity of the hippocampus (Fig. 2D). Both the PFC and MTL differences occurred bilaterally, speaking to the robustness of the effects. While there is extensive evidence of white-matter deterioration in OAs (Gunning-Dixon et al. 2009), very little evidence is available regarding the localization of white-matter deficits mediating different aspects of cognitive aging. Most DTI studies of aging have focused on a single cognitive measure, such as executive (e.g., Grieve et al. 2007) or memory function (e.g., Charlton et al. 2010), so comparisons between the white-matter correlates of different cognitive measures are very scarce (Sasson et al. 2012). The DTI tractography study by Davis et al. (2009) found that executive scores in OAs correlated with FA in anterior white-matter regions (e.g., UF, genu of corpus callosum), whereas memory scores correlated with FA in posterior white matter (e.g., posterior section of the ILF, splenium of corpus callosum). The current findings also show an anterior–posterior difference but they localize the memory effects to white-matter regions close the hippocampus.
Similar to our first finding (fMRI), our second finding (DTI) supports a multifactorial view of cognitive aging whereby different forms of age-related cognitive decline and compensation are mediated by different neural mechanisms. This multifactorial view differs from views that emphasize a common factor for age-related cognitive deficits, such as processing speed (Salthouse 1996) or sensory decline (Lindenberger and Baltes 1994). Within the memory domain, our results support a 2-factor model which explains age-related memory deficits in terms of an executive/PFC factor and a memory/MTL factor. As noted before, this 2-factor model has been supported by factor analyses of neuropsychology test batteries (Glisky et al. 1995; Glisky 2007). There is also evidence that a similar 2-factor model can account for memory changes not only in old age but also in childhood, providing a parsimonious view of memory development across the lifespan (Shing et al. 2008).
DTI-fMRI: Impaired White-Matter is Associated with Greater Success-Related Activity
Combining our first 2 findings, our third result was that, both within PFC and within MTL, white-matter deficits were directly associated with success-related activations. Within PFC, FA in a frontostriatal white-matter tract was negatively correlated with RSA in a nearby dorsolateral gray matter region (Fig. 3A). Within MTL, FA in a temporal lobe tract was negatively correlated with RSA in a nearby hippocampal area (Fig. 3B). To our knowledge, this is the first neuroimaging evidence directly linking impaired white matter and greater activity in OAs.
Our third finding is consistent with rodent electrophysiology evidence that an age-related decrease in the number of afferent fibers is coupled with greater neural firing in the remaining synapses (Barnes and McNaughton 1980; Burke and Barnes 2010). A reasonable interpretation of this finding is that neurons compensate for an age-related loss of local connectivity with an increase in synaptic responsiveness (i.e., less wiring, more firing). Extrapolating this idea to system-level measures in humans, we proposed the hypothesis that greater activity in older OAs may compensate for a decline in white-matter integrity. Our third finding provides direct support to this hypothesis: greater activations occurred not only in close proximity to impaired white-matter regions but the 2 effects were negatively correlated.
Although our finding is broadly consistent with the results from rodent studies (Barnes and McNaughton 1980; Burke and Barnes 2010), it is important to acknowledge that human neuroimaging and animal electrophysiology measure neural activity and connectivity at very different scales. In terms of neural activity, fMRI provides measure blood flow changes associated with activity of hundreds of neurons, whereas single-cell recording can measure spikes in individual neurons (Logothetis 2008). Regarding connectivity, DTI provides a measure of the integrity of white-matter tracts between different brain regions, whereas staining techniques in rodent studies allow visualization of direct fiber connections between individual neurons. It is also important to acknowledge our limited knowledge of how greater firing of a reduced number of fibers translates into changes in fMRI activity. Although we assumed a net increase in fMRI activity, it is unclear whether the reduced number of fibers would offset the greater firing resulting in no net change in fMRI activity. However, we do find a clear negative coupling between FA and fMRI values, which supports the less firing, more wiring idea. The gap between human neuroimaging and rodent electrophysiology could be closed in the future by animal neuroimaging studies. At any rate, our finding converges with the rodent electrophysiology data on the basic idea that age-related connectivity deficits may lead to a compensatory increase in neuronal activity. Future studies could investigate whether connectivity deficits can be ameliorated by enhancing neurotransmission using drugs known to modulate age-related changes in brain activity.
Conclusion
In summary, in the current study, we investigated the hypothesis inspired by rodent electrophysiology that over-recruitment in OAs may compensate for white matter decline (less wiring, more firing). We measured executive and memory abilities in our OA participants using a neuropsychological battery, linked executive, and memory scores to white-matter integrity measured with DTI and to success-related memory activity measured using event-related fMRI. The study yielded 3 main findings. First, low-executive OAs showed enhanced success-related activity in PFC, and low-memory OAs, in MTL. Second, low-executive OAs displayed impaired white matter in PFC, and low-memory OAs, in MTL. Finally, consistent with the less-wiring–more-firing hypothesis, in both PFC and MTL, white matter integrity and success-related activity were negatively correlated. This hypothesis provides a testable account of the unknown neural mechanisms for the common finding of greater activations in OAs.
Funding
This work was supported by the National Institutes of Health (NIH) (grant numbers R01 AG019731 and R01 AG23770 awarded to R.C. and grant number F32 AG029738 awarded to S.M.H.), the Department of Veterans Affairs (VA), Rehabilitation Research & Development Service (grant number E7822W awarded to S.M.H.), and the National Science Foundation (NSF) Graduate Research Fellowship (grant number 110640 awarded to V.I.). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH, VA, or NSF.
Notes
The authors thank Elsa Baena, Michael White, and Odera Umeano for assistance with data collection and Norbou Buchler for contributions to study design. Conflict of Interest: None declared.
References
- Aggleton JP, O'Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31:2292–2307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33:153–161. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037/0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Nathan PJ, Hammond G, Robbins TW, Hodges JR, Michael A, Semple JM, Bullmore ET, Sahakian BJ. Differential cognitive deterioration in dementia: a two year longitudinal study. J Alzheimers Dis. 2011;24:125–136. doi: 10.3233/JAD-2010-100450. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, Markus HS, Morris RG. The relationship between episodic long-term memory and white matter integrity in normal aging. Neuropsychologia. 2010;48:114–122. doi: 10.1016/j.neuropsychologia.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Daselaar S, Cabeza R. 3.28—Episodic memory decline and healthy aging. In: John HB, editor. Learning and memory: a comprehensive reference oxford. Academic Press; 2008. pp. 577–599. [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. NeuroImage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, Pantelis C. Normative data from the CANTAB. I: development of executive function over the lifespan. J Clin Exp Neuropsychol. 2003;25:242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Dennis N, Cabeza R. Frontal lobes and aging: deterioration and compensation. In: Stuss D, Knight R, editors. Principles of frontal lobe function. 2nd ed. New York: Oxford University Press; 2012. [Google Scholar]
- de Rover M, Pironti VA, McCabe JA, Acosta-Cabronero J, Arana FS, Morein-Zamir S, Hodges JR, Robbins TW, Fletcher PC, Nestor PJ, et al. Hippocampal dysfunction in patients with mild cognitive impairment: a functional neuroimaging study of a visuospatial paired associates learning task. Neuropsychologia. 2011;49:2060–2070. doi: 10.1016/j.neuropsychologia.2011.03.037. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb Cortex. 2009;19:733–744. doi: 10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, editor. Brain aging: models, methods, and mechanisms. Florida: CRC Press; 2007. [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. doi: 10.1037/0894-4105.9.2.229. [DOI] [Google Scholar]
- Grady CL, McIntosh AR, Craik FIM. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. Am J Neuroradiol. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Dubno JR, Keren NI, Ahlstrom JB, Eckert MA. Speech recognition in younger and older adults: a dependency on low-level auditory cortex. J Neurosci. 2009;29:6078–6087. doi: 10.1523/JNEUROSCI.0412-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Kučera H, Francis WN. Providence, RI: Brown University Press; 1967. Computational analysis of present-day American English. [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037/0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126:1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Martin A. Automatic activation of the medial temporal lobe during encoding: lateralized influences of meaning and novelty. Hippocampus. 1999;9:62–70. doi: 10.1002/(SICI)1098-1063(1999)9:1&lt;62::AID-HIPO7&gt;3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Mather M, Johnson MK, Leonardis DMD. Stereotype reliance in source monitoring: age differences and neuropsychological test correlates. Cogn Neuropsychol. 1999;16:437–458. doi: 10.1080/026432999380870. [DOI] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prull MW, Dawes LL, Martin AM, 3rd, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: adult age differences and neuropsychological test correlates. Psychol Aging. 2006;21:107–118. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, Yesavage JA, Gabrieli JD. Variable effects of aging on frontal lobe contributions to memory. Neuroreport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295X.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y. Structural correlates of cognitive domains in normal aging with diffusion tensor imaging. Brain Struct Funct. 2012;217:503–515. doi: 10.1007/s00429-011-0344-7. [DOI] [PubMed] [Google Scholar]
- Saunders NL, Summers MJ. Attention and working memory deficits in mild cognitive impairment. J Clin Exp Neuropsychol. 2010;32:350–357. doi: 10.1080/13803390903042379. [DOI] [PubMed] [Google Scholar]
- Shing YL, Werkle-Bergner M, Li SC, Lindenberger U. Associative and strategic components of episodic memory: a life-span dissociation. J Exp Psychol Gen. 2008;137:495–513. doi: 10.1037/0096-3445.137.3.495. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010;20:2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage. 2003;18:293–309. doi: 10.1016/S1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Wang TH, Kruggel F, Rugg MD. Effects of advanced aging on the neural correlates of successful recognition memory. Neuropsychologia. 2009;47:1352–1361. doi: 10.1016/j.neuropsychologia.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Proffitt T, Mahony K, Smith DJ, Buchanan JA, Brewer W, Stuart GW, Velakoulis D, McGorry PD, Pantelis C. Visuospatial memory and learning in first-episode schizophreniform psychosis and established schizophrenia: a functional correlate of hippocampal pathology? Psychol Med. 2002;32:429–438. doi: 10.1017/s0033291702005275. [DOI] [PubMed] [Google Scholar]